Abstract
Interleukin (IL) 4 is a potent immunomodulatory cytokine secreted by T-helper 2 (Th2) cells and Th2 mast cells that promotes the commitment of cells. However, unregulated production and release of IL-4 can exacerbate allergic reactions and increase susceptibility to infectious organisms and viruses. Here, we present evidence that AG-490, a Janus tyrosine kinase (JAK) 2-JAK3 inhibitor, effectively blocked IL-4 gene expression and secretion in the Th2 cell line D10 that was not occurring after anti-CD3 antibody stimulation, whereas AG-490 had no inhibitory effect on production of other Th2 cytokines or cytokines synthesized by the corresponding Th1 cell line clone 29. AG-490 potently inhibited IL-4–mediated proliferation of both D10 and the IL-4–dependent cell line CT.4S. Moreover, AG-490 markedly inhibited IL-4 activation of JAK3 and blocked the downstream activation of signal transducer and activator of transcription 6, as judged by tyrosine phosphorylation, DNA binding, and transcription assays. In contrast, AG-490 did not affect tumor necrosis factor activation of NF-κB at similar concentrations of drug. These data suggest that tyrosine kinase inhibitors that inhibit JAK3 may have previously unrecognized and selective clinical potential as immunotherapeutic drugs to treat Th2-mediated diseases driven by IL-4.
CD4+ T lymphocytes can be divided into 3 subsets of T-helper (Th) cells1: Th1, Th2 (defined by their distinct cytokine-secretion profile), and Th0 cells, which produce low levels of most cytokines. Th1 cells synthesize interleukin (IL) 2, interferon (IFN) γ, and lymphotoxin, which are cytokines that regulate cell-mediated immune responses. Th2 cells, on the other hand, release IL-4, IL-5, IL-10, and IL-13 and promote B-cell growth and differentiation, which serves to coordinate humoral-type immune responses.2-4 Aberrant activation of CD4+ T cells initiates a variety of cell-mediated disease states in humans. Several T-cell–based disease conditions can be accentuated by a preferentially activated Th subset.5 These syndromes are believed to be due partly to dysregulated production and release of cytokines, soluble mediators that promote the recruitment, growth, and differentiation of immune responsive cells.6 7
Many cytokines, hormones, and growth factors activate a family of receptors that use Janus tyrosine kinases (JAKs), which then activate 1 or more of the 7 members of the signal transducers and activators of transcription (STAT) family.8 STATs regulate gene expression and are fundamentally important for T-cell function. Indeed, STAT1-deficient mice are highly sensitive to viral or bacterial infection and lack responsiveness to IFN-γ or IFN-α,8,9whereas mice without STAT4 show a loss of Th1 cell function and mice without STAT6 have a loss of Th2 cell function.10 11 Thus, pharmaceutical agents that inhibit the production of Th cytokines or their signaling pathways would have a profound impact on T-cell activity and possess therapeutic potential.
It is well known that cytokines secreted by one Th subset often promote their own growth and expansion while exerting opposing effects on the other Th subset, thereby accentuating cell polarization and cytokine production of a particular Th subset. For example, release of Th1 cytokines can promote autoimmune diseases, including experimental allergic encephalomyelitis and multiple sclerosis, but it can also trigger insulin-dependent diabetes mellitus and allograft rejection.12,13 In contrast, overproduction and circulation of Th2 cells are commonplace during allergic disorders such as asthma, allergic rhinitis, and atopic dermatitis by means of their ability to activate and recruit monocytes, basophils, mast cells, and eosinophils.14 These findings suggest that imbalance of Th1 or Th2 might be corrected by an IL-2 inhibitor that could disrupt Th1 diseases, whereas an inhibitor that blocked IL-4 signal could inhibit Th2-type diseases.
AG-490 is a recent addition to the synthetically derived tyrphostin family of tyrosine kinase inhibitors.15-17 At relatively low concentrations, AG-490 blocked hyperactive forms of JAK2 found in B-cell precursors of acute lymphoblastic leukemia (ALL)17and in JAK2 constructed hyperactive variants.18 We demonstrated that AG-490 could directly inhibit JAK3 catalytic activity.19 Moreover, AG-490 potently inhibited consequent JAK3-mediated STAT1 and STAT3 activation by IL-2.20 AG-490 also has been found to effectively prevent the accumulation of leukocytes in the brain and the development of experimental autoimmune encephalomyelitis.21-23
In the study described in this paper, we compared the effect of tyrphostin AG-490 on Th1 and Th2 cytokine production, respectively, in murine cell lines clone 29 and D10. We found evidence that AG-490 potently and effectively inhibits production of IL-4 but not other Th2 and Th1 cytokines. IL-4–mediated JAK3 activity was potently inhibited by AG-490, as was the downstream activation of STAT6. These data suggest that AG-490 (or its analogues) may represent a previously unrecognized therapeutic agent that can be used to selectively treat a variety of Th2-derived disorders by disrupting an autocrine-regulated loop mediated by IL-4.
Materials and methods
Cell culture and treatment
The murine Th2 cell line D10 was maintained in RPMI-1640 medium containing 10% fetal-calf serum (FCS), 2 mmol/Ll-glutamine, penicillin (50 IU/mL)-streptomycin (μg/mL), 2 U/mL IL-1 (PeproTech, Rocky Hill, NJ), 25 U/mL recombinant human IL-2 (Hoffmann-LaRoche, Nutley, NJ), 2 μg/mL concanavalin A (Sigma, St Louis, MO), 35 μmol/L β-mercaptoethanol, and 6 mmol/L HEPES. The Th1 cell line clone 29 was maintained in the above media without concanavalin A or IL-1. Both Th clones were gifts from Dr Minute Li-Weber. The IL-4–dependent murine T-cell line CT.4S,24 25 provided by W. E. Paul's laboratory, was maintained in Dulbecco modified Eagle medium supplemented with 500 U/ml IL-4, 10% FCS, 2 mmol/Ll-glutamine, penicillin (50 IU/mL)-streptomycin (50 μg/mL), 1 mmol/L sodium pyruvate, and 0.05 mmol/L β-mercaptoethanol. All reactions with AG-490 (Calbiochem, San Diego, CA) were kept in the dark to avoid inactivation of the tyrphostin.
Cytokine analysis measured with enzyme-linked immunosorbent assay (ELISA)
D10 and clone 29 cells were washed and grown in the normal medium described above or stimulated by coated anti-CD3 antibodies and treated in the presence or absence of AG-490. The final concentration of dimethyl sulfoxide (DMSO) in control and AG-490 treatments was below 0.1%. After 16 hours of treatment, supernatants were collected and assayed for murine cytokines (IL-2, IL-4, IL-5, IL-10, IL-13, tumor necrosis factor (TNF) α, TNF-β, and IFN-γ) with Endogen kits (Wolburn, MA), according to the manufacturer's instructions, by the Clinical Services Department of the Frederick Cancer Research and Development Center.
Proliferation assays
Quiescent D10 cells (5 × 104/well) were plated in flat-bottomed 96-well microtiter plates in 200 μL of growth media with 5% FCS and cultured with IL-4 (1 nmol/L) or media alone. Cells were treated for 16 hours with AG-490 or DMSO, pulsed for the remaining 4 hours of the assay with tritium thymidine (18.5 × 102 Bq/200 μL), and harvested onto glass-fiber filters. Tritium-thymidine incorporation was analyzed by liquid scintillation counting.26
Solubilization of membrane proteins, immunoprecipitation, and Western blotting
Cells were solubilized in lysis buffer (108 cells/mL) containing 10 mmol/L Tris-hydrochloric acid (pH 7.6), 5 mmol/L EDTA, 50 mmol/L sodium chloride, 30 mmol/L sodium pyrophosphate, 50 mmol/L sodium fluoride, 200 mmol/L sodium orthovanadate, 1% Triton-X 100, 1 mmol/L phenylmethylsulfonyl fluoride, 5 μg/mL aprotinin, 1 μg/mL pepstatin A, and 2 μg/mL leupeptin. Immunoprecipitation of the selected protein was performed by using STAT6 antibodies (R&D Systems, Minneapolis, MN) or JAK3 antibodies (Upstate Biotechnology, Lake Placid, NY) as described previously.20 JAK3 or STAT6 captured proteins were separated on 7.5% sodium dodecyl sulfate–polyacrylamide electrophoresis under reducing conditions. All proteins were transferred to Immobilon-P membrane and blotted with appropriate antibodies that were diluted 1:1000 in blocking buffer as described previously.27
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from D10 cells treated with AG-490 or the DMSO control stimulated in the presence or absence of cytokine. The sequences of the oligonucleotides used as probes were 5′ GATCAAGACCTTTTCCCAAGAAATCTAT 3′, corresponding to the human immunoglobulin heavy-chain germ-line ε promoter Cε, for STAT628,29 and 5′ AGTTGAGGGGACTTTCCCAG 3′ for NF-κB.30 The probes were filling-labeled with phosphorus 32 (32P) deoxyadenosine triphosphate. EMSA was performed as described previously.20
Ribonuclease protection assays (RPAs)
For RPAs,20 D10 cells were treated as described above with AG-490 or the DMSO control. Total RNA was isolated from parallel sets of treated cells by using Trizol. Cytokine or receptor message RNA was examined by RPA using 20 μg total RNA hybridized to phosphorus 33 (33P)–labeled probe corresponding to mCK-1 or mCR-1 (PharMingen, San Diego, CA). Unhybridized RNA was digested with RNase T1 and RNase A and then with proteinase K. Hybridized RNA probes were denatured and electrophoresed on a 5% polyacrylamide gel. The dried gels were exposed to x-ray film.
Construction of STAT6 binding element Cɛ–luciferase reporter construct
An oligonucleotide consisting of 3 copies of IL-4 nuclear-activated factor STAT6 binding promoters of the murine Cε (−119 to −104)31 in a direct repeat was synthesized withSacI and XhoI overhangs and ligated into pGL3 luciferase reporter vector. The correct reporter construct sequence was confirmed by DNA sequencing. Plasmid DNA was prepared by using the Wizard Maxipreps DNA purification system.
Transient transfection and luciferase assays
Transient transfections of Th2 cells were performed with the dimethylaminoethanol-dextran method.20 Cells were treated with or without AG-490 for 16 hours and stimulated in the presence or absence of IL-4 for 10 hours at 37°C. Cells were lysed and subjected to luciferase assay (Promega, Madison, WI). Luciferase activity was normalized against protein concentration.
Results
AG-490 selectively inhibits synthesis of IL-4 but not other Th2 or Th1 cytokines
Selective inhibition of cytokines produced by T cells would have great therapeutic potential. Therefore, we investigated whether the tyrphostin AG-490 could inhibit Th2 cell function, which is mediated by the JAK3-activating cytokine IL-4. For this analysis, we chose the corresponding pair of Th1 and Th2 cell lines, clone 29 and D10, as model systems. D10 or clone 29 cells were stimulated or not stimulated by anti-CD3 antibodies in the presence of AG-490 for 16 hours. The supernatants were collected and assayed for cytokines by ELISA. As shown in Figure 1, constitutive IL-4 production was dramatically reduced (approximately 90%) by AG-490, whereas little effect on the remaining Th2 cytokines (IL-5, IL-10, and IL-13) or Th1 cytokines (IFN-γ, TNF-α, TNF-β, and IL-2) was detected. Furthermore, anti-CD3 significantly induced production of cytokines. However, AG-490 did not block any cytokine synthesis during the 16 hours of the experiment. This observation suggests that AG-490 could selectively and specifically inhibit the production of IL-4 in T-cell subsets that is not occurring at the initiation of activation mediated by the T-cell antigen receptor. We thus next investigated whether the loss of IL-4 production in this Th2 cell line occurred at the level of protein synthesis and secretion or transcription.
Th (T-helper) 2 and Th1 clonal cell lines treated with AG-490 show preferential inhibition of interleukin (IL) 4 protein but not after anti-CD3 stimulation.
(A) Th2 clone D10 or Th1 cells (clone 29) were treated with AG-490 or vehicle alone at 37°C for 16 hours. Enzyme-linked immunosorbent analysis (ELISA) results for each cytokine (indicated on ordinate) were plotted on the abscissa as the percentage of inhibition of the mean (± SE; n = 6) from ELISA values as follows: IL-4–dimethyl sulfoxide (DMSO), 497 ± 91 pg/mL, and AG-490, below detection (< 60 pg/mL); IL-5–DMSO, 34 984 ± 4422 pg/mL, and AG-490, 34 585 ± 8504 pg/mL; IL-10–DMSO, 54 689 ± 1554 pg/mL, and AG-490, 38 038 ± 3518 pg/mL; and IL-13–DMSO, 53 907 ± 6398 pg/mL, and AG-490, 66 439 ± 1995 pg/mL. Effects of AG-490 on clone 29 Th1 cytokine values (n = 6) were as follows: interferon γ–DMSO, 1062 ± 96 pg/mL, and AG-490, 1011 ± 18 pg/mL; tumor necrosis factor (TNF) α–DMSO, 1523 ± 108 pg/mL, and AG-490, 1378 ± 96 pg/mL; TNF-β–DMSO 375 ± 23 pg/mL, and AG-490, 307 ± 21 pg/mL; and IL-2–DMSO 13 ± 1 pg/mL, and AG-490, 12.8 ± 1 pg/mL. (B) AG-490 did not block production of IL-4 by D10 and IL-2 by clone 29 after stimulation with anti-CD3. D10 and clone 29 cells were washed, stimulated by coated anti-CD3 antibodies, and treated in the presence or absence of 50 μmol/L AG-490 for 16 hours.
Th (T-helper) 2 and Th1 clonal cell lines treated with AG-490 show preferential inhibition of interleukin (IL) 4 protein but not after anti-CD3 stimulation.
(A) Th2 clone D10 or Th1 cells (clone 29) were treated with AG-490 or vehicle alone at 37°C for 16 hours. Enzyme-linked immunosorbent analysis (ELISA) results for each cytokine (indicated on ordinate) were plotted on the abscissa as the percentage of inhibition of the mean (± SE; n = 6) from ELISA values as follows: IL-4–dimethyl sulfoxide (DMSO), 497 ± 91 pg/mL, and AG-490, below detection (< 60 pg/mL); IL-5–DMSO, 34 984 ± 4422 pg/mL, and AG-490, 34 585 ± 8504 pg/mL; IL-10–DMSO, 54 689 ± 1554 pg/mL, and AG-490, 38 038 ± 3518 pg/mL; and IL-13–DMSO, 53 907 ± 6398 pg/mL, and AG-490, 66 439 ± 1995 pg/mL. Effects of AG-490 on clone 29 Th1 cytokine values (n = 6) were as follows: interferon γ–DMSO, 1062 ± 96 pg/mL, and AG-490, 1011 ± 18 pg/mL; tumor necrosis factor (TNF) α–DMSO, 1523 ± 108 pg/mL, and AG-490, 1378 ± 96 pg/mL; TNF-β–DMSO 375 ± 23 pg/mL, and AG-490, 307 ± 21 pg/mL; and IL-2–DMSO 13 ± 1 pg/mL, and AG-490, 12.8 ± 1 pg/mL. (B) AG-490 did not block production of IL-4 by D10 and IL-2 by clone 29 after stimulation with anti-CD3. D10 and clone 29 cells were washed, stimulated by coated anti-CD3 antibodies, and treated in the presence or absence of 50 μmol/L AG-490 for 16 hours.
AG-490 blocks mRNA expression of IL-4 in D10 cells
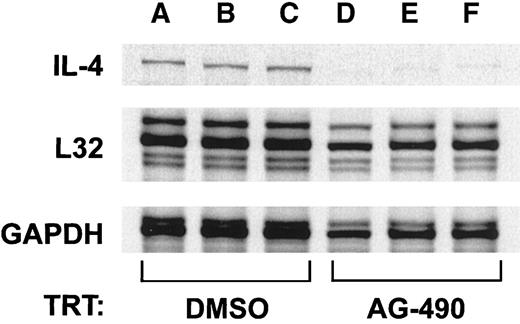
IL-4 is a highly tissue-specific gene expressed in Th2 cells but not in Th1 cell subsets.32-34 Because the D10 cell line represents the prototypic Th2 cell model,35 we used this cell line to determine whether loss of IL-4 protein (Figure 1) was due to AG-490 inhibition of IL-4 messenger RNA (mRNA) expression. For this analysis, total RNA was isolated from corresponding AG-490–treated cells and untreated cells and then probed for IL-4 message with an RPA (Figure 2). The IL-4 transcript was greatly reduced in replicates of tyrphostin-treated cells (Figure 2, lanes D-F) compared with controls (Lanes A-C). Densitometric analysis of IL-4 mRNA message normalized against the housekeeping geneL32/GAPDH indicated that the IL-4 transcript was reduced by more than 79% (n = 3). These findings support the idea that the tyrphostin AG-490 blocks the transcriptional regulation of IL-4 rather than protein synthesis or intracellular trafficking and secretion.
AG-490 inhibits IL-4 messenger RNA (mRNA) expression.
Results of RNase protection assay analysis of IL-4 mRNA isolated from AG-490 (100 μmol/L)–treated or DMSO-treated D10 cells are shown. The mRNA was isolated and examined for expression of murine IL-4 orL32 and GAPDH control genes by using a phosphorus 33 (33P)–labeled probe. Densitometric analysis of IL-4 RNA message compared with L32/GAPDH indicated a greater than 79% reduction in the AG-490–treated samples (n = 3).
AG-490 inhibits IL-4 messenger RNA (mRNA) expression.
Results of RNase protection assay analysis of IL-4 mRNA isolated from AG-490 (100 μmol/L)–treated or DMSO-treated D10 cells are shown. The mRNA was isolated and examined for expression of murine IL-4 orL32 and GAPDH control genes by using a phosphorus 33 (33P)–labeled probe. Densitometric analysis of IL-4 RNA message compared with L32/GAPDH indicated a greater than 79% reduction in the AG-490–treated samples (n = 3).
AG-490 inhibits IL-4–mediated proliferation of both D10 and CT.4S cells
IL-4 is a unique cytokine in that it drives proliferation and differentiation of Th2 cells and also up-regulates its synthesis in an autocrine fashion.36 Therefore, we examined the dose-dependent inhibitory effects of AG-490 on IL-4–mediated growth of Th2 cells by coculturing D10 cells with increasing concentrations of AG-490 for 16 hours and assaying them for tritium-thymidine incorporation. As shown in Figure 3A, AG-490 dramatically inhibited IL-4–induced proliferation (concentration that inhibited 50%, 20 μmol/L). A-1, the inactive analogue of AG-490, exerted little inhibitory effect on cell proliferation at the same concentration (< 10%; data not shown). Overall, these cells were judged to be more than 85% viable on the basis of trypan blue dye exclusion and fluorescence-activated cell sorter analysis using propidium iodide and annexin V staining as indicators of apoptosis (data not shown). To determine whether these effects were unique to D10 cells, we tested the IL-4–dependent cell clone CT.4S. As shown in Figure 3B, AG-490 had a similar inhibitory effect on the IL-4–induced proliferation. Together, these findings suggest that AG-490 markedly inhibits IL-4 transcription and IL-4–mediated growth-promoting signals.
AG-490 inhibits IL-4–induced proliferation in both D10 and CT.4S cells in a dose-dependent manner.
Proliferation of quiescent D10 (A) or CT.4S (B) cells (5 × 104/well) was examined after treatment with the DMSO control or increasing concentrations of AG-490 (ordinate) for 16 hours at 37°C in the presence (solid box) or absence of 1 nmol/L IL-4 (hatched box). Cells were then pulsed with tritium-thymidine (18.5 × 102 Bq/200μL) for 4 hours. Incorporation of radiolabeled probe is plotted on the abscissa, expressed as total counts per minute (n = 6).
AG-490 inhibits IL-4–induced proliferation in both D10 and CT.4S cells in a dose-dependent manner.
Proliferation of quiescent D10 (A) or CT.4S (B) cells (5 × 104/well) was examined after treatment with the DMSO control or increasing concentrations of AG-490 (ordinate) for 16 hours at 37°C in the presence (solid box) or absence of 1 nmol/L IL-4 (hatched box). Cells were then pulsed with tritium-thymidine (18.5 × 102 Bq/200μL) for 4 hours. Incorporation of radiolabeled probe is plotted on the abscissa, expressed as total counts per minute (n = 6).
AG-490 does not alter mRNA expression of IL-4 receptors
To investigate whether AG-490–mediated effects on IL-4 signaling were due to reduced expression of IL-4 receptor, we performed RPAs to identify the level of expression of the IL-4Rα and IL-2Rγ chains. For this analysis, total mRNA was isolated from 2 different sets of control (DMSO-treated) cells or AG-490–treated cells and hybridized against 33P-labeled receptor probes. As shown in Figure4, IL-4–receptor message RNA from control cells (lanes A and B) and D10 cells treated with AG-490 (lanes C and D) failed to show a significant change in mRNA expression in comparison with the control housekeeping gene L32. From this data, we conclude that the loss of IL-4–mediated cell growth and IL-4 message after AG-490 treatment was not due to a significant reduction in IL-4Rα and IL-2Rγ expression but occurred at a site distal to the receptor.
Ribonuclease protection assay of AG-490–treated D10 cells does not result in altered mRNA expression of γ chain receptors.
Complementary DNA was generated from freshly isolated mRNA obtained from 2 sets of D10 cells treated in the absence (lanes A and B) or presence (lanes C and D) of 100 μmol/L AG-490 for 16 hours. D10 RNA was then probed with 33P-labeled RNA probes corresponding to transcripts for individual murine γ chain receptors (mCR-1), according to the PharMingen protocol. The autoradiographs of the RNase-protected fragments were separated by 5% polyacrylamide gel electrophoresis.
Ribonuclease protection assay of AG-490–treated D10 cells does not result in altered mRNA expression of γ chain receptors.
Complementary DNA was generated from freshly isolated mRNA obtained from 2 sets of D10 cells treated in the absence (lanes A and B) or presence (lanes C and D) of 100 μmol/L AG-490 for 16 hours. D10 RNA was then probed with 33P-labeled RNA probes corresponding to transcripts for individual murine γ chain receptors (mCR-1), according to the PharMingen protocol. The autoradiographs of the RNase-protected fragments were separated by 5% polyacrylamide gel electrophoresis.
AG-490 ablates IL-4–dependent JAK3 and STAT6 tyrosine phosphorylation in vivo
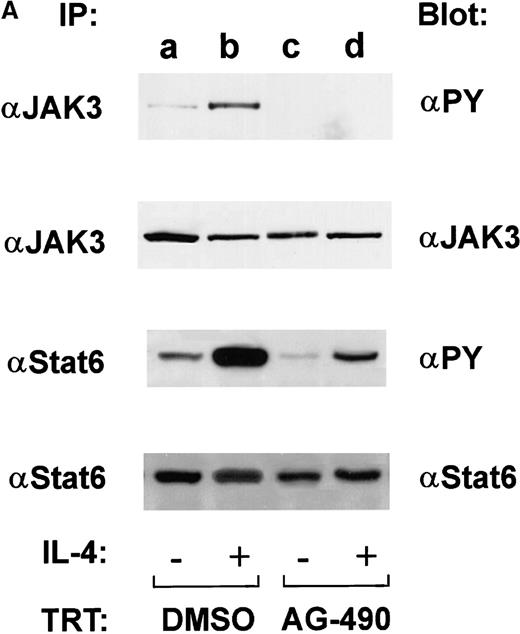
Studies in JAK3 and STAT6 knockout mice have established that these 2 proteins play a key role in T-cell development, IL-4 responsiveness, and Th2 cell commitment.37,38 Because JAKs demonstrate tyrosine autophosphorylation after activation and tyrosine phosphorylation of STATs is required for their dimerization, before nuclear translocation and subsequent transcriptional activity, the effects of AG-490 on inhibition of these 2 events were measured.39 In this experiment, D10 cells were treated with AG-490, stimulated in the presence or absence of IL-4, and assayed by Western blot analysis for tyrosine phosphorylated JAK3 or STAT6. Constitutively tyrosine phosphorylated STAT6 was observed in the unstimulated control (Figure 5A, lane a), and further phosphorylation occurred when exogenous IL-4 was added (Figure 5A, lane b). However, STAT6 tyrosine phosphorylation was significantly reduced in AG-490–treated cells stimulated in the presence or absence of IL-4 (Figure 5A, lanes c and d). Similarly, we found that AG-490 inhibited basal and IL-4–induced tyrosine phosphorylation of the STAT6 activator JAK3 (Figure 5A). Reblotting for both STAT6 and JAK3 showed equivalent loading and demonstrated that changes in tyrosine phosphorylation were not due to a loss of protein expression (Figure 5, lower panel).
AG-490 inhibits tyrosine phosphorylation of Janus tyrosine kinase 3 (JAK3) and signal transducer and activator of transcription 6 (STAT6).
(A) D10 cells were treated with DMSO (lanes a and b) or 100 μmol/L AG-490 (lanes c and d) for 16 hours and then stimulated with or without 100 nmol/L IL-4 at 37°C for 10 minutes. Cells were lysed, immunoprecipitated with anti-JAK3 (α-J3; upper panel) or anti-STAT6 (α-STAT6; lower panel), subjected to Western blotting with α-PY (antiphosphotyrosine), and then reprobed with anti-JAK3 or anti-STAT6 (indicated beneath phosphorylation blots) to verify equivalent loading. Arrow indicates migrational location of either JAK3 or STAT6. (B) Tyrosine phosphorylated proteins were normalized against total protein. The ratio of phosphorylated protein to unphosphorylated protein is plotted on the abscissa (arbitrary units) against AG-490 treatment (ordinate).
AG-490 inhibits tyrosine phosphorylation of Janus tyrosine kinase 3 (JAK3) and signal transducer and activator of transcription 6 (STAT6).
(A) D10 cells were treated with DMSO (lanes a and b) or 100 μmol/L AG-490 (lanes c and d) for 16 hours and then stimulated with or without 100 nmol/L IL-4 at 37°C for 10 minutes. Cells were lysed, immunoprecipitated with anti-JAK3 (α-J3; upper panel) or anti-STAT6 (α-STAT6; lower panel), subjected to Western blotting with α-PY (antiphosphotyrosine), and then reprobed with anti-JAK3 or anti-STAT6 (indicated beneath phosphorylation blots) to verify equivalent loading. Arrow indicates migrational location of either JAK3 or STAT6. (B) Tyrosine phosphorylated proteins were normalized against total protein. The ratio of phosphorylated protein to unphosphorylated protein is plotted on the abscissa (arbitrary units) against AG-490 treatment (ordinate).
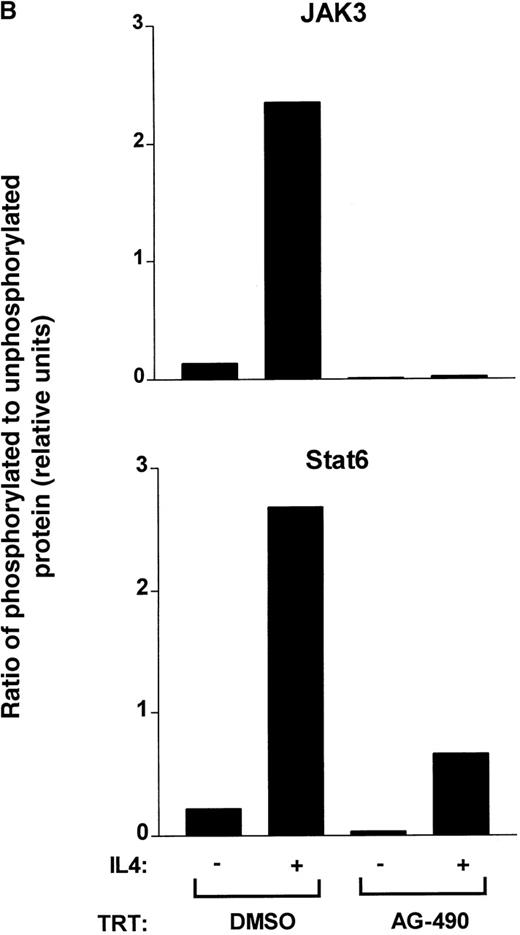
Quantitation of decreased tyrosine phosphorylation signal intensity was done by normalizing densitometric traces against total JAK3 or STAT6 protein. As shown in Figure 5B, AG-490 potently inhibited basal and IL-4–induced JAK3 tyrosine phosphorylation. However, basal STAT6 tyrosine phosphorylation was completed inhibited and IL-4–induced STAT6 tyrosine phosphorylation was inhibited by greater than 87%. Although there may be several plausible explanations for this finding, we believe that it could have been due to a slower turnover rate of activated tyrosine phosphorylated STAT6 compared with JAK3. On the basis of these data, we propose that AG-490 blocks transcription of IL-4 mRNA in Th2 cells by disrupting the IL-4 autocrine-activation loop involving the JAK3-STAT6 signaling cascade.
AG-490 inhibits IL-4–induced STAT6 DNA binding but not TNF-–induced NF-κB DNA-binding transactivation
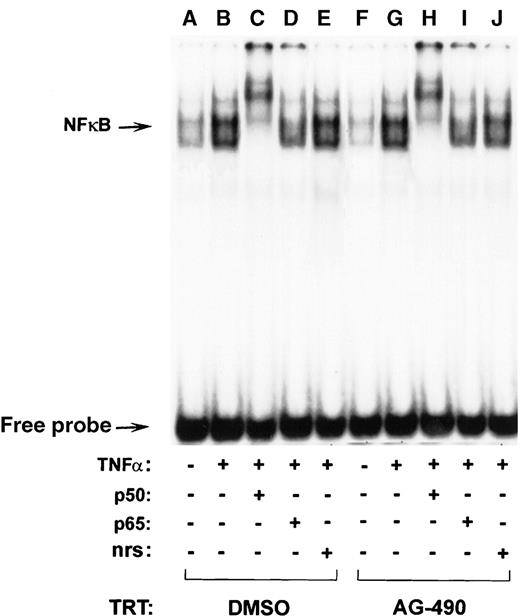
To verify that AG-490 indeed blocks STAT6 transactivation potential, its ability to bind DNA and drive transcription was assessed. For the DNA-binding assay, nuclear extracts from cells treated with AG-490 and untreated cells were compared for their ability to bind a radiolabeled STAT6 binding element, Cε oligonucleotide probe, and EMSA was performed (Figure 6). Although control extracts displayed IL-4–modulated STAT6 binding (Figure 6, lanes A-D), equivalent nuclear extracts from AG-490–treated cells showed diminished binding capacity (lanes E-H). To confirm that the reduced binding efficiency was due specifically to STAT6, the complexes were supershifted with anti-STAT6 antibody. In supershifted samples (Figure6, lanes C and G) or nonsupershifted samples (lanes B and F), the IL-4–induced complexes could completely compete with anti-STAT6. In contrast, AG-490 failed to inhibit NF-κB binding activity induced by TNF-α a Th1 cytokine, in D10 cells (Figure7). These findings suggest that AG-490 could selectively inhibit IL-4–induced STAT6 DNA binding.
Pretreatment of D10 cells with AG-490 inhibits IL-4–induced STAT6 DNA binding as demonstrated by electrophoretic mobility shift analysis.
D10 cells were treated with the DMSO control (lanes A-D) or 100 μmol/L AG-490 (lanes E-H) and incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts corresponding to 5 μg of protein were incubated in the absence of antibody (lanes A, B, E, and F), α-STAT6 (lanes C and G), or normal rabbit serum (nrs; lanes D and H) and then with a phosphorus 32 (32P)–labeled oligonucleotide probe corresponding to the Cε gene promoter. Arrow indicates migrational location of each nonsupershifted STAT6-DNA complex or free probe.
Pretreatment of D10 cells with AG-490 inhibits IL-4–induced STAT6 DNA binding as demonstrated by electrophoretic mobility shift analysis.
D10 cells were treated with the DMSO control (lanes A-D) or 100 μmol/L AG-490 (lanes E-H) and incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts corresponding to 5 μg of protein were incubated in the absence of antibody (lanes A, B, E, and F), α-STAT6 (lanes C and G), or normal rabbit serum (nrs; lanes D and H) and then with a phosphorus 32 (32P)–labeled oligonucleotide probe corresponding to the Cε gene promoter. Arrow indicates migrational location of each nonsupershifted STAT6-DNA complex or free probe.
AG-490 fails to inhibit TNF-–induced NF-κB DNA binding.
D10 cells were treated with the DMSO control (lanes A-E) or 100 μmol/L AG-490 (lanes F-J) and incubated with medium (−) or 20 ng/mL TNF-α (+) for 10 minutes at 37°C. Nuclear extracts corresponding to 5 μg of protein were incubated in the absence (lanes A, B, F, and G) or presence of antibody α-NF-κB p50 (lanes C and H), p65 (lanes D and I), or normal rabbit serum (nrs; lanes E and J) and then with a 32P-labeled oligonucleotide probe. Arrow indicates migrational location of each nonsupershifted NF-κB–DNA complex or free probe.
AG-490 fails to inhibit TNF-–induced NF-κB DNA binding.
D10 cells were treated with the DMSO control (lanes A-E) or 100 μmol/L AG-490 (lanes F-J) and incubated with medium (−) or 20 ng/mL TNF-α (+) for 10 minutes at 37°C. Nuclear extracts corresponding to 5 μg of protein were incubated in the absence (lanes A, B, F, and G) or presence of antibody α-NF-κB p50 (lanes C and H), p65 (lanes D and I), or normal rabbit serum (nrs; lanes E and J) and then with a 32P-labeled oligonucleotide probe. Arrow indicates migrational location of each nonsupershifted NF-κB–DNA complex or free probe.
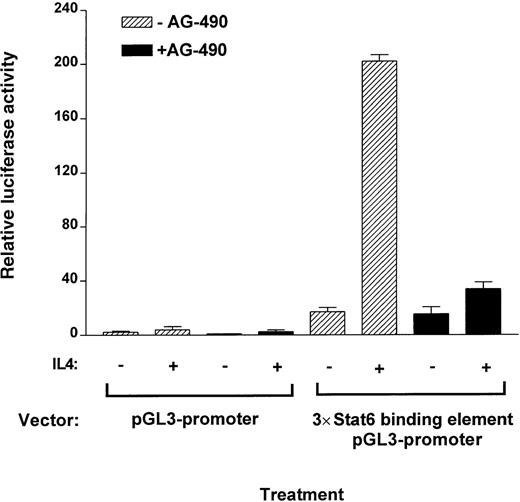
AG-490 inhibits IL-4–induced STAT6 transactivation
To assess quantitatively whether AG-490 blocked the transactivation potential of STAT6 in Th2 cells compared with control cells, we examined the ability of the cells to drive a pGL3 luciferase reporter containing the Cε STAT6 binding element. We found that the luciferase activity of IL-4–stimulated cells was substantially reduced in AG-490–treated samples (>90%) compared with untreated controls also transfected with the luciferase reporter (Figure8). Interestingly, basal luciferase transactivation of the reporter construct was also inhibited in non–cytokine-stimulated controls treated with AG-490. These findings are similar to results shown in Figure 5, ie, AG-490 blocked basal tyrosine phosphorylation of STAT6. These observations suggest that AG-490 inhibits JAK3, which in turn blocks STAT6 tyrosine phosphorylation and activation and subsequent transcriptional activity necessary for driving IL-4–responsive genes.
AG-490 ablates IL-4–induced transcriptional activity of STAT6 binding element of a luciferase reporter construct.
D10 cells were stably transfected with a 3 copies of a STAT6 binding element–pGL3 promoter–luciferase construct or with pGL3 promoter–luciferase construct alone. Cells were then treated in the absence or presence of AG-490 for 16 hours and incubated with or without IL-4 (100 nmol/L) for 10 hours. Luciferase activity of lysed cells was measured and normalized against protein concentration.
AG-490 ablates IL-4–induced transcriptional activity of STAT6 binding element of a luciferase reporter construct.
D10 cells were stably transfected with a 3 copies of a STAT6 binding element–pGL3 promoter–luciferase construct or with pGL3 promoter–luciferase construct alone. Cells were then treated in the absence or presence of AG-490 for 16 hours and incubated with or without IL-4 (100 nmol/L) for 10 hours. Luciferase activity of lysed cells was measured and normalized against protein concentration.
Discussion
In the current study, AG-490 inhibited IL-4 production but not expression of its receptor chains IL-4Rα and IL-2Rγ. Moreover, this tyrphostin selectively modified exogenous and basal IL-4–mediated cell signaling. AG-490 inhibited IL-4–induced cell proliferation of a Th2 cell clone, D10, and an IL-4–responsive T-cell line, CT.4S. Although AG-490 blocked JAK3-STAT6 activation, it failed to inhibit TNF-α–induced NF-κB DNA-binding activity. Moreover, IL-4 message was completely disrupted in D10 cells. These results were supported by the finding that AG-490 failed to inhibit production of IL-2, IL-3, and TNF-α. Th2-mediated diseases are known to promote secretion of IL-4, IL-5, IL-10, and IL-13. To identify a mechanism to disrupt such conditions, we sought to disrupt the principal Th2 cytokine, IL-4, and more specifically, the transcription machinery required for its expression.
Transcriptional mechanisms that regulate IL-4 synthesis are not completely understood; however, it has been proposed that the cooperation of several transcription factors is required.36,40 STAT6 is unique in that it is critical for Th2 development, IL-4 proliferation, and Th2 cytokine production.36-41 To inactive this protein, we focused our efforts on its upstream regulator, JAK3. As expected, IL-4–induced STAT6 and JAK3 tyrosine phosphorylation were similarly inhibited by AG-490. Moreover, AG-490 inhibited constitutive STAT6 tyrosine phosphorylation in actively growing D10 cells. Concomitantly, STAT6 transcriptional activity was greatly depressed by this drug, as judged by EMSA and luciferase reporter assays (Figures 6 and 8). In contrast, AG-490 did not affect TNF-α–mediated transcriptional factor NF-κB (p50 and p65) binding complexes (Figure 7). Taken together, these data suggest that AG-490 modifies Th2 cell responsiveness (ie, IL-4 mRNA synthesis and proliferation) by selectively blocking STAT6 transactivation in addition to inhibiting JAK3.
Current drug therapies cannot neutralize cytokines selectively in humans, unlike cytokine-specific antibodies (eg, anti-TNF), which have been effective in mice and humans. This deficiency has prompted development of pharmacologic agents that can alter cytokine synthesis or release. TAK-603 has been shown to have potential as an antirheumatic drug by blocking Th1 cytokines,10 whereas pentoxifylline42 and roquinimex (Linomide)43can block synthesis of both sets of Th1 and Th2 cytokines. Because of studies with ALL cells, AG-490 has been considered to be a JAK-specific inhibitor.17 One conclusion that can be drawn from our studies is that AG-490 may have broader specificities than previously described, as is often the case with studies of kinase inhibitors performed by many laboratories. Meydan et al17 demonstrated that this tyrphostin had no effect on other lymphocytic tyrosine kinases (such as Lck, Lyn, Btk, Syk, and Src) and found that the lack of reactivity against JAK3 was difficult to assess because of the relatively low level of its expression in ALL cells. Moreover, they observed that AG-490 was well tolerated by mice.17 Such results would not be expected for a general kinase inhibitor.
Although anti-CD3 stimulation resulted in efficient production of IL-2 by Th1 cells and of IL-4 by Th2 cells, we did not find that AG-490 inhibited cytokine production in Th2 cells and Th1 cells during initiation of the T-cell–receptor (TCR) signal. We showed previously that AG-490 did not block anti-CD3 activation of Zap70 or p56 Lck in human T cells or early activation of T-cell clones stimulated with tetanus toxoid or peptide.19 Therefore, the selective effect of AG-490 on the production of Th2 cytokine compared with Th1 cytokine may result from a selective ability of AG-490 to inhibit JAK tyrosine kinases. As pointed out by Naora et al,3 cytokine gene expression in D10 cells is regulated by TCR-dependent and TCR-independent signaling mechanisms. This suggests that AG-490 may affect activated IL-4–STAT6 signaling but not TCR or costimulatory pathways. Furthermore, because AG-490 can also inhibit the IL-2– and IL-4–mediated signaling pathway, it seems plausible to expect that an imbalance of these T-cell subsets may be inhibited by AG-490. Therefore, these findings suggest that AG-490 inhibits both Th1 and Th2 responses at different levels, depending on the activation status of these immune cells.
In summary, we have provided evidence that the negative effect of AG-490 on Th2 cell activity is due to inhibition of the IL-4 signaling pathway that disrupts JAK3-STAT6 activities. We propose that inhibition of IL-4 message in actively growing Th2 cells may occur through inactivation of an autocrine-regulated loop. Our results support the idea that the small-molecule drug AG-490 can selectively modulate Th2 cell function and cytokine production. These findings also highlight the therapeutic potential of AG-490 and its analogues as pharmaceutical agents to inhibit the progression of Th cell–mediated disease states driven by unwarranted expression of IL-4.
Acknowledgments
We thank Dr Minute Li-Weber for generously providing clone D10 and clone 29 Th subsets, Dr William Paul for kindly providing the CT.4S cell line, Jennifer Brown for skillful preparation of the figures, Dr Gerald Evans for densitometric analysis, and Dr Joost Oppenheim for critical review of the manuscript.
Supported by the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Reprints:William L. Farrar, National Cancer Institute, PO Box B, Bldg 560, Room 31-68, Frederick, MD 21702; e-mail:farrar@mail.ncifcrf.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal