Abstract
Allogeneic bone marrow transplantation (BMT) induces 2 closely associated immune responses: graft-versus-tumor (GVT) activity and graft-versus-host disease (GVHD). We have previously shown that pretransplant immunization of allogeneic BMT donors with a recipient-derived tumor cell vaccine increases both GVT activity and lethal GVHD because of the priming of donor T cells against putative minor histocompatibility antigens (mHAgs) on the tumor vaccine cells. The work reported here tested the hypothesis that tumor cell vaccination after BMT would produce an increase in GVT activity without exacerbating GVHD. C3H.SW donor bone marrow and splenocytes were transplanted into major histocompatibility complex-matched, mHAg-mismatched C57BL/6 recipients. One month after BMT, recipients were immunized against either a C57BL/6 myeloid leukemia (C1498) or fibrosarcoma (205). Immunized recipients had a significant increase in survival and protection against tumor growth in both tumor models, and significant tumor protection was seen even in recipients with preexisting micrometastatic cancer before immunization. Alloreactivity appeared to contribute to the in vitro anti-tumor cytolytic activity, but in vivo immunity was tumor specific, and no exacerbation of GVHD was observed. Although the immunodominant mHAg B6dom1 was shown to be expressed by all B6 tumors tested and was largely responsible for the alloreactivity resulting from tumor immunization of donors, the in vitro alloreactivity of immune recipients was more restricted and was not mediated by recognition of B6dom1. In conclusion, post-transplant tumor immunization of allogeneic BMT recipients against either a leukemia or a solid tumor can increase GVT activity and survival without exacerbating GVHD.
Allogeneic bone marrow transplantation (BMT) is associated with an unequivocal graft-versus-tumor (GVT) immune response mediated by donor T cells, but the benefit of GVT activity is often offset by graft-versus-host disease (GVHD), a potentially fatal immune response caused primarily by mature donor T cells attacking normal cells in the recipient.1 Although it is commonly believed that the target antigens of GVHD in MHC-matched BMT are minor histocompatibility antigens (mHAgs), only a few have been identified because of their polymorphic and heterogeneous nature.2,3 Similarly the target antigens for GVT activity are unknown. In theory they could include some or all of the following categories: (i) ubiquitously expressed immunodominant host mHAgs that are the targets of GVHD, (ii) ubiquitously expressed nonimmunodominant host mHAgs, (iii) tissue-restricted host mHAgs not expressed in physiologically critical GVHD target organs, (iv) differentiation stage- and tissue-restricted host mHAgs, or (v) tumor-specific antigens. The simplest hypothesis that would explain “classical” graft versus tumor activity that occurs without specific immune manipulation of either donor or host is that the targets are ubiquitous, immunodominant mHAgs. However, at present it is unclear to what extent the donor T-cell populations mediating GVT activity and GVHD overlap or are identical in terms of either antigen specificity or effector mechanism 2 3
Previous work in our laboratory has shown that recipient-derived tumor cell vaccines given to donors before BMT cause not only increased GVT activity but also lethal GVHD,4 which we postulated was caused by the recognition of immunodominant mHAgs expressed by tumor cells and target organs of GVHD. Nevertheless, both clinical and experimental evidence suggests that some of the effector cells mediating GVT activity may be distinct from those mediating GVHD.5-9
Unlike solid organ transplantation, BMT does not require life-long immunosuppression. This is most likely because of the development of unresponsiveness or tolerance to immunodominant mHAgs after BMT. If the spectrum of antigens on GVHD target tissues and tumor cells are not identical, it is conceivable that in this setting tumor cell vaccines could expand the donor-derived T-cell populations recognizing antigens on tumor cells (eg, theoretical antigen categories ii to v), but not those unresponsive or absent populations that recognize immunodominant mHAgs present on most normal cells. Therefore, the experiments described in this study tested the hypothesis that post-transplant immunization of BMT recipients with a tumor cell vaccine would substantially increase “nonclassical” donor GVT activity and extend the survival of BMT recipients without exacerbating GVHD.
Materials and methods
Animals
Female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD), and female C3H.SW-H2b/SnJ (C3H.SW) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). They were used for experiments at 6 to 12 weeks of age. Mice were housed in conventional rooms with food and water ad libitum. From 2 or 3 days before BMT until day 14, water was acidified (pH 2.5) and supplemented with 2 g/L neomycin sulfate (Sigma, St Louis, MO).
Cell lines
The cell line 205 is a weakly immunogenic methylcholanthrene-induced C57BL/6 fibrosarcoma cell line.10 This tumor is not spontaneously metastatic but reproducibly forms multiple lung nodules when at least 1 × 104 cells are injected intravenously into C57BL/6 mice. 205IL-2/TK is a 205 cell line modified to express both the interleukin-2 (IL-2) gene and the herpes simplex virus thymidine kinase (TK) suicide gene using the LXSN and pBabePuro retroviral vectors, respectively. The pBabePuro vector has a puromycin resistance gene,11 and the LXSN vector contains a neomycin resistance gene.12 Transduced cells were selected in 2.5 μg/mL puromycin, 1 mg/mL G418, or both. C1498 is a C57BL/6 myelomonocytic leukemia cell line of spontaneous origin (ATCC, Rockville, MD). When injected intravenously, 1 × 104 cells is a near-uniformly lethal dose of cells; animals die with gross evidence of leukemia, including hepatomegaly and splenomegaly. Both 205 and C1498 express major histocompatibility complex (MHC) class I but not MHC class II. B16F10 is a spontaneous, weakly immunogenic C57BL/6 melanoma cell line (a gift from Dr I. J. Fidler, M.D. Anderson Cancer Center). EL4 is a C57BL/6 lymphoma cell line (ATCC). P815 is a lymphokine activated killer (LAK)-sensitive DBA/2 mastocytoma cell line (ATCC). Yac-1 is a natural killer (NK)-sensitive A/Sn lymphoma cell line (ATCC). Cells were grown in tissue culture using RPMI-1640 (RPMI) supplemented with 5% heat-inactivated fetal bovine serum (Biowhittaker, Walkersville, MD), and 2 mmol/L L-glutamine.
BMT recipient immunization
205IL-2/TK + ganciclovir immunization.
We have shown that ganciclovir-mediated ablation of live 205IL-2/TK cells induces systemic immunity to unmodified 205 in C57BL/6 mice13 and in C3H.SW donors.4 In the initial experiments recipients were injected subcutaneously in the flank with 3 to 5 × 106 live 205IL-2/TK cells in 0.2 mL Hank's balanced salt solution (HBSS) 1 month after BMT and then received 1 mg ganciclovir intraperitoneally in 0.2 mL phosphate-buffered saline daily for 1 week starting 3 days after the cell injection. Subsequent vaccines were given as above at 2-week intervals.
Irradiated 205IL-2/TK or 205 immunization.
Some recipients (and control C3H.SW donors) were injected subcutaneously in the flank with 3 to 5 × 106irradiated 205IL-2/TK or 205 cells in 0.2 mL HBSS 1 month after BMT. Vaccines were given at 1-week intervals.
C1498 tumor immunization.
Mice were injected subcutaneously in the flank with 10 × 106 irradiated C1498 cells in 0.2 mL HBSS starting 1 month after BMT. Vaccines were given at a 1-week interval unless the vaccine was given at the time of intravenous leukemia challenge, which was 10 days after the previous vaccine.
In vivo tumor inoculation
Micrometastatic lung tumors were established by injecting C57BL/6 mice with 1 × 105 205 tumor cells intravenously in 0.2 mL HBSS. C1498 leukemia was established by injection of 1 to 1.5 × 104 cells intravenously. The timing of tumor challenge in relation to the vaccine schedule is specified in the text and legends.
Bone marrow transplantation
BMT recipients received 850 cGy total body irradiation using a60Co source 1 day before BMT. On the day of BMT, 2 to 4 × 106 bone marrow cells and 5 to 10 × 106 spleen cells were injected intravenously together in a total volume of 0.2 mL HBSS. Bone marrow was isolated from donors by flushing each femur and tibia with RPMI. Spleen cells were isolated by macerating the spleens between 2 frosted glass slides, followed by lysis of erythrocytes. Fewer than 5% of recipients were excluded from these studies before the initiation of immunization because of signs of severe GVHD.
Enumeration of pulmonary tumor nodules
After death, lungs from mice injected with 205 tumor were stained black by suffusion with India ink instilled through the trachea. Lungs were fixed, and white tumor nodules on the black lung surface were counted without magnification.
Evaluation of GVHD
Recipients were weighed weekly and observed daily for signs of GVHD (weight loss, alopecia, dermatitis, hunched posture, and death). In some experiments histologic examination of livers for GVHD was performed. Liver sections stained with hematoxylin and eosin were examined for characteristic mononuclear cell infiltrates in portal triads.
Cytotoxicity assays
For use as effector cells, spleen cells were cultured in 6-well plates at 1 × 106 cells/mL and 10 mL/well in RPMI supplemented with 10% FBS (Summit Biotech, Fort Collins, CO), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 100 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acids, and 50 μmol/L 2-mercaptoethanol (complete medium). In assays for alloreactivity, 30 Gy-irradiated C57BL/6 spleen cells or B6dom1 peptide-pulsed C3H.SW spleen cells were used as stimulators at a concentration of 1 × 106 cells/mL or 1.25 × 105cells/mL, respectively. Peptide-pulsed stimulators were incubated for 1 hour at 37°C with 35 μg/mL B6dom1 peptide (AAPDNRETF, synthesized by the Peptide Synthesis Core Laboratory of M.D. Anderson Cancer Center). In assays for combined tumor-reactivity and alloreactivity, 250 Gy-irradiated 205 tumor cells or 100 Gy-irradiated C1498 leukemia cells were used as stimulators at a concentration of 5 × 103 cells/mL or 5 × 104cells/mL, respectively. After 4 to 5 days in culture with the appropriate stimulator cells, effector cells were harvested and plated in triplicate with 5 × 10351Cr-labeled target cells per well at effector:target ratios ranging from 200:1 to 12.5:1. Target cells were labeled by combining 5 × 106 cells in 0.1 mL complete medium with 20 μL FBS (Summit) and 0.1 mL (approximately 100μCi) sterile isotonic Na251CrO (Amersham, Arlington Heights, IL) for 60 minutes at 37°C. Concanavalin A (ConA) lymphoblast (CAB) targets were generated by stimulating C57BL/6 or C3H.SW spleen cells for 2 days with 2 μg/mL. ConA at 2 × 106 cells/mL in complete medium. They were labeled with 51Cr as above for 45 minutes. Some C3H.SW CAB were loaded with B6dom1 peptide by incubation for 90 minutes with 40 μg peptide (45 minutes before adding 51Cr plus 45 minutes after adding 51Cr). Labeled targets were washed 3 times before plating with effectors in a total volume of 0.2 mL/well in 96-well round-bottom plates. Plated cells were incubated for 4 hours at 37°C, after which 0.1 mL supernatant was counted in a gamma counter (Wallac, San Francisco, CA). Percentage lysis was calculated as 100 × (experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)]. Spontaneous release was usually less than 20% and always less than 30% of the maximum release.
Statistical analysis
Prism 3.0 software (GraphPad Software for Scientists, Sorrento, CA) was used for statistical evaluation of data. When more than 2 groups of lung nodules were compared, a nonparametric 1-way analysis of variance (Kruskal-Wallis test) was performed. If P < .05 overall, then the groups were compared using a Dunn's multiple comparison test. When only 2 groups were compared, a Mann-Whitney U test was used. To compare numbers of mice with no lung nodules, the Fisher exact test was used. To compare Kaplan-Meier survival curves, the log-rank test was used.
Results
Tumor immunization of allogeneic BMT recipients increases GVT activity
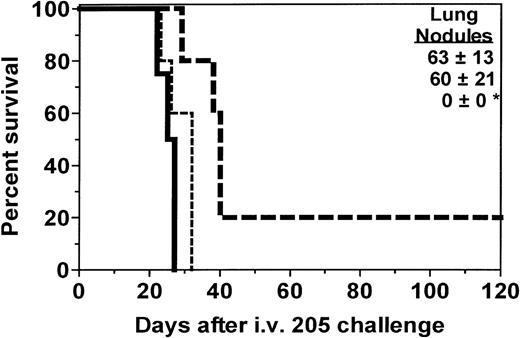
Experiments were conducted to test the hypothesis that tumor immunization of allogeneic BMT recipients could protect against the growth of metastatic cancer in BMT recipients. We first assessed the kinetics of immune function recovery and found that recipients in this model could not mount a detectable cytolytic immune response at 2 weeks after BMT but began responding to tumor cell antigens by 1 month after BMT and began responding to influenza nucleoprotein antigen by 5 weeks after BMT, as assessed by in vitro cytotoxicity assays (data not shown). Therefore, in the experiments described in this study, we initiated vaccination at least 1 month after BMT. C3H.SW→ C57BL/6 recipients in the initial experiments were immunized with 205IL-2/TK cells plus ganciclovir. As seen in Figure 1, recipients immunized only a single time were not protected against tumor growth, but those immunized again at the time of tumor challenge had complete protection against growth of pulmonary tumor nodules (P < .05) and significantly enhanced survival (P = .0040) compared to nonimmunized recipients. All deaths among recipients immunized twice resulted from growth of a nonpulmonary metastasis. Nonpulmonary metastases usually consisted of tumors in the connective tissue of the sacral/pelvic/paraspinal region or ovary. Nonpulmonary tumors also grew in some nonimmunized recipients.
Immunization of BMT recipients increases survival and GVT activity.
One month after BMT, SW→B6 recipients were immunized with 205IL-2/TK cells and ganciclovir. Two weeks after the first vaccine, micrometastases were established by intravenous injection of 1 × 105 205 tumor cells, at which time 1 group of recipients received a second vaccine. Lung nodules were counted at the time of death or after day 100 for each recipient. The deaths that occurred in the recipient group immunized twice resulted from the growth of a nonpulmonary metastasis (sacral/pelvic mass) that necessitated sacrifice. Groups and sizes were: no vaccine, n = 4; 1 vaccine, n = 5; and 2 vaccines, n = 5. *P < .05 for lung nodules compared to 1 or no vaccine controls. **P = .004 compared to nonimmunized recipient survival.
Immunization of BMT recipients increases survival and GVT activity.
One month after BMT, SW→B6 recipients were immunized with 205IL-2/TK cells and ganciclovir. Two weeks after the first vaccine, micrometastases were established by intravenous injection of 1 × 105 205 tumor cells, at which time 1 group of recipients received a second vaccine. Lung nodules were counted at the time of death or after day 100 for each recipient. The deaths that occurred in the recipient group immunized twice resulted from the growth of a nonpulmonary metastasis (sacral/pelvic mass) that necessitated sacrifice. Groups and sizes were: no vaccine, n = 4; 1 vaccine, n = 5; and 2 vaccines, n = 5. *P < .05 for lung nodules compared to 1 or no vaccine controls. **P = .004 compared to nonimmunized recipient survival.
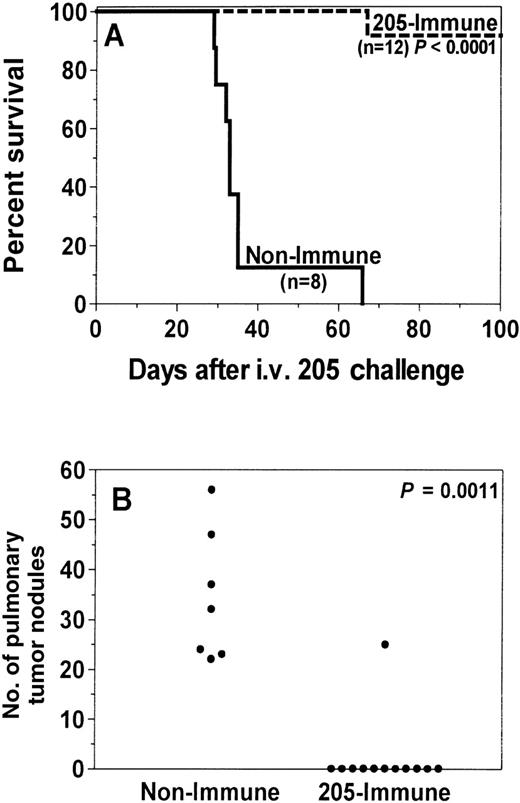
Because of evidence of greater efficacy with multiple vaccine treatments, recipients in subsequent experiments were immunized 4 times. Recipients immunized 4 times with irradiated 205-IL-2/TK cells had markedly extended survival (P < .0001; Figure2A) and often had complete prevention of lung nodule growth (P = .0011; Figure 2B). Furthermore, 92% (11 of 12) survived more than 100 days without signs of tumor or GVHD. Recipients immunized 4 times with irradiated, unmodified 205 cells also had enhanced survival (P = .0016; Figure3A) and significant reduction of lung nodules (P = .0022; Figure 3B). Fifty percent (3 of 6) survived more than 100 days, and 83% (5 of 6) were completely protected against lung nodules.
Recipient immunization with an irradiated tumor cell vaccine increases GVT activity and prevents death from tumor.
One month after C3H.SW→C57BL/6 BMT, recipients (205-immune, n = 12) were immunized with 50 Gy-irradiated 205IL-2/TK cells. Control recipients (nonimmune, n = 8) were not immunized. Ten days after the first vaccine, micrometastases were established in all recipients by intravenous injection of 1 × 105 205 tumor cells, and 205-immune recipients received 3 more vaccines at weekly intervals. Lung nodules were counted after death or after day 100 for each recipient; pulmonary nodule counts were not available for 1 mouse in the nonimmune group because the carcass was severely cannibalized before autopsy. 205-immune recipients had significantly enhanced survival (A, P < .0001) and a significant reduction of lung nodules (B, P = .0011) compared to nonimmune recipients. Results were pooled from 2 independent experiments.
Recipient immunization with an irradiated tumor cell vaccine increases GVT activity and prevents death from tumor.
One month after C3H.SW→C57BL/6 BMT, recipients (205-immune, n = 12) were immunized with 50 Gy-irradiated 205IL-2/TK cells. Control recipients (nonimmune, n = 8) were not immunized. Ten days after the first vaccine, micrometastases were established in all recipients by intravenous injection of 1 × 105 205 tumor cells, and 205-immune recipients received 3 more vaccines at weekly intervals. Lung nodules were counted after death or after day 100 for each recipient; pulmonary nodule counts were not available for 1 mouse in the nonimmune group because the carcass was severely cannibalized before autopsy. 205-immune recipients had significantly enhanced survival (A, P < .0001) and a significant reduction of lung nodules (B, P = .0011) compared to nonimmune recipients. Results were pooled from 2 independent experiments.
Recipient immunization with irradiated unmodified 205 cells increases GVT activity and prevents death from tumor.
One month after C3H.SW→ C57BL/6 BMT, recipients (205-immune, n = 6) were immunized with 50 Gy-irradiated unmodified 205 cells. Control recipients (nonimmune, n = 6) were not immunized. One week after the first vaccine, micrometastases were established in all recipients by intravenous injection of 1 × 105 205 tumor cells, and 205-immune recipients received 3 more vaccines at weekly intervals. Lung nodules were counted after death or after day 100 for each recipient. 205-immune recipients had significantly enhanced survival (A, P = .0016) and significant reduction of lung nodules (B,P = .0022) compared to nonimmune recipients.
Recipient immunization with irradiated unmodified 205 cells increases GVT activity and prevents death from tumor.
One month after C3H.SW→ C57BL/6 BMT, recipients (205-immune, n = 6) were immunized with 50 Gy-irradiated unmodified 205 cells. Control recipients (nonimmune, n = 6) were not immunized. One week after the first vaccine, micrometastases were established in all recipients by intravenous injection of 1 × 105 205 tumor cells, and 205-immune recipients received 3 more vaccines at weekly intervals. Lung nodules were counted after death or after day 100 for each recipient. 205-immune recipients had significantly enhanced survival (A, P = .0016) and significant reduction of lung nodules (B,P = .0022) compared to nonimmune recipients.
We next tested the influence of post-BMT vaccines in a second tumor model, the C57BL/6 C1498 model of acute myelogenous leukemia. Using the immunization method shown by Boyer et al14 to induce a T-cell-mediated immune response in syngeneic C57BL/6 mice, C3H.SW → C57BL/6 BMT recipients were immunized 4 times with irradiated C1498 cells. Immunization significantly extended survival (P < .0001; Figure 4) compared to nonimmunized recipients, and 71% (5 of 7) of recipients achieved long-term survival for more than 100 days without signs of leukemia or GVHD. In all recipients that died, there was gross evidence of leukemia, including hepatomegaly, but not GVHD.
Tumor immunization of BMT recipients increases GVT activity against leukemia.
One month after BMT, SW→ B6 recipients (C1498-immune, n = 8) were immunized twice with irradiated C1498 leukemia cells at a 1-week interval. Control recipients (nonimmune, n = 8) were not immunized. 10 days after the 2nd vaccine, 1 × 104 C1498 cells were injected intravenously to simulate relapse after BMT, and C1498-immune recipients were immunized twice more at a 1-week interval. Immunization significantly enhanced survival (P < .0001) compared to nonimmune control recipients.
Tumor immunization of BMT recipients increases GVT activity against leukemia.
One month after BMT, SW→ B6 recipients (C1498-immune, n = 8) were immunized twice with irradiated C1498 leukemia cells at a 1-week interval. Control recipients (nonimmune, n = 8) were not immunized. 10 days after the 2nd vaccine, 1 × 104 C1498 cells were injected intravenously to simulate relapse after BMT, and C1498-immune recipients were immunized twice more at a 1-week interval. Immunization significantly enhanced survival (P < .0001) compared to nonimmune control recipients.
GVT activity is induced by tumor cell vaccines in BMT recipients with preexisting micrometastatic cancer
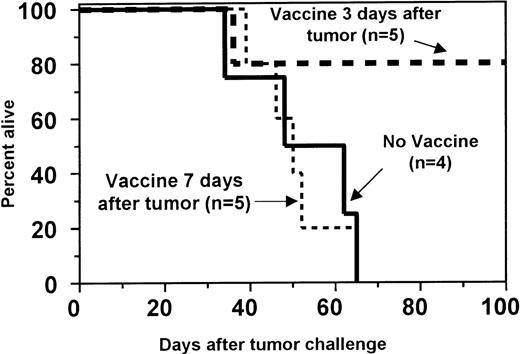
Both 205 and C1498 tumors progress rapidly in vivo, often leading to death within 1 month. Because the induction of primary T-cell responses usually does not occur until 7 to 10 days after the administration of vaccines, we predicted that vaccine efficacy would be reduced in mice with established, progressively growing tumors. Indeed, in mice inoculated with C1498 leukemia cells 1 week before immunization, survival was not prolonged by tumor vaccination (Figure 5). However, in recipients with C1498 leukemia established 3 days before the initiation of vaccination, survival was significantly increased compared to nonimmunized recipients (P = .0351; Figure 5). In the 205 tumor model, recipients that began vaccine treatment 3 days after the establishment of 205 metastases had a significant reduction in pulmonary tumor nodules (P = .0140, Figure6). In a separate experiment in which long-term survival was the endpoint, the observed increase in median survival after 205 tumor challenge (from 31 to 41 days) was not statistically significant (data not shown). Death in these vaccine-treated animals resulted from growth of either nonpulmonary tumors or progressive growth of a small number of pulmonary nodules.
Tumor immunization of BMT recipients increases GVT activity against preexisting leukemia.
One month after BMT, SW→ B6 recipients were challenged intravenously with 1.5 × 104 C1498 cells to induce preexisting leukemia. Recipients were then either not immunized (no vaccine; solid line) or immunized 4 times with 10 × 106 irradiated C1498 leukemia cells at a 1-week interval starting 3 days (day 3 vaccine; thick dashed line) or 7 days (day 7 vaccine; thin dashed line) after tumor challenge. Although immunization beginning on day 7 did not improve survival, immunization beginning on day 3 significantly enhanced survival (P = .0351) compared to the nonimmune controls.
Tumor immunization of BMT recipients increases GVT activity against preexisting leukemia.
One month after BMT, SW→ B6 recipients were challenged intravenously with 1.5 × 104 C1498 cells to induce preexisting leukemia. Recipients were then either not immunized (no vaccine; solid line) or immunized 4 times with 10 × 106 irradiated C1498 leukemia cells at a 1-week interval starting 3 days (day 3 vaccine; thick dashed line) or 7 days (day 7 vaccine; thin dashed line) after tumor challenge. Although immunization beginning on day 7 did not improve survival, immunization beginning on day 3 significantly enhanced survival (P = .0351) compared to the nonimmune controls.
Recipient immunization with a tumor cell vaccine increases GVT activity against preexisting 205 tumor.
One month after SW→ B6 BMT, micrometastatic fibrosarcoma was established by intravenous injection of 1 × 105 205 tumor cells. Recipients were either not immunized (nonimmune) or immunized 4 times with 50 Gy-irradiated 205IL-2/TK cells (205-immune) at a weekly interval starting on day 3 after challenge. Lung nodules were counted 1 month after tumor challenge. The day 3 205-immune group had a significant reduction in lung nodules (P = .0140) compared to the nonimmune recipients.
Recipient immunization with a tumor cell vaccine increases GVT activity against preexisting 205 tumor.
One month after SW→ B6 BMT, micrometastatic fibrosarcoma was established by intravenous injection of 1 × 105 205 tumor cells. Recipients were either not immunized (nonimmune) or immunized 4 times with 50 Gy-irradiated 205IL-2/TK cells (205-immune) at a weekly interval starting on day 3 after challenge. Lung nodules were counted 1 month after tumor challenge. The day 3 205-immune group had a significant reduction in lung nodules (P = .0140) compared to the nonimmune recipients.
Tumor immunization of allogeneic BMT recipients does not exacerbate GVHD
Recipients surviving tumor challenge in the above experiments did not develop signs of acute GVHD (eg, weight loss, fur loss, diarrhea, dermatitis, or histologic evidence of GVHD in the liver). This suggested that tumor cell vaccines administered after BMT did not increase GVHD. To test this hypothesis directly, we also conducted 3 experiments in which recipients were immunized 4 times against 205 but were not challenged with tumor. All immunized recipients in both experiments (n = 16) survived more than 100 days without signs of GVHD, including weight loss.
GVT activity induced by tumor immunization of recipients is tumor-specific in vivo
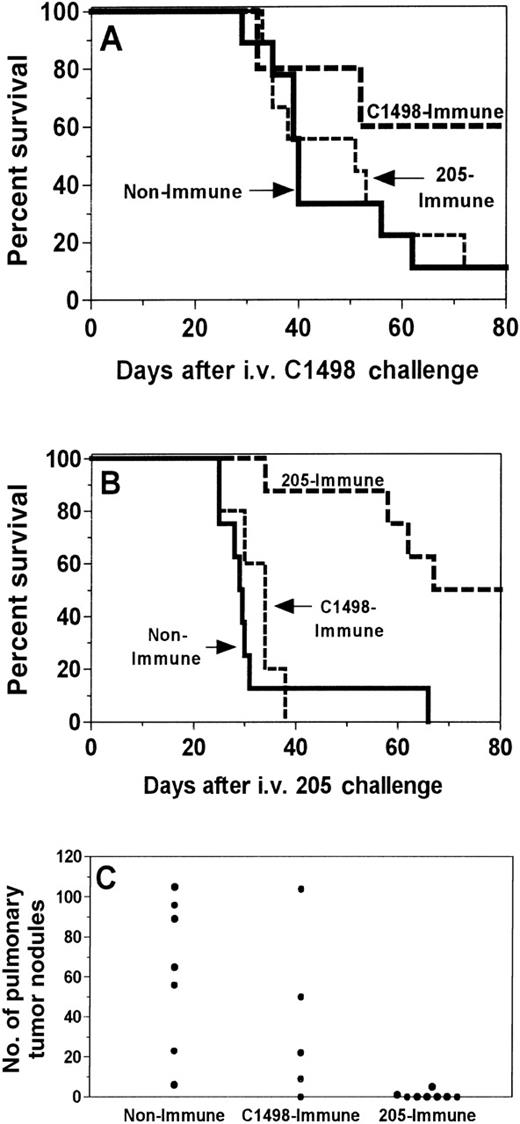
We have previously shown that the GVT activity induced by tumor immunization of allogeneic BMT donors is mediated by alloreactive T cells that cross-react broadly with recipient tumors and nonmalignant tissues.4 Therefore, experiments were performed to determine whether recipient immunization would induce alloreactivity. If so, tumor vaccination should induce cross-protection against another immunologically unrelated recipient tumor. Recipients were either not immunized (nonimmune) or immunized against 205 (205-immune) or C1498 (C1498-immune) and then challenged with either 205 or C1498. As seen in Figure7, the GVT activity of 205-immune recipients was tumor specific. The 205 vaccine again induced protection against 205 (P = .0006; Figure 7B) but did not protect against C1498 challenge compared to nonimmune controls (Figure 7A). Similarly, vaccination against C1498 increased survival after challenge with C1498 immunization (Figure 7A) but not after challenge with 205 (Figure 7B). Reduction in 205 pulmonary metastases was seen with the use of 205 vaccines but not with the use of C1498 vaccine (Figure 7C).
GVT activity after tumor immunization of BMT recipients is tumor-specific in vivo.
One month after BMT, SW→ B6 recipients were immunized twice with irradiated C1498 leukemia cells (C1498-immune) or were irradiated 205IL-2/TK cells (205-immune) at a 1-week interval. Control recipients (nonimmune) were not immunized. Ten days after the 2nd vaccine, 1.5 × 104 C1498 cells (A) or 1 × 105 205 cells (B, C) were injected intravenously to simulate relapse after BMT, and immune recipients were immunized twice more at 1-week intervals. Lung nodules were counted in the 205-challenged mice at the time of death or after 100 days. Although tumor immunization against either 205 or C1498 induced protection (P < .05) against the immunizing cell type, it did not induce cross-protection against the other tumor (P > .05 for both survival tests and lung nodules). Results were pooled from 2 independent experiments.
GVT activity after tumor immunization of BMT recipients is tumor-specific in vivo.
One month after BMT, SW→ B6 recipients were immunized twice with irradiated C1498 leukemia cells (C1498-immune) or were irradiated 205IL-2/TK cells (205-immune) at a 1-week interval. Control recipients (nonimmune) were not immunized. Ten days after the 2nd vaccine, 1.5 × 104 C1498 cells (A) or 1 × 105 205 cells (B, C) were injected intravenously to simulate relapse after BMT, and immune recipients were immunized twice more at 1-week intervals. Lung nodules were counted in the 205-challenged mice at the time of death or after 100 days. Although tumor immunization against either 205 or C1498 induced protection (P < .05) against the immunizing cell type, it did not induce cross-protection against the other tumor (P > .05 for both survival tests and lung nodules). Results were pooled from 2 independent experiments.
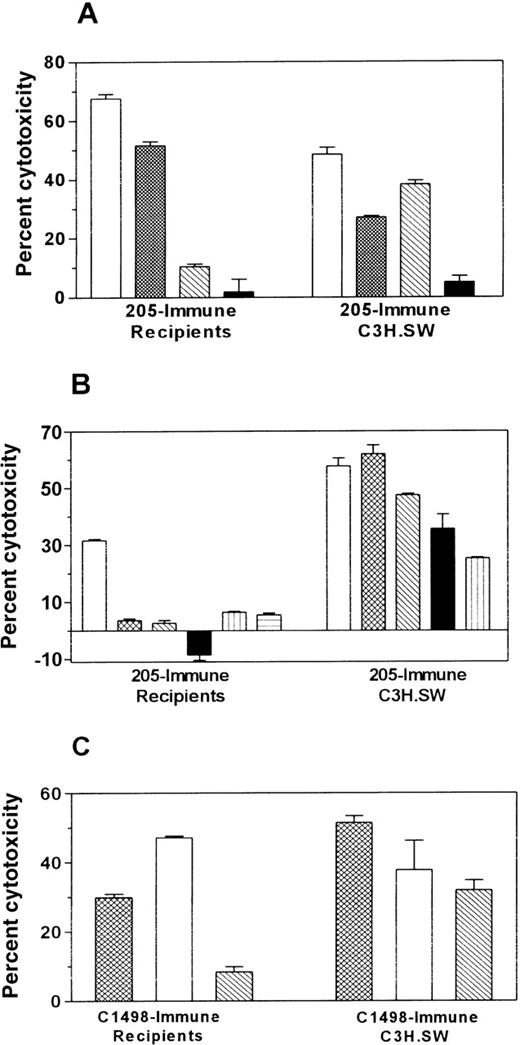
Tumor-immunized allogeneic BMT recipients exhibit in vitro cytolytic antitumor activity but only limited alloreactivity
To evaluate further the specificity of the antitumor response, in vitro cytotoxicity assays were performed. Splenocytes from immunized BMT recipients or control immunized donors (C3H.SW) were stimulated in vitro with tumor cells and tested for their ability to kill various target cells (Figure 8). Immunization of C3H.SW donors with either 205 or C1498 vaccines generated broadly alloreactive CTL activity, lysing all C57BL/6 targets. In contrast, BMT recipients immunized with 205 or C1498 generated cytotoxic cells with a different target specificity profile. In 3 independent experiments, BMT recipients generated cytotoxic cells that killed the immunizing tumor, but cross-reactivity was only seen against B16F10 in the case of 205-immune recipients and against 205 for C1498-immune recipients. Neither immune recipients nor control immunized C3H.SW donor splenocytes killed Yac-1 or P815, indicating that the cytolytic response was not caused by nonspecific NK or LAK activity.
Tumor immunization of BMT recipients increases antitumor cytolytic activity and induces limited alloreactivity compared to immune C3H.SW controls.
Three months (A) or 1 month (B, C) after BMT, C3H.SW→ B6 recipients were immunized twice with irradiated 205IL-2/TK cells (205-immune) or irradiated C1498 leukemia cells (C1498-immune) at a 1-week interval. Control C3H.SW donors were also immunized in an identical manner. Ten days after the 2nd vaccine, spleens were harvested, and spleen cells were stimulated for 4 days in vitro with irradiated 205 (A, B) or C1498 (C). A 51Cr-release assay was performed using targets specified in the legends. B6 CAB are C57BL/6 ConA lymphoblasts. A, B, and C represent independent experiments with different panels of targets; neither P815 nor Yac cells were used as targets in experiment C because other experiments had demonstrated little or no LAK or NK activity. Data shown are at an E:T ratio of 200:1, and each effector:target condition was performed in triplicate using splenocytes pooled from 2 or 3 mice.
Tumor immunization of BMT recipients increases antitumor cytolytic activity and induces limited alloreactivity compared to immune C3H.SW controls.
Three months (A) or 1 month (B, C) after BMT, C3H.SW→ B6 recipients were immunized twice with irradiated 205IL-2/TK cells (205-immune) or irradiated C1498 leukemia cells (C1498-immune) at a 1-week interval. Control C3H.SW donors were also immunized in an identical manner. Ten days after the 2nd vaccine, spleens were harvested, and spleen cells were stimulated for 4 days in vitro with irradiated 205 (A, B) or C1498 (C). A 51Cr-release assay was performed using targets specified in the legends. B6 CAB are C57BL/6 ConA lymphoblasts. A, B, and C represent independent experiments with different panels of targets; neither P815 nor Yac cells were used as targets in experiment C because other experiments had demonstrated little or no LAK or NK activity. Data shown are at an E:T ratio of 200:1, and each effector:target condition was performed in triplicate using splenocytes pooled from 2 or 3 mice.
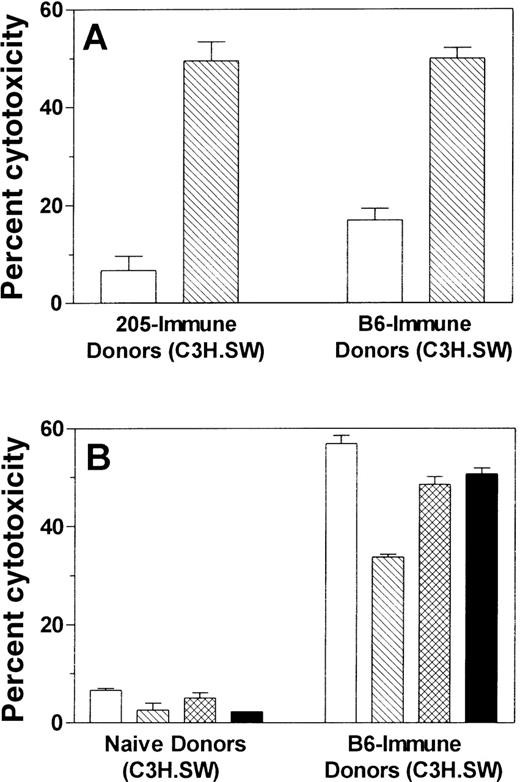
Unresponsiveness against the immunodominant recipient minor histocompatibility antigen B6dom1 is not reversed by post-transplant tumor immunization of recipients
Perreault et al15-17 have identified an immunodominant peptide mHAg (B6dom1), which is presented by H-2Db on normal C57BL/6 cells and is at least partially responsible for GVHD in the C3H.SW → C57BL/6 BMT model. Therefore, we wanted to determine whether tumor cell vaccines induce a response to B6dom1 in C3H.SW mice, whether C57BL/6 tumor cell lines express B6dom1, and whether BMT recipients fail to respond to B6dom1, which would help explain their limited alloreactivity compared to immunized donors. First, C3H.SW mice were immunized with either C57BL/6 spleen cells (B6-immune) or irradiated 205IL-2/TK tumor cells (205-immune). Control mice were not immunized (naı̈ve SW). After stimulation of immune splenocytes with B6dom1 peptide in vitro, we tested their ability to specifically kill C3H.SW ConA lymphoblasts loaded with B6dom1 peptide (B6dom1/SW CAB) versus control unpulsed SW CAB. As seen in Figure9A, both 205-immune and control B6-immune donors developed a specific and almost identical response to B6dom1, demonstrating that 205 tumor cells do express B6dom1 as an immunodominant antigen. We next tested the ability of B6-immune, B6dom1-stimulated C3H.SW cells to kill various C57BL/6 tumor cell lines (Figure 9B). All lines tested (205, C1498, B16F10, and EL4) were killed efficiently and therefore express B6dom1, explaining why 205-immune C3H.SW splenocytes were broadly cross-reactive against all of them as seen in Figure 8.
Tumor immunization of C3H.SW BMT donors induces a response to the mHAg B6dom1 found on all C57BL/6 tumor targets tested. C3H.SW (donor strain) mice were immunized twice with 5 × 106irradiated 205IL-2/TK cells (205-immune) or 20 × 106 C57BL/6 spleen cells (B6-immune) at a weekly interval, or they were not immunized (naı̈ve). Ten days after the 2nd vaccine, their splenocytes were stimulated for 5 days in vitro with C3H.SW spleen cells loaded exogenously with B6dom1 peptide. A 51Cr-release assay was performed using the targets specified in the graphs. B6dom1/SW CAB indicates C3H.SW ConA lymphoblast targets that were loaded exogenously with B6dom1 peptide, whereas SW CAB were not loaded with peptide. Data shown are at an E:T ratio of 100:1, and each effector:target condition was performed in triplicate using splenocytes pooled from 2 or 3 mice. Figures 9A and 9B represent data from the same experiment.
Tumor immunization of C3H.SW BMT donors induces a response to the mHAg B6dom1 found on all C57BL/6 tumor targets tested. C3H.SW (donor strain) mice were immunized twice with 5 × 106irradiated 205IL-2/TK cells (205-immune) or 20 × 106 C57BL/6 spleen cells (B6-immune) at a weekly interval, or they were not immunized (naı̈ve). Ten days after the 2nd vaccine, their splenocytes were stimulated for 5 days in vitro with C3H.SW spleen cells loaded exogenously with B6dom1 peptide. A 51Cr-release assay was performed using the targets specified in the graphs. B6dom1/SW CAB indicates C3H.SW ConA lymphoblast targets that were loaded exogenously with B6dom1 peptide, whereas SW CAB were not loaded with peptide. Data shown are at an E:T ratio of 100:1, and each effector:target condition was performed in triplicate using splenocytes pooled from 2 or 3 mice. Figures 9A and 9B represent data from the same experiment.
Because tumor-immunized recipients do not have broad cross-reactivity against all C57BL/6-derived targets (unlike tumor-immunized C3H.SW), it is possible that they do not mount a substantial response to B6dom1. To test this hypothesis we examined the relative ability of 205-immune BMT recipient splenocytes to kill B6dom1-loaded SW CAB and regular SW CAB (Figure10). Although 205-immune C3H.SW controls again mounted a strong response to B6dom1, 205-immune BMT recipients had virtually no response to B6dom1.
Unresponsiveness to the recipient mHAg B6dom1 is not reversed by post-transplant tumor immunization of recipients.
One month after BMT, allogeneic BMT recipients or control C3H.SW donors were immunized twice with irradiated 205IL-2/TK cells at a 1-week interval. Ten days after the second vaccine, their splenocytes were stimulated for 5 days in vitro with B6dom1-loaded C3H.SW spleen cells. A 51Cr-release assay was then performed using C3H.SW ConA lymphoblast targets that were either loaded with B6dom1 peptide (B6dom1/SW CAB) or not loaded with peptide (SW CAB). Data shown are at an E:T ratio of 50:1, and each effector:target condition was performed in triplicate using splenocytes pooled from 2 or 3 mice.
Unresponsiveness to the recipient mHAg B6dom1 is not reversed by post-transplant tumor immunization of recipients.
One month after BMT, allogeneic BMT recipients or control C3H.SW donors were immunized twice with irradiated 205IL-2/TK cells at a 1-week interval. Ten days after the second vaccine, their splenocytes were stimulated for 5 days in vitro with B6dom1-loaded C3H.SW spleen cells. A 51Cr-release assay was then performed using C3H.SW ConA lymphoblast targets that were either loaded with B6dom1 peptide (B6dom1/SW CAB) or not loaded with peptide (SW CAB). Data shown are at an E:T ratio of 50:1, and each effector:target condition was performed in triplicate using splenocytes pooled from 2 or 3 mice.
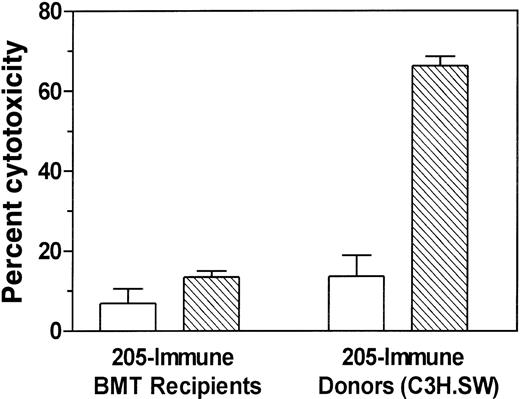
Unresponsiveness against recipient mHAgs responsible for GVHD is not reversed by secondary adoptive transfer of lymphocytes from tumor-immunized BMT chimeras into new irradiated C57BL/6 hosts
Although use of tumor vaccines after BMT did not increase severe acute GVHD (as measured by death, weight loss, dermatitis, and histology), it is possible that tumor vaccines may have produced subclinical exacerbation of GVHD. To test this possibility, we transferred tumor-immunized BMT recipient splenocytes into newly irradiated C57BL/6 hosts. This is potentially a more sensitive bioassay for the detection of GVHD because in it the secondary hosts may generate the total body irradiation-induced cytokine storm, a critical cofactor in the generation of severe GVHD.18-22 Long-term survivors of C3H.SW → C57BL/6 BMT and irradiated 205IL-2/TK immunization were reimmunized twice before transferring their bone marrow and spleen cells to irradiated C57BL/6 recipients. Secondary transfer of 205-immune BMT recipient (chimera) splenocytes did not induce any signs of GVHD in any of the recipients tested (Table1).
Secondary adoptive transfer of 205-immune BMT chimera lymphocytes into irradiated recipients does not induce GVHD
| Donor . | Vaccine Treated? . | # Dead/N . | # With Signs of Acute GVHD . | Final Weight Avg. ± SEM . |
|---|---|---|---|---|
| C3H.SW | No | 0/5 | 0/5 | 25 ± 0.9 |
| C3H.SW | Yes | 2/3* | 2/3 | 16 ± 3† |
| BMT chimera | No | 0/4 | 0/4 | 25 ± 0.9 |
| BMT chimera | Yes | 0/4 | 0/4 | 25 ± 1.5 |
| Donor . | Vaccine Treated? . | # Dead/N . | # With Signs of Acute GVHD . | Final Weight Avg. ± SEM . |
|---|---|---|---|---|
| C3H.SW | No | 0/5 | 0/5 | 25 ± 0.9 |
| C3H.SW | Yes | 2/3* | 2/3 | 16 ± 3† |
| BMT chimera | No | 0/4 | 0/4 | 25 ± 0.9 |
| BMT chimera | Yes | 0/4 | 0/4 | 25 ± 1.5 |
C3H.SW or long-term surviving C3H.SW → B6 BMT chimeras were either not immunized (nonimmune) or immunized (205-immune) with 5 × 106 irradiated 205IL-2/TK cells twice at a weekly interval. Ten days later, their splenocytes were adoptively transferred to C57BL/6 recipients that were given 850 cGy total body irradiation 1 day earlier. Survival was monitored until day 108. Final weights represent the weight of the surviving mice at day 108 or the weight at death for the 2 dead mice. Secondary transfer recipients were engrafted with C3H.SW T-cells as assessed by flow cytometry, which showed that the majority of Thy1.2+ cells in the spleen after sacrifice were CD5.1+.
P = .0431 compared to survival of recipients of nonimmune C3H.SW.
P < .01 compared to final weight of recipients of nonimmune C3H.SW.
Discussion
This work tested the hypothesis that immunization of MHC-matched allogeneic BMT recipients with a tumor cell vaccine would substantially increase GVT activity and prolong tumor-free survival without exacerbating GVHD. Tumor vaccination of recipients against either a fibrosarcoma or a myeloid leukemia produced a substantial increase in GVT activity, which was capable of complete protection against tumor growth and of preventing the growth of preexisting micrometastatic cancer cells. Furthermore, BMT recipients did not develop signs of acute GVHD after tumor cell vaccination, demonstrating that GVT activity can occur independent of GVHD.
This study shows that tumor cells of diverse histology are capable of both expression of a minor histocompatibility antigen (mHAg) (B6dom1) and induction of an immune response against this mHAg, which is an immunodominant GVHD target antigen in this BMT model.15-17 This finding confirms at the molecular level our previous findings in the allogeneic donor immunization model4 and elucidates the mechanism whereby tumor-immunized donor lymphocytes can kill various recipient targets and cause GVHD. Specific restimulation of tumor-immune donor cells with the H-2Db-restricted B6dom1 peptide in vitro caused a similar level of tumor cell lysis compared to tumor cell restimulation, indicating that much of the alloreactive cytolytic T-cell activity seen after donor immunization with tumor cells is caused by the recognition of B6dom1.
Although the same vaccines used in this study have been shown to increase both GVT activity and lethal GVHD when used to treat donors before BMT,4 they did not cause GVHD when administered after BMT. This is consistent with the hypothesis that after transplantation, naive donor-derived T cells eventually become unresponsive to the immunodominant mHAgs that are potential targets for GVHD. Because the antitumor response of recipients after BMT does not exacerbate GVHD, it is likely that much of the immune response is directed against other antigens, such as nonimmunodominant mHAgs, with restricted tissue distribution or possibly even tumor-specific antigens. This is supported by the observation that in vivo tumor protection in this study was tumor specific; the fibrosarcoma and leukemia vaccines induced immunity that did not cross-protect against growth of the other tumor (Figure 7), despite the presence of common mHAgs on both tumors (Figure 9).4 The hypothesis that allogeneic BMT recipients become unresponsive to immunodominant mHAgs that are targets of GVHD is also supported by the finding that although donor immunization with recipient tumor cells induced a potent response to B6dom1, immunization of recipients 1 month after BMT failed to induce a significant response to B6dom1. Such T-cell unresponsiveness to B6dom1 explains why immune recipient cytolytic activity is not broadly cross-reactive against all recipient targets. The mechanism for this unresponsiveness is unknown. These data are consistent with either clonal deletion, anergy, or the generation of suppressor cells. Further studies are being conducted to help elucidate the mechanism of unresponsiveness to recipient mHAgs, which will be helpful in learning how to better apply tumor vaccination strategies to human BMT recipients.
The limited pattern of cytolytic activity seen in vitro was likely caused by activation and expansion of some T-cell clones that recognized tumor antigens and other T cells that recognized mHAgs other than B6dom1. These other putative mHAgs appear to be expressed on some but not all tumor cell lines and with limited or no expression on normal lymphoblasts or targets of GVHD. For example, 205-immune recipient splenocytes recognized both 205 and B16F10 tumors (Figure 8A). (205 and B16F10 do not induce cross-reactivity in normal, syngeneic C57BL/6 mice and therefore do not share tumor-specific antigens.13) That this cross-reactivity was attributable to the recognition of shared mHAgs is supported by the observation of lysis of 205 and B16F10 after in vitro allostimulation of 205-immune recipient splenocytes with normal C57BL/6 splenocytes that do not express tumor antigens (data not shown). Nevertheless, the mHAgs recognized by post-transplant BMT recipients must be different than those recognized by tumor-immunized donors because of the lack of broad cross-reactivity against all recipient targets, which was seen after donor immunization (Figure 8). The lack of expression of the target antigens of alloreactivity by target cells of GVHD is further supported by the observation that the alloreactive T cells did not induce GVHD even when transferred to secondary irradiated recipients (Table 1).
Although useful in many respects, the experimental BMT model used in these studies does not exactly replicate the clinical situation in which the BMT recipient is in clinical remission but harbors minimal residual tumor at the time of BMT that may reappear clinically in months or years. As was shown, the experimental murine tumors showed rapid growth producing death in approximately 1 month. In these murine transplants, as in human transplants, the capacity of the host to respond to immunization to antigens in the first month is very poor. Therefore, it was not practical within this model to test the efficacy of the tumor vaccines in the setting of preexisting tumor. The results must be viewed with this in mind. It is possible that the results of post-BMT tumor vaccination would be different in such a setting. One could hypothesize that minimal residual tumor at time of BMT could make the donor-derived lymphocytes tolerant to antigens on host tumor cells. We are developing BMT models to test this hypothesis.
Use of post-BMT tumor vaccines in human trials will necessarily require careful scrutiny of the potential of such vaccines to exacerbate GVHD. Because of the complex and uncharacterized expression of most mHAgs on tumor cells, caution must be exercised about using whole tumor cells as vaccines until methods are developed for identifying mHAgs, their pattern of tissue distribution, and their relative ability to induce GVT activity and GVHD. However, in this study we showed that the induction of GVT activity can occur independent of GVHD if recipients are immunized with cancer cells after the development of unresponsiveness to immunodominant mHAgs. In some human malignancies, such as melanoma, tumor-specific antigens and antigens with relatively restricted tissue distribution have been identified.23 24Perhaps the identification of such tumor antigens for other malignancies may lead to the ability to immunize BMT donors or recipients without the risk for exacerbating GVHD.
Supported in part by a Clinical Oncology Career Development Award (CDA-96-61) from the American Cancer Society (C.A.M.), a Research Project Grant (RPG-98-035-01-CIM) from the American Cancer Society (C.A.M.), a grant from the Leukemia Research Foundation (C.A.M.), and a Rosalie B. Hite Fellowship (L.D.A.). Support for Veterinary and Peptide Synthesis core laboratory facilities was provided by NIH Cancer Center Support Grant CA16672.
Reprints:Craig A. Mullen, Department of Experimental Pediatrics, The University of Texas M. D. Anderson Cancer Center, Box 88, Room B7.4518, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: mullen@mdacc.tmc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal