The antigen-mediated activation of mast cells by means of IgE antibodies bound to the cell surface leads to direct interactions between FcɛRI receptor cytoplasmic domains and various intracellular proteins. These interactions initiate diverse signal-transduction pathways, and the activation of these pathways results in the immediate release of proinflammatory agents. A delayed response also occurs and includes the release of various cytokines. It is clear that the activation of kinases is a requirement for the exocytosis observed in mast cells. In addition to the tyrosine phosphorylation of the affected system by soluble tyrosine kinases, activity of protein kinase C (PKC) results in serine or threonine phosphorylation of multiple protein substrates. In this study, we found that mast cells derived from PKCβ-deficient mice produce less interleukin 6 in response to IgE-Ag. The inhibition of exocytosis in the PKCβ-deficient mast cells occurred whether the stimuli were due to the aggregation of the mast cell surface FcɛRI or to the calcium ionophore, ionomycin. However, no significant changes were observed in the proliferative response of the mast cells to interleukin 3 (IL-3) or in their apoptotic rate after IL-3 depletion.
Protein kinase C (PKC) enzymes constitute a family of regulatory proteins that play a major role in receptor-mediated signal transduction and are involved in the regulation of a variety of physiologic processes, including differentiation, proliferation, and apoptosis.1 The role of a specific PKC isozyme in exocytosis was first described in studies in rat pituitary cells, in which secretion of luteinizing hormone was reconstituted by PKCα and PKCβ.2 It was observed that the rat mucosa-like mast cell line, RBL-2H3, contains at least 5 species of PKC isozymes: α, β, δ, ε, and ζ.3 Both calcium-dependent and calcium-independent PKC isoenzymes are activated in RBL cells in response to FcεRI-receptor aggregation.3 It was also shown that PKCβ and, to some extent, PKCδ are the isonymous involved in the secretory response to antigen activation in reconstituted permeabilized RBL cells.3 Moreover, PKCδ was implicated as a key element in the early events of phosphorylation of the mast cell FcεRI.4
Evidence for a requirement of PKC activity in gene expression has accumulated during the past 20 years. Studies in our laboratory implicated PKCs in the regulation of mast cell growth and in the expression of transcription factors in response to the aggregation of FcεRI.5,6 These studies suggested a role for PKC activity in the regulation of the transcription factor complex activating protein 1 (AP-1). We also reported that PKCβ and PKCε could serve as a link between the aggregation of FcεRI and the subsequent expression of c-fos and c-jun.7 Moreover, we found that PKCβ could serve as a link for upstream stimulating factor 2 expression in response to aggregation of FcεRI.8
Interleukin-6 (IL-6) is a multifunctional cytokine that is produced by activated mast cells9 and regulates cellular function in many cell lineages. A clear association between PKC activity and IL-6 synthesis has been found in studies using various culture systems.10-12 It was reported that up-regulation of PKCβ in mast cells was associated with a significant increase in the accumulation of IL-6 messenger RNA (mRNA).13 The IL-6 promoter contains the AP-1 binding site, and it was shown that a specific JunD-JunD AP-1 complex is essential for IL-6 induction by transforming growth factor-β (TGF-β).14 In the current study, we analyzed the level of JunD mRNA in PKCβ-deficient cells to provide insights into the possible role of this member of the AP-1 family in IL-6 gene regulation by PKCβ.
Mice lacking PKCβ were produced and used to demonstrate specific effects on B and T lymphocytes.15 The phenotype of these mice was similar to that of Bruton tyrosine kinase (Btk)-deficient mice in several ways. Both have a greatly reduced number of peritoneal B1B cells, lowered IgM and IgG-3 levels, a drastic reduction in the immune response to T-cell–independent antigen, and a markedly reduced proliferative response of B cells to IgM and other stimulants.15-17 One explanation for this similarity in phenotype is that it is due to the direct binding of Btk and PKCβ and the involvement of PKCβ in the Btk signal-transduction pathway.17
In the present work, bone marrow-derived mast cells (BMMC) from PKCβ-deficient mice were analyzed with respect to their proliferation, survival rate, degranulation capability, and IL-6 mRNA accumulation.
Materials and methods
Animals and cell culture
The production of mice lacking PKCβ was previously described.15 The mice had a mixed 129/Sv/129/Ola background, with controls derived from litter mates of heterozygous matings. In the current study, PKCβ-deficient mice and control litter mates were killed on the same day. Mouse BMMC were obtained as previously described.18 Briefly, bone marrow cells were obtained from the femurs and tibias of control and PKCβ-deficient 2-month-old mice. We used bone marrow from 1 or 2 mice per flask. Cells from control and PKCβ-deficient mast cells were grown in individual flasks according to the date the mice were killed. The cells were cultured at a starting density of 0.1 × 106 cells/mL at 37°C in a humidified atmosphere containing 5% carbon dioxide (CO2). The culture medium was RPMI 1640 supplemented with 2 mmol/L of L-glutamine, 2 mmol/L of nonessential amino acids, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 50 μmol/L of β-2-mercaptoethanol, 10% fetal-calf serum, and 100 U/mL of yeast-derived interleukin 3 (IL-3; Genzyme Diagnostics, Cambridge, MA). The nonadherent cells from the bone marrow cultures were transferred every 7 days into fresh IL-3–containing medium after verification that there were similar cell densities for the control and PKCβ-deficient cells. The cultures were maintained for 3 to 5 weeks. Mast cells were identified by toluidine blue staining.
Obtaining and staining peritoneal mast cells
Mice were injected with 10 mL of normal saline intraperitoneally. After gentle abdominal massage, the peritoneal fluid was aspirated, 250 μL of the fluid was centrifuged, and the pellet was resuspended in 1 mL of normal saline. Then, 200 μL of this suspension was spun for 5 minutes in a cytospin centrifuge. The cells were stained by using a modification of the original Enerbac protocol19 that uses 0.5% Alcian blue and 0.3% acetic acid. They were then rinsed with water and counterstained with 0.1% safranin and 0.1% acetic acid.
IgE-Ag and ionomycin-mediated mast cell degranulation
IgE sensitization was carried out by incubating 2 × 106 cells with 30 μg of monoclonal IgE against dinitrophenol (DNP) (provided by F.-T. Liu, La Jolla, CA) for 2 hours at 37°C in RPMI medium. The cells were washed once with 1 mL of Tyrode buffer and resuspended in the same buffer. Fifty-microliter aliquots of this cell suspension were put into microfuge tubes containing increasing amounts of human serum albumin (HSA) conjugated to DNP (DNP-HSA) or ionomycin (Sigma, St Louis, MO) for 45 minutes. The release of β-hexosaminidase was then determined in triplicate in a 96-well plate. The results were expressed as the net percentage release of hexosaminidase, as previously described.20
For experiments investigating cytokine mRNA production and release, the cells were preincubated for 4 days with IgE at the same concentration described above and then incubated with 100 ng/mL of HSA-DNP in tissue-culture medium for 8 to 10 hours. This incubation time was based on the work by Zhu et al21 and Marshall et al,22 in which the production of IL-6 in activated mast cells was determined on the level of mRNA and of protein after overnight incubation. The cells were then centrifuged and the supernatants were collected and frozen at −70°C for later cytokine determination. The pellets containing the activated mast cells were used for RNA preparation. The IL-6 secreted into the medium was measured by an enzyme-linked immunosorbent assay kit (Endogen, Woburn, MA).
Proliferation assay
The proliferation assay used was described previously.17Cells were cultured in 200 μL of medium supplemented with doses of mouse IL-3 defined according to the requirements of each experiment. Cells from each treatment were divided into 4 replicates and were incubated for 24 hours at 37°C in 5% CO2 in round-bottomed 96-well microtiter plates (Nunc, Denmark) at a density of 2 × 105 cells per well. The cells were labeled with 0.037 MBq of tritium-thymidine for the last 4 hours at 37°C and transferred onto glass-fiber filter paper (Titerek, United Kingdom). Thymidine incorporation was measured as described previously.18
Measurement of apoptosis
BMMC were analyzed for apoptosis by using 2-color flow cytometry. The cells were stained with annexin V antibody (Boehringer Mannheim, Germany)23 and then with biotin-labeled antimouse antibody, followed by avidin-phycoerythrin (BD-Pharmingen, San Diego, CA). The cells were also stained with the fluorescent DNA binding agent 7-amino actinomycin D (7AAD), which was used as a substitute for propidium iodide to mark the cells in the last phase of apoptosis.24The percentage of cell survival was calculated by subtracting the number of cells stained with 7AAD, which stains DNA in dead cells, from the total cell count.
Reverse transcriptase-polymerase chain reaction (RT-PCR) of cytokines
Cell pellets were lysed directly with 1 mL of Tri-Reagen, and total RNA was isolated and purified according to the manufacturer's instructions. The following primer pairs were designed from the mouse complementary DNA sequences available in GenBank: for IL-6, 5′-CTGGTGACAACCACGGCCTCCCCT-3′ and the 3′ primer 5′-TGGATCACGCAATACGGATTCGTA-3′, which amplify a 620 base pair (bp); for interleukin 10 (IL-10), 5′-CCAGTTTTACCTGGTAGAAGTGATG-3′ and the 3′ primer 5′-TGTCTAGGTCCTGGAGTCCAGCAGACTCAA-3′, which amplify a 440 bp; for β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and the 3′ primer 5′-GCCTGACAATGACTCGACGCAAA-3′, which amplify a 348 bp; and for JunD, 5′-ATGGAAACGCCCTTCTATGGC-3′ and the 3′ primer 5′-CGTTCTTGCGTGTCCATGTCG-3′, which amplify a 783 bp.
Reverse transcription and polymerase chain reaction (PCR) were done by using the Titan one-tube RT-PCR System (Boehringer Mannheim). The linear range of amplification was determined by measuring gene expression after 25 to 35 cycles (nonsaturated PCR product). After the PCR reaction, loading buffer was added to each 5-μL reaction and aliquots were resolved on 2% agarose gel containing 0.008% ethidium bromide. DNA molecular-weight standards were run in parallel.
Results
Peritoneal mast cells
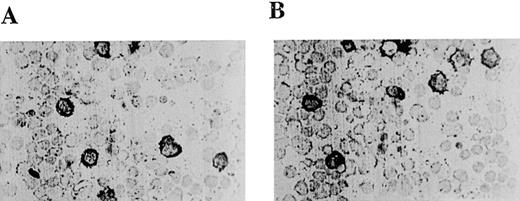
Peritoneal mast cells are different in many respects from cultured BMMC. For instance, they contain heparin in their granules that is stained strongly by safranin, whereas BMMC contain chondroitin sulfate E in their granules that is stained by Alcian blue.19 In vitro, BMMC can differentiate into heparin-containing mast cells if coincubated with fibroblasts or c-kit ligand.25 26Thus, primary mast cell cultures like BMMC are now considered precursors of heparin-containing mast cells. If heparin-containing mast cells are dependent on PKCβ for their differentiation, one would not expect to see peritoneal mast cells from PKCβ-deficient mice stained with safranin. However, we found that the peritoneal mast cells in cytospin preparations from mouse peritoneum from both control and PKCβ-deficient mice were stained with safranin (Figure1A and Figure 1B). Hence, PKCβ-deficient mice, in contrast to mouse strains such as c-kit-deficient mice, contain large numbers of mast cells that can differentiate into heparin-containing mast cells.
Light micrographs of peritoneal mast cells from control mice (A) and mice deficient in protein kinase Cβ(B).
Peritoneal exudates were stained with Alcian blue and counterstained with safranin.
Light micrographs of peritoneal mast cells from control mice (A) and mice deficient in protein kinase Cβ(B).
Peritoneal exudates were stained with Alcian blue and counterstained with safranin.
Replication and survival
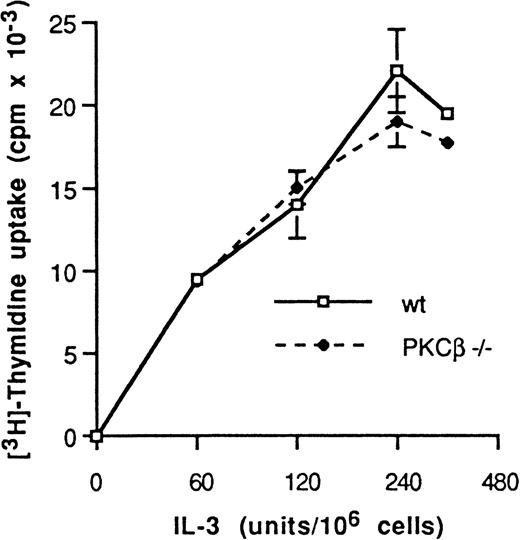
Our initial assessment of BMMC cell growth was by cell count after 2, 3, and 4 weeks of cell culture. At this stage, most of the cells were mast cells (as determined by toluidine blue staining). BMMC cell density increased in similar proportions in control and PKCβ-deficient mast cells (data not shown). We then measured cell proliferation in 3 individual experiments. BMMC derived from wild-type and from PKCβ-deficient mice were incubated for 48 hours in the presence of increasing concentrations of IL-3 and were assessed for their rate of proliferation by tritium-thymidine incorporation into DNA and cell number (Figure 2). A similar pattern of IL-3 dose-dependent proliferation rate was observed in these 2 types of BMMC. Thus, the deficiency of PKCβ in these cells did not affect their rate of proliferation.
Dose response to interleukin 3 of mast cell DNA synthesis.
Protein kinase C (PKC) β-deficient and wild-type mast cells were incubated for 24 hours with medium containing increasing doses of interleukin 3 (IL-3) and then with tritium-thymidine for 4 hours. The level of incorporation of radioactivity into DNA (mean ± SE) was quantified for each condition in quadruplicate. One representative experiment of 3 is shown.
Dose response to interleukin 3 of mast cell DNA synthesis.
Protein kinase C (PKC) β-deficient and wild-type mast cells were incubated for 24 hours with medium containing increasing doses of interleukin 3 (IL-3) and then with tritium-thymidine for 4 hours. The level of incorporation of radioactivity into DNA (mean ± SE) was quantified for each condition in quadruplicate. One representative experiment of 3 is shown.
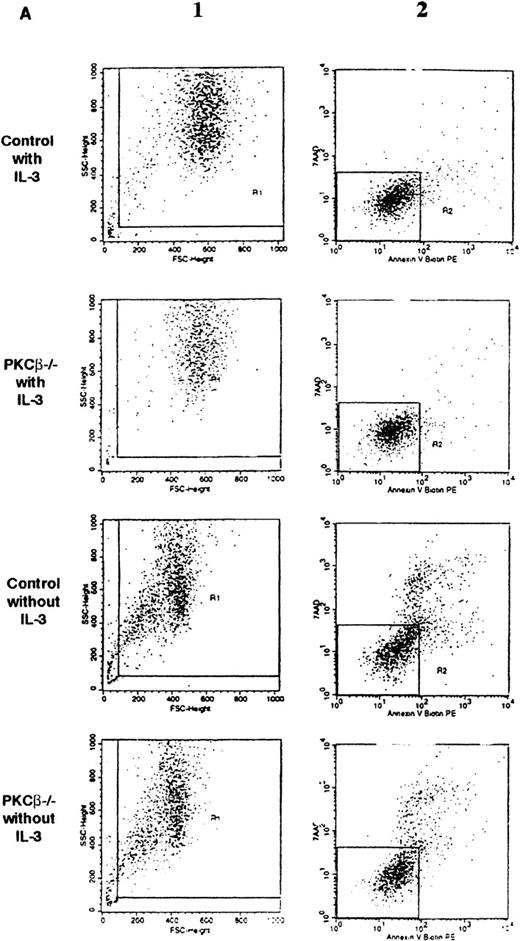
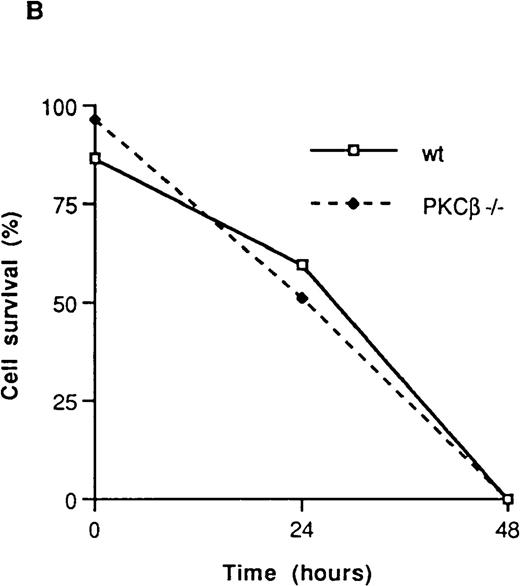
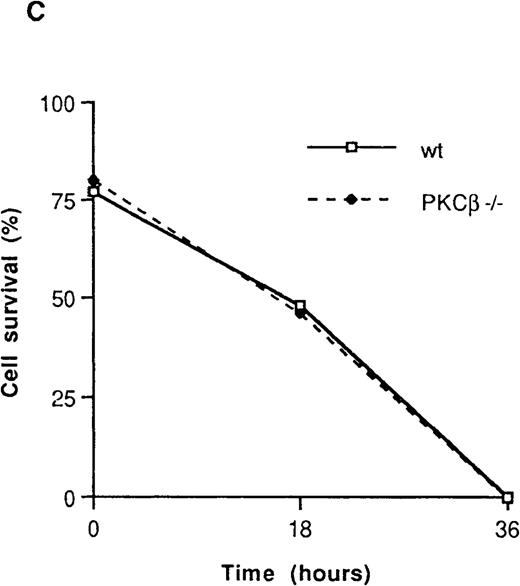
The effect of IL-3 deprivation on apoptosis of BMMC derived from the PKCβ-deficient mice was also assessed in 3 separate experiments. These experiments were carried out with 2 different staining reagents: annexin V, which stains phosphatidylserine and thus determines an early event in apoptosis23; and 7AAD, which stains DNA and so reveals apoptotic cells at a later stage.20PKCβ-deficient and wild-type mast cells were incubated with or without IL-3 for 18 hours and then stained with annexin V and 7AAD. As shown in Figure 3A and Figure 3B, the rates of apoptosis after IL-3 deprivation were similar in PKCβ-deficient and control BMMC.
Fluorescence flow cytometry analysis of apoptotic mast cells.
(A) PKCβ-deficient and wild-type mast cells were incubated with IL-3 (upper panels) or without IL-3 for 18 hours and stained with annexin V and 7-amino actinomycin D (7AAD). The left-hand column shows the forward- and side-scatter analysis of the cells. The right-hand column shows a 2-dimensional representation of cells derived from the left-hand column by using annexin V and 7AAD. (B) Graph based on results obtained by fluorescence-activated cell separation analysis done in a manner similar to the analysis shown in Figure 2A for PKCβ-deficient mast cells compared with wild-type cells after growth factor deprivation. (C) A similar graph for cells treated with mitomycin C. Here, the cells were grown with only 20 U of IL-3.
Fluorescence flow cytometry analysis of apoptotic mast cells.
(A) PKCβ-deficient and wild-type mast cells were incubated with IL-3 (upper panels) or without IL-3 for 18 hours and stained with annexin V and 7-amino actinomycin D (7AAD). The left-hand column shows the forward- and side-scatter analysis of the cells. The right-hand column shows a 2-dimensional representation of cells derived from the left-hand column by using annexin V and 7AAD. (B) Graph based on results obtained by fluorescence-activated cell separation analysis done in a manner similar to the analysis shown in Figure 2A for PKCβ-deficient mast cells compared with wild-type cells after growth factor deprivation. (C) A similar graph for cells treated with mitomycin C. Here, the cells were grown with only 20 U of IL-3.
Mitomycin C is a chemotherapeutic agent that has been shown to induce apoptosis in a variety of cells. Therefore, the effect of mitomycin C on both wild-type and PKCβ-deficient BMMC was determined. Cells were grown in medium containing a suboptimal concentration of IL-3 (20 U/mL) that was found to prevent BMMC apoptosis and 30 μmol/L of mitomycin C. No significant difference in the apoptotic rate was observed between wild-type and PKCβ-deficient BMMC (Figure 3C). Thus, deficiency of PKCβ in mast cells did not affect their rate of proliferation or apoptosis.
Degranulation
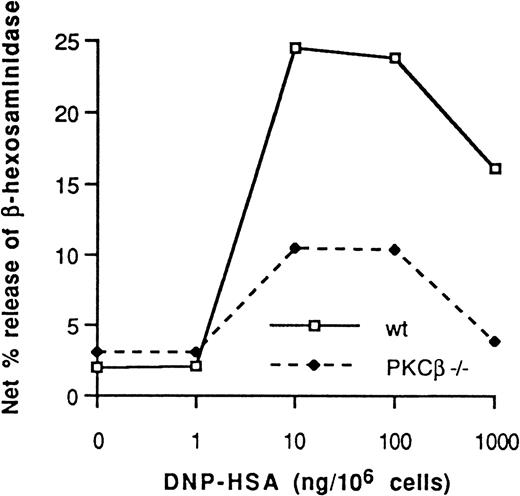
Wild-type and PKCβ-deficient BMMC were sensitized with monoclonal mouse IgE against HSA-DNP and challenged with increasing doses of HSA-DNP–released β-hexosaminidase (Figure4). In 4 consecutive experiments, degranulation was 52% ± 6% (mean ± SE) lower in BMMC derived from the PKCβ-deficient mice than in wild-type BMMC.
Dinitrophenol-human serum albumin antigen dose-dependent net percentage release of β-hexosaminidase from IgE-sensitized PKCβ-deficient mast cells and wild-type cells.
One representative experiment of 4 is shown.
Dinitrophenol-human serum albumin antigen dose-dependent net percentage release of β-hexosaminidase from IgE-sensitized PKCβ-deficient mast cells and wild-type cells.
One representative experiment of 4 is shown.
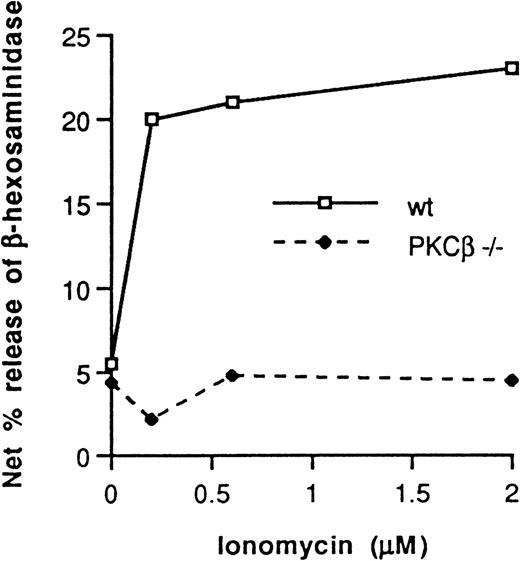
In 3 separate experiments, cells were incubated for 20 minutes at 37°C with increasing concentrations of ionomycin. Degranulation was 75% ± 7% (mean ± SE) lower in the PKCβ-deficient BMMC than in the wild-type BMMC (Figure 5).
Ionomycin dose-dependent net percentage release of β-hexosaminidase from PKCβ-deficient mast cells and wild-type cells.
One representative experiment of 3 is shown.
Ionomycin dose-dependent net percentage release of β-hexosaminidase from PKCβ-deficient mast cells and wild-type cells.
One representative experiment of 3 is shown.
PKCβ-deficient BMMC and cytokine mRNA accumulation
Our initial experiments to assess cytokine release from BMMC failed to detect important amounts of cytokines in the medium, probably because the preincubation period with IgE was only 2 hours. Because several articles reported up-regulation of IgE receptors by IgE,27-29 we preincubated the cells for 4 days with IgE before stimulating them with the antigen for 8 to 10 hours. The culture medium was then collected and the mRNA was extracted from the cells.
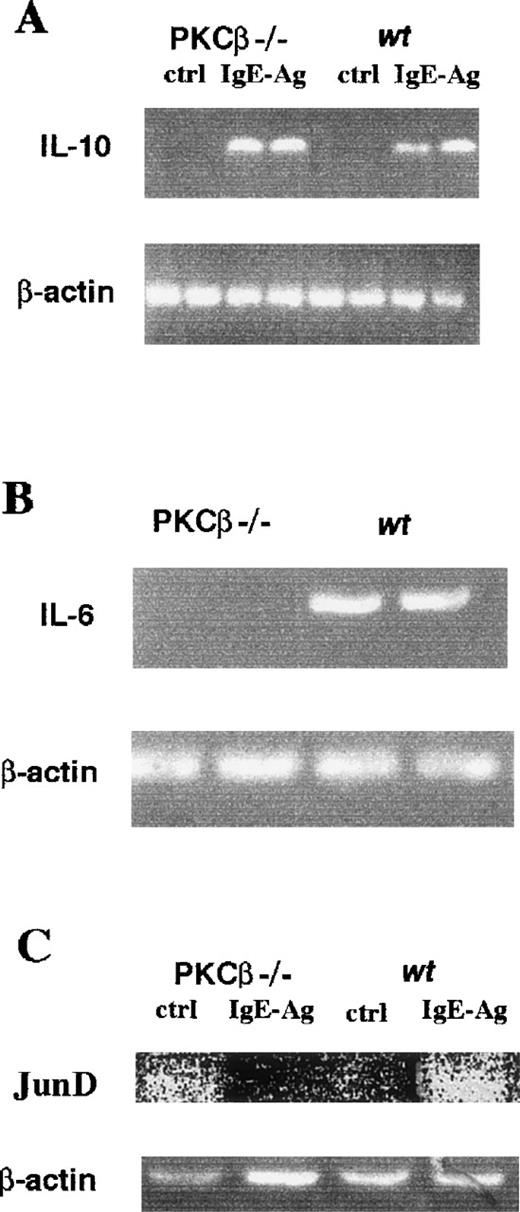
Because it was demonstrated that IL-10 is induced in human mast cells by IgE-Ag stimulation,30 we investigated IL-10 mRNA accumulation in PKCβ-deficient and wild-type BMMC with and without IgE-Ag activation, with β-actin as the control. The level of induction of IL-10 was similar in PKCβ-deficient and wild-type cells (Figure 6A), showing that there is probably no general defect in IgE-Ag induction in PKCβ-deficient BMMC.
IgE-Ag–mediated interleukin 10 (A), interleukin 6 (B), and JunD (C) mRNA accumulation in bone marrow mast cells from PKCβ-deficient and wild-type mice.
RNA isolated from IgE-Ag–stimulated mast cells was reverse transcribed with specific primers and then amplified. Polymerase chain reaction products were resolved on 2% agarose gel containing 0.008% ethidium bromide.
IgE-Ag–mediated interleukin 10 (A), interleukin 6 (B), and JunD (C) mRNA accumulation in bone marrow mast cells from PKCβ-deficient and wild-type mice.
RNA isolated from IgE-Ag–stimulated mast cells was reverse transcribed with specific primers and then amplified. Polymerase chain reaction products were resolved on 2% agarose gel containing 0.008% ethidium bromide.
Overexpression of PKCβ in RBL cells induces the accumulation of IL-6 mRNA.13 Therefore, we measured the accumulation of IL-6 mRNA in IgE-Ag–activated PKCβ-deficient BMMC. Figure 6B shows that the mRNA level of IL-6 was significantly reduced in the PKCβ-deficient BMMC compared with the wild-type cells.
The homodimer of JunD/JunD has been suggested to be a regulator of IL-6 gene expression.14 We showed that, in mast cells, the expression of JunD on the level of mRNA is dependent on PKC.5 We therefore decided to determine whether the level of JunD mRNA accumulation is different in BMMC derived from PKCβ-deficient mice compared with wild-type BMMC. As shown in Figure6C, compared with the findings in wild-type BMMC, there was indication of a reduction in the level of JunD mRNA in PKCβ-deficient BMMC after activation.
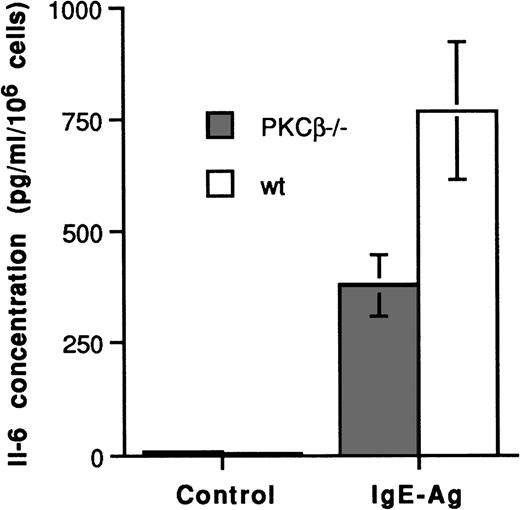
To determine whether the PKCβ deficiency in BMMC also inhibited IL-6 production, we measured the FcεRI-dependent synthesis of IL-6 in these BMMC (Figure 7). No IL-6 protein was detected in the resting BMMC. However, the average results from 3 separate experiments showed that IL-6 was significantly reduced in the PKCβ-deficient BMMC (381 ± 66 pg/mL per 106cells) compared with the wild-type BMMC (769 ± 154 pg/mL per 106 cells). Thus, the PKCβ deficiency in these cells significantly affected their capacity to produce IL-6.
IgE-Ag mediated interleukin 6 synthesis in bone marrow mast cells from PKCβ-deficient and wild-type mice.
Cells were incubated with IgE for 4 days and with antigen for 8 to 10 hours. Supernatants were frozen at −70°C until assayed for interleukin 6 by enzyme-linked immunosorbent assay. Results (mean ± SE) are shown for 3 separate experiments.
IgE-Ag mediated interleukin 6 synthesis in bone marrow mast cells from PKCβ-deficient and wild-type mice.
Cells were incubated with IgE for 4 days and with antigen for 8 to 10 hours. Supernatants were frozen at −70°C until assayed for interleukin 6 by enzyme-linked immunosorbent assay. Results (mean ± SE) are shown for 3 separate experiments.
Discussion
The finding of peritoneal mast cells stained with safranin in peritoneal exudates from PKCβ-deficient mast cells shows that PKCβ-deficient mice contain mast cells that can differentiate into heparin-containing cells. Because no previous studies indicated that PKCβ is essential for mast cell proliferation or differentiation, this result was not unexpected, and we therefore focused on the analysis of the specific functions of mast cells by using BMMC.
Our hypotheses regarding specific differences between control and PKCβ-deficient mast cells were based on 2 kinds of evidence—that obtained by using mice lacking in Btk, which display an immunophenotype very similar to PKCβ-deficient mice15; and that obtained with in vitro cellular studies. The similarity between deficiency of Btk and deficiency of PKCβ has been shown in studies in knockout mice. One explanation for the similarity in phenotype could be an association between Btk and PKCβ. However, since signal-transduction pathways can vary substantially between different cell types, finding a difference in the phenotype of these 2 mutant cell types was not surprising. Our observation that PKCβ-deficient mast cells undergo apoptosis at the same rate as control cells in response to growth-factor deprivation is in contrast to the situation in Btk-deficient mast cells. Btk-deficient mast cells undergo apoptosis at a lower rate than control mast cells in response to growth-factor deprivation.16 Several explanations for the difference in phenotype between Btk-deficient and PKCβ-deficient mast cells could be postulated, such as the use of a different kind of PKC for pathways involving Btk for the induction of apoptosis in PKCβ-deficient mast cells. Additional work is needed to explain the differences in the phenotypes.
Most chemotherapeutic agents cause cell death by inducing apoptosis. Interestingly, safingol, a PKC inhibitor, was shown to greatly enhance mitomycin C-induced apoptosis of gastric tumor cells.31Others reported that PKCβ inhibition reduced the apoptotic rate of B lymphocytes.32 We therefore tried to assess the chemotherapy-induced apoptotic response of PKCβ-deficient mast cells. However, even in this context, we observed no significant difference between PKCβ-deficient BMMC and wild-type BMMC in the mitomycin C-induced death rate.
Our observations that the total number of mast cells grown with IL-3 and the proliferation rates were not different in control and PKCβ-deficient mast cells (Figure 2) indicate that PKCβ has a nonessential role (if any) in mast cell growth. This is in disagreement with the evidence from studies in a rat mast cell line that overexpressed PKCβ and had an increase in cellular proliferation rate.13 Unless these results are due to dissimilarities of the mast cell subtypes assessed in the different studies, it seems that although an abnormal signal from PKCβ can increase cellular proliferation rate, this enzyme does not have an essential role in this process. In contrast to this discrepancy, our finding of a substantial decrease in mast cell degranulation in PKCβ-deficient mast cells compared with control cells (Figure 4) is in agreement with results from reconstitution and overexpression experiments. Although PKCδ could also allow degranulation in reconstitution experiments, it could not totally replace the absence of PKCβ in cells lacking this enzyme. Hence, PKCβ has an essential role in degranulation.
Because ionomycin was unable to rescue the degranulation defect in the PKCβ-deficient mice, it appears that PKCβ might function further downstream of the FcεRI receptor itself. It has been shown that calcium ionophore-induced secretion from mast cells correlates with myosin light-chain phosphorylation by PKC33; thus, myosin light-chain might present a target for PKCβ. Alternatively, it has been reported that PKC is involved in the regulation of coat protein assembly in mast cells and therefore in the modulation of secretion.34 Additional experiments are needed to elucidate the physiologic target of PKCβ in mast cells.
IgE-Ag induction of 2 cytokine mRNAs, IL-6 and IL-2, was increased in mast cells that overexpressed PKCβ.13 We detected a decrease in IL-6 released by activated PKCβ-deficient mast cells compared with control cells. An even greater decrease in the amount of IL-6 mRNA was observed in activated PKCβ-deficient mast cells compared with control cells. It is almost impossible to determine whether the decrease in the level of IL-6 protein in mast cells activated overnight was due to a decay in the level of IL-6 mRNA. Additional work must be performed to find out whether the control of IL-6 synthesis is on the transcriptional, translation, or mRNA accumulation level. Experiments to investigate calcium flux in these cells would be of great interest because degranulation is dependent on calcium, and IL-6 production is probably dependent on calcium by means of the activation of an NF-AT or NF-AT–like factor, although a classical NF-AT site is not present in the IL-6 promoter.35Interestingly, there was no significant change in the amount of mRNA for IL-10 between the different cell types, which suggests that there is no general defect in IgE-receptor signal transduction in these cells. In contrast, mRNA levels of JunD, which has been shown to be a regulator of the IL-6 gene,14 were lower in activated PKCβ-deficient mast cells than in wild-type cells. This suggests that JunD induction by PKCβ is important for IgE-Ag induction of the IL-6 gene. In previous work, we demonstrated that JunD mRNA accumulation is dependent on PKC.5 We also showed that the PKCβ isozyme is the predominant PKC isozyme in BMMC.8 Therefore, it is not surprising that in this study we found indication that PKCβ-deficient BMMC had lower levels of IgE-induced JunD mRNA.
Thus, our study not only supports the main conclusions from reconstitution and overexpression experiments regarding the role of PKCβ in signal transduction leading to degranulation and cytokine induction, but it also shows that under normal conditions the role of this enzyme is not redundant and cannot be totally replaced by other PKC isozymes. Specific PKC isozyme inhibitors are currently being formulated. Studies using different PKC knockout mice should prove to be invaluable not only for assessing the possible effects of such inhibitors but also for increasing our understanding of the complex role of different PKC isozymes in signal transduction in different cell types.
Acknowledgments
We thank the members of the A. Tarakhovsky's laboratory, especially I. Mecklenbrauker.
Supported by grants from the Israel Academy for Science (to E.R.) and the Israeli Ministry of Health (to E.R.). H.N. was supported by a short-term EMBO fellowship.
Reprints:Ehud Razin, Department of Biochemistry, Hebrew University-Hadassah Medical School, PO Box 12272, Jerusalem, 91120, Israel; e-mail: ehudr@cc.huji.ac.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal