Abstract
Lysophosphatidic acid (LPA) is a lipid-derived second messenger that mobilizes many cells of the circulatory and vascular systems to assist in thrombus development and wound healing. LPA, however, has not been tested on human erythrocytes, largely because erythrocytes are considered to be both biologically inert and inactive in intercellular communication. To test this presumption, we have examined the impact of LPA on signaling reactions within the human red blood cell (RBC). Using both 45Ca++ and a Ca++-sensitive fluorescent probe (Fluo-3), we demonstrated that LPA, but not phosphatidic acid or the closely related sphingosine-1–phosphate, stimulates the influx of micromolar quantities of extracellular Ca++ into fresh RBCs. This Ca++ influx was shown to be channel mediated rather than leak promoted because the influx was observed at LPA concentrations too low to perturb membrane integrity, it was inhibited by P-type but not L-type Ca++ channel blockers, it was inhibited by broad-specificity protein kinase inhibitors, and it was not induced by inactive analogues of LPA. Further characterization reveals that only approximately 25% of the RBCs participate in LPA-induced Ca++ entry and that within this active population, Ca++ gating occurs in an all-or-nothing manner. Because the stimulation of Ca++ uptake occurs at LPA concentrations (1-5 μmol/L) known to occur near a developing thrombus and because the internalized Ca++can potentially promote prothrombic properties in the stimulated RBCs, we conclude that RBCs are not insensitive to signals released from other cells.
When platelets are activated, they release varying compounds that recruit the assistance of neighboring cells in clot formation and wound healing. Included among these extracellular messengers/mediators are prostaglandins, adenine nucleotides, platelet-activating factor, serotonin, epinephrine, a variety of adhesive molecules, platelet-derived growth factor, several proinflammatory cytokines, and lysophosphatidic acid.1-5Responses to these signal molecules include expression of adhesion receptors on endothelial cells,6,7 recruitment of resting platelets into active clot formation,8,9 induction of the coagulation cascade,10 constriction of smooth muscle cells in the microvasculature,11,12 and activation of nearby inflammatory cells.13 Notably absent from the cells reported to respond to molecules released by activated platelets are erythrocytes.
The presumed insensitivity of erythrocytes to signals released by activated platelets may stem primarily from the perceived absence of signaling organelles/enzymes in red blood cells (RBCs). Thus, the loss of an RBC nucleus precludes the cell from synthesizing new proteins in response to extracellular stimuli, and the lack of an endoplasmic reticulum prevents the erythrocyte from discharging intracellular stores of Ca++. The absence of mitochondria compromises the cell's ability to engage in apoptotic pathways, and the loss of Golgi and related trafficking organelles precludes any major membrane remodeling. As a consequence, the RBC has acquired a possibly undeserved reputation as a biologically inactive container for hemoglobin with no important functions outside the arena of gas transport. Peculiarly, this reputation has survived despite abundant evidence that the RBC is replete with signaling components such as G proteins, gated ion channels, phospholipases, adenylate and guanylate cyclases, phosphatases, calmodulin and Ca++-activated enzymes, and Ser/Thr and Tyr protein kinases.14-16
To evaluate the responsiveness of the erythrocyte to the needs of other cells in the circulatory system, we have begun to examine the impact of various signal molecules released by activated platelets on erythrocyte biochemistry. In earlier articles by others17,18 and us,19 prostaglandin E2 was shown to induce K+ efflux, RBC shrinkage, and reduced red cell filterability. Although some of these responses could be argued to assist in clot formation, a plausible case for a direct molecular communication between platelets and erythrocytes during hemostasis has not yet been established. Indeed, for this to be achieved, multiple signals discharged by activated platelets must be shown to induce hemostatically meaningful changes in resting RBCs.
Lysophosphatidic acid (LPA) is a prominent second messenger generated in copious amounts by activated platelets.5,20-22Consistent with its biosynthesis by stimulated platelets, LPA is also observed to recruit cells of the circulatory system to assist in clot formation and wound healing. For example, LPA has been shown to activate resting platelets,23 induce the narrowing of intercellular gaps between endothelial cells,24 contract the microvasculature,25 promote proliferation of vascular smooth muscle cells, endothelial cells, and fibroblasts,26,27 induce cell surface binding of fibronectin,28 and mobilize monocytes, lymphocytes, and neutrophils.29-31 Curiously, the impact of LPA on erythrocytes, the cells that constitute approximately 99% of the blood cell mass, has never been examined. The purpose of this article, therefore, is to evaluate whether the human RBC is stimulated by concentrations of LPA that activate other cells of the circulatory system. The data provided below demonstrate that concentrations of LPA that are generated near a developing thrombus indeed activate a signaling pathway in human erythrocytes that changes the Ca++ content of the cell. Because intraerythrocytic Ca++ can induce cell dehydration, phosphatidylserine flip-flop, and changes in membrane skeletal structure, it is conceivable that stimulation by LPA could enlist RBC participation in clot formation.
Materials and methods
Materials
L-α-lysophosphatidic acid was obtained from Sigma Chemical (St. Louis, MO). The LPA was immediately dissolved in phosphate-buffered saline (PBS) at a final concentration of 500 μmol/L, aliquoted into 250-μL fractions, and stored at −20°C until use. A fresh vial was used each week because loss of activity was observed during exposure to air. A23 187, 4-bromo-A23 187, verapamil, nifedipine, sphingosine-1–phosphate (Sph-1–P), ω-agatoxin and Fluo-3 AM were purchased from Calbiochem (San Diego, CA). Phosphatidic acid was from Avanti Polar Lipids (Alabaster, AL), 45Ca++ was from Dupont NEN (Wilmington, DE), and Quin 2/AM was from Molecular Probes (Eugene, OR).
Buffers used were PBS (140 mmol/L NaCl, 5 mmol/L glucose, 5 mmol/L sodium phosphate, pH 7.4), and HEPES (125 mmol/L NaCl, 3 mmol/L KCl, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 16 mmol/L HEPES, 1.2 mmol/L sodium phosphate, and 10 mmol/L glucose, pH 7.4).
Preparation and labeling of red blood cells with Fluo-3/AM
Blood was drawn by venipuncture into acid citrate dextrose solution from healthy human volunteers on the day of each experiment. Red blood cells were washed twice in PBS and twice in HEPES buffer to equilibrate the cells in a Ca++-containing medium. During these washing steps, essentially all the white cells and platelets were aspirated away. Centrifugation steps were conducted at room temperature for 5 minutes at 750g.
Fluo-3/AM loading of erythrocytes32 was performed in the dark using a stock solution of Fluo-3/AM dissolved in dimethyl sulfoxide (2 mg/mL). Washed RBCs suspended at 2 μL packed cells/mL HEPES buffer were incubated in 3 μmol/L Fluo-3/AM at 37°C with shaking for 1 hour. After incubation, the cells were washed with 0.5% bovine serum albumin in PBS, then PBS alone, and finally twice in HEPES buffer (to remove all extracellular Fluo-3). The cells were then resuspended in HEPES buffer at approximately 0.07% hematocrit in preparation for spectroscopic and flow cytometer analyses of LPA-induced Ca++ influxes. Because the formaldehyde generated during hydrolysis of the acetoxymethyl esters of Ca++ chelators (Fluo-3/AM, Quin 2/AM) can gradually deplete erythrocytes of adenosine triphosphate (ATP),33 all Ca++ uptake experiments were conducted within 4 hours of chelator uptake, when ATP levels were still within 80% of their normal value.
Uptake of 45Ca++ by lysophosphatidic acid-stimulated RBCs
Freshly drawn erythrocytes were washed free of contaminating blood cells and suspended at 0.5% hematocrit in HEPES buffer, as described above. To reduce the ability of the erythrocyte's Ca++ATPase from pumping the influxed 45Ca++ rapidly back out of the cell, the RBCs were preincubated with the permeant Ca++ chelator, Quin 2/AM (5 μmol/L final concentration added from a 4 mg/mL stock dissolved in dimethyl sulfoxide) at 37°C with shaking for 1 hour. The cells were then washed with PBS containing 5 mg/mL bovine serum albumin, once with PBS, and twice with HEPES buffer to remove extracellular Quin2. The cells were resuspended in HEPES buffer at 5% hematocrit and equilibrated with 6 to 8 μCi/mL45Ca++ at 37°C with shaking for 10 minutes before the addition of 5μmol/L LPA (dissolved in PBS) or PBS alone (negative control). At the desired times after stimulation, 200-μL aliquots of the shaken cell suspension were removed and centrifuged to pellet the erythrocytes. Cell pellets were washed first in PBS containing 5 mmol/L EGTA and then 3 times in PBS before lysis in deionized water. Twenty microliters lysed suspension were removed for quantitation of hemoglobin using a Drabkin's assay kit from Sigma Chemical, and the remaining suspension was decolorized with H2O2 before liquid scintillation analysis of the 45Ca++ content.
Results
Lysophosphatidic acid induces Ca++ influx into human erythrocytes
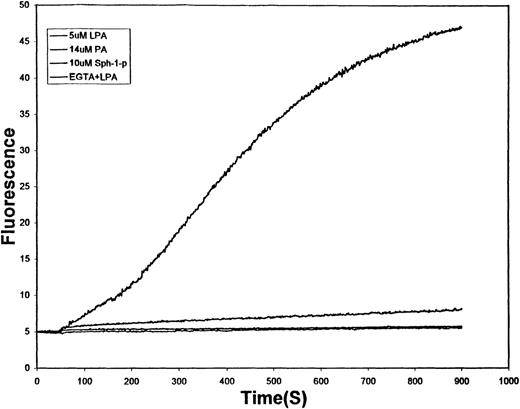
One of the more universal effects of platelet-derived LPA on target cells in the vasculature is the influx of extracellular Ca++.20,27 34 To evaluate whether a similar response might be evoked in human erythrocytes, RBCs were loaded with Fluo-3/AM, a long-wavelength, cell-permeant, Ca++-sensitive fluorophore with an affinity for Ca++ in the high nanomolar to micromolar range. As shown in Figure 1, the stimulation of Fluo-3 containing erythrocytes with 5 μmol/L LPA induces a slow increase in intracellular Ca++ that approaches a maximum approximately 15 minutes after LPA administration. In contrast, related lipid-derived second messengers commonly generated by other cell types in the course of their signaling functions (14 μmol/L phosphatidic acid, which differs from LPA only in the presence of an acyl chain at the sn-2 position, and 10 μmol/L Sph-1-P, which is the sphingolipid analogue of LPA) promoted little increase in Fluo-3 fluorescence. To ensure that the LPA-stimulated rise in Fluo-3 fluorescence was indeed a consequence of Ca++ entry, 5 mmol/L EGTA was added to the cell suspension immediately before LPA was added. Under these conditions, no change in erythrocyte Fluo-3 fluorescence could be detected (see lowest tracing, Figure 1). These data demonstrate that LPA promotes the influx of extracellular Ca++ into RBCs by a pathway that can distinguish between closely related, commonly induced lipid second messengers.
Effect of lipid-derived second messengers on the kinetics of Ca++ influx into human erythrocytes.
Washed human erythrocytes loaded with Fluo-3/AM and suspended in HEPES buffer (125 mmol/L NaCl, 3 mmol/L KC1, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 1.2 mmol/L sodium phosphate, 10 mmol/L glucose, 16 mmol/L HEPES, pH 7.4) were stirred at 0.07% hematocrit in a fluorescence spectrophotometer and treated at time (t = 60 seconds) with 5 μmol/L LPA (top tracing), 14 μmol/L PA (middle tracing), 10 μmol/L Sph-1-P (lower tracing), or 5 mmol/L EGTA followed by 5 μmol/L LPA (lowest tracing). All lipid stimulants were added from stock solutions prepared in PBS. The increase in fluorescence (λex = 506 nm, λem = 530 nm) caused by Ca++ binding to Fluo-3 was then monitored as a function of time. Labeled RBCs not treated with any stimulant displayed no change in Fluo-3 fluorescence.
Effect of lipid-derived second messengers on the kinetics of Ca++ influx into human erythrocytes.
Washed human erythrocytes loaded with Fluo-3/AM and suspended in HEPES buffer (125 mmol/L NaCl, 3 mmol/L KC1, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 1.2 mmol/L sodium phosphate, 10 mmol/L glucose, 16 mmol/L HEPES, pH 7.4) were stirred at 0.07% hematocrit in a fluorescence spectrophotometer and treated at time (t = 60 seconds) with 5 μmol/L LPA (top tracing), 14 μmol/L PA (middle tracing), 10 μmol/L Sph-1-P (lower tracing), or 5 mmol/L EGTA followed by 5 μmol/L LPA (lowest tracing). All lipid stimulants were added from stock solutions prepared in PBS. The increase in fluorescence (λex = 506 nm, λem = 530 nm) caused by Ca++ binding to Fluo-3 was then monitored as a function of time. Labeled RBCs not treated with any stimulant displayed no change in Fluo-3 fluorescence.
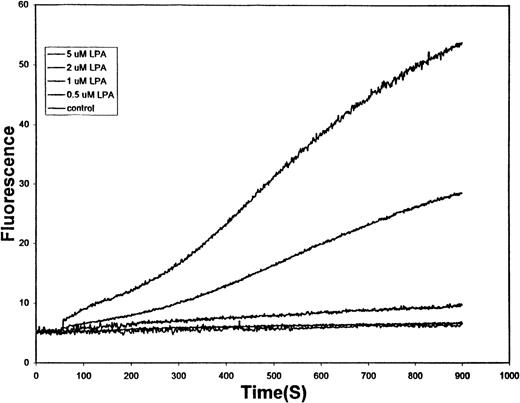
It has been reported that LPA concentrations in coagulated serum generally range from 1 to 10 μmol/L5,20-22 and that in the direct vicinity of a clot, the corresponding levels can reach as high as 20 μmol/L.21 To determine whether RBCs are sensitive to LPA concentrations commonly generated near the site of a wound, we conducted the influx assay described above as a function of the LPA content in the medium. As seen in Figure2, all LPA concentrations between 1 and 5 μmol/L induced measurable entry of Ca++ into cells. Indeed, a monotonic increase in Ca++ uptake could be seen as LPA levels increased through the physiological range. Higher LPA concentrations were observed to promote still higher rates of Ca++ entry, but these tracings were often truncated because of saturation of the intracellular Ca++ fluorophore (data not shown).
Effect of physiologic concentrations of LPA on the kinetics of Ca++ entry into human RBCs.
Erythrocytes prepared and loaded with Fluo-3, as described in the legend to Figure 1, were treated with (top to bottom tracing) 5 μmol/L LPA, 2 μmol/L LPA, 1 μmol/L LPA, 0.5 μmol/L LPA, or no LPA at time t = 60 seconds. The change in Fluo-3 fluorescence caused by Ca++ entry into the cells was then monitored by fluorescence spectroscopy.
Effect of physiologic concentrations of LPA on the kinetics of Ca++ entry into human RBCs.
Erythrocytes prepared and loaded with Fluo-3, as described in the legend to Figure 1, were treated with (top to bottom tracing) 5 μmol/L LPA, 2 μmol/L LPA, 1 μmol/L LPA, 0.5 μmol/L LPA, or no LPA at time t = 60 seconds. The change in Fluo-3 fluorescence caused by Ca++ entry into the cells was then monitored by fluorescence spectroscopy.
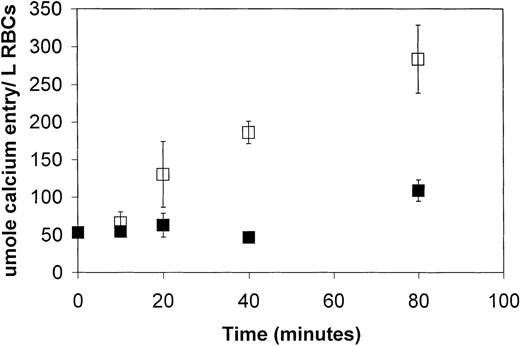
To obtain a more quantitative evaluation of the capacity of LPA to promote Ca++ influx into RBCs,45Ca++ was used to monitor the cation's influx instead of the above fluorescent dye. In this case, however, the RBCs were preloaded with a Ca++ chelating agent (Quin-2/AM) to retard the otherwise rapid expulsion of influxed45Ca++ by the their Ca++-ATPase.35 Under these conditions, 5 μmol/L LPA was seen to promote a 4.5-fold increase in the rate of Ca++ uptake by the RBCs (Figure3). By 80 minutes' incubation this influx could be seen to have increased the Ca++ content of the cell by approximately 170 μmol/L RBCs over unstimulated controls. Because most of the influxed 45Ca++ was likely complexed by Quin2 and other endogenous Ca++ chelators, it was difficult to predict the level of free Ca++ that could be induced by LPA. Certainly, with the Ca++-ATPase still active, elevated free Ca++ levels would not have been stable for any extended duration.
LPA stimulation of 45Ca++entry into fresh human erythrocytes as a function of time.
RBCs were washed, loaded with Quin-2 (a Ca++ chelator), and suspended in HEPES buffer containing tracer amounts of45Ca++. After a 30-minute equilibration, RBCs were stimulated with 5 μmol/L LPA (open squares) or buffer alone (closed squares). At the desired times after stimulation, the cells were washed and assayed for hemoglobin content and radioactivity, as described in “Materials and methods.” The data points represent the mean ± SD, where n = 6.
LPA stimulation of 45Ca++entry into fresh human erythrocytes as a function of time.
RBCs were washed, loaded with Quin-2 (a Ca++ chelator), and suspended in HEPES buffer containing tracer amounts of45Ca++. After a 30-minute equilibration, RBCs were stimulated with 5 μmol/L LPA (open squares) or buffer alone (closed squares). At the desired times after stimulation, the cells were washed and assayed for hemoglobin content and radioactivity, as described in “Materials and methods.” The data points represent the mean ± SD, where n = 6.
Not all erythrocytes respond to lysophosphatidic acid
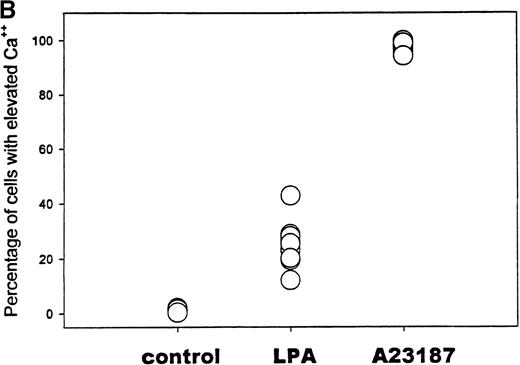
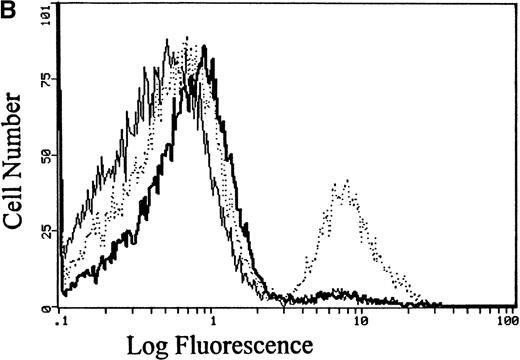
Because the above analyses of Ca++ influx report only the average response of the entire erythrocyte suspension, no information could be extracted from these data on the fraction of RBCs that actually participates in the LPA-triggered Ca++ entry. To resolve this question, Fluo-3–loaded erythrocytes were stimulated with 5 μmol/L LPA and then examined 10 minutes later by flow cytometry. As seen in Figure 4A, unstimulated cells remained essentially nonfluorescent, whereas A23 187 ionophore-treated cells, as anticipated, sorted strongly with the fluorescent population. Peculiarly, LPA-incubated cells displayed a biphasic distribution; approximately 25% of the cells exhibited a fluorescence intensity comparable to that of ionophore-treated cells, and the remaining population of erythrocytes displayed only slightly elevated fluorescence. Similar results were obtained in multiple replicates using blood samples from a variety of donors (Figure 4B). These data suggest that a large fraction of the erythrocytes is insensitive to LPA stimulation and that response to LPA is largely an all-or-nothing process for any individual cell. The data further suggest that the internalized Ca++ quantitated in Figure 3must be concentrated in the cytosol of only one fourth of the total population of treated RBCs, resulting in an average elevation of approximately 680 μmol Ca++/L RBC in participating cells.
Flow cytometric analysis of the fraction of RBCs that respond to stimulation with LPA.
(A) RBCs loaded with Fluo-3 were treated with buffer only (thin line), 5 μmol/L LPA (dotted line), or 2 μmol/L of the Ca++ionophore, A23 187 (thick line). After 10 minutes' incubation at 37°C, the cell suspension was evaluated for fluorescent cells using an EPICS Elite flow cytometer. (B) Summary of the flow cytometry data collected on 10 separate blood samples, showing the percent positively sorted cells in each sample. Where all 10 data points are not visible, the data points overlap.
Flow cytometric analysis of the fraction of RBCs that respond to stimulation with LPA.
(A) RBCs loaded with Fluo-3 were treated with buffer only (thin line), 5 μmol/L LPA (dotted line), or 2 μmol/L of the Ca++ionophore, A23 187 (thick line). After 10 minutes' incubation at 37°C, the cell suspension was evaluated for fluorescent cells using an EPICS Elite flow cytometer. (B) Summary of the flow cytometry data collected on 10 separate blood samples, showing the percent positively sorted cells in each sample. Where all 10 data points are not visible, the data points overlap.
What is the nature of the Ca++ influx pathway?
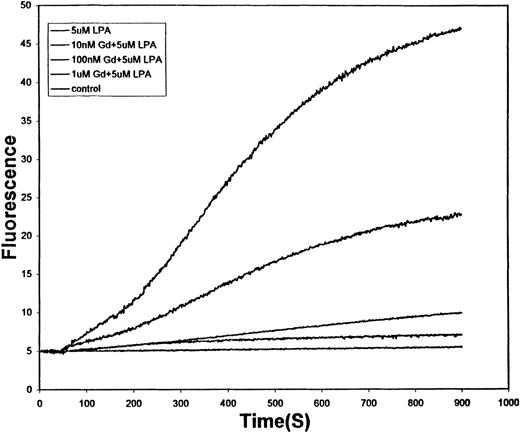
Numerous Ca++ influx pathways have been identified in mammalian cells,36,37 and at least 2 have been inferred from kinetic data in human erythrocytes.36,38,39 To obtain a clearer understanding of the nature of the LPA-stimulated Ca++ influx pathway, we have examined the sensitivity of the pathway to a variety of Ca++ influx inhibitors. As shown in Figure 5, the relatively nonselective Ca++ channel blocker, Gd+3, diminished Ca++ entry over the concentration range (10-1000 nmol/L), where it commonly inhibits other well-characterized mammalian Ca++ channels.40 This observation confirmed that LPA-induced Ca++ uptake is mediated by a specific pathway rather than a nonspecific leak.
Effect of various concentrations of Gd+3 on the LPA-stimulated influx of Ca++ into fresh human RBCs.
Conditions are the same as those described in Figure 1, except 5 μM LPA was used as the stimulus in all tracings other than the untreated control (lowest tracing). Gadolinium concentrations used from top to bottom are 0 nmol/L, 10 nmol/L, 100 nmol/L, 1 μmol/L, and 0 μmol/L (control).
Effect of various concentrations of Gd+3 on the LPA-stimulated influx of Ca++ into fresh human RBCs.
Conditions are the same as those described in Figure 1, except 5 μM LPA was used as the stimulus in all tracings other than the untreated control (lowest tracing). Gadolinium concentrations used from top to bottom are 0 nmol/L, 10 nmol/L, 100 nmol/L, 1 μmol/L, and 0 μmol/L (control).
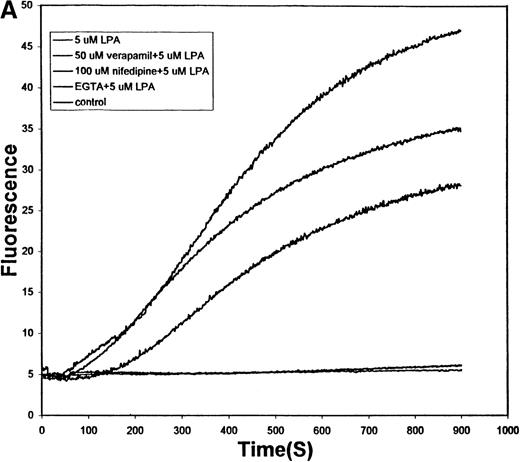
Figure 6A displays the effect of 2 L-type Ca++ channel blockers on the same LPA-induced Ca++ uptake. In contrast to Gd+3, verapamil and nifedipine only partially reduce Ca++ entry into the RBCs, and this reduction is seen only at abnormally high inhibitor concentrations. These data argue that the LPA-stimulated influx pathway does not involve a classical L-type Ca++ channel.
Effect of L-type and P-type Ca++ channel blockers on the LPA-induced influx of Ca++ into human RBCs.
(A) Conditions are the same as those described in Figure 5, except that the RBCs were pretreated for 10 minutes with (top to bottom tracings) buffer only, 50 μmol/L verapamil, 100 μmol/L nifedipine, or 5 mmol/L EGTA before stimulation with 5 μmol/L LPA. The bottom tracing displays the response of unstimulated red cells. (B) RBCs were prepared as in Figure 6A, but the pretreatment involved a 20-minute exposure to buffer only before induction with 5 μmol/L LPA (dotted line), a 20-minute exposure to 1 μmol/L ω-agatoxin before stimulation with 5 μmol/L LPA (thick line), or treatment with buffer only (thin line). After the desired LPA stimulation (approximately 10 minutes), the cells were analyzed by flow cytometry as in Figure 4. Flow cytometry was used instead of fluorescence spectroscopy to reduce the amount of ω-agatoxin required to conduct the study. Agatoxin pretreatment reduced the fraction of fluorescent cells (Ca++ positive) from 21% to 4% in this sample. The unstimulated control RBCs contained 3% fluorescent cells.
Effect of L-type and P-type Ca++ channel blockers on the LPA-induced influx of Ca++ into human RBCs.
(A) Conditions are the same as those described in Figure 5, except that the RBCs were pretreated for 10 minutes with (top to bottom tracings) buffer only, 50 μmol/L verapamil, 100 μmol/L nifedipine, or 5 mmol/L EGTA before stimulation with 5 μmol/L LPA. The bottom tracing displays the response of unstimulated red cells. (B) RBCs were prepared as in Figure 6A, but the pretreatment involved a 20-minute exposure to buffer only before induction with 5 μmol/L LPA (dotted line), a 20-minute exposure to 1 μmol/L ω-agatoxin before stimulation with 5 μmol/L LPA (thick line), or treatment with buffer only (thin line). After the desired LPA stimulation (approximately 10 minutes), the cells were analyzed by flow cytometry as in Figure 4. Flow cytometry was used instead of fluorescence spectroscopy to reduce the amount of ω-agatoxin required to conduct the study. Agatoxin pretreatment reduced the fraction of fluorescent cells (Ca++ positive) from 21% to 4% in this sample. The unstimulated control RBCs contained 3% fluorescent cells.
Figure 6B examines the ability of ω-agatoxin, a P-type Ca++ channel blocker, to inhibit the same pathway. In contrast to the dihydropyridine derivatives tested above, this spider venom peptide strongly reduces Ca++ influx through the LPA-activated channel. Thus, whereas 5 μmol/L LPA induced approximately 21% of the Fluo-3–loaded RBCs to shift into the positively sorted (fluorescent) fraction, only background numbers of the ω-agatoxin–treated RBCs joined the same population. Based on these comparisons, we propose that the Ca++ influx proceeds through a pathway more closely related to P-type than L-type Ca++ channels.
LPA stimulates Ca++ influx by a kinase-regulated signaling cascade
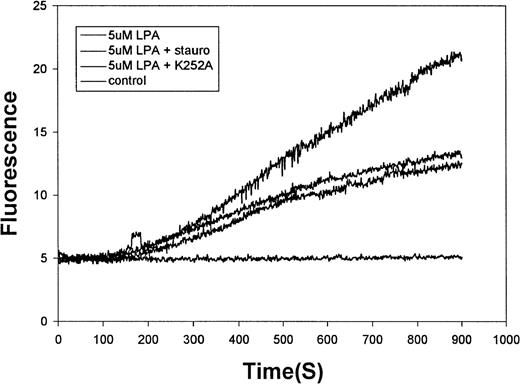
The molecular identity of a Ca++ channel can also be partly defined by its mode of activation. That is, certain Ca++ channels are directly ligand gated, whereas others require a signaling pathway to stimulate their open state. As a preliminary evaluation of the involvement of signaling intermediates in the molecular mechanism of channel opening, we investigated the impact of 2 broad-specificity protein kinase inhibitors on the LPA-induced fluorescence increase. As shown in Figure7, both staurosporine (1 μmol/L) and K-252a (1 μmol/L) modestly inhibited LPA-induced Ca++entry, suggesting the possible involvement of RBC kinases in regulating the activity of the channel. Because these inhibitors suppress the activities of a number of red cell kinases, it is not currently possible to identify the kinase involved.
Effect of kinase inhibitors on the LPA-stimulated influx of Ca++ into human RBCs.
Erythrocytes prepared as in Figure 1 were preincubated for 10 minutes with (top to bottom tracings) buffer only, 1 μmol/L staurosporine, or 1 μmol/L K252a before stimulation with 5μmol/L LPA. The bottom tracing displays the response of unstimulated red cells.
Effect of kinase inhibitors on the LPA-stimulated influx of Ca++ into human RBCs.
Erythrocytes prepared as in Figure 1 were preincubated for 10 minutes with (top to bottom tracings) buffer only, 1 μmol/L staurosporine, or 1 μmol/L K252a before stimulation with 5μmol/L LPA. The bottom tracing displays the response of unstimulated red cells.
Discussion
We have demonstrated that LPA, a water-soluble lipid second messenger released from activated platelets, induces Ca++influx into human erythrocytes. Several lines of evidence argue that the Ca++ uptake is mediated by a well-defined Ca++ channel rather than by a nonspecific Ca++leak. First, the influx is stimulated at LPA concentrations well below its critical micelle concentration (approximately 70 μmol/L to 1 mmol/L),5 where detergent-like effects might be anticipated. Indeed, in studies of LPA action on other cell types, LPA concentrations significantly exceeding 10 μmol/L are not uncommon.41-43 Second, the channel is inhibited by Ca++ channel blockers that would not be expected to obstruct a nonspecific membrane leak. Third, other membrane-active agents (phosphatidic acid, Sph-1–P) exert no effect on RBC Ca++ uptake, even at higher concentrations than the LPA levels used in this study. Finally, the LPA stimulation is at least partially dependent on the activity of an erythrocyte kinase. Because Ca++ channel activation is seen at LPA concentrations thought to exist near a developing clot, these results suggest that an activated platelet may be capable of communicating its “wound alert” to red cells in its immediate vicinity.
Although no signaling intermediates were characterized in this article, the rudimentary signaling data presented here are not inconsistent with the known actions of LPA on other hematopoietic cells. Thus, in a number of these cell types, LPA is believed to bind to a G protein-coupled receptor that activates a C-type phospholipase20 21 that in turn generates diacylglycerol and 1,4,5-inositoltrisphosphate. These second messengers are then believed to activate protein kinase C and to promote the release of Ca++ into the cell. Although Ca++influx and kinase activation are clearly implicated in the red cell response, it will now be important to examine the signaling intermediates in LPA-treated RBCs to determine whether they correspond to those stimulated by LPA in other blood cells.
As noted in the “Introduction,” the effects of LPA on other cells of the circulatory system can be reasonably argued to enhance either development of the thrombus or recovery of the tissue surrounding the wound. The question, therefore, arises regarding what meaningful functions might be promoted by the ability of RBCs to respond to LPA. Three possible observations come to mind, but their expression in vivo must obviously await further investigation. First, an influx of Ca++ could conceivably activate the phospholipid scramblase leading to phosphatidylserine exposure on the RBC's external leaflet32,44 and conversion of the RBC to a procoagulant state.45 Second, Ca++ entry can activate the Gardos channel, resulting in K+ efflux and the consequent reduction in RBC volume that causes impaired RBC filterability.17,46 Third, although molecular mechanisms have not been defined, the RBC has been shown to enhance the ability of stimulated platelets to aggregate and release adenosine diphosphate, serotonin, and other prothrombic agents.47 48 For the erythrocyte to render this assistance, it must somehow be notified of the need for platelet stimulation and then respond to this notification. Conceivably, LPA could mediate this intercellular communication. It is also possible, however, that the LPA-gated Ca++ is pumped from the RBC as rapidly as it enters, resulting in no change in erythrocyte properties.
Finally, it should be noted that another platelet-generated signal, prostaglandin E2, may also elicit its changes in RBC behavior through the activation of Ca++ influx.19Although the magnitude of the PGE2 effect is smaller than that observed with LPA, it is possible that recruitment of a resting RBC to the clotting process requires multiple concurrent signals, all inducing the same biologic changes. The mounting evidence supporting a role for the erythrocyte in thrombus formation48 suggests that this type of intercellular communication warrants further scrutiny.
Supported in part by National Institutes of Health grants GM24417-20 and K08HL03350-04.
Reprints:Philip S. Low, Department of Chemistry, Purdue University, 1393 Brown Bldg, West Lafayette, IN 47907-1393; e-mail:lowps@omni.cc.purdue.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal