The thrombocytopenia and absent radii (TAR) syndrome is a rare disease associating bilateral radial agenesis and congenital thrombocytopenia. Here, we investigated in vitro megakaryocyte (MK) differentiation and expression of c-mpl in 6 patients. Using blood or marrow CD34+ cells, the colony-forming unit (CFU)-MK number was markedly reduced. CD34+ cells were also cultured in liquid medium in the presence of a combination of 3 cytokines (stem cell factor, interleukin-3, and interleukin-6) or megakaryocyte growth and development factor (PEG-rHuMGDF) with or without SCF. In the presence of PEG-rHuMGDF, the majority of mature megakaryocytes (CD41 high, CD42 high) underwent apoptosis. This phenomenon was also observed in cultures stimulated by three cytokines. However, this last combination of cytokines allowed a more complete terminal MK differentiation. Surprisingly, a homogeneous population of CD34-CD41+CD42- cells accumulated during the cultures. This population was unable to differentiate along the myeloid pathways. This result suggests that a fraction of MK cells is unable to differentiate in the TAR syndrome. We subsequently investigated whether this could be related to an abnormality in c-mpl. No mutation or rearrangement in the c-mpl gene was found by Southern blots or by sequencing of the c-mpl coding region and its promoter in any of the patients. Using Western blot analysis, a decreased level of Mpl was found in patient platelets. A decreased level of c-mpl messenger RNA in TAR platelets was also detected with a lower c-mpl-P to c-mpl-K ratio in comparison to adult platelets. Altogether, these results demonstrate that the thrombocytopenia of the TAR syndrome is associated with a dysmegakaryocytopoiesis characterized by cells blocked at an early stage of differentiation.
The thrombocytopenia and absent radii (TAR) syndrome is a rare disease occurring with an approximate prevalence of 1 case in 500 000 to 1 million births. The TAR syndrome is characterized by the association of skeletal malformations and hematologic abnormalities.1-3 Among the skeletal defects, bilateral absence of the radii and the presence of thumbs are the most typical features. Thrombocytopenia is present in all cases; it is extremely profound at birth and during the first 4 months of life, with platelet counts below 10 × 109/L.1-3Thrombocytopenia has 2 main characteristics: (1) according to most authors, it is due to the absence of megakaryocytes (MK) in the marrow, with a profound defect in the growth of colony-forming units (CFU)-MK in vitro4,5; according to others, it may be due to a defect in megakaryocytopoiesis with the presence of small MKs in the marrow as well as in CFU-MK–derived colonies6; and (2) the thrombocytopenia improves during the first 2 years of life, and platelet counts may reach nearly normal values in adulthood.1-3
The pathophysiology of TAR syndrome is poorly understood. One of the possible candidate genes was a HOX gene. The HOX gene family is known to play a key role during embryogenesis and cell differentiation, including hematopoietic lineages. Moreover, targeted disruption of the HOXA11 and HOXD11 genes resulted in radio-ulnar aplasia in mice.7 Furthermore, HOXA10is the principal HOX gene expressed during MK differentiation, and its overexpression in murine hematopoietic cells increases megakaryocytic differentiation.8,9 However, HOXA10, HOX A11, and HOXD11 genes had a normal nucleotide sequence, andHOXA10 expression could be detected in patient cells by reverse transcriptase-polymerase chain reaction (RT-PCR), thus ruling out a direct involvement of these HOX genes in the TAR syndrome.10 Recently, Ballmaier et al4 have shown that the thrombocytopenia in the TAR syndrome may be related to a defect in the thrombopoietin (TPO) signaling pathway. For this reason, we reinvestigated in vitro megakaryocytopoiesis from 6 patients with TAR syndrome. We found a profound defect in MK progenitors associated with a blockage in MK differentiation with accumulation of cells expressing CD41 without CD42. MK differentiation was poorly stimulated by TPO and remained extremely abnormal, even when TPO was replaced by a combination of cytokines. This abnormal MK differentiation was associated with a decrease in c-mpl transcripts and Mpl protein. An increased c-mpl-K to c-mpl-P ratio was also found.
Materials and methods
Patients
Nine patients with a TAR syndrome, including 1 fetus, were studied. The clinical features and the investigations performed with each samples are detailed in Table 1. Informed consent was obtained in all cases (patients, parents, and controls) in accordance with the institutional guidelines of the Committee on Human Investigation.
Clinical and laboratory features of TAR patients
| Patient . | Age . | Platelet Count (109/L) . | Thrombopoietin (pg/L) . | Western Blot MPL . | c-mpl Sequencing . |
|---|---|---|---|---|---|
| P1 | 5 | 10 | ND | ND | + |
| P2 | 53 | <50* | 125 | ND | + |
| P3 | Abortion | 70 | ND | ND | + |
| P4 | 13 | 53 | 43 | + | + |
| P5 | 10 | 69 | 36 | ND | + |
| P6 | 7 | 55 | 58 | + | + |
| P7 | 8 | 43 | 120 | + | + |
| P8 | 55 | 78 | 112 | + | ND |
| P9 | 17 | 58 | ND | ND | + |
| Patient . | Age . | Platelet Count (109/L) . | Thrombopoietin (pg/L) . | Western Blot MPL . | c-mpl Sequencing . |
|---|---|---|---|---|---|
| P1 | 5 | 10 | ND | ND | + |
| P2 | 53 | <50* | 125 | ND | + |
| P3 | Abortion | 70 | ND | ND | + |
| P4 | 13 | 53 | 43 | + | + |
| P5 | 10 | 69 | 36 | ND | + |
| P6 | 7 | 55 | 58 | + | + |
| P7 | 8 | 43 | 120 | + | + |
| P8 | 55 | 78 | 112 | + | ND |
| P9 | 17 | 58 | ND | ND | + |
Patient ages ranged from 5 to 55 years; all patients had recovered a platelet count higher than 43 × 109/L when studied. Serum thombopoietin levels were evaluated by ELISA.
ND indicates no data.
Patent P2 received platelet transfusions.
Antibodies and reagents
Directly conjugated R-phycoerythrin (R-PE) anti-CD34 (HPCA-2, Becton Dickinson, Mountain View, CA), R-PE-CY5 anti-CD34 (Immunotech, Lumigny, France), R-PE anti-CD41a (anti-GPIIb/IIIa, Pharmingen, San Diego, CA), fluorescein isothiocyanate (FITC) anti-CD42a (GPIX, Immunotech), R-PE anti-glycophorin A (Dako, Glostrup, Denmark), and FITC anti-CD15 (Lewisx, Dako) monoclonal antibodies (MoAb) were used for flow cytometry. FITC–, R-PE–, and R-PE-CY5–conjugated immunoglobulin G1 (IgG1) MoAb (obtained from Becton Dickinson and Dako) were used as isotype controls.
Unconjugated Y2/51 MoAb (anti-CD61, GPIIIa) was a generous gift from D. Mason (Oxford, UK). Alkaline phosphatase–coupled polyclonal goat antibody against mouse immunoglobulin (Caltag Laboratories, Burlingame, CA) was purchased from Tebu (Le Perray-en-Yvelines, France).
Different antibodies were used for Western blot analysis: a rabbit IgG anti-human Mpl provided by T. Kato (Kirin, Tokyo, Japan), a rabbit anti-human CD41 antibody11 obtained from D. Pidard (INSERM U485, Institut Pasteur, Paris, France), and a rabbit anti-human glycocalicin (GPIbα, CD42b) antibody.12 Lastly, a horseradish peroxidase–conjugated donkey anti-rabbit IgG antibody was purchased from Amersham (Life Sciences, Buckinghamshire, UK).
FITC-annexin-V (Immunotech) and 7 actinomycin D (7AAD) (Sigma, St Louis, MO) were used for apoptosis analysis and cell death evaluation. Phorbol 12-myristate 13-acetate (PMA) and 13-cis retinoic acid were obtained from Sigma.
Thrombopoietin ELISA
The enzyme-linked immunosorbent assay (ELISA) for the detection of human TPO was the Human TPO Quantikine kit (R & D Systems, Minneapolis, MN), which was used according to the manufacturer's instructions. The ELISA sensitivity limit was 25 ng/L of TPO.
Purification of CD34+ cells
CD34+ cells were purified either from bone marrow or blood of TAR syndrome patients. Controls were aliquots of leukapheresis obtained from mobilized patients, blood from normal controls, and bone marrow from patients undergoing hip surgery. Informed consent was obtained from all these donors. CD34+ cells were purified using a magnetic cell-sorting system (mini MACS, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Cells from leukapheresis samples and normal bone marrow were passed twice through the column. Purity evaluated by flow cytometry was about 90%. For blood or bone marrow samples from TAR syndrome patients and blood from normal controls, the immunomagnetic cell-sorting system was used to enrich the samples for CD34+ cells by passing them only once on the column. Cells eluted from the column were stained by the R-PE anti-CD34 MoAb and sorted by means of a FACS (fluorescence-activated cell sorter) Vantage (Becton Dickinson) equipped with a 100-μm nozzle and an argon laser (Coherent Radiation, Palo Alto, CA) tuned to 488 nm and operating at 500 mW.
Quantitation of clonogenic progenitors in semisolid cultures
Serum-free fibrin clot assays.
Cultures were performed in serum-free fibrin clot assays in the presence of cytokines as previously reported.13 Target cells were either low-density blood or marrow cells (1 × 105 to 2.5 × 105 cells/mL) or CD34+ cells (1 × 103 to 2 × 103 cells/mL). Three different cytokine conditions were used: (1) megakaryocyte growth and development factor (PEG-rHuMGDF; 10 ng/mL, a pegylated truncated form of human TPO, a generous gift from J. L. Nichol, Amgen; Thousand Oaks, CA) alone; or (2) with stem cell factor (SCF; 50 ng/mL, Amgen) or (3) a combination of 3 cytokines: SCF (50 ng/mL); interleukin (IL)-6 (100 U/mL), a generous gift from Dr S. Burstein (Oklahoma City, OK); and IL-3 (100 U/mL), kindly provided by Novartis (Basel, Switzerland). Cultures were incubated at 37°C in a fully humidified atmosphere containing 5% carbon dioxide in air. MK colonies were enumerated after 10 to 12 days by an indirect immuno-alkaline phosphatase–labeling technique.13
Methylcellulose assays.
Erythroid (burst-forming unit, erythroid [BFU-E]) and granulocytic (CFU-GM [granulocyte macrophage]) progenitors were quantitated using previously described methylcellulose assays.14CD34+ cells were plated at a concentration varying from 0.5 × 103 to 2 × 103 cells/mL of complete methylcellulose medium (0.8% methylcellulose in Iscove's modified Dulbecco's medium (IMDM; Gibco Life Technologies, Cergy-Pontoise, France), 30% fetal calf serum (FCS; Stem Cell, Vancouver, BC), 1% deionized bovine serum albumin (Cohn fraction V, Sigma) and 10-4 mol/L β-mercaptoethanol. Cultures were carried out in the presence of recombinant human growth factors: PEG-rHuMGDF (10 ng/mL), SCF (50 ng/mL), granculocyte colony-stimulating factor (G-CSF, 20 ng/mL; Amgen), IL-6 (100 U/mL), IL-3 (100 U/mL), and human erythropoietin (Epo, 1000 IU/L; Janssen-Cilag, Issy les Moulineaux, France). Hematopoietic progenitors were scored on day 12 using an inverted microscope.
In vitro liquid cultures of megakaryocytes from CD34+cells
CD34+ cells were grown for 6 to 12 days in serum-free medium supplemented with a combination of 2 cytokines (PEG-rHuMGDF and SCF) or 3 cytokines (SCF, IL-3, and IL-6).13
Immunolabeling and flow cytometric analysis
Cultured cells were labeled with different combinations of fluorescent-conjugated antibodies by a 30-minute incubation at 4°C. Flow cytometric analyses were performed on a FacSort cytometer (Becton Dickinson).
Cell sorting of megakaryocyte subsets
MKs at different stages of differentiation were obtained after 6 days of culture and sorted on the basis of expression of CD34, CD41a, and CD42a. Cells were incubated with a mixture of FITC anti-CD42a, R-PE anti-CD41a, and R-PE-CY5 anti-CD34 MoAbs for 30 minutes at 4°C in their culture medium. Cells were sorted according to their immunophenotype into 2 populations: CD34-CD41a+CD42a- and CD42a-CD41a++CD42a++. Individual CD34-CD41a+CD42a- cells were sorted with the automatic cloning unit device of the cell sorter into 96-well plates.13 Serum-free cultures were performed in the presence of different combinations of the following 6 cytokines: PEG-rHuMGDF (10 ng/mL), SCF (50 ng/mL), IL-3 (100 U/mL), IL-6 (100 U/mL), G-CSF (12 ng/mL), and Epo (1 IU/mL). Plates were examined at days 7 and 12 after incubation at 37°C in an air atmosphere supplemented with 5% carbon dioxide.
Western blot analysis
Platelet lysate preparation.
Peripheral blood was obtained from 4 TAR syndrome patients; platelet controls included 2 healthy donors and 2 different cord blood samples. Platelet-rich plasma was prepared. The platelet pellet was washed twice with phosphate buffered-saline (PBS; Gibco, Paisley, Scotland) containing 0.1% ethylenediaminetetraacetic acid (EDTA; Sigma). Platelets were then lysed in a lysis buffer supplemented with protease inhibitors (Complete, Boehringer Mannheim, Meylan, France); the soluble material was collected after centrifugation at 12 000g for 15 minutes. Protein concentration was determined for each sample using the Bio-Rad DC Protein colorimetric assay (Bio-Rad, Hercules, CA).
Western blotting.
Platelet lysates were diluted in an equal volume of Laemmli buffer and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 8% polyacrylamide gels); 20 μg of total protein lysate was deposited in each lane with prestained molecular weight markers (Bio-Rad). Proteins were then electrophoretically transferred onto a nitrocellulose membrane (Bio-Rad). Residual nonspecific protein binding sites were blocked by incubating the filter for 2 hours at room temperature in PBS with 0.1% Tween 20, containing 5% (w/v) dried milk. For immunoblotting, either rabbit IgG anti-human Mpl or rabbit anti-human GPIIb polyclonal or rabbit anti-glycocalicin (GPIbα) antibodies were diluted at 1:500, 1:2000, and 1:1000, respectively, in PBS-Tween-milk buffer and were incubated with the membrane for 1 hour at room temperature. After washes, the filter was incubated with a donkey anti-rabbit polyclonal antibody coupled to horseradish peroxidase diluted at 1:5000. Antibody binding was visualized with the enhanced chemoluminescence system (ECL kit, Amersham). After scanning, the intensity in each lane was measured by pixel quantitation using the MacBas v2.2 software.
Quantitative titration of messenger RNA by RT-PCR
RNA and complementary DNA preparation.
Total RNA was isolated using RNA PLUS (Bioprobe Systems, Quantum, Montreuil sous Bois, France). RNA was reverse transcribed using random hexamers (Gibco BRL/Life Technologies) with Superscript reverse transcriptase.
Construction of double internal standard for c-mpl-P and c-mpl-K.
An internal standard was constructed that contains the specific sequence of c-mpl-P and c-mpl-K. In a first step, we amplified 124 base pairs (bp) ranging from the exon 10 common to c-mpl and c-mpl-K to the exon 11 specific to c-mpl-P (sense primer: 5-GATCTCCTTGGTGACCGCTC-3; antisense primer: 5-AAGTGAGGGCCACAGGGC-3) from a human erythroleukemia cell line complementary DNA (cDNA). A 19-bp oligonucleotide (5-CTGGTCCACCGCCAGTCT-3) that corresponds to a sequence present on the intron 10, specific to c-mpl-K, was synthetized and subsequently ligated to the previous product by PCR. This standard was cloned, and its sequence was checked.
Real-time quantitative RT-PCR.
To study more precisely the ratio between Mpl-P and Mpl-K, a quantitative approach based on the fluorescent Taqman methodology and real-time PCR on the ABI Prism 7700 sequence detection system (Perkin-Elmer, Foster City, CA) was performed. An internal control containing the c-mpl-P and c-mpl-K was constructed. The steady state level of GPIIb, c-mpl-P (the main c-mpl splicing variant), and c-mpl-K messenger RNAs (mRNAs) in the specimens was compared using a relative quantitative approach as just described. Relative quantitation was performed using the standard curve method. Amplification of an endogenous control (18S ribosomal RNA) was performed to standardize the amount of sample cDNA added to the reaction.
Primers and probes were designed using the Primer Express (Perkin-Elmer) and Oligo 4 (National Biosciences, Plymouth, MN) softwares and purchased from Perkin-Elmer.
PCR amplifications were performed using the Taqman core kit (ABI) in standard conditions according to the manufacturer's instructions. Briefly, reactions were performed in 50-μL reactions containing the cDNA equivalent to 25 ng of total RNA, 1 × Taqman buffer A, 5 mM MgCl2, 200 μM dATP, dCTP, dGTP, and 400 μM dUTP, 1.25 U AmpliTaq Gold, and 0.5 U UNG (uracil N-glycosylase [AmpErase], 250 nM each primer and 100 nM of the probe). Each reaction was performed in duplicate. ABI 7700 sequence detection system was set up in the manufacturer's standard thermal cycling conditions. The SDS software was used to analyze fluorescent signals and calculate the cycle threshold.
DNA sequencing
Genomic DNA was isolated from peripheral blood cells (leukocytes and mononuclear cells), bone marrow cells, or patient Epstein-Barr virus cell lines using the proteinase K/SDS treatment and phenol/chloroform extraction.15 Each of the individual 12 exons of the c-mpl gene was amplified and sequenced using a PCR technique previously described16 with the same primers (Table 2). The gene promoter, a 903-bp fragment that includes GATA and its binding sites, was also sequenced. PCR was performed with the primers described in Table 2.
Oligonucleotide primers for sequencing c-mplgene
| Exon . | Sense . | Antisense . |
|---|---|---|
| 1 | 5′-GGCACACAGTGGCGGAGAAG-3′ | 5′-TCCCAGGGCTCCCTCTTCCT-3′ |
| 2 | 5′-ACATGCCTGGGAGGACCCAG-3′ | 5′-GGACTCAGCTAAGTGCAGGG-3′ |
| 3 | 5′-GTCCTCAGGCGTCCGCATGG-3′ | 5′-ATTCCGGGAGCTGGACTGGG-3′ |
| 4 | 5′-GGCTGAGCCATAGACTGTGG-3′ | 5′-CCTGGGGCAAGATTGAAGGT-3′ |
| 5 | 5′-CTCTCTCAGCTGACAGGCAG-3′ | 5′-GCCCAGGCTTCCCTAGAGAT-3′ |
| 6 | 5′-TATACAGTAGGGGCACACGG-3′ | 5′-TGTGGCTCACTCCCATGACA-3′ |
| 7 | 5′-GGATTAGTCTCTGAGGCAGG-3′ | 5′-CACAGGGTCAGATTCAGTGG-3′ |
| 8 | 5′-TGGCTCTGGTGGCACAATGC-3′ | 5′-GCGTAGTGAGGTCTGTGGGC-3′ |
| 9 | 5′-GACGCTGGGCTATCGAAGCC-3′ | 5′-AGTCCCTGCGCAGGCGCTGT-3′ |
| 10 | 5′-GTGGGCCGAAGTCTGACCCT-3′ | 5′-ACACCGGTTCGGCTCCACCT-3′ |
| 11 | 5′-CCTGCCAATCCACTGCCATG-3′ | 5′-AGGATCCAGTACCAGGCAGG-3′ |
| 12 | 5′-TTCCTGTACAGTCCAGCCCC-3′ | 5′-GAGAAGTCTCGAGAGTTTAGC-3′ |
| Exon . | Sense . | Antisense . |
|---|---|---|
| 1 | 5′-GGCACACAGTGGCGGAGAAG-3′ | 5′-TCCCAGGGCTCCCTCTTCCT-3′ |
| 2 | 5′-ACATGCCTGGGAGGACCCAG-3′ | 5′-GGACTCAGCTAAGTGCAGGG-3′ |
| 3 | 5′-GTCCTCAGGCGTCCGCATGG-3′ | 5′-ATTCCGGGAGCTGGACTGGG-3′ |
| 4 | 5′-GGCTGAGCCATAGACTGTGG-3′ | 5′-CCTGGGGCAAGATTGAAGGT-3′ |
| 5 | 5′-CTCTCTCAGCTGACAGGCAG-3′ | 5′-GCCCAGGCTTCCCTAGAGAT-3′ |
| 6 | 5′-TATACAGTAGGGGCACACGG-3′ | 5′-TGTGGCTCACTCCCATGACA-3′ |
| 7 | 5′-GGATTAGTCTCTGAGGCAGG-3′ | 5′-CACAGGGTCAGATTCAGTGG-3′ |
| 8 | 5′-TGGCTCTGGTGGCACAATGC-3′ | 5′-GCGTAGTGAGGTCTGTGGGC-3′ |
| 9 | 5′-GACGCTGGGCTATCGAAGCC-3′ | 5′-AGTCCCTGCGCAGGCGCTGT-3′ |
| 10 | 5′-GTGGGCCGAAGTCTGACCCT-3′ | 5′-ACACCGGTTCGGCTCCACCT-3′ |
| 11 | 5′-CCTGCCAATCCACTGCCATG-3′ | 5′-AGGATCCAGTACCAGGCAGG-3′ |
| 12 | 5′-TTCCTGTACAGTCCAGCCCC-3′ | 5′-GAGAAGTCTCGAGAGTTTAGC-3′ |
Results
Selective defect in megakaryocyte progenitors of TAR syndrome patients
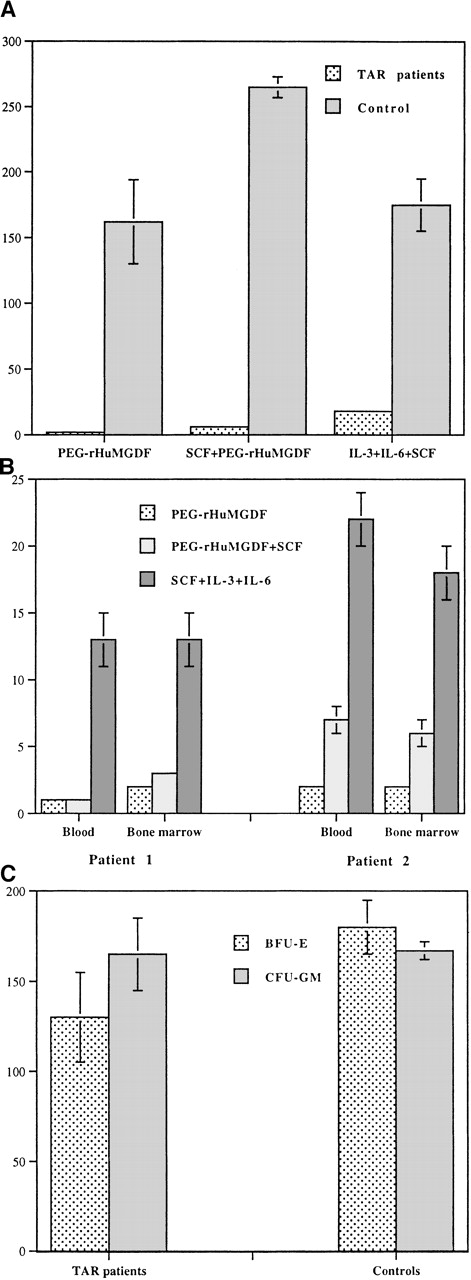
In a first set of experiments, CFU-MK was grown from low-density marrow or blood cells from 3 TAR syndrome patients. MK colonies were almost undetectable in serum-free fibrin clot assays after 12 days of culture. Therefore, further experiments were performed using purified CD34+ cells to obtain a much higher number of colonies. Because most experiments in TAR syndrome patients were performed with blood samples, we evaluated the number of CFU-MK present in the peripheral blood CD34+ cells from 2 healthy donors obtained in steady state conditions. Three conditions of cytokine stimulation were compared: (1) PEG-rHuMGDF alone, (2) SCF plus PEG-rHuMGDF, and (3) combination of 3 cytokines (SCF, IL-3, and IL-6) (Figure1A). The best growth of normal progenitors was obtained with the combination of PEG-rHuMGDF and SCF, leading to 255 and 271 CFU-MK per 2000 CD34+ control cells.
Colony formation in the TAR syndrome.
(A) CFU-MK colony formation from peripheral blood CD34+cells of TAR syndrome patients. CD34+ cells from blood of 4 TAR patients and 2 normal controls were purified by an immunomagnetic procedure followed by cell sorting and were cultured in the serum-free fibrin clot assay; 2000 CD34+ cells were seeded in the presence of PEG-rHuMGDF alone, SCF plus PEG-rHuMGDF, or a combination of 3 cytokines (IL-3, SCF, and IL-6) in triplicate. Colonies were scored at day 12 after staining with an anti-CD61 MoAb. CFU-MK was considered as aggregates of more than 2 CD61+ cells. A marked reduction in CFU-MK colony formation was observed in TAR syndrome patients compared to controls. In addition, colonies were composed of aggregates of a maximum of 5 cells. (B) Comparison of CFU-MK colony formation from blood and marrow CD34+ cells in 2 other TAR syndrome patients. The same technique was used as in Figure 1A. A marked parallel decrease in CFU-MK colony formation was observed with both blood and marrow CD34+ cells. Combination of 3 cytokines was more efficient than PEG-rHuMGDF alone or SCF plus PEG-rHuMGDF in the induction of CFU-MK growth. (C) BFU-E and CFU-GM colony formation from peripheral blood CD34+ cells of TAR syndrome patients. Peripheral blood CD34+ cells were purified as described above and were plated in methylcellulose in the presence of a combination of PEG-rHuMGDF, SCF, G-CSF, IL-6, IL-3, and Epo for 14 to 16 days. Colonies were scored under an inverted microscope. No alteration in CFU-GM and BFU-E colony formation was found in 4 TAR syndrome patients. All results are expressed per 2000 CD34+ cells.
Colony formation in the TAR syndrome.
(A) CFU-MK colony formation from peripheral blood CD34+cells of TAR syndrome patients. CD34+ cells from blood of 4 TAR patients and 2 normal controls were purified by an immunomagnetic procedure followed by cell sorting and were cultured in the serum-free fibrin clot assay; 2000 CD34+ cells were seeded in the presence of PEG-rHuMGDF alone, SCF plus PEG-rHuMGDF, or a combination of 3 cytokines (IL-3, SCF, and IL-6) in triplicate. Colonies were scored at day 12 after staining with an anti-CD61 MoAb. CFU-MK was considered as aggregates of more than 2 CD61+ cells. A marked reduction in CFU-MK colony formation was observed in TAR syndrome patients compared to controls. In addition, colonies were composed of aggregates of a maximum of 5 cells. (B) Comparison of CFU-MK colony formation from blood and marrow CD34+ cells in 2 other TAR syndrome patients. The same technique was used as in Figure 1A. A marked parallel decrease in CFU-MK colony formation was observed with both blood and marrow CD34+ cells. Combination of 3 cytokines was more efficient than PEG-rHuMGDF alone or SCF plus PEG-rHuMGDF in the induction of CFU-MK growth. (C) BFU-E and CFU-GM colony formation from peripheral blood CD34+ cells of TAR syndrome patients. Peripheral blood CD34+ cells were purified as described above and were plated in methylcellulose in the presence of a combination of PEG-rHuMGDF, SCF, G-CSF, IL-6, IL-3, and Epo for 14 to 16 days. Colonies were scored under an inverted microscope. No alteration in CFU-GM and BFU-E colony formation was found in 4 TAR syndrome patients. All results are expressed per 2000 CD34+ cells.
In the case of TAR syndrome patients, a profound decrease in colony number, from 10-fold to 25-fold, was observed in all culture conditions depending on the cytokines used (Figure 1A). In contrast to what was observed with normal cells, the best cytokine combination (SCF, IL-3, and IL-6) did not include PEG-rHuMGDF, and the combination of SCF plus PEG-rHuMGDF was slightly superior to PEG-rHuMGDF alone. In addition, colonies were composed of only a few cells (from 3 to 5 cells).
To confirm that this profound quantitative defect in MK progenitors was not related to abnormal migration of MK progenitors in blood, CFU-MK growth of marrow and blood CD34+ cells from 2 patients was compared (Figure 1B). Similar numbers of MK progenitors and responses to cytokines were found in blood and marrow CD34+ cells.
To investigate if this quantitative defect was restricted to the MK lineage, other hematopoietic progenitors were assayed in methylcellulose in the presence of 5 cytokines (SCF, IL-3, IL-6, Epo, and G-CSF). Growth of BFU-E– and CFU-GM–derived colonies from peripheral blood CD34+ cells was similar in TAR syndrome and controls (Figure 1C).
We next investigated if this abnormal MK colony formation was due to a pure quantitative defect in the number of MK progenitors or to a maturation defect that impairs MK colony formation.
Presence of an in vitro MK maturation defect in the TAR syndrome
To precisely study MK maturation, cultures from CD34+cells were performed in liquid medium in the presence of PEG-rHuMGDF, or SCF plus PEG-rHuMGDF, or the combination of 3 cytokines (SCF, IL-3, and IL-6). In the case of normal CD34+ cells, these experimental conditions induced terminal MK differentiation, including platelet-shedding MKs, on day 9-10 of culture.17
With PEG-rHuMGDF alone, no cell amplification was observed at day 8 with TAR syndrome samples. When SCF was added to PEG-rHuMGDF, only a 2.5-fold increase in cell number was noted, whereas the combination of 3 cytokines (SCF, IL-3, and IL-6) elicited a 5-fold increase in cell number. No terminal MK maturation was noted except in the presence of 3 cytokines, a condition in which rare proplatelet-bearing MKs (less than 1%) were observed. These experiments demonstrate impaired in vitro MK maturation in the TAR syndrome and an abnormal response to cytokines, particularly to TPO.
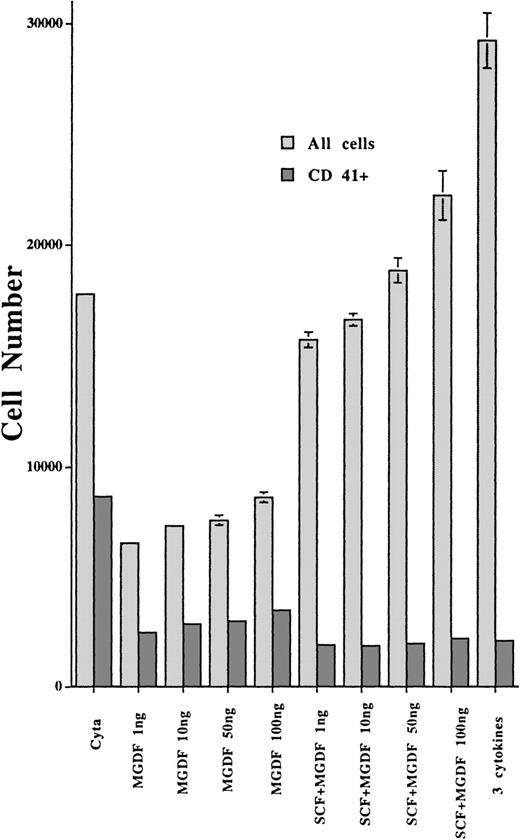
To better investigate if this poor response to TPO was due to a decreased sensitivity, we studied the effect of increasing concentrations of PEG-rHuMGDF in the absence or presence of SCF. CD34+ cells (105 cells/mL) were grown in 96-well plates in the presence of 4 different PEG-rHuMGDF concentrations (1, 10, 50, and 100 ng/mL supplemented or not with SCF 25 ng/mL), each condition being performed in triplicate. For a control, normal CD34+ cells were cultured in presence of 10 ng/mL PEG-rHuMGDF and 25 ng/mL SCF. After 10 days of culture, each well was harvested separately and cells were labeled by FITC anti-CD42a and R-PE anti-CD41a MoAbs. Dead cells were excluded by 7AAD. Total cell numbers and CD41a+ cells were directly quantitated by flow cytometry through a time-dependent acquisition and analyzed after removing dead cells by gating 7AAD-positive events. Figure2 illustrates the results obtained in 1 patient. The dose-response assay did not differ from normal controls, with a plateau obtained at 1 ng/mL of PEG-rHuMGDF for CD41+cells.18 SCF had no synergistic effects with PEG-rHuMGDF on the growth of MK. In contrast, it markedly increased the number of non-MK cells (CD34+ and myeloid cells). Interestingly, patient cells grown in the presence of 3 cytokines exhibited a higher proliferation rate, but the content in CD41a+ cells was very similar to that of cultures stimulated by PEG-rHuMGDF.
Effects of PEG-rHuMGDF in liquid culture medium on the growth of MKs from CD34+ cells of TAR syndrome patients.
PEG-rHuMGDF was tested at different concentrations, alone or in combination with SCF. CD34+ cells (2000 cells) from bone marrow of a TAR syndrome patient were grown in a 96-well plate (100 μL) in triplicate. At day 10, the numbers of viable cells and viable CD41+ cells were quantitated by flow cytometry using staining with an R-PE anti-CD41a MoAb and 7AAD.
Effects of PEG-rHuMGDF in liquid culture medium on the growth of MKs from CD34+ cells of TAR syndrome patients.
PEG-rHuMGDF was tested at different concentrations, alone or in combination with SCF. CD34+ cells (2000 cells) from bone marrow of a TAR syndrome patient were grown in a 96-well plate (100 μL) in triplicate. At day 10, the numbers of viable cells and viable CD41+ cells were quantitated by flow cytometry using staining with an R-PE anti-CD41a MoAb and 7AAD.
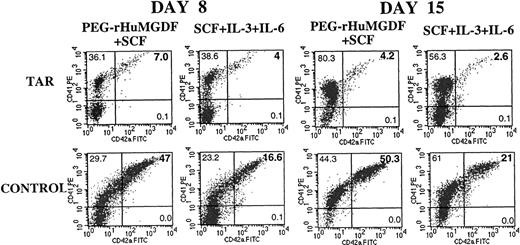
In these experiments, we observed a dissociation between the expression of CD41 and CD42 (data not shown). To further characterize the cellular abnormality associated with the TAR syndrome, we studied the expression of CD41a and CD42a by flow cytometry in MK liquid cultures from 6 different patients. CD34+ cells from mobilized leukapheresis samples, normal bone marrow, or normal peripheral blood grown in similar conditions were used as a control. Analyses were performed on different days of culture. At day 8, a very low proportion of cells expressed CD42a in comparison to the controls. More surprisingly, cultures were extremely depleted in cells coexpressing CD41a and CD42a, corresponding to maturing MKs (Figure3). At day 15, unlike the situation observed in normal cultures, the proportion of CD41a+CD42a+ cells decreased in the TAR syndrome cultures, and a well-defined subset of CD41a+CD42a- cells appeared (Figure 3). This MK subpopulation seemed to be blocked at an early stage of maturation. In control cultures, most cells coexpressed CD41a and CD42a because a shift along the X axis (CD42a, FITC) was observed for CD41a+ cells, including most of those expressing a low level of CD41a (Figure 3). Only rare cells with low expression of CD41a appeared to be negative for CD42a. In contrast, cells with an intermediate and low level of CD41a did not express CD42a in the TAR syndrome cultures (Figure 3). Similar results were observed in the 6 patients studied, either in the presence of PEG-rHuMGDF or 3 cytokines. However, some differences were observed depending on the cytokines used. When PEG-rHuMGDF alone was used, most of the cells expressed the CD41a antigen, but no terminal maturation was observed. In addition, marked apoptosis detected by binding of annexin V to CD41a+cells was present (Figure 4). Three cytokines appeared to be the best cytokine mix to support TAR syndrome MK cultures.
Double staining by anti-CD41a and CD42a MoAbs of CD34+ cells cultured for 8 and 15 days in liquid culture medium.
Blood CD34+ cells from a TAR syndrome patient were grown in liquid medium in the presence of SCF plus PEG-rHuMGDF or a combination of 3 cytokines (IL-3, SCF, and IL-6). At days 8 and 15, cells were labeled by an R-PE anti-CD41a MoAb and FITC anti-CD42a MoAb and analyzed by flow cytometry. This figure illustrates the results obtained in 1 patient. Similar results were obtained in the 4 other patients studied.
Double staining by anti-CD41a and CD42a MoAbs of CD34+ cells cultured for 8 and 15 days in liquid culture medium.
Blood CD34+ cells from a TAR syndrome patient were grown in liquid medium in the presence of SCF plus PEG-rHuMGDF or a combination of 3 cytokines (IL-3, SCF, and IL-6). At days 8 and 15, cells were labeled by an R-PE anti-CD41a MoAb and FITC anti-CD42a MoAb and analyzed by flow cytometry. This figure illustrates the results obtained in 1 patient. Similar results were obtained in the 4 other patients studied.
Quantitative analysis of apoptosis in CD41a+ cells from TAR syndrome patients.
Blood CD34+ cells were grown in liquid culture in the presence of PEG-rHuMGDF (A), SCF plus PEG-rHuMGDF (B), or a combination of 3 cytokines (SCF, IL-3, and IL-6) (C). At day 9, cells were stained by annexin V-FITC, R-PE anti-CD41a MoAb, and 7AAD. Dead cells were excluded on the basis of 7-AAD staining (FL3). Viable cells were studied for CD41a and annexin V staining. The horizontal axis shows cells stained by annexin V and the vertical axis cells stained by the anti-CD41a MoAb. This figure illustrates a typical experiment from 1 patient. Similar results were obtained in 3 other patients.
Quantitative analysis of apoptosis in CD41a+ cells from TAR syndrome patients.
Blood CD34+ cells were grown in liquid culture in the presence of PEG-rHuMGDF (A), SCF plus PEG-rHuMGDF (B), or a combination of 3 cytokines (SCF, IL-3, and IL-6) (C). At day 9, cells were stained by annexin V-FITC, R-PE anti-CD41a MoAb, and 7AAD. Dead cells were excluded on the basis of 7-AAD staining (FL3). Viable cells were studied for CD41a and annexin V staining. The horizontal axis shows cells stained by annexin V and the vertical axis cells stained by the anti-CD41a MoAb. This figure illustrates a typical experiment from 1 patient. Similar results were obtained in 3 other patients.
The rare maturing MKs, ie, CD41a+CD42a+ cells, were also sorted and plated in liquid culture to investigate terminal maturation. Cultures were examined under an inverted microscope, and platelet-shedding MKs were scored daily for 5 days. In 3 different patients, very few platelet-shedding MKs were observed in culture with PEG-rHuMGDF alone, whereas the best results were obtained with the combination of 3 cytokines (SCF, IL-3, and IL-6) (data not shown).
All of these results suggest that MK progenitors mature abnormally, with a partial block in maturation at a cellular stage characterized by the presence of CD41 and absence of CD42. Rare cells escaped to this block.
Characterization of the CD41a+CD42a- cell subset
Cells were first characterized by their immunophenotype using 3-color labeling. CD41a+CD42a- cells did not express CD34 or other lineage markers, such as glycophorin A, CD15, and CD14 (data not shown).
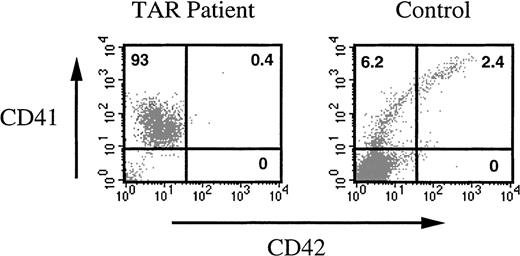
To further investigate their biologic properties, these cells were sorted and plated either in semisolid or in liquid medium in the presence of different cytokine combinations. Clonogenic and limiting dilution assays failed to detect any progenitors among CD41a+CD42a- TAR syndrome cells (data not shown). In liquid cultures, cells were immunophenotyped to investigate their differentiation in response to different growth factor combinations (including SCF, IL-3, IL-6, Epo, G-CSF, and PEG-rHuMGDF). Even after prolonged cultures, no phenotypic changes were observed in sorted cells, except that very few cells (less than 0.1%) acquired CD42 before undergoing apoptosis (Figure5). Cells did not acquire markers of other myeloid cell lineages (glycophorin A, CD15, CD14). Addition of phorbol ester (PMA 20 and 40 nM) to CD41+CD42- cells did not induce any differentiation (data not shown). In contrast, PMA markedly increased the expression of CD41a and CD42a when added to purified CD41+CD42- cells obtained after a 7-day stimulation by PEG-rHuMGDF and SCF of normal CD34+cells (data not shown).
Differentiation of CD41a+CD42a− cells in the presence of 6 cytokines.
CD34+ cells from a TAR syndrome patient and a control were grown in the presence of SCF plus PEG-rHuMGDF for 8 days. CD41a+CD42a- cells were sorted and cultured for 10 additional days in the presence of 6 cytokines (SCF, IL-3, IL-6, G-CSF, PEG-rHuMGDF, and Epo). At the end of the culture, cells were restained with an R-PE anti-CD41a MoAb and an anti-CD42a MoAb. The TAR syndrome patient cells remained CD41a+ and did not acquire CD42a or new markers of differentiation. The majority of normal CD41a+CD42a- cells lost the CD41 antigen and acquired other markers of differentiation, in particular, glycophorin A (data not shown), and the remaining differentiated along the MK pathway with the appearance of CD42.
Differentiation of CD41a+CD42a− cells in the presence of 6 cytokines.
CD34+ cells from a TAR syndrome patient and a control were grown in the presence of SCF plus PEG-rHuMGDF for 8 days. CD41a+CD42a- cells were sorted and cultured for 10 additional days in the presence of 6 cytokines (SCF, IL-3, IL-6, G-CSF, PEG-rHuMGDF, and Epo). At the end of the culture, cells were restained with an R-PE anti-CD41a MoAb and an anti-CD42a MoAb. The TAR syndrome patient cells remained CD41a+ and did not acquire CD42a or new markers of differentiation. The majority of normal CD41a+CD42a- cells lost the CD41 antigen and acquired other markers of differentiation, in particular, glycophorin A (data not shown), and the remaining differentiated along the MK pathway with the appearance of CD42.
No genomic alteration in c-mpl gene of TAR syndrome patients
Because c-mpl, the TPO receptor, plays a crucial role in MK differentiation, we searched for an abnormality of c-mpl in TAR syndrome. We first investigated the c-mpl gene.
A Southern blot was performed that excluded a large deletion in the 32-kilobase long genomic region that was analyzed (data not shown). Then, a subtle genomic alteration was sought by sequencing the coding region of the gene, the intron-exon junctions and, finally, the c-mpl promoter. Neither mutation nor recurrent polymorphisms could be detected in 8 patients tested, leading to the conclusion that the defect in MK differentiation was not the result of an alteration in thec-mpl gene. However, this result does not exclude a defect in c-mpl expression.
Impaired expression of Mpl in TAR syndrome platelets
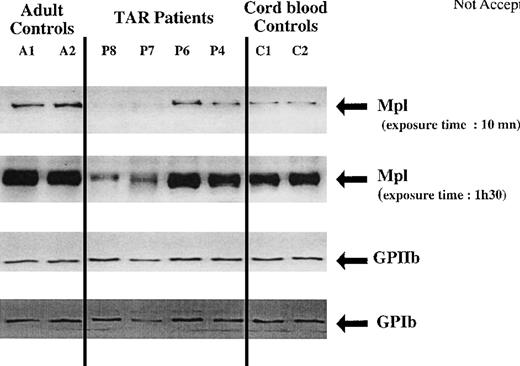
Mpl expression was studied by Western blot analysis in platelets from 4 different patients (ages ranging from 7 to 55 years, platelet counts from 50 × 109/L to 80 × 109/L). Platelets from adult and cord blood were used as controls. Mpl was revealed first by probing with an IgG polyclonal antibody (Figure 6); filters were stripped and reprobed with an anti-GPIIb antibody for normalization. Mpl and GPIIb bands were scanned and submitted to pixel quantitation. Figure 7 shows that normalized Mpl expression in TAR syndrome platelets was significantly lower than in adult control platelets. Two patients (P7 and P8) had extremely low levels of Mpl in their platelets. The 2 other patients also had a decreased level of Mpl in comparison to adult platelets, as shown in Figure 7, at a 10-minute exposure time. It is noteworthy that cord blood platelets expressed much lower amounts of Mpl than platelets from adults. On average, the levels of Mpl in neonatal and TAR syndrome platelets were quite similar. Using our technique of Western blot, we could not differentiate the Mpl-P and Mpl-K isoforms.
Western blot analysis of Mpl, GPIIb, and GPIb expression in platelets from normal adult controls, newborns, and TAR syndrome patients.
Platelet lysates (20 μg) were separated by SDS-PAGE and probed with polyclonal antibodies against Mpl, GPIIb, and GPIbα.
Western blot analysis of Mpl, GPIIb, and GPIb expression in platelets from normal adult controls, newborns, and TAR syndrome patients.
Platelet lysates (20 μg) were separated by SDS-PAGE and probed with polyclonal antibodies against Mpl, GPIIb, and GPIbα.
Comparison of Mpl expression in platelets from normal adult controls, newborns, and TAR syndrome patients.
Western blots were scanned, and the intensity of each lane was measured by pixel quantitation using the MacBas v2.2 software. Mpl levels were normalized to GPIIb expression and compared. Results are expressed in arbitrary units resulting from the ratio of the Mpl to GPIIb band. Four patients with a TAR syndrome, 4 adult controls, and 4 newborns were studied.
Comparison of Mpl expression in platelets from normal adult controls, newborns, and TAR syndrome patients.
Western blots were scanned, and the intensity of each lane was measured by pixel quantitation using the MacBas v2.2 software. Mpl levels were normalized to GPIIb expression and compared. Results are expressed in arbitrary units resulting from the ratio of the Mpl to GPIIb band. Four patients with a TAR syndrome, 4 adult controls, and 4 newborns were studied.
GPIb was normally expressed in TAR syndrome, further demonstrating that platelet production arises from CD41+CD42+cells and not from the CD41+CD42- cell subset.
Expression level of c-mpl mRNA in platelets and MKs from TAR syndrome patients
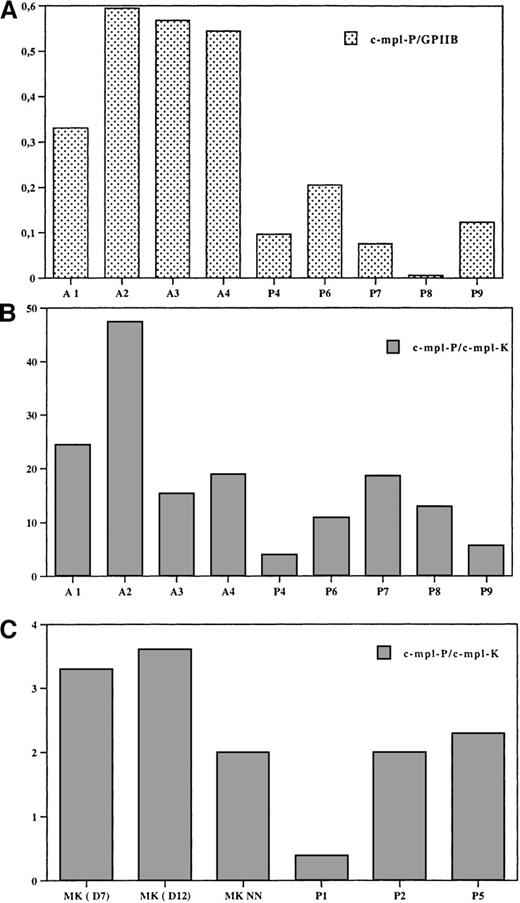
To investigate the mechanisms of the low level of Mpl in platelets from TAR patients, we studied the expression of c-mpl by means of a real-time quantitative RT-PCR assay. We studied 2 isoforms of c-mpl—c-mpl-P and c-mpl-K—which arise by alternative splicing with a premature termination of transcription in intron 10, and normalized their expression to that of GPIIb and 18S mRNA. GPIIb was expressed at the same level in TAR, adult, and cord blood platelets in comparison to 18S. Thus, the GPIIb transcript was used for normalization. The ratio between c-mpl and GPIIb is not the real ratio because in these experiments we have not used an internal standard for each marker, but it can be used to compare the difference in c-mpl content in each platelet samples. As illustrated in Figure8A, c-mpl-P in TAR platelets was markedly decreased in comparison to adult platelets (a 3-fold decrease, n = 6). c-mpl-P was also expressed at a lower level in cord blood platelets than in adult platelets, and only a 50% decrease was observed in TAR platelets in comparison to neonate platelets. The c-mpl-P to c-mpl-K ratio was studied by using a single standard that contains both forms and thus allows to precisely determine the real ratio between c-mpl-P and c-mpl-K in each cell sample. c-mpl-P was the predominant form of c-mpl in platelets, as previously demonstrated.19 20 The c-mpl-P to c-mpl-K ratio was reduced in TAR syndrome platelets in comparison to adult platelets (Figure 8B) or neonate platelets but with some variation among patients (2 patients having a 5-fold decreased level). The c-mpl-P to c-mpl-K ratio was also studied in cultured MKs from adult cord blood and TAR MKs. Surprisingly, this ratio was a 6-fold decrease in normal MKs in comparison to platelets. Thus, this result suggests that during normal MK differentiation expression of c-mpl-P increased at the expense of c-mpl-K. In the TAR syndrome, only 3 patients could be studied. In 2 of them, the ratio was in the same range as normal adult or cord blood MKs (Figure 8C); in the third, the ratio was extremely low.
Expression of c-mpl-P and c-mpl-K RNA by real-time RT-PCR in platelets and MKs from normal adult controls, newborns, and TAR syndrome patients.
Expression of c-mpl-P, c-mpl-K, GPIIb, and 18S was studied by a quantitative RT-PCR assay (see “Materials and Methods”). (A) Comparison of the expression of c-mpl-P in normal adults and TAR platelets. Expression of c-mpl-P was normalized in each sample to the expression of GPIIb. This is expressed by a ratio that does not correspond to the real ratio between the 2 different transcripts because we did not use a standard. This ratio allows the comparison of the c-mpl-P transcript expression in normal and TAR platelets. (B) Ratio between c-mpl-P and c-mpl-K in platelets. To calculate the real ratio between the 2 isoforms, a standard was constructed that contains the 2 amplified sequences permitting precise evaluation of the efficiency of each PCR. (C) Ratio between c-mpl-P and c-mpl-K in MKs. MKs were obtained from culture of normal adult bone marrow at day 7 and day 12, from cord blood (NN) at day 12, and TAR patients at day 12.
Expression of c-mpl-P and c-mpl-K RNA by real-time RT-PCR in platelets and MKs from normal adult controls, newborns, and TAR syndrome patients.
Expression of c-mpl-P, c-mpl-K, GPIIb, and 18S was studied by a quantitative RT-PCR assay (see “Materials and Methods”). (A) Comparison of the expression of c-mpl-P in normal adults and TAR platelets. Expression of c-mpl-P was normalized in each sample to the expression of GPIIb. This is expressed by a ratio that does not correspond to the real ratio between the 2 different transcripts because we did not use a standard. This ratio allows the comparison of the c-mpl-P transcript expression in normal and TAR platelets. (B) Ratio between c-mpl-P and c-mpl-K in platelets. To calculate the real ratio between the 2 isoforms, a standard was constructed that contains the 2 amplified sequences permitting precise evaluation of the efficiency of each PCR. (C) Ratio between c-mpl-P and c-mpl-K in MKs. MKs were obtained from culture of normal adult bone marrow at day 7 and day 12, from cord blood (NN) at day 12, and TAR patients at day 12.
Plasma TPO level in 6 TAR patients
Finally, we measured plasma TPO levels in 6 TAR patients with platelet counts ranging between 50 × 109/L and 110 × 109/L. The TPO level was slightly but significantly elevated in these patients (mean 75 ng/L versus 40 ng/L in adult and 50 ng/L in cord blood), as previously reported,4 suggesting that there is no major abnormality in the synthesis of TPO in this disease.
Discussion
The TAR syndrome is a hereditary disease associated with skeletal malformations, including thrombocytopenia and a bilateral absence of radii.1-3 The thrombocytopenia has very peculiar characteristics: it is due to a defect in platelet production, and it is extremely severe during the first years of life, but it progressively improves, with the platelet count returning to near normal values in adulthood. Progressive correction of the thrombocytopenia after birth suggests that the gene(s) involved in defective megakaryocytopoiesis is developmentally regulated.
To better understand the defects in MK differentiation in the TAR syndrome, we studied 6 patients with this disease. All patients were studied in infancy or adulthood at a time when thrombocytopenia was moderate (50 × 109/L to 110 × 109/L). Nevertheless, we found that in vitro megakaryocytopoiesis was profoundly altered. Confirming a previous study,5 in vitro MK colony formation from either blood or bone marrow CD34+ cells was 10-fold to 20-fold reduced in comparison to that of normal controls, whatever cytokine was used in the culture. In contrast, growth of BFU-E– and CFU-GM–derived colonies was normal. Impaired MK colony formation could be related to a true absence of MK progenitors or to a maturation defect leading to a subsequent block in MK differentiation. To solve this problem, we investigated the immunophenotype of MKs derived in vitro from CD34+ cells after cytokine stimulation in liquid medium. Very few mature MKs characterized by high levels of CD41 and CD42 were present in the cultures, whatever cytokine was used. Surprisingly, in cultures of TAR syndrome patients, accumulation of cells with a peculiar phenotype was detected. These cells expressed CD41 antigen in the absence of CD42 and CD34 antigens. They began to be detectable after day 6 of culture and, thereafter, increased in number. They had the morphology of blasts cells and could not be induced to undergo terminal MK differentiation by cytokines or PMA treatment. These cells were long-lived and could be kept in culture for several weeks without proliferation or modification of their immunophenotype. However, their survival was dependent on the presence of cytokines in the medium. Cells with a similar phenotype could be observed in low numbers in liquid cultures of normal adult CD34+ cells and to a higher degree in cultures of cord blood CD34+ cells (data not shown). These cells from normal controls expressed much lower amounts of CD41 than their TAR syndrome counterparts; higher levels of CD41 were associated with expression of CD42 in normal controls. These CD41+CD42- cells could be the first commitment step toward terminal MK differentiation because it has been demonstrated that CD41 expression precedes CD42 expression during MK differentiation.21,22 However, there is increasing evidence that CD41 antigen is not specific for the MK lineage and can be expressed early during myeloid, erythroid, and T-cell differentiation.23-27 In the TAR syndrome, CD41+CD42- cells do not seem identical to those cells because they could not be induced toward erythroid or granulocytic differentiation in the presence of Epo or G-CSF. For this reason, in the TAR syndrome, CD41+CD42- cells are likely, although not totally demonstrated, MK precursors blocked in their differentiation. It is noteworthy that these CD41+CD42- cells did not contribute to platelet formation because they do not shed platelets in vitro and, also, that GPIb was normally expressed in TAR syndrome platelets. In a patient with the TAR syndrome, de Alarcon et al reported a normal number of CFU-MK–derived colonies. Cells making up the colonies had marked morphologic abnormalities and were of small size.6 These cells might be the equivalent of the CD41+CD42-cells found in our assays.
Therefore, one might speculate that dysmegakaryocytopoiesis in the TAR syndrome is the consequence of an intrinsic cellular defect related to an abnormality in a protein that regulates late MK differentiation. The TAR syndrome can be compared in many ways to Diamond-Blackfan anemia, in which skeletal and hematologic abnormalities are both present. In this syndrome, the anemia is associated with an erythroblastopenia and a variable number of erythroid progenitors, suggesting a blockage in the erythroid differentiation at a CFU-E stage of differentiation.28,29 Recently, a mutation in the gene encoding ribosomal protein S19 has been demonstrated in 30% of the patients.30
A reduced response to TPO was also found in addition to maturation defects: (1) unlike normal controls, a higher number of colonies were obtained using a combination of cytokines that did not include PEG-rHuMGDF; (2) in liquid culture, platelet-shedding MKs were observed essentially with a combination of 3 cytokines (IL-3, IL-6, and SCF); and (3) most maturing MKs stimulated by PEG-rHuMGDF underwent apoptosis. These findings support the results of Ballmaier et al that showed that platelets from TAR syndrome patients did not respond to TPO, suggesting that this defect could be related to abnormal signal transduction downstream of Mpl.4 A point mutation in the c-mpl gene was not ruled out. We searched for an abnormality of the c-mpl gene in the TAR syndrome. Our results ruled out the presence of a point mutation in the c-mplgene and its proximal promoter, in agreement with the recent report of Strippoli et al.31 However, we found 2 abnormalities:
(1) The level of Mpl was decreased in platelets from TAR syndrome patients in comparison to that of adult platelets, and this reduction was heterogeneous but quite marked in 2 patients. It is noteworthy that the Mpl content of cord blood platelets was markedly reduced in comparison to that of adult platelets, suggesting that c-mpl is regulated during development. Levels of Mpl in neonatal and TAR syndrome platelets were quite similar. This was not due to the age of the TAR syndrome patients because 2 of them were adults.
(2) c-mpl RNA levels studied by quantitative RT-PCR assay were markedly reduced in TAR syndrome platelets in comparison to those of adult platelets but identical to those of neonatal platelets. In addition, we studied the 2 main c-mpl isoforms (c-mpl-P and c-mpl-K).32 33 The c-mpl-P to c-mpl-K ratio was reduced in TAR platelets but heterogeneous among patients. However, this ratio surprisingly varied during MK differentiation. It was much lower in normal MKs than in platelets. The c-mpl-P to c-mpl-K ratio in TAR MKs was only slightly diminished in comparison to normal MKs, suggesting that the relative change in isoforms did not occur during MK differentiation in the TAR syndrome.
Diminished expression of c-mpl has been reported in myeloproliferative diseases, including essential thrombocythemia, polycythemia vera, and myelofibrosis.19,34 As in the TAR syndrome, this decrease in Mpl protein is associated with a parallel reduction in c-mpl RNA. However, in essential thrombocythemia it was shown that the relative expression of the different c-mpl isoforms was normal.20 In the TAR syndrome, the decrease in c-mpl expression associated with a marked reduction in the c-mpl-P to c-mpl-K ratio may have some biologic consequences. Indeed, Mpl-K is an isoform that has an extracellular sequence that is identical to that of Mpl-P but has an extensive deletion in its intracellular domain with absence of signaling boxes 1 and 2.32,33,35 Thus, Mpl-K is theoretically unable to transduce a signal. At present, it is not known if Mpl-K is normally expressed on the surface of MKs and platelets. If it is expressed on their surface, it should be able to bind TPO without transducing a signal. Furthermore, it may behave as a dominant negative molecule because Mpl requires homodimerization to transduce a signal.36-38 If it is the case, the low c-mpl-P to c-mpl-K ratio observed during the late MK differentiation in the TAR syndrome may play a role in the defective megakaryocytopoiesis. Further experiments to study the precise role of Mpl-K will be important to support this hypothesis.
On the other hand, it seems unlikely that the low level of Mpl-P can entirely explain the MK differentiation defect in the TAR syndrome, for the following reasons: (1) the maturation defect persists, although to a lesser extent, when cultures are stimulated by a combination of 3 cytokines, and (2) c-mpl-/- mice have decreased numbers of MK progenitors as well as other hematopoietic progenitors,39 40 a finding not present in the TAR syndrome, and they have no MK maturation defect. This suggests that the abnormal c-mpl expression in TAR syndrome may be the consequence of an intrinsic cellular defect. However, it cannot be excluded that it participates in the mechanism of thrombocytopenia. Future experiments to induce c-mpl-P overexpression in TAR syndrome MK progenitors may help to solve this question.
Acknowledgments
We thank C. Cailliot (Amgen, Neuilly, France) and J. L. Nichol (Amgen, Thousand Oaks, CA) for the gift of PEG-rHuMGDF and stem cell factor; T. Kato (Kirin Brewery Co, Ltd, Tokyo, Japan) for the gift of polyclonal antibodies against human Mpl; and D. Pidart (Institut Pasteur, Paris, France) for the gift of anti-GPIIb antibody. We are grateful to Dr F. Beaujean and F. Norol (Hôpital Henri Mondor, Créteil, France) for providing the cytapheresis samples. We are indebted to B. Forget for editing the English in the manuscript.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale. N.V. was supported by a fellowship from Amgen and R.L. by a fellowship from the Comité de Recherche Clinique (Institut Gustave Roussy, Villejuif, France).
Reprints:Najet Debili, INSERM U 362, Laboratoire associéno. 5 du comité de Paris de la Ligue Nationale, Institut Gustave Roussy, Villejuif 94805, Cedex, France; e-mail: denali@igr.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal