Recipient IgG immunity against leukoreduced donor platelets is dependent on indirect T-cell allorecognition and is suppressed in vivo by inhibitors (aminoguanidine, AMG) of inducible nitric oxide synthase (iNOS). To examine recipient processing pathways of donor platelet antigens, enriched macrophages (antigen-presenting cells [APC]) from BALB/c (H-2d) mice were pulsed with allogeneic C57BL/6 (H-2b) platelets and transfused weekly into naive BALB/c mice. Platelet-pulsed APC stimulated IgG antidonor antibody production in 45% of recipients by the second transfusion and in 100% by the sixth transfusion; this response was enhanced by pulsing in the presence of interferon-γ. By the sixth transfusion, high-titer IgG1 (mean titer 4990) and IgG2a (1933) isotypes specific for donor major histocompatibility complex (MHC) class I antigens were detected. Platelet pulsing in the presence of AMG or colchicine significantly inhibited the ability of APC to stimulate IgG alloantibodies; only 50% (P < .005) and 20% (P < .0001) of recipients, respectively, produced antibodies by the sixth transfusion. AMG inhibition was reversed by the addition of l-arginine, the substrate for iNOS. In contrast, pulsing in the presence of chloroquine, the proteasome inhibitory peptide MG115, or Brefeldin A enhanced APC immunity (70-100% of recipients antibody positive by the second transfusion [P < .05]); these agents allowed the pulsed APC to stimulate IgG2a but inhibited IgG1 production and this correlated with a reduction in serum interleukin (IL)-4 levels. The results suggest that for donor platelet antigens to stimulate IgG alloantibodies, recipient APC use the essential generation of nitric oxide and a noncytosolic, pH-independent processing pathway, which can be exploited as an effective immunotherapy target to further inhibit alloimmunization against leukoreduced platelets.
Production of IgG antibodies is critically dependent on T-cell recognition and activation.1,2 T cells recognize protein antigens, which are degraded or processed and combined with molecules encoded by the major histocompatibility complex (MHC).3,4 Antigen processing is critical for generating protein determinants that can be loaded and bound within the antigen-binding grooves of either MHC class I or II molecules.5 The spectrum of antigen processing ranges from the simple unfolding of conformational determinants to the proteolytic exposure of primary structure by pH-dependent enzymes (eg, cathepsins).6-9 Exogenous proteins (eg, bacterial glycoproteins) are generally processed by antigen-presenting cells (APC) via endosomal compartments and are shunted to intracellular compartments rich in MHC class II molecules.10 This pathway is necessary for the activation of CD4+ T-helper cells and help for eventual IgG antibody production.10 Endogenous antigens (eg, virally derived proteins), on the other hand, are processed by large molecular weight proteasomes within the APC cytosol and are subsequently transported to the luminal surface of the endoplasmic reticulum for loading onto MHC class I molecules.9,10 This pathway is responsible for the stimulation of CD8+ cytotoxic T cells (CTL).11Experimentally, the major distinction between these pathways has been that exogenous antigen processing is generally susceptible to pH-raising lysomotrophic agents such as chloroquine and NH4Cl, whereas the endogenous or nonendosomal pathway is not.9 12-14 Understanding antigen-processing pathways of clinically relevant protein antigens such as platelet alloantigens may be fundamental to developing efficacious antigen-specific therapies for alloimmunization.
Two recipient T-cell recognition mechanisms have been shown to initiate alloimmunity. The direct pathway occurs when recipient T-helper cells directly interact with MHC class II molecules on donor APC, whereas the indirect pathway is analogous to the normal immune response.15 Indirect recognition occurs when allogeneic non-APC are administered to a recipient and involves the processing and presentation of allelic donor antigens (eg, MHC class I molecules) by recipient APC to recipient T-helper cells. The indirect pathway of allorecognition has been implicated in rejection responses in various transplantation models of cardiac,19 kidney,20and skin15,21,22 grafts. Within the context of indirect allorecognition, interactions between donor antigen and self-APC are critical to T-cell activation and subsequent antibody formation.23-25 In 1995, 2 laboratories using different animal models (murine versus rat) of platelet immunity suggested that allogeneic platelets stimulated IgG antidonor immunity via indirect recognition.17,27 We subsequently demonstrated that the indirect alloimmunity against platelets was dependent on the activation of inducible nitric oxide synthase (iNOS) within recipient macrophages.17,18 26 These results suggested that recipient macrophages may mediate platelet alloimmunity via their known roles as a phagocyte and APC. However, the mechanisms by which donor platelets are engulfed, processed, and presented to the recipient's immune system to stimulate IgG antidonor immunity remain unknown. To study the antigen-processing pathways of allogeneic platelet antigens, adherent APC from recipient mice were pulsed with donor platelets in the presence of various metabolic inhibitors and then examined for their ability to stimulate alloantibodies in naive recipient mice. The results show that recipient APC use unique intracellular pathway(s) to process allogeneic platelet MHC antigens for the stimulation of recipient immunity and suggest that manipulating these pathways may be an effective form of immunotherapy.
Materials and methods
Animals and cell lines
Inbred female BALB/c (H-2d) mice, 8 to 12 weeks of age, were used as the transfusion recipients and as the source of APC; female C57BL/6 (H-2b) mice, 6 to 12 weeks of age, were used as platelet donors; the mice were purchased from Harlan-Sprague Dawley (Indianapolis, IN). EL-4 (H-2b) C57BL/6 thymoma, P815 (H-2d) DBA mastocytoma, and RT 1.1 (H-2k) CBA lymphoma cell lines were used for serologic typing of the recipient sera. All cell lines and cell culture assays were maintained in RPMI-1640 with 5% fetal calf serum (FCS), 100 μg/mL penicillin/streptomycin/fungizone, 100 mM l-glutamine, and 5 × 10−5 mol/L 2-mercaptoethanol (cRPMI).
Chemicals
Aminoguanidine (AMG), l-arginine (l-arg), colchicine, chloroquine, NH4Cl, Brefeldin A, and the proteasome inhibitor peptide carbobenzoxy-l-leucyl-l-leucyl-l-norvalinal (MG115) were obtained from the Sigma Chemical Co. (St. Louis, MO). Recombinant murine interferon (IFN)-γ was obtained from Genzyme Corp. (Cambridge, MA). All of the in vitro incubations were maintained in cRPMI.
Antibodies
Fluorescein isothiocyanate (FITC)-and phycoeyrthrin (PE)-conjugated antibodies against CD45, CD4, CD8, CD61, F4/80, H-2 I-Ad/I-Ed, H-2Dd, and B220 were obtained from Cedarlane Laboratories (Hornby, Ontario, Canada) and used to phenotype the adherent APC populations.
Platelet preparation
C57BL/6 donor mice were bled and leukoreduced platelets were prepared as previously described.25 Briefly, mice were bled via the tail vein into EDTA-microvettes (Sarstedt, St. Laurent, Canada), the blood was pooled and centrifuged at 220g, and the platelet-rich plasma (PRP) collected; care was taken not to disturb or aspirate the buffy coat. The platelets were washed 3 times in 1% EDTA-saline and adjusted to a concentration of 109/mL (stock solution, this concentration approximates a 300-mL platelet concentrate of 3 × 1011 platelets). White blood cells (WBC) were enumerated by flow cytometry as previously described.26 WBC levels in the stock platelet solution were 1.9 ± 1.7 WBC/μL. At these levels, allogeneic WBC on their own did not generate alloantibodies or appear to affect recipient immunity.23 26 For incubation with APC, platelets were resuspended in cRPMI.
Platelet-pulsed APC
In vitro platelet-pulsed APC were prepared according to Oh et al,24 with modifications. Briefly, BALB/c mice were killed and their spleens were teased into single cell suspensions and 108 spleen cells in 10 mL cRPMI were incubated in plastic tissue culture-treated Petri dishes (Falcon, Franklin Lakes, NJ) for 2 hours at 37°C to allow for adherence. The supernatants were removed and the dishes were gently rinsed 3 times with Hank's balanced salt solution (HBSS) to remove nonadherent cells. The number of adherent cells/dish was estimated by subtracting the nonadherent cell counts from the seed dose of the dish (approximately 30% of the spleen cells adhered). The phenotype of the adherent cells (APC) is summarized in Table 1. Based on the number of adherent APC/dish, platelets were added to pulse the APC at a ratio of 10:1 (platelets/APC) and were incubated for 18 hours at 37°C. The pulsed APC were gently scraped from the dish with a Teflon scraper and centrifuged at 330g to reduce free platelets. The pellet was then washed twice with 1 × HBSS and the concentration was adjusted to 107 APC/mL for transfusion. Mice received 100-μL transfusions. The transfusion contained less than 5 × 106 free intact platelets; at these levels, intact allogeneic platelets were not immunogenic within the 6-week transfusion protocol.26 The number of adherent donor WBC in the transfusion product was determined to be ≈10 WBC per transfusion; this number of allogeneic WBC per transfusion was not immunogenic on its own (J.W.S., unpublished data). Cell viability was measured as 80% to 95% by trypan blue dye exclusion. Incubation of the adherent APC with either syngeneic or allogeneic platelets for 18 hours caused only a slight increase in the percentage of CD45+/CD61+ cells (Table 1). Where indicated, IFN-γ (100 U/mL final) or the chemical inhibitors (0.5 or 1.0 mM AMG; 1 μg/mL colchicine; 0.1 mM chloroquine, 50 mM NH4Cl, 1 μg/mL Brefeldin A, or 5 μM MG115, Table2) were added to the platelet-pulsing step.
Immunophenotype of BALB/c spleen cell leukocyte populations
| Subpopulation . | Whole Spleen . | 2 h Non-adh. APC . | 2 h Adh. APC . | 18 h Adh. APC + Platelets . |
|---|---|---|---|---|
| CD4+ T cells | 12.3 ± 4.8* | 10.6 ± 0.8 | 3.0 ± 1.2 | 2.6 ± 1.0 |
| CD8+ T cells | 6.1 ± 3.0 | 5.5 ± 1.4 | 3.0 ± 2.0 | 1.9 ± 0.4 |
| B220+ B cells | 17.6 ± 4.5 | 15.6 ± 6.0 | 24.1 ± 7.5 | 32.3 ± 9.2 |
| F4/80 macrophages | 4.5 ± 4.0 | 4.5 ± 1.3 | 33.2 ± 3.0 | 26.7 ± 0.9 |
| MHC class I+ (H-2Dd) | 49.1 ± 9.7 | 45.7 ± 11.3 | 67.4 ± 7.5 | 67.7 ± 4.5 |
| MHC class II+ (I-Ad/I-Ed) | 24.7 ± 6.9 | 24.3 ± 4.9 | 63.0 ± 2.1 | 58.5 ± 3.9 |
| CD45+ cells | 44.0 ± 0.4 | 39.4 ± 10.0 | 56.1 ± 13.6 | 58.5 ± 9.9 |
| CD45+/CD61+ cells | < 1.0 | < 1.0 | 1.5 ± 0.1 | 5.1 ± 0.1† |
| Subpopulation . | Whole Spleen . | 2 h Non-adh. APC . | 2 h Adh. APC . | 18 h Adh. APC + Platelets . |
|---|---|---|---|---|
| CD4+ T cells | 12.3 ± 4.8* | 10.6 ± 0.8 | 3.0 ± 1.2 | 2.6 ± 1.0 |
| CD8+ T cells | 6.1 ± 3.0 | 5.5 ± 1.4 | 3.0 ± 2.0 | 1.9 ± 0.4 |
| B220+ B cells | 17.6 ± 4.5 | 15.6 ± 6.0 | 24.1 ± 7.5 | 32.3 ± 9.2 |
| F4/80 macrophages | 4.5 ± 4.0 | 4.5 ± 1.3 | 33.2 ± 3.0 | 26.7 ± 0.9 |
| MHC class I+ (H-2Dd) | 49.1 ± 9.7 | 45.7 ± 11.3 | 67.4 ± 7.5 | 67.7 ± 4.5 |
| MHC class II+ (I-Ad/I-Ed) | 24.7 ± 6.9 | 24.3 ± 4.9 | 63.0 ± 2.1 | 58.5 ± 3.9 |
| CD45+ cells | 44.0 ± 0.4 | 39.4 ± 10.0 | 56.1 ± 13.6 | 58.5 ± 9.9 |
| CD45+/CD61+ cells | < 1.0 | < 1.0 | 1.5 ± 0.1 | 5.1 ± 0.1† |
Data are expressed as mean percentage ± SD of total cells (n = 3).
CD45+/CD61+ phenotype was similar if either syngeneic or allogeneic platelets were used to pulse the APC.
Summary of metabolic inhibitors used during the platelet pulsing
| Cytokine/Inhibitor . | Biochemical Effect . | Ref . |
|---|---|---|
| IFN-γ | Up-regulates nitric oxide production | 41, 42 |
| Up-regulates MHC class II expression and iNOS | 32 | |
| Aminoguanidine | Inhibits iNOS production of NO | 43, 44 |
| L-arginine | Amino acid substrate for iNOS. | 43, 44 |
| Colchicine | Inhibits tubulin polymerization | 31 |
| NH4Cl | Prevents endosomal/lysosomal acidification | 45 |
| Chloroquine | Prevents endosomal/lysosomal acidification | 46, 47 |
| Strips β2M from MHC class I | 27, 28 | |
| Brefeldin A | Inhibits nascent protein transport from E/R to golgi. | 48 |
| MG115 | Inhibits cytosolic proteasomes | 49 |
| Cytokine/Inhibitor . | Biochemical Effect . | Ref . |
|---|---|---|
| IFN-γ | Up-regulates nitric oxide production | 41, 42 |
| Up-regulates MHC class II expression and iNOS | 32 | |
| Aminoguanidine | Inhibits iNOS production of NO | 43, 44 |
| L-arginine | Amino acid substrate for iNOS. | 43, 44 |
| Colchicine | Inhibits tubulin polymerization | 31 |
| NH4Cl | Prevents endosomal/lysosomal acidification | 45 |
| Chloroquine | Prevents endosomal/lysosomal acidification | 46, 47 |
| Strips β2M from MHC class I | 27, 28 | |
| Brefeldin A | Inhibits nascent protein transport from E/R to golgi. | 48 |
| MG115 | Inhibits cytosolic proteasomes | 49 |
Chloroquine pretreatment of platelets and APC
Where indicated, platelets and APC were pretreated with chloroquine. Briefly, 109 platelets/mL were prepared in phosphate-buffered saline (PBS) containing 0.1 mM chloroquine and 0.4% bovine serum albumin (BSA) and incubated for 2 hours in the dark at room temperature. The platelets were then washed twice in PBS (containing 1% EDTA) and readjusted to 109/mL in cRPMI medium. For chloroquine pretreatment of APC, the adherent APC were incubated with the chloroquine solution on the Petri dishes and then washed as above.
Transfusion protocol and blood preparation
In each transfusion protocol, all mice were prebled 48 hours before the first transfusion and injected with 100 uL of the platelet-pulsed APC solution (107/mL) weekly via the tail vein. Each week, blood was collected from the mice into red top microvettes (Starstedt, Montreal, Quebec, Canada) and immediately placed on ice until clot formation. A portion of the fresh sera was used to determine antibody titers and the remainder was frozen at −80°C and used for cytokine determinations.
Flow cytometric analysis
For detection of IgG antidonor antibodies, 106 donor spleen cells were incubated with serial dilutions of fresh recipient sera for 45 minutes at 4°C, washed once, and labeled with FITC-conjugated goat antimouse IgG (Fc specific, Cedarlane Laboratories) for 45 minutes at 4°C in the dark. Cells were analyzed by flow cytometry using a FACSort flow cytometer (Becton Dickinson, San Jose, CA) equipped with an argon ion laser, operating at 15 mW; 10,000 events were acquired using an electronic cellular (lymphocyte) gate based on forward and side scatter and were analyzed using LYSYS II software (Becton Dickinson). Matched prebleed serum was used as the negative control in all experiments. Antidonor MHC specificity of the antibodies was confirmed by positive reactivity with donor cells but absence of reactivity with recipient or third-party cells. Isotype characterization of the antidonor antibodies was performed using FITC-conjugated goat antimouse IgG1 and 2a (Cedarlane Laboratories). For phenotypic analysis of adherent APC, scraped cells were stained with the indicated FITC-labeled or PE-labeled antibodies for 45 minutes in the dark, washed, and analyzed as above.
Cytokine determinations
Sera from the transfused mice or controls were tested for the presence of IL-4 and IL-12 using an ultrasensitive commercial solid-phase enzyme-linked immunosorbent assay (ELISA) kit (OptEIA Mouse IL-4 and IL-12 sets, PharMingen, San Diego, CA). The IL-4 kit had a sensitivity of more than 0.2 pg/mL and the IL-12 assay had a sensitivity of more than 5 pg/mL.
Statistical analysis
Chi square test for unpaired proportions was used to compare the number of antibody-positive recipients between 2 transfusion groups at each week of transfusion.
Results
Allogeneic platelet-pulsed APC immunity
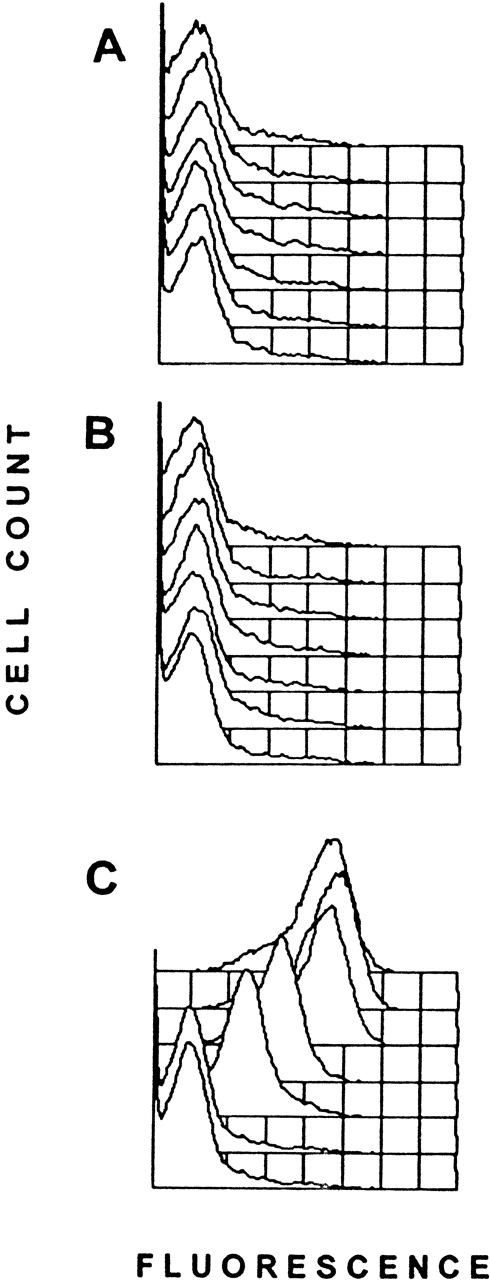
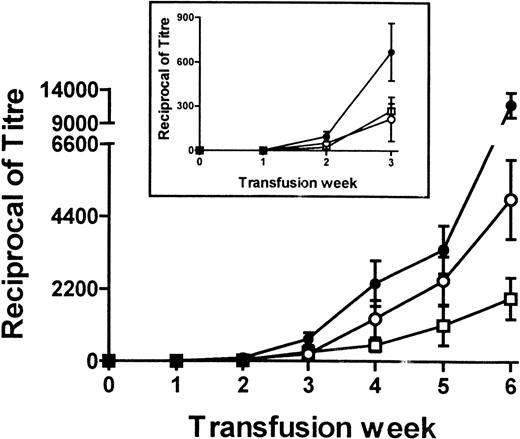
To determine the immunogenicity of platelet-pulsed APC, 106were transfused weekly and the sera of the recipient mice were tested for the presence of antidonor IgG antibodies by flow cytometry. Control transfusions with syngeneic APC alone or syngeneic platelet-pulsed APC did not induce an antidonor IgG response in any mice tested (Figure 1A and B) nor did APC pulsed with the donor WBC amounts found in the allogeneic platelet population (not shown). In contrast, allogeneic platelet-pulsed APC induced detectable IgG antidonor antibody production by the second transfusion (Figure 1C); 45% of recipients had antidonor antibodies, and by the sixth transfusion 100% of recipients became antibody positive (Table 3). Thus, recipient APC became immunogenic when pulsed with donor platelets. Characterization of the serum IgG antibodies showed that they reacted strongly with donor MHC-matched cells (EL-4, H-2b) but not with recipient (P815, H-2d) or third-party (R1.1, H-2k) MHC cell lines. Isotype analysis of the IgG molecules showed that the pulsed APC induced the production of high-titer IgG1 (4990 ± 1213, mean ± SEM) and IgG2a (1933 ± 629) antidonor antibodies (Figure 2). In preliminary experiments, sera from immunized recipients could induce thrombocytopenia when infused into naive platelet donor mice; platelet counts dropped from 974 ± 160 to 225 ± 240 ( × 109/L) by 1 hour after infusion.
IgG antidonor antibody production in BALB/c mice with weekly transfusions of 106 allogeneic platelet-pulsed BALB/c APC.
Each panel represents overlaid fluorescence histograms of C57BL/6 WBC incubated with sera (1:25 dilution) from a representative transfused mouse and labeled with FITC-goat antimouse IgG. The front histogram in each panel represents the prebleed serum, and each of the subsequent histograms are consecutive transfusions (weeks 1-6). A shift of the fluorescence to the right represents presence of antibody. (A) 106 unpulsed BALB/c APC transfused into BALB/c recipients. (B) 106 BALB/c platelet-pulsed APC transfused into BALB/c recipients. (C) 106 C57BL/6 platelet-pulsed APC transfused into BALB/c recipients.
IgG antidonor antibody production in BALB/c mice with weekly transfusions of 106 allogeneic platelet-pulsed BALB/c APC.
Each panel represents overlaid fluorescence histograms of C57BL/6 WBC incubated with sera (1:25 dilution) from a representative transfused mouse and labeled with FITC-goat antimouse IgG. The front histogram in each panel represents the prebleed serum, and each of the subsequent histograms are consecutive transfusions (weeks 1-6). A shift of the fluorescence to the right represents presence of antibody. (A) 106 unpulsed BALB/c APC transfused into BALB/c recipients. (B) 106 BALB/c platelet-pulsed APC transfused into BALB/c recipients. (C) 106 C57BL/6 platelet-pulsed APC transfused into BALB/c recipients.
Percentage of mice with detectable antidonor alloantibody3-150
| Agent Added to Pulse . | N . | Transfusion Week . | |||
|---|---|---|---|---|---|
| Pre . | 2 . | 4 . | 6 . | ||
| None | 38 | 03-151 | 45 — | 70 — | 100 — |
| IFN-γ (100 U/mL) | 10 | 0 | 80 (P < .05)3-152 | 100 (P < .05) | 100 (n/s) |
| AMG (1 mM) | 19 | 0 | 10 (P < .01) | 30 (P < .05) | 50 (P < .0005) |
| Colchicine (1 μg/mL) | 15 | 0 | 15 (P < .05) | 20 (P < .01) | 20 (P < .0001) |
| NH4Cl (50 mM) | 10 | 0 | 40 (n/s) | 80 (n/s) | 100 (n/s) |
| Chloroquine (0.1 mM) | 31 | 0 | 70 (P < .05) | 100 (P < .01) | 100 (n/s) |
| Brefeldin A (1 μg/mL) | 9 | 0 | 100 (P < .05) | 100 (n/s) | 100 (n/s) |
| MG115 (5 μM) | 9 | 0 | 100 (P < .05) | 100 (n/s) | 100 (n/s) |
| Agent Added to Pulse . | N . | Transfusion Week . | |||
|---|---|---|---|---|---|
| Pre . | 2 . | 4 . | 6 . | ||
| None | 38 | 03-151 | 45 — | 70 — | 100 — |
| IFN-γ (100 U/mL) | 10 | 0 | 80 (P < .05)3-152 | 100 (P < .05) | 100 (n/s) |
| AMG (1 mM) | 19 | 0 | 10 (P < .01) | 30 (P < .05) | 50 (P < .0005) |
| Colchicine (1 μg/mL) | 15 | 0 | 15 (P < .05) | 20 (P < .01) | 20 (P < .0001) |
| NH4Cl (50 mM) | 10 | 0 | 40 (n/s) | 80 (n/s) | 100 (n/s) |
| Chloroquine (0.1 mM) | 31 | 0 | 70 (P < .05) | 100 (P < .01) | 100 (n/s) |
| Brefeldin A (1 μg/mL) | 9 | 0 | 100 (P < .05) | 100 (n/s) | 100 (n/s) |
| MG115 (5 μM) | 9 | 0 | 100 (P < .05) | 100 (n/s) | 100 (n/s) |
APC were pulsed with donor platelets at a ratio of 10 platelets: 1 APC in the presence of the agents indicated, washed twice and 106 APC were transfused weekly.
Data are expressed as the percentage of recipients with detectable antidonor antibody by flow cytometry (rounded to nearest 5%).
The χ2 test of independent proportions was used to compare the agent-treated pulsed APC groups against the nontreated pulsed APC group (n/s indicates not significant).
Titers of total IgG (•), IgG1 (○), and IgG2a (□) antidonor antibodies in BALB/c mice after receiving weekly transfusions of 106 donor platelet-pulsed BALB/c APC.
Fresh sera from the recipient mice at each transfusion week were titrated and analyzed for reactivity against donor cells by flow cytometry. The data are expressed as the mean ± SEM of the reciprocal titers. The insert shows rescaled data for the first 3 weeks of transfusion.
Titers of total IgG (•), IgG1 (○), and IgG2a (□) antidonor antibodies in BALB/c mice after receiving weekly transfusions of 106 donor platelet-pulsed BALB/c APC.
Fresh sera from the recipient mice at each transfusion week were titrated and analyzed for reactivity against donor cells by flow cytometry. The data are expressed as the mean ± SEM of the reciprocal titers. The insert shows rescaled data for the first 3 weeks of transfusion.
The adherent APC were then pulsed with donor platelets in the presence of 100 U/mL IFN-γ, a potent stimulator of MHC class II expression and iNOS activation. By the second transfusion, 80% of the recipients were antibody positive and 100% were immune by the fourth transfusion (Table 3). Thus, IFN-γ significantly enhanced the immunogenicity of donor platelet-pulsed APC (P < .05).
The role of microtubules in platelet antigen processing
To determine the role of tubulin in platelet antigen processing, APC were pulsed with donor platelets in the presence of 1 μg/mL colchicine. Compared with nontreated pulsed APC, colchicine significantly inhibited the IgG antibody response; antidonor antibodies were detected in 15% of recipients by the second transfusion (P < .05, Table 3) and only 20% by the sixth transfusion (P < .0001, Table 3).
Recipient APC iNOS activation plays a critical role in platelet antigen processing
Previously we observed that when AMG was administered to recipient mice, it completely prevented formation of IgG antidonor antibodies against transfusions of intact platelets.25 To test the role of this inhibitor in affecting platelet antigen processing, recipient APC were pulsed with donor platelets in the presence of 1 mM AMG. Compared with AMG untreated-pulsed APC immunity, after 2 transfusions of AMG treated-pulsed APC, only 10% of recipients became antibody positive and 50% were antibody positive after 6 transfusions (P < .0005, Table 3). Flow cytometric analysis of recipient sera revealed that AMG significantly reduced the titers of total IgG, IgG1, IgG2a, alloantibodies (not shown).
Kinetic characteristics of the AMG effects on antibody production were studied by pulsing in the presence of either 0.5 mM AMG, 1 mM AMG, or 1 mM AMG plus 1 mM l-arg, the endogenous substrate for iNOS (Table 4). Both doses of AMG reduced the ability of pulsed APC to stimulate an IgG immune response. As expected, compared with the lower dose, 1 mM AMG caused an earlier inhibition because only 10% of the recipients were antibody positive after 2 transfusions (P < .02, Table 4). In contrast, co-incubation of AMG with an equimolar concentration (1 mM) of l-arg induced antibody production in all recipients by the second transfusion, that is, l-arg rescued and enhanced the AMG-mediated antibody inhibition (P < .05, Table 4). These results confirmed that modulation of iNOS activity within recipient APC significantly affects the alloantibody response.
Effects of in vitro AMG and L-arginine on the pulsed APC immunity4-150
| Treatment . | N . | Transfusion Week . | |||
|---|---|---|---|---|---|
| Pre . | 2 . | 4 . | 6 . | ||
| None | 33 | 04-151 | 45 — | 70 — | 90 — |
| AMG (0.5 mM) | 8 | 0 | 50 (n/s)‡ | 25 (P < .1) | 50 (P < .05) |
| AMG (1 mM) | 19 | 0 | 10 (P < .02) | 30 (P < .05) | 50 (P < .01) |
| AMG (1 mM) + L-Arg (1 mM) | 8 | 0 | 100 (P < .05) | 100 (n/s) | 100 (n/s) |
| Treatment . | N . | Transfusion Week . | |||
|---|---|---|---|---|---|
| Pre . | 2 . | 4 . | 6 . | ||
| None | 33 | 04-151 | 45 — | 70 — | 90 — |
| AMG (0.5 mM) | 8 | 0 | 50 (n/s)‡ | 25 (P < .1) | 50 (P < .05) |
| AMG (1 mM) | 19 | 0 | 10 (P < .02) | 30 (P < .05) | 50 (P < .01) |
| AMG (1 mM) + L-Arg (1 mM) | 8 | 0 | 100 (P < .05) | 100 (n/s) | 100 (n/s) |
APC were pulsed with donor platelets at a ratio of 10 platelets: 1 APC in the presence of the agents indicated, washed twice and 106 APC were transfused weekly.
Data are expressed as the percentage of recipients with detectable antidonor antibody by flow cytometry (rounded to nearest 5%).
The χ2 test of independent proportions was used to compare the agent-treated pulsed APC groups against the nontreated pulsed APC group. (n/s indicates not significant).
Endosomal and nonendosomal pathways in APC differentially affect platelet antigen immunity
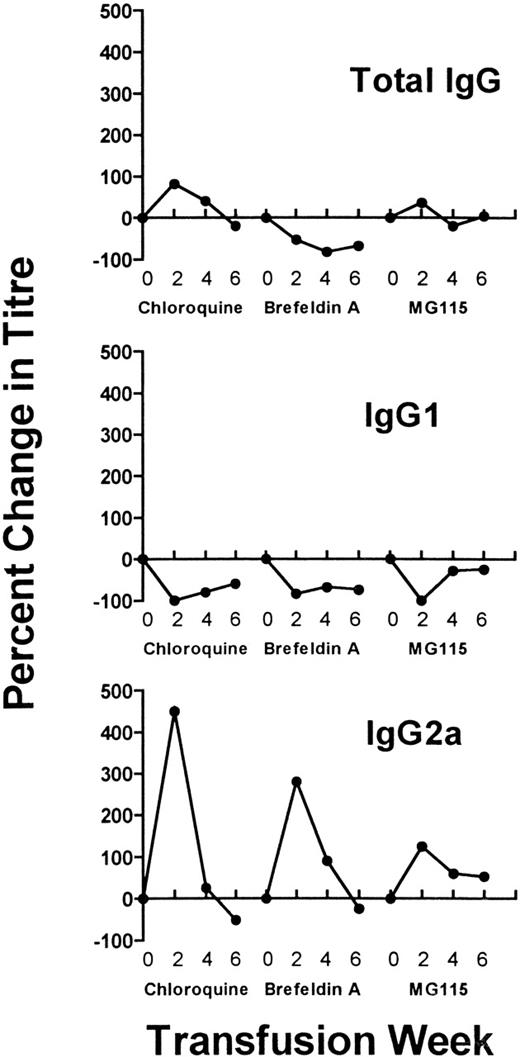
To distinguish between endosomal (pH dependent) and nonendosomal (pH independent) processing pathways, the lysomotropic agents NH4Cl and chloroquine were incubated with the platelet/APC cultures. Although the antibody response after NH4Cl exposure was similar to responses seen with control transfusions (ie, without inhibitor), chloroquine significantly accelerated the IgG response in that 70% of recipients were antibody positive after the second transfusion (P < .05, Table 3) and all mice were positive by the fourth transfusion (P < .01, Table 3). Flow cytometric analysis of serum IgG revealed that chloroquine initially increased total IgG production, which subsequently declined (Figure3). Concurrently, the chloroquine-treated pulsed APC markedly increased the production of IgG2a by the second transfusion (Figure 3), whereas the production IgG1 alloantibodies was inhibited throughout the entire transfusion protocol (Figure 3). Thus, endosomal or chloroquine-sensitive (pH dependent) processing pathways of platelet antigens favor the production of IgG1 (Figure 2), whereas chloroquine-insensitive (pH independent) pathways are associated with elevated IgG2a responses (Figure 3).
Analysis of IgG isotype patterns.
Recipient mice were transfused with donor platelet-pulsed APC that were additionally exposed to inhibitors during the pulsing step (chloroquine, Brefeldin A, and MG115). Sera sampled at the second, fourth, and sixth transfusions were analyzed for total IgG, IgG1, and IgG2a antibodies by flow cytometry. Each data point represents the percent change in antibody titer compared to APC not exposed to inhibitor, that is, the mean IgG1 or IgG2a titers obtained in the inhibitor groups divided by the mean titers in the noninhibitor group (as shown in Figure 2).
Analysis of IgG isotype patterns.
Recipient mice were transfused with donor platelet-pulsed APC that were additionally exposed to inhibitors during the pulsing step (chloroquine, Brefeldin A, and MG115). Sera sampled at the second, fourth, and sixth transfusions were analyzed for total IgG, IgG1, and IgG2a antibodies by flow cytometry. Each data point represents the percent change in antibody titer compared to APC not exposed to inhibitor, that is, the mean IgG1 or IgG2a titers obtained in the inhibitor groups divided by the mean titers in the noninhibitor group (as shown in Figure 2).
Because chloroquine can strip β2M from the surface of platelets,27 28 we wanted to ensure that the chloroquine treatments were only affecting the adherent APC function. Donor platelets or APC were first pretreated with the 0.1-mM chloroquine dose in the pulsing step. Compared with nontreated platelets, pretreatment of platelets with 0.1 mM chloroquine did not affect the ability of the platelet-pulsed APC to induce IgG antibodies (Table 5). On the other hand, pretreatment of APC with 0.1 mM chloroquine before platelet pulsing enhanced its ability to stimulate IgG antibodies similarly to when chloroquine was added concurrently with the platelets; all recipients became antibody positive after the second transfusion (Table 5). Thus, the 0.1 mM dose of chloroquine in the APC pulsing cultures primarily affected APC function.
Effects of chloroquine pretreatment on antidonor alloantibody production5-150
| Untreated Cell . | Pretreated Cell . | Chloroquine Concentration . | N . | Transfusion Week . | ||
|---|---|---|---|---|---|---|
| Pre . | 2 . | 4 . | ||||
| APC/platelets5-151 | — | 0 | 33 | 05-152 | 45 — | 70 — |
| APC | Platelets | 0.1 mM | 14 | 0 | 55 (n/s)5-153 | 85 (n/s) |
| Platelets | APC | 0.1 mM | 8 | 0 | 100 (P < .01) | 100 (P < .1) |
| Untreated Cell . | Pretreated Cell . | Chloroquine Concentration . | N . | Transfusion Week . | ||
|---|---|---|---|---|---|---|
| Pre . | 2 . | 4 . | ||||
| APC/platelets5-151 | — | 0 | 33 | 05-152 | 45 — | 70 — |
| APC | Platelets | 0.1 mM | 14 | 0 | 55 (n/s)5-153 | 85 (n/s) |
| Platelets | APC | 0.1 mM | 8 | 0 | 100 (P < .01) | 100 (P < .1) |
APC or donor platelets were pretreated with chloroquine and then used in the APC pulsing assay, washed twice and 106 APC were transfused weekly.
Data for the untreated APC/platelet cultures (n = 33) are included from Table 2 for statistical comparison.
Data are expressed as the percentage of recipients with detectable antidonor antibody by flow cytometry (rounded to nearest 5%).
The χ2 test of independent proportions was used to compare the agent-treated pulsed APC groups against the nontreated pulsed APC group. (n/s indicates not significant).
The role of cytosolic pathways in donor platelet antigen processing
To determine if the donor platelet antigens could be processed within the cytosolic compartment, the APC were pulsed with donor platelets in the presence of the proteasome inhibitory peptide MG115. In a similar fashion to chloroquine, MG115 significantly accelerated the IgG response so that 100% of recipients were antibody positive after the second transfusion (P < .05, Table 3), whereas it increased IgG2a titers during the entire transfusion protocol and decreased titers of IgG1 (Figure 3). Pulsing the APC in the presence of Brefeldin A caused similar results (Table 3 and Figure 3). Thus, inhibiting cytosolic pathways (proteasomes and nascent protein synthesis) increased the pulsed APC immunity and affected its ability to stimulate T-helper 1–associated and T-helper 2–associated IgG isotypes.
Inhibitor-mediated IgG2a production is associated with changes in serum IL-4 levels
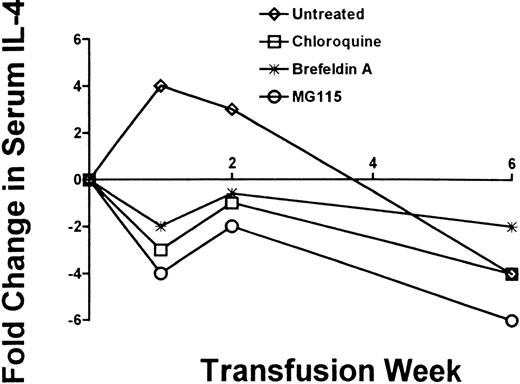
Because murine IgG1 and IgG2a isotypes are associated with T-helper 1 and T-helper 2 cytokine patterns, respectively, serum IL-4 and IL-12 levels were analyzed by ELISA. None of the platelet-pulsed APC populations significantly affected IL-12 levels within the recipients (not shown). On the other hand, platelet-pulsed APC stimulated an initial rise in IL-4 sera levels at 1 and 2 weeks of transfusion, which subsequently declined to nontransfused serum levels by the sixth transfusion (Figure 4). However, when chloroquine, Brefeldin A, or MG115 was added to the APC/platelet cultures, a decrease in serum IL-4 levels was detected by the first and second transfusion (Figure 4).
Analysis of serum IL-4.
Recipient mice were transfused with donor platelet-pulsed APC that were additionally exposed to inhibitors during the pulsing step (chloroquine, Brefeldin A, and MG115). Sera at the first, second, and sixth transfusions were analyzed for IL-4 and IL-12 cytokines by ELISA. Data are expressed as the fold change in IL-4 and were calculated by dividing the mean pg/mL values obtained at each transfusion week with the mean pg/mL values in the prebleed sera. There were no significant changes in IL-12 levels.
Analysis of serum IL-4.
Recipient mice were transfused with donor platelet-pulsed APC that were additionally exposed to inhibitors during the pulsing step (chloroquine, Brefeldin A, and MG115). Sera at the first, second, and sixth transfusions were analyzed for IL-4 and IL-12 cytokines by ELISA. Data are expressed as the fold change in IL-4 and were calculated by dividing the mean pg/mL values obtained at each transfusion week with the mean pg/mL values in the prebleed sera. There were no significant changes in IL-12 levels.
Discussion
The recipient immune mechanisms that result in platelet alloimmunization remain relatively poorly understood. Our results suggest that platelet alloimmunization is fundamentally related to the antigen-processing and presentation mechanisms within recipient APC, which stimulate recipient T-helper cells and eventual alloantibody production. We previously showed that allogeneic leukoreduced platelet transfusions stimulate alloantibody formation together with a transient and early stimulation of splenic macrophage-mediated cytotoxicity.23 Both responses could be completely suppressed in vivo by inhibitors of iNOS such as NG-monomethyl-l-arginine23 and AMG.25 This suggested that iNOS may be associated with platelet antigen-processing mechanism(s) within the APC, which was responsible for platelet immunity. To study the processing pathways in recipient APC, we used an in vitro system in which adherent APC from recipient mice were pulsed with allogeneic platelets and then transfused weekly into naive recipient mice. Our results show that recipient APC pulsed with donor platelets produced high-titer IgG antidonor MHC class I antibodies when transfused, and that iNOS activation was an essential APC processing event leading to alloantibody formation. In addition, both endosomal and nonendosomal processing compartments within the APC were used to process platelet antigens, and these pathways affected the isotype profile of the alloantibody response.
The IgG antibody response induced by the platelet-pulsed APC was specific for intact MHC class I molecules on donor leukocytes. The only source of intact allogeneic MHC class I within the washed pulsed APC that could prime antigen-reactive B cells for T-cell help would be those present on free donor platelets. We estimated that less than 5 × 106 free platelets (including both donor-derived and recipient-derived platelets) from the pulsing step were actually transfused. Although these numbers are too low to induce an antibody response themselves,26 they could be in sufficient quantity to prime antigen-specific B cells to become reactive to the T-cell help generated by the pulsed APC. Furthermore, small numbers of donor WBC from the pulsing step may have been potentially transfused. We estimated that up to approximately 10 donor WBC could potentially have been transfused, but in control experiments these numbers of donor WBC could not stimulate immunity on their own. Thus, the observed immunity generated was primarily due to the recipient APC pulsed with donor platelets. The ability of the donor platelet pulsing step to induce antibody (ie, the immunostimulatory platelet antigen-processing pathway) was sensitive to AMG, a selective inhibitor of iNOS,29,30 and colchicine, an inhibitor of tubulin formation.31 The colchicine sensitivity suggests that platelets require tubulin-dependent processes (eg, phagocytosis), possibly to be taken up and transported to phagolysosomes for destruction. With respect to AMG, our previous results showed that when AMG was administered to recipient mice, it completely inhibited their ability to mount an IgG alloantibody response against intact donor platelet transfusions.25 Our current results are consistent with this in that AMG significantly inhibited the ability of APC to stimulate alloantibody production (Table 3). Thus, it appears that iNOS activation is an essential platelet-processing step within APC responsible for IgG alloantibody production. However, how iNOS and its product nitric oxide (NO) actually mediate platelet antigen processing is unknown, but 2 possibilities exist. Because iNOS is known to associate with phagolysosome membranes,32,33 perhaps NO itself, or its conversion to peroxynitrite (ONOO−) in the presence of superoxide, directly causes platelet membrane glycoprotein damage (eg, due to nitration, unfolding, or cleavage) to generate MHC class II-binding motifs within the phagolysosomes. Because endosomes containing MHC class II molecules normally fuse with phagolysosomes,34 it may allow for the NO-processed platelet antigens to be loaded into the antigen-binding grooves of the MHC molecules for subsequent transport to the surface. Alternatively, NO significantly affects F-actin rearrangements and intracellular membrane movements, which could physically shunt platelet antigens to sites rich in MHC molecules.35 36 It is possible that a combination of both mechanisms could affect platelet antigen processing and ultimately antibody production. We are currently studying these possibilities. The current results support the concept that manipulating NO levels within recipient APC may be an effective and selective immunotherapy for the total reduction of alloimmunization against leukoreduced platelets.
Classically, for MHC class II presentation and subsequent stimulation of antibody production, exogenous protein antigens need to be shunted to endosomal compartments within the APC for processing by pH-dependent (eg, sensitive to the lysomotrophic agents, chloroquine and NH4Cl) proteases such as the cathepsins.3,5 The experiments using lysomotrophic agents, particularly chloroquine, showed that it actually enhanced the recipients' ability to produce IgG more quickly against the platelet-pulsed APC (Table 3). This suggests that processing of exogenously added donor platelet antigens can occur via a chloroquine insensitive or nonendosomal cellular pathway. Paradoxically, we found that chloroquine treatment of the recipient APC reduced their ability to stimulate non–complement-fixing IgG1 and transiently, but significantly, enhanced the production of complement-fixing IgG2a antibodies (Figure 3). Thus, both endosomal and nonendosomal processing pathways can act on platelet antigens; endosomal (chloroquine-sensitive) processing may be responsible for IgG1 alloantibody responses, whereas both endosomal and particularly nonendosomal (chloroquine insensitive) pathways regulate IgG2a production. These data are one of the few examples in which an exogenous protein antigen can be handled by an APC for subsequent MHC class II-dependent antibody production without the requirement for endosomal (chloroquine-independent) processing; to our knowledge, this has only been shown to occur for influenza matrix protein13and measles virus protein.14 Several laboratories have confirmed that T-cell responses to exogenous antigen can be chloroquine insensitive if the protein antigens have been previously degraded into peptide fragments.3,37 For example, chemically modified myoglobin,4 denatured lysozyme,5 and the unfolded carboxy-terminal epitope of fibrinogen6 all can be presented to MHC class II–restricted T-cell clones, even in the presence of enough chloroquine to completely block the presentation of the native protein. Because chloroquine can potentially modify MHC by stripping β2M, we ensured it was not directly acting on the platelets in the pulsing step by showing that pretreating the platelets with 0.1 mM chloroquine did not affect the platelets' ability to convert adherent APC into immunogenic cells (Table 5). The significance of chloroquine-insenstive (nonendosomal) processing of platelet antigens is not yet known, but because IgG1 and IgG2a isotypes are closely associated with T-helper 2 and T-helper 1 activation, respectively, it may suggest that the different processing pathways (endosomal versus nonendosomal) affect the isotype patterns via differential T-cell activation. In support of this, chloroquine-treated APC significantly reduced the serum levels of IL-4 in the recipient mice by the second transfusion (Figure 4). Furthermore, the results (Figure 3) showing that Brefeldin A also enhanced IgG2a production while suppressing IgG1 antibodies additionally suggests that nascent protein synthesis (eg, MHC class II) within the APC may be required for IgG1 but not IgG2a production. Perhaps IgG2a production uses recycled MHC class II molecules from the APC surface, as has been shown to occur for other protein antigens.38 39 Taken together, our data suggest the intriguing possibility that different processing pathways may shunt platelet antigens to different intracellular compartments or MHC class II molecules, which can induce differential stimulation of IgG isotypes.
It is generally thought that exogenous antigens do not enter the cytosolic (proteasome) pathway of antigen processing that is mainly responsible for the generation of MHC class I peptide complexes for recognition by CTL. We previously reported4 that allogeneic platelet transfusions are associated with the generation of CD8+ CTL and immune nonresponsiveness in C57BL/6 recipients is reversed when CD8+ T cells are deleted (eg, CD8 knockout mice).40 However, the proteasome inhibitory peptide MG115 actually enhanced the ability of pulsed APC to stimulate IgG2a production and argues that if CD8+ T cells are being activated by the MHC class I processing pathway, they do not appear to inhibit the immune response (eg, by activated CTL lysing recipient APC as they are presenting platelet antigens). The fact that MG115 elevated IgG2a production indicates that exogenous platelet antigens can reach the cytosolic MHC class I pathway, and its inhibition may make more antigen available for the MHC class II pathways.
In summary, allogeneic platelet antigens have unique antigen-processing requirements to stimulate antidonor MHC class I antibody formation. Within the recipient APC, platelet-derived MHC class I molecules need exposure to iNOS activation and can use either an endosomal or nonendosomal processing pathway to generate immunogenic motifs that control the stimulation of IgG alloantibody formation. Our results may have application for those clinical situations where platelet recipients become alloimmunized despite receiving leukoreduced platelets or potential application in those already immunized recipients (eg, due to prior pregnancy or transfusion) who receive leukoreduced platelet products. Overall, our results suggest that platelet processing pathways, particularly related to iNOS, may be targets for the development of specific immunotherapies to prevent alloimmunization in these patients.
Acknowledgment
The authors would like to thank Dr Jung H. Oh (Department of Experimental Pathology, Emory University, Atlanta, GA) for his helpful discussions and encouragement.
Supported by a grant (XT008) from the Canadian Blood Services R&D Fund. K.W.A.B. is the recipient of a Research Studentship from the Canadian Blood Services.
Reprints:John W. Semple, Department of Laboratory Medicine, St. Michael's Hospital, 30 Bond St, Toronto, Ontario, Canada, M5B 1W8.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal