Abstract
Expression of CD134 (OX40) on activated CD4+ T cells has been observed in acute graft-versus-host disease (GVHD) after human and rat allogeneic bone marrow transplantation (BMT). We investigated the role of interaction between CD134 and CD134 ligand (CD134L) in a murine model of acute GVHD by using a newly established monoclonal antibody (mAb) against murine CD134L. Acute GVHD was induced by transfer of bone marrow cells and spleen cells into lethally irradiated recipients in a parent (C57BL/6) to first filial generation (C57BL/6 crossed with DBA/2) BMT. Administration of anti-CD134L mAb significantly reduced the lethality of acute GVHD and other manifestations of the disease, such as loss of body weight, hunched posture, diarrhea, and patchy alopecia. The survival rate 80 days after BMT in mice treated with the mAb was about 70%, whereas all mice treated with control antibodies died within 43 days. Histologic examinations revealed that inflammatory changes in target organs such as the liver, gut, and skin were also ameliorated in mice treated with the mAb compared with control mice. An in vitro assay of T-cell proliferation showed a marked hyporesponsiveness to host alloantigen in samples from mice treated with anti-CD134L mAb. In addition, low levels of interferon γ and transiently elevated levels of interleukin 4 and IgE in serum samples were found in mice treated with anti-CD134L mAb. These results suggest that CD134-CD134L interactions have an important role in the pathogenesis of acute GVHD.
Allogeneic bone marrow transplantation (BMT) is a potentially curative therapy for patients with several disorders of hematopoiesis and certain hematologic malignant diseases.1,2 However, acute graft-versus-host disease (GVHD), one of the major obstacles to allogenic BMT, occurs in up to 75% of recipients of unmanipulated HLA-matched marrow.3Decreasing the number of T cells in the donor marrow by a variety of purging methods can decrease the incidence and the severity of acute GVHD,4-8 but the overall usefulness of T-cell depletion has been questioned because of its association with higher rates of relapse, graft failure, lethal infection, and Epstein-Barr virus–related lymphoproliferative disorders after BMT.9-11Current clinical approaches to decreasing the occurrence of acute GVHD are targeted on selective manipulation of alloreactive T cells. It has been reported that immunotoxin to the interleukin-2 (IL-2) receptor prevented acute GVHD without affecting entire T-cell populations.12-15
Tumor necrosis factor (TNF) α has been found to participate in the pathogenesis of acute GVHD.16-19 Administration of anti-TNF antibody ameliorated acute GVHD in a murine model.16 Other molecules belonging to the TNF-TNF receptor (TNFR) family are also involved in the pathogenesis of acute GVHD. Blockade of CD40-CD154 (CD40 ligand [L]) interaction by administration of anti-CD154 monoclonal antibody (mAb) ameliorated murine acute and chronic GVHD.20,21 CD95 (Fas, Apo-1)-CD95L interaction was found to be responsible for acute GVHD in studies using functionally CD95L-defective gld mice as donors22-24 and in investigations using anti-CD95L mAb.19
CD134 (OX40) is also a member of the TNFR family, and its ligand, CD134L, belongs to the TNF family. CD134 is expressed on recently activated CD4+ T cells, and CD134+CD4+ T cells have been reported to increase in human25 and rat26 BMT recipients with acute GVHD. In addition, a study found that anti-CD134 mAb treatment resulted in conversion of chronic GVHD to acute GVHD.27
In the study described here, we found that administration of anti-CD134L mAb successfully ameliorated lethal acute GVHD in mice after BMT, with induction of hyporesponsiveness to host alloantigen. This treatment also affected the serum cytokine levels in BMT recipients and alleviated various manifestations of GVHD. Anti-CD134L treatment may be useful for selectively manipulating alloreactive T-cell populations in patients with acute GVHD.
Materials and methods
Mice
Female 6- to 8-week-old C57BL/6 (B6, H-2b), DBA/2 (H-2d), C3H/HeJ (H-2k), and (C57BL/6 crossed with DBA/2) first filial generation (BDF1, H-2b/d) mice were purchased from Japan SLC (Shizuoka, Japan) and cared for in our animal facilities.
Monoclonal antibodies
Rat antimouse CD134L mAb (RM134L, rat IgG2bκ) was produced in our laboratory as described previously.28Purified antimouse-Thy 1.2 mAb (30-H12), control rat IgG, fluorescein isothiocyanate-conjugated (FITC) anti-CD19 (1D3), anti-CD4 (RM4-5), anti-H-2Kb (AF6-88.5), phycoerythrin (PE)-conjugated anti-CD3 (145-2C11), anti-CD8 (53-6.7), anti-H-2Kd (SF1-1.1), and PE-conjugated avidin were obtained from PharMingen (San Diego, CA). Biotinylated antimouse CD134 mAb (MRC OX86) was prepared as described previously.28
BMT
Recipient BDF1 mice (10 mice in each group) were lethally irradiated (12 Gy) by using a cobalt 60 irradiator (MBR 1505 R2; Hitachi, Japan). Bone marrow (BM) cells were flushed from the shafts of femurs and tibias of donor mice, and single-cell suspensions were prepared. The BM cells were treated with anti-Thy1.2 mAb and rabbit complement for preparation of T-cell–depleted BM (TCD-BM) cells. Single-cell suspensions of spleen cells from donor mice were used as the source of GVHD effector cells. Recipients received 2 × 107 BM cells with 2.5 × 107 spleen cells (BMS) in 0.5 mL of phosphate-buffered saline administered into the tail vein. To create GVHD-negative controls, TCD-BM cells were transplanted without spleen cells. The day of the BMT was designed as day 0. For the anti-CD134L mAb treatment, 1 mg of the mAb was administered intraperitoneally on the day before BMT (day −1) and on day 0, and then 0.5 mg of the mAb was given intraperitoneally twice a week until day 14 after BMT. We concluded that this dose and treatment period would be the most optimal after titrating the mAb from 0.25 mg per mouse to 1 mg per mouse or extending the treatment period up to 4 weeks after BMT. Rat IgG was also administered to GVHD-positive control recipients. All BMT recipients were given sterilized water containing antibiotics (2 mg/mL of neomycin sulfate) from 2 days before BMT to 14 days afterward. The mice were monitored for clinical signs of GVHD, including death, weight loss, diarrhea, alopecia, and hunched posture. Representative mice were killed at various times after BMT to obtain tissues for histologic examinations.
Histopathologic assessment
Liver, intestine, and skin tissue obtained from mice BMT recipients were fixed in 10% buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin and examined microscopically.
Flow cytometric analysis
Spleen cells were obtained from BDF1 mice, recipients of B6 TCD-BM, recipients of B6 BMS plus rat IgG, and recipients of B6 BMS plus anti-CD134L mAb on days 14 and 28 after BMT. Cells were stained with FITC-conjugated CD19, CD4, H-2Kb, PE-conjugated CD3, CD8, H-2Kd, and biotinylated anti-CD134 followed by PE-conjugated avidin and analyzed by means of fluorescence-activated cell sorter (FACScan and Cell Quest program; Becton Dickinson, San Jose, CA). Recipient and donor lymphocytes were identified as H-2Kb+d+ and H-2Kb+d−, respectively.
Mixed lymphocyte reaction (MLR)
Responder spleen cells (2 × 105/well) obtained from recipients of TCD-BM, GVHD controls, and recipients of anti-CD134L 14 days after BMT were cultured with irradiated (30 Gy) stimulator DBA/2 (H-2d) or C3H/HeJ (H-2k) spleen cells (2 × 105/well) in 96-well round-bottomed microtiter plates at 37°C in a 5% carbon dioxide humidified atmosphere. Cells were also cultured in the presence of 200 U/mL of recombinant interleukin 2 (IL-2) (Shionogi, Osaka, Japan) to examine their reactivity to alloantigen. After 5 days, the cultures were pulsed for 18 hours with 0.019 MBq per well of tritium thymidine and harvested (Micro 96 Harvester; Skartron, Lier, Norway). Incorporated radioactivity was measured in a β counter (Micro Beta Plus; Wallac, Turku, Finland).
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were obtained from the retro-orbital plexus of mice under ether anesthesia on representative days after BMT. Serum samples were individually divided into aliquots and stored at −40°C until testing. Serum IgE levels were determined by solid-phase ELISA using rat antimouse IgE mAb as described previously.29 Serum interferon γ (IFN-γ) and serum interleukin 4 (IL-4) were evaluated with commercial ELISA kits (PharMingen, San Diego, CA, and Amersham, Buckinghamshire, England, respectively).
Statistical analyses
Data were analyzed by using the Statview program (Brainpower Inc, Calabasas, CA). Comparison assessments included analysis of variance and the Student t test. P values < .05 were considered to represent statistical significance.
Results
Effect of anti-CD134L mAb treatment on acute GVHD-induced mortality and weight loss
Lethal acute GVHD was induced by intravenous inoculation of BMS cells (2 × 107 B6 BM cells plus 2.5 × 107 B6 spleen cells) into lethally irradiated BDF1 mice. As shown in Figure 1A, all recipients given control IgG died within 43 days. In these mice, clinical symptoms of acute GVHD, such as weight loss, hunched posture, diarrhea, and patchy alopecia, were apparent beginning 10 days after BMT. Administration of anti-CD134L mAb significantly improved the survival of BMT recipients; the survival rate among mice given the was 70% on day 80 (Figure 1A). Recovery of body weight in this group was slightly impaired compared with that in recipients of TCD-BM (Figure1B).
Prevention of lethal acute graft-versus-host disease (GVHD) by anti-CD134L monoclonal antibody (mAb).
Lethal acute GVHD was induced by transfer of B6 bone marrow (BM) cells with 2.5 × 107 spleen cells (B6 BMS) into lethally irradiated BDF1 mice. Ten mice in each group were given intraperitoneal injections of 1 mg of anti-CD134L mAb on the day before bone marrow transplantation (BMT) (day −1) and the day of BMT (day 0) and then 0.5 mg of anti-CD134L mAb twice a week until day 14 after BMT. For an acute-GVHD–positive control, control rat IgG was administered instead of anti-CD134L mAb. For an acute-GVHD–negative control, B6 T-cell–depleted BM was transplanted without spleen cells. Administration of anti-CD134L mAb to the B6 BMS group ameliorated both mortality (A) and loss of body weight (B) due to acute GVHD. The survival rate of the recipients treated with anti-CD134L mAb was 70% on day 80 after BMT, whereas all mice in the control-IgG group died within 43 days. Pooled results of 3 similar experiments are shown (total n = 30).
Prevention of lethal acute graft-versus-host disease (GVHD) by anti-CD134L monoclonal antibody (mAb).
Lethal acute GVHD was induced by transfer of B6 bone marrow (BM) cells with 2.5 × 107 spleen cells (B6 BMS) into lethally irradiated BDF1 mice. Ten mice in each group were given intraperitoneal injections of 1 mg of anti-CD134L mAb on the day before bone marrow transplantation (BMT) (day −1) and the day of BMT (day 0) and then 0.5 mg of anti-CD134L mAb twice a week until day 14 after BMT. For an acute-GVHD–positive control, control rat IgG was administered instead of anti-CD134L mAb. For an acute-GVHD–negative control, B6 T-cell–depleted BM was transplanted without spleen cells. Administration of anti-CD134L mAb to the B6 BMS group ameliorated both mortality (A) and loss of body weight (B) due to acute GVHD. The survival rate of the recipients treated with anti-CD134L mAb was 70% on day 80 after BMT, whereas all mice in the control-IgG group died within 43 days. Pooled results of 3 similar experiments are shown (total n = 30).
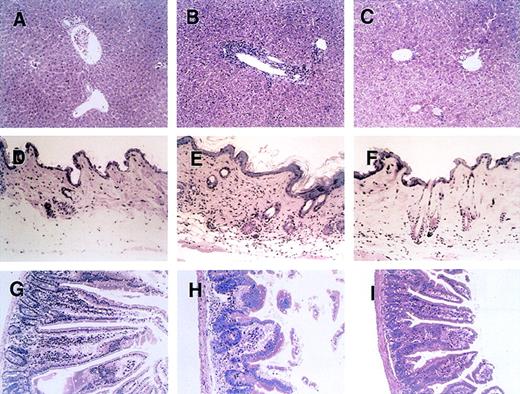
Effect of anti-CD134L mAb treatment on histopathologic features of acute GVHD
Figure 2 shows a histopathologic analysis of tissues obtained on day 28 after BMT from representative acute-GVHD–positive control mice, mice treated with anti-CD134, and acute-GVHD–negative control mice. In liver tissue from control mice with acute GVHD, a massive infiltration of mononuclear cells was observed mainly in the periportal areas (Figure 2B). In contrast, such inflammatory changes were minimal in liver tissue from mice treated with anti-CD134L (Figure 2C). Skin from control mice with acute GVHD showed severe inflammatory infiltrates with intraepidermal lymphocytes, dyskeratotic cells, ulceration, and loss of hair follicles (Figure 2E). Such changes were not observed in mice given anti-CD134L (Figure 2F). Gut tissue from control mice with acute GVHD showed dilatation, flattening of the villi, and elevation of crypts, all of which are characteristics of intestinal acute GVHD (Figure 2H). Such findings were minimal in mice treated with anti-CD134L (Figure 2I). Recipients of TCD-BM had almost no signs of GVHD in tissues (Figure 2A, 2D, and2G). These results suggest that administration of anti-CD134L mAb prevented development of acute GVHD in recipients of BMS.
Histopathologic examination of liver, skin, and intestine tissues from mice with acute GVHD treated with anti-CD134L mAb.
Induction of lethal acute GVHD (B, E, and H) and administration of anti-CD134L mAb (C, F, and I) were performed and acute-GVHD–negative controls (A, D, and G) were generated as described in the legend for Figure 1. On day 28 after BMT, 3 mice in each group were killed. Paraffin sections of the liver (A-C), skin (C-F), and intestine (G-I) were stained with hematoxylin and eosin. The specimens shown are from 3 representative mice in each group with similar histologic features. Original magnification, ×200.
Histopathologic examination of liver, skin, and intestine tissues from mice with acute GVHD treated with anti-CD134L mAb.
Induction of lethal acute GVHD (B, E, and H) and administration of anti-CD134L mAb (C, F, and I) were performed and acute-GVHD–negative controls (A, D, and G) were generated as described in the legend for Figure 1. On day 28 after BMT, 3 mice in each group were killed. Paraffin sections of the liver (A-C), skin (C-F), and intestine (G-I) were stained with hematoxylin and eosin. The specimens shown are from 3 representative mice in each group with similar histologic features. Original magnification, ×200.
Effect of anti-CD134L mAb treatment on cellular recovery
To assess immune-system recovery in BMT recipients, 3 representative mice in each study group were killed on days 14 and 28 after BMT, and flow cytometry was used to analyze total cell numbers and surface phenotypes (Table 1). The total number of lymphocytes in the spleen of recipients treated with anti-CD134L was lower than that in recipients given TCD-BM. However, the numbers did not decrease progressively and a certain number of CD19+ B cells were preserved. In contrast, in control IgG-treated BMT recipients, the number of spleen cells decreased successively and the decrease in the number of B cells was remarkable. The percentages of CD134+CD4+ T cells were higher in control IgG-treated recipients, suggesting that CD4+ T cells were more activated in that group than in the other 2 groups. Analysis of H-2K haplotype showed greater than 95% of donor haplotype (H-2Kb+d−) in BMT recipients treated with anti-CD134L, suggesting that the mAb did not inhibit engraftment of donor cells. The spleen cells harvested on day 14 were also used as responder cells in MLR to assess their alloreactivity.
Cell numbers and populations of lymphocytes in the spleen of mice recipients of bone marrow transplantation (BMT) 14 and 28 days after BMT
| Mouse group . | Day . | No. of cells (×107) . | Cell population (% of total spleen cells) . | |||||
|---|---|---|---|---|---|---|---|---|
| CD3 . | CD19 . | CD4 . | CD134+CD4 . | CD8 . | H-2Kb+d+/H-2Kb+d− . | |||
| BDF1 (control) | 7.5 ± 1.6 | 52.1 ± 7.4 | 36.4 ± 8.1 | 35.6 ± 7.2 | 3.2 ± 0.8 | 17.8 ± 4.2 | 100/0 | |
| B6 TCD-BM | 14 28 | 6.5 ± 1.2 6.8 ± 1.4 | 14.9 ± 2.2 15.2 ± 3.5 | 44.2 ± 8.8 47.0 ± 7.9 | 11.5 ± 2.4 11.9 ± 2.2 | 4.1 ± 1.9 4.2 ± 1.2 | 2.4 ± 0.8 2.6 ± 1.1 | 15.2 ± 2.4/83.8 ± 4.3 12.8 ± 3.1/86.2 ± 5.1 |
| B6 BMS + rat IgG | 14 28 | 1.2 ± 0.6 0.62 ± 0.2 | 68.6 ± 8.8 79.2 ± 8.4 | 6.4 ± 3.5 8.9 ± 3.6 | 30.1 ± 5.4 46.3 ± 6.1 | 16.2 ± 2.4 24.5 ± 3.3 | 31.7 ± 5.1 25.1 ± 4.5 | 1.4 ± 0.8/98.5 ± 1.1 6.3 ± 2.2/93.2 ± 4.5 |
| B6 BMS + anti-CD134L mAb | 14 28 | 1.0 ± 0.3 1.3 ± 0.2 | 32.1 ± 4.1 48.4 ± 5.3 | 29.8 ± 3.5 20.5 ± 4.6 | 14.1 ± 2.7 25.5 ± 4.3 | 4.6 ± 2.1 8.8 ± 3.1 | 21.5 ± 3.5 26.2 ± 3.8 | 1.5 ± 0.8/97.9 ± 2.1 1.8 ± 1.1/97.1 ± 3.1 |
| Mouse group . | Day . | No. of cells (×107) . | Cell population (% of total spleen cells) . | |||||
|---|---|---|---|---|---|---|---|---|
| CD3 . | CD19 . | CD4 . | CD134+CD4 . | CD8 . | H-2Kb+d+/H-2Kb+d− . | |||
| BDF1 (control) | 7.5 ± 1.6 | 52.1 ± 7.4 | 36.4 ± 8.1 | 35.6 ± 7.2 | 3.2 ± 0.8 | 17.8 ± 4.2 | 100/0 | |
| B6 TCD-BM | 14 28 | 6.5 ± 1.2 6.8 ± 1.4 | 14.9 ± 2.2 15.2 ± 3.5 | 44.2 ± 8.8 47.0 ± 7.9 | 11.5 ± 2.4 11.9 ± 2.2 | 4.1 ± 1.9 4.2 ± 1.2 | 2.4 ± 0.8 2.6 ± 1.1 | 15.2 ± 2.4/83.8 ± 4.3 12.8 ± 3.1/86.2 ± 5.1 |
| B6 BMS + rat IgG | 14 28 | 1.2 ± 0.6 0.62 ± 0.2 | 68.6 ± 8.8 79.2 ± 8.4 | 6.4 ± 3.5 8.9 ± 3.6 | 30.1 ± 5.4 46.3 ± 6.1 | 16.2 ± 2.4 24.5 ± 3.3 | 31.7 ± 5.1 25.1 ± 4.5 | 1.4 ± 0.8/98.5 ± 1.1 6.3 ± 2.2/93.2 ± 4.5 |
| B6 BMS + anti-CD134L mAb | 14 28 | 1.0 ± 0.3 1.3 ± 0.2 | 32.1 ± 4.1 48.4 ± 5.3 | 29.8 ± 3.5 20.5 ± 4.6 | 14.1 ± 2.7 25.5 ± 4.3 | 4.6 ± 2.1 8.8 ± 3.1 | 21.5 ± 3.5 26.2 ± 3.8 | 1.5 ± 0.8/97.9 ± 2.1 1.8 ± 1.1/97.1 ± 3.1 |
Phenotypic analysis was performed with use of fluorescence-activated cell sorter. Plus-minus values are the mean ± SD for 3 mice in each group. TCD-BM indicates T-cell-depleted bone marrow; BMS, bone marrow cells and spleen cells; and mAb, monoclonal antibody.
Effect of anti-CD134L mAb treatment on MLR
To clarify the mechanisms of amelioration of acute GVHD by anti-CD134L mAb, we examined the alloreactivity of lymphocytes from BMT recipients treated with anti-CD134L. Representative mice from each of the 3 study groups were killed 14 days after BMT, and single-cell suspensions of spleen cells were prepared as responder cells in MLR assessments. Spleen cells from DBA/2 (H-2d) and C3H/HeJ (H-2k) mice were also prepared as stimulator cells in MLR assessments as the host alloantigen and the third-party stimulator, respectively (Figure3). Spleen cells from mice with acute GVHD were highly reactive against host alloantigen (H-2d), whereas spleen cells from mice that received TCD-BM showed little response to H-2d. In mice treated with anti-CD134L, the proliferative response to H-2d was significantly suppressed compared with that in acute-GVHD–positive controls, while the response to the third-party H-2k was preserved. These responses were more evident in the presence of exogenous IL-2. These results suggest strongly that treatment with anti-CD134L induced host-alloantigen–specific hyporesponsiveness in our murine model of acute GVHD.
Proliferative response to host alloantigen in mice with acute GVHD treated with anti-CD134L mAb.
Representative mice in each indicated group were killed on day 14 after BMT, and single-cell suspensions of spleen cells were prepared for responder cells in a mixed lymphocyte reaction. Responder cells were cultured with irradiated (30 Gy) stimulator DBA/2 (H-2d) or C3H/He (H-2k) spleen cells (2 × 105/well) in the presence or absence of IL-2 (200 U/mL) in 96-well round-bottomed microtiter plates. After 5 days, the cultures were pulsed for 18 hours with tritium thymidine and the incorporated radioactivity was measured in a β counter. Data are the mean ± SD results from triplicate samples. Responder cells from mice with acute GVHD were highly reactive against host alloantigen (H-2d), whereas responder cells from mice treated with anti-CD134L mAb showed little response to H-2d.
Proliferative response to host alloantigen in mice with acute GVHD treated with anti-CD134L mAb.
Representative mice in each indicated group were killed on day 14 after BMT, and single-cell suspensions of spleen cells were prepared for responder cells in a mixed lymphocyte reaction. Responder cells were cultured with irradiated (30 Gy) stimulator DBA/2 (H-2d) or C3H/He (H-2k) spleen cells (2 × 105/well) in the presence or absence of IL-2 (200 U/mL) in 96-well round-bottomed microtiter plates. After 5 days, the cultures were pulsed for 18 hours with tritium thymidine and the incorporated radioactivity was measured in a β counter. Data are the mean ± SD results from triplicate samples. Responder cells from mice with acute GVHD were highly reactive against host alloantigen (H-2d), whereas responder cells from mice treated with anti-CD134L mAb showed little response to H-2d.
Effect of anti-CD134L treatment on serum cytokines and IgE
It has been reported that IFN-γ is responsible for Th1-type responses such as acute forms of GVHD, whereas IL-4 is related to Th2-type responses such as chronic forms of GVHD.30-32 We evaluated IFN-γ and IL-4 levels in serum samples from representative mice killed 7, 14, 28, and 56 days after BMT (Table2). In mice with acute GVHD, IFN-γ levels were elevated early after BMT and decreased gradually until day 28. Serum IFN-γ levels on day 7 were lower in BMT recipients that received anti-CD134L mAb than in those given control antibody (P < .05) and were undetectable in recipients given TCD-BM. Interestingly, serum IL-4 levels were markedly higher on day 28 in BMT recipients given anti-CD134L mAb than in those that received control antibody or TCD-BM.
Serum interferon γ (IFN-γ) and interleukin (IL-4) levels detected by enzyme-linked immunosorbent assay (ELISA) in mice BMT recipients on various days after BMT
| . | Mouse group . | Day 7 . | Day 14 . | Day 28 . | Day 56 . |
|---|---|---|---|---|---|
| IFN-γ, pg/mL* | B6 TCD-BM | <31.3 | <31.3 | <31.3 | <31.3 |
| B6 BMS + rat IgG | 3870 ± 1647† | 494 ± 112 | 519 ± 107 | — | |
| B6 BMS + anti-CD134L mAb | 1406 ± 617† | <31.3 | <31.3 | <31.3 | |
| IL-4, pg/mL | B6 TCD-BM | 460 ± 55 | 1254 ± 108 | 1303 ± 124 | 976 ± 101 |
| B6 BMS + rat IgG | 451 ± 64 | 578 ± 157 | 689 ± 168† | — | |
| B6 BMS + anti-CD134L mAb | 657 ± 78 | 741 ± 104 | 95 782 ± 12 038† | 1215 ± 248 |
| . | Mouse group . | Day 7 . | Day 14 . | Day 28 . | Day 56 . |
|---|---|---|---|---|---|
| IFN-γ, pg/mL* | B6 TCD-BM | <31.3 | <31.3 | <31.3 | <31.3 |
| B6 BMS + rat IgG | 3870 ± 1647† | 494 ± 112 | 519 ± 107 | — | |
| B6 BMS + anti-CD134L mAb | 1406 ± 617† | <31.3 | <31.3 | <31.3 | |
| IL-4, pg/mL | B6 TCD-BM | 460 ± 55 | 1254 ± 108 | 1303 ± 124 | 976 ± 101 |
| B6 BMS + rat IgG | 451 ± 64 | 578 ± 157 | 689 ± 168† | — | |
| B6 BMS + anti-CD134L mAb | 657 ± 78 | 741 ± 104 | 95 782 ± 12 038† | 1215 ± 248 |
Blood samples were obtained from recipients of B6 TCD-BM, B6 BMS plus rat IgG, and B6 BMS plus anti-CD134L on days 7, 14, 28, and 56 after BMT. Serum samples were divided into aliquots and stored in at −40°C for ELISA. Plus-minus values are the mean ± SD for 5 mice in each group. Recipients of B6 BMS plus rat IgG were not examined on day 56 because none had survived.
A concentration of 31.3 pg/mL was the lowest detection limit of the assay.
P < .05 for comparison of values in the 2 groups on the specified day.
We also analyzed serum IgE levels as a marker of murine chronic GVHD. Serum IgE levels, similar to IL-4 levels, were increased in BMT recipients given anti-CD134L mAb (Table 3). The recipients treated with anti-CD134L mAb that survived for longer than 80 days after BMT had no typical manifestations of chronic GVHD as described previously.29 These results suggest that anti-CD134L treatment inhibited IFN-γ production and consequently enhanced IL-4 and IgE production transiently.
Serum IgE levels detected by ELISA in mice BMT recipients on various days after BMT
| Mouse Group . | Serum IgE (pg/mL) . | ||
|---|---|---|---|
| Day 14 . | Day 28 . | Day 56 . | |
| B6 TCD-BM | 468 ± 135 | 556 ± 154 | 578 ± 178 |
| B6 BMS + rat IgG | 572 ± 1213-150 | 586 ± 1373-151 | — |
| B6 BMS + anti-CD134L mAb | 2152 ± 2133-150 | 13 534 ± 34513-151 | 1134 ± 215 |
| Mouse Group . | Serum IgE (pg/mL) . | ||
|---|---|---|---|
| Day 14 . | Day 28 . | Day 56 . | |
| B6 TCD-BM | 468 ± 135 | 556 ± 154 | 578 ± 178 |
| B6 BMS + rat IgG | 572 ± 1213-150 | 586 ± 1373-151 | — |
| B6 BMS + anti-CD134L mAb | 2152 ± 2133-150 | 13 534 ± 34513-151 | 1134 ± 215 |
Plus-minus values are the mean ± SD for 5 mice in each group. Recipients of B6 BMS plus rat IgG were not examined on day 56 because none had survived.
P < .05 for comparison of values in the 2 groups on day 14.
P < .01 for comparison of values in the 2 groups on day 14.
Discussion
It has been reported that expression of CD134 on CD4+ T cells is up-regulated in patients with acute GVHD after BMT.25 Tittle et al26 also found that CD134 is expressed on alloreactive CD4+ T cells derived from donor grafts. These observations suggest that CD134-CD134L interaction may be involved in T-cell functions responsible for acute GVHD. In this study, we examined the ameliorating and preventive effects on acute GVHD of a newly established mAb against murine CD134L in a murine model of lethal acute GVHD.
Administration of anti-CD134L mAb ameliorated the lethality of acute GVHD (Figure 1) and the pathologic manifestations of acute GVHD in target tissues such as the liver, intestine, and skin (Figure 2). CD134 is known to be expressed on CD4+ T cells after activation and ligation of CD134 with CD134L further activates these T cells expressing CD134.33,34 Therefore, the mAb against CD134L might inhibit this costimulatory process of CD134+ T cells. Because the anti-CD134L mAb used in the current study did not deplete the target cells or possess the capacity for signaling,28we could conclude that amelioration of acute GVHD by administration of the mAb was due to in vivo blockade of CD134-CD134L interaction. However, we cannot completely exclude the presence of other mechanisms. Studies using monovalent mAb fragments might be required to clarify this issue.
We also demonstrated that host-alloantigen–specific hyporesponsiveness was induced by administration of anti-CD134L mAb (Figure 3). Because the response to the third-party alloantigen was preserved, the mAb specifically inhibited the response to host alloantigen in vivo. The host-alloantigen–specific hyporesponsiveness in BMT recipients treated with anti-CD134L may have been due to cell death rather than anergy of responsible T cells because the number of CD3 T cells decreased and the decreased response observed on MLR assessment could not be overcome by IL-2. Addition of the mAb to the MLR in vitro failed to inhibit the response to host alloantigen (data not shown). Thus, these findings suggest that CD134-CD134L interactions contribute minimally to the direct presentation of host alloantigen in MLR in vitro but contribute to a large extent to the indirect presentation of host alloantigen in vivo. These results indicate that APC has a potentially important role in antigen presentation and that T-cell priming occurs in the early phase of immune responses. In fact, administration of anti-CD134L mAb after BMT failed to ameliorate the lethality of acute GVHD (data not shown). Therefore, it appears that early blockade of CD134-CD134L interactions in the process of host-alloantigen recognition and subsequent activation of donor T cells is required to suppress development of acute GVHD. CD134-CD134L interactions might be involved in the induction of tolerance. We found previously28that CD134L expressed on the surface of activated B cells can provide CD28-independent costimulatory signals to T cells. In addition, CD134L has been reported to be expressed on dendritic cells35; thus, the mAb might block the interaction between CD134+ T cells and CD134L-expressing APCs. This idea is in agreement with studies showing an ameliorating effect of CD134-Ig in both murine experimental allergic encephalomyelitis (EAE) and inflammatory bowel disease models.36,37 Weinberg et al36 demonstrated that CD134L is expressed on CD11b+ APC derived from the central nervous system of mice with active EAE and that CD134-Ig can inhibit the interaction between antigen-specific T cells and CD11b+APCs. However, it should be noted that there are other molecular pairs known to be responsible for the pathogenesis of acute GVHD, such as CD28-CD152 (CTLA4) and their ligands,37-40 and CD40 and its ligand.20,21 40 Thus, the interaction between CD134 and its ligand may play an important role in the pathogenesis or pathophysiologic aspects of acute GVHD either independently or synergistically with other costimulatory molecular pairs, suggesting the presence of complex in vivo interactions between T cells and APCs in disease states.
In this study, anti-CD134L mAb inhibited IFN-γ production in the early phase after BMT and elevated IL-4 and IgE production in the relatively late phase, suggesting that the Th1-type response that normally develops in mice with acute GVHD was suppressed and might have been transiently converted to a Th2-type response. However, it has been reported that ligation of CD134 expressed on CD4+ T cells with CD134L can induce a Th2-type response.41 These results disagree with our finding that administration of the mAb suppressed the Th1-type response and led to a transient Th2-type response. If our mAb had blocked the induction of the Th2-type response, acute GVHD would have been exacerbated. At present, we do not have direct evidence that CD134-CD134L interaction induces Th1-type responses in our acute-GVHD model. Whether the apparently opposite results were caused by different assay systems (ie, in vivo compared with vitro) or different mouse strains (C57BL/6 or DBA/2 compared with BALB/c or C57BL/6) remains to be determined.
Studies have found that acute GVHD is ameliorated by administration of interleukin 10,42 which is known to be one of the Th2-type cytokines, or by transfer of CD4+ or CD8+ T cells polarized to type 2.43 It has also been reported that administration of interleukin 12, which is known to be one of the Th1-type cytokines, converted Th2-type chronic GVHD to Th1-type acute GVHD.44 On the other hand, Murphy et al,45 in a study using mice deficient in IFN-γ or IL-4 as donors, clearly demonstrated that donor T cells lacking IFN-γ accelerated acute GVHD, whereas those lacking IL-4 prevented acute GVHD. According to their results, IFN-γ prevents the occurrence of acute GVHD and IL-4 exacerbates acute GVHD, contrary to our speculation. It must still be determined whether the decreased IFN-γ and increased IL-4 levels in the mice in our study that were given anti-CD134L were the primary cause of amelioration of acute GVHD or a secondary consequence of the ameliorating effect.
Imura et al46 reported that CD134L is expressed on vascular endothelial cells and related to tissue infiltration of CD134-expressing adult T-cell leukemia-lymphoma cells. It has also been reported that expression of CD134L on vascular endothelial cells is related to various skin diseases.47 Thus, it is possible that administration of the mAb could suppress the infiltration of T cells into CD134L-expressing tissue.
In summary, we believe that anti-CD134L treatment could ameliorate acute GVHD by (1) inhibition of antigen presentation and T-cell priming, (2) modulation of balance between Th1 and Th2 immune responses, (3) inhibition of T-cell migration to target tissue, or (4) a combination of these mechanisms. Our results should help to reveal additional aspects of the complex regulatory mechanisms of acute GVHD and contribute to development of a novel strategy for treatment of this disease.
Acknowledgments
We thank T. Hirano, H. Miyajima, and Y. Hayakawa for technical assistance and helpful suggestions.
Supported by grants from the Ministry of Education, Science and Culture, and the Ministry of Health, Japan.
Reprints:Tetsuji Kobata, Division of Immunology, Institute for Medical Science, Dokkyo University School of Medicine, 880 Kitakobayashi, Mibu, Tochigi 321-0293, Japan; e-mail:tkobata@dokkyomed.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal