Abstract
The role of interleukin-1β (IL-1β) as a regulator of the immune response, although extensively investigated, is still debated. We then studied the expression of IL-1β by human dendritic cells (DCs), the professional antigen presenting cells, and its modulation during immune reactions in vitro. Our results show that, on maturation or tetanus toxoid presentation to specific CD4+ CD40L+T lymphocytes, DCs begin to accumulate IL-1β precursor (pro–IL-1β) but do not secrete bioactive IL-1β. In contrast, interaction with alloreactive T cells results in both stimulation of pro–IL-1β synthesis and secretion of processed isoforms of the cytokine, that display biologic activity. Both CD4+ and CD8+ subsets of allospecific T lymphocytes are required: CD4+ T cells drive the synthesis of pro–IL-1β through CD40 engagement but have no effects on pro–IL-1β processing; CD8+ T cells, unable to induce synthesis of pro–IL-1β per se, are responsible for the generation of mature IL-1β by pro–IL-1β–producing DCs. Interleukin-1β–converting enzyme (ICE) inhibitors do not prevent the recovery of IL-1β bioactivity after allorecognition, indicating that allospecific CD8+ T cells may induce the release of bioactive IL-1β via mechanism(s) other than ICE activation. Altogether, these findings suggest that CD4+ and CD8+ T-lymphocyte subsets have distinct roles in the induction of IL-1β secretion by DCs and support the hypothesis that IL-1β plays a role in cell-mediated immune responses.
Dendritic cells (DCs) are professional antigen-presenting cells,1,2 able to capture and process antigens, to migrate into lymphoid organs, and to present antigenic peptides to naive T cells, inducing the immune response. In most tissues, DCs are present in the immature state, unable to stimulate T cells but highly efficient in the uptake of antigens. Bacterial products, cytokines such as tumor necrosis factor-α (TNF-α)3 or the engagement of the DC-surface receptor CD40 by CD40L expressed by activated T cells,4 induce maturation, with a rapid decline in the skills to capture antigens and an increase in the capacity of eliciting a strong T-cell response. DCs express, constitutively or upon maturation, a number of cytokines that display biologic activities that are borderline between mediators of inflammation and modulators of immune response.1 These include, among others, IL-12,1 TNF-α,1 and IL-18.5 Instead, it is not clear whether DCs express IL-1 and whether this cytokine plays a role during antigen presentation. IL-1 is the prototypic multifunctional cytokine produced mainly by activated mononuclear phagocytes.6 Although this soluble factor was formerly discovered as a costimulator of T lymphocytes, it soon became clear that its major function is as mediator of host inflammatory response in natural immunity. However, recent experimental evidences and clinical trials with IL-1 inhibitors point to a role for IL-1 in antigen-specific immunity as well as in the pathogenesis of autoimmune diseases.6 Indeed, even if IL-1 seems dispensable for mounting an immune response, it appears to regulate the expansion of different functional T-cell subsets.6-9 In addition, the inhibition of IL-1 activity, which seems to have little or no effects on antigen-specific T-cell proliferation,10,11 results in a block of chemokine production in mixed lymphocyte reaction (MLR).12Furthermore, a reduction in the severity of a number of immune-mediated diseases has been observed on treatment with the IL-1 receptor antagonist.6 Two forms of IL-1 exist, α and β; in humans, the major extracellular form is IL-1β, whereas IL-1α remains mainly cell associated.6 Despite its extracellular localization and function, IL-1β lacks a secretory signal sequence,13 and is released through a nonclassical pathway that avoids the ER-Golgi route.14,15 The cytokine is synthesized as a 35-kd precursor, devoid of biologic activity,6 and is secreted in the mature bioactive form of 17 kd.6,14,15 The IL-1β–converting enzyme (ICE) cleaves pro–IL-1β into the 17-kd form16,17: however, although ICE is essential for lypopolysaccharide (LPS)-driven IL-1β maturation,18,19 induction of bioactive IL-1β by different stimuli may occur independently of this caspase.20,21 Indeed, pro–IL-1β can be cleaved by cathepsin G,20 chymotrypsin,20elastase,20 a mast cell chimase,22 different matrix metalloproteinases,23 and granzyme A24: These proteases are present in inflammatory fluids and may thus contribute to extracellular processing of pro–IL-1β with amplification of the inflammatory response.
To clarify the role of IL-1β in the immune response, we approached the question of whether the cytokine is produced by immature or mature DCs and how its release is regulated in the course of interactions with antigen-specific T cells. Our results indicate that: (i) DCs synthesize but do not secrete IL-1β on maturation or CD40 triggering; (ii) secretion of biologically active IL-1β follows an antigen-specific cell-to-cell contact between DCs and alloreactive T cells; in contrast, no release of IL-1β occurs on the presentation of a soluble antigen such as tetanus toxoid to specific CD4+ T cells; and (iii) in the absence of inflammatory stimuli, both the CD4+ and the CD8+ subsets of responder T lymphocytes are required.
Materials and methods
Derivation of dendritic cells from adherent cells
DCs were obtained by culturing adherent cells from peripheral blood mononuclear cells (PBMCs) from healthy donors for 8 days in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 1 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Biochrom), 10% heat-inactivated fetal calf serum (FCS) (PAA Labour, Linz, Austria), 40 ng/mL recombinant granulocyte-monocyte colony-stimulating factor (GM-CSF, Schering-Plough, Milan, Italy), and 1000 U/mL IL-4 as described.25,26 The cell population obtained was more than 95% CD14−, CD80+, CD86+, and HLA-DR+, as reported.25,26 Media were endotoxin free as shown by the Limulus lysate colorimetric assay (PBI, Milan, Italy). LPS (1 μg/mL; Sigma Chemical Co, St. Louis, MO) or TNF-α (50 ng/mL, Genzyme, Cinisello B., Milan, Italy) were added during the last 40 hours of culture to induce DC maturation.3 Purified T cells were obtained after 2 rounds of plastic adherence, followed by immunodepletion of CD14+ and HLA-DR+cells with immunomagnetic beads (Dynal, Milan, Italy).27
Human monocytes enriched by adherence from PBMCs were activated with 10 μg/mL of LPS for 1 hour in RPMI medium, supplemented with 10% FCS as described.15
Establishment of tetanus toxoid– or dermatophagoides pteronissinus–specific T-cell lines and mixed lymphocyte reaction
Tetanus toxoid (tetox)-specific or dermatophagoides pteronissinus (dph)-specific T-cell lines were obtained as described27 by stimulation of autologous PBMCs with tetox (10 μg/mL, Pasteur-Marieux Connaught, North York, Ontario, Canada) or with dph (5 μg/mL, a kind gift of Lofarma Allergeni, Milan, Italy), followed by Percoll (Amersham Italia, Milan, Italy) gradient separation of T-cell blasts after 7 days, culture in 10% T-cell growth factor (Lymphocult, Biotest Diagnostics Inc, Dreieich, Germany), and weekly restimulation with the antigen. Allospecific T cells were obtained by coculturing, for 1 week, purified T cells with allogenic irradiated PBMCs (4000 rad) as described.27 The percentage of CD8+ and CD4+ T lymphocytes after 7 days of MLR was evaluated by FACS analysis. The antigen specificity of dph- or tetox-specific autologous T cells and of allogeneic T cells was assessed as described.5,27 CD8+ and CD4+ T lymphocytes were negatively selected from 7-day MLR by immunodepletion with anti-CD4 or anti-CD8 monoclonal antibodies as described.27 After removal of CD8+ or CD4+ cells, respectively, the percentage of CD4+ or CD8+ T cells in the remaining cell population (evaluated by FACS analysis) varied from 80% to 95% in the different experiments.
Culture conditions
DCs, cultured for 6 days in 6-well plates with GM-CSF and IL-4 plus 2 days in the absence or presence of LPS or TNF-α, were incubated with RPMI medium, supplemented with 1% Nutridoma-HU (Boheringer, Mannheim, Germany) at 37°C for 16 hours with 10 μg/mL of tetox, followed by 8 hours in the presence or absence of autologous tetox-specific T-cell lines or dph-specific T-cell lines as control at a 10:1 ratio5 27 or at a different T-cell:DC ratio as indicated.
DCs, prepared as above, were incubated with autologous T cells activated in a 7-day MLR against an unrelated donor, or allospecific T cells from 7-day MLR (whole T cells or CD4+ or CD8+ purified T cells) at a T-cell:DC ratio of 10:1.5 27 Cocultures were carried out for different periods. When indicated, the ICE inhibitor AcYVAD-CMK (10 μmol/L, Biochem, Milan, Italy) was added to the cultures. In some experiments, allospecific T cells were treated with anti-CD40L monoclonal antibody (10 μg/mL, Ancell, Bayport, MN) for 30 minutes before coculture with DCs.
In other experiments, DCs were challenged with CD40L transfected cells28 (kind gift of P. Lane, Basel Institute for Immunology, Basel, Switzerland) at a 1:1 ratio for different periods as indicated.
At the end of the incubations, supernatants were concentrated by 10% trichloroacetic acid; cells were detached by scraping and lysed in 1% Triton X-100–containing buffer.29 Aliquots of cell lysates corresponding to 105 DCs (or 200 μg of proteins, protein dosage performed with the Bio-Rad kit based on the colorimetric Lowry method [Bio-Rad, Milan, Italy]) and the correspondent trichloroacetic acid-concentrated supernatants, were solubilized in sample buffer and resolved on 12% SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions.29 Gels were electrotransferred onto nitrocellulose filters (Hybond ECL, Amersham Italia), stained with Ponceau Red (Sigma) to confirm equal protein loading (not shown), and destained before blocking overnight with 10% nonfat dry milk in phosphate-buffered saline (PBS). Filters were hybridized with the anti–IL-1β monoclonal antibody 3ZD (provided by NCI Biological Resources Branch, Frederick, MD), followed by a horseradish peroxidase-conjugated goat antimouse IgG (HRP-GAM, DAKO, Milan, Italy), or with the goat antihuman ICE antibody (Santa Cruz Biotechnology, Segrate, Milan, Italy), followed by a horseradish peroxidase-conjugated rabbit antigoat IgG (DAKO), and developed by ECL-Plus (Amersham), according to the manufacturer's instructions.
Determination of IL-1β biologic activity
To test the biologic activity of IL-1β secreted by DCs under the different culture conditions, human umbilical vein endothelial cells (HUVECs) were grown at confluence in 96-well plates and incubated for 20 hours with the different supernatants, in the presence or absence of 20 pg/mL of recombinant IL-1 receptor antagonist (IRAP, R&D System, Milan, Italy). Recombinant IL-1β (R&D System) was used as a positive control at 200 pg/mL. At the end of incubation, the amount of surface intercellular adhesion molecule-1 (ICAM1) was determined by enzyme-linked immunosorbent assay (ELISA) with anti-ICAM1 antibodies. Plates were read with an ELISA reader (Packard) at λ = 450 nm. Results are expressed as optical density (OD) at 450 nm.
Results
Synthesis of IL-1β by dendritic cells induced by maturational stimuli
DCs were cultured with GM-CSF/IL-4 for 8 days in the presence or absence of LPS or TNF-α as maturative stimuli for the last 40 hours,3,5 and synthesis and secretion of IL-1β were investigated in both immature and mature DCs. As shown in Figure1A, pro–IL-1β (migrating as a 35-kd band) is absent in DCs and is induced by treatments with LPS or TNF-α. Interestingly, unlike activated monocytes, which mostly secrete the fully processed 17-kd form of IL-1β (lane 8), mature DCs release variable amounts of pro–IL-1β, but no traces of 17-kd IL-1β (Figure 1A, lanes 4 to 6). Pro–IL-1β is known to be biologically inactive6: in keeping with this, we failed to detect IL-1β activity in the supernatants of TNF-α– or LPS-stimulated DCs (not shown). However, the inactive precursor form of the pro–IL-1β convertase ICE is present in cell lysates from DCs either unstimulated or after treatment with LPS or TNF-α (Figure 1B, lanes 1 to 3) in comparable amounts to LPS-stimulated monocytes (lane 4), thus ruling out that the failure of pro–IL-1β processing by immature and mature DCs is due to the lack of expression of this enzyme.
Synthesis of pro–IL-1β by DCs is induced by maturative stimuli.
(A) Cell lysates (lanes 1 to 3, c) and supernatants (lanes 4 to 6, s) from DCs cultured for 6 days in the presence of GM-CSF and IL-4 followed by 40 hours with GM-CSF/IL-4 alone (−, lanes 1 and 4) or in the presence of LPS (lanes 2 and 5) or TNFα (lanes 3 and 6) were incubated for 8 hours in serum-free medium supplemented with 1% nutridoma. Lanes 7 and 8: cell lysates (c) and supernatants (s) from freshly isolated monocytes stimulated for 3 hours with LPS. Samples were analyzed by SDS-PAGE, followed by Western blotting with anti–IL-1β antibodies. (B) Cell lysates from DCs (lanes 1 to 3) or monocytes (lane 4) untreated (lane 1) or treated with LPS (lanes 2 and 4) or TNF-α (lane 3) as indicated. Blots were hybridized with anti ICE antibodies.
Synthesis of pro–IL-1β by DCs is induced by maturative stimuli.
(A) Cell lysates (lanes 1 to 3, c) and supernatants (lanes 4 to 6, s) from DCs cultured for 6 days in the presence of GM-CSF and IL-4 followed by 40 hours with GM-CSF/IL-4 alone (−, lanes 1 and 4) or in the presence of LPS (lanes 2 and 5) or TNFα (lanes 3 and 6) were incubated for 8 hours in serum-free medium supplemented with 1% nutridoma. Lanes 7 and 8: cell lysates (c) and supernatants (s) from freshly isolated monocytes stimulated for 3 hours with LPS. Samples were analyzed by SDS-PAGE, followed by Western blotting with anti–IL-1β antibodies. (B) Cell lysates from DCs (lanes 1 to 3) or monocytes (lane 4) untreated (lane 1) or treated with LPS (lanes 2 and 4) or TNF-α (lane 3) as indicated. Blots were hybridized with anti ICE antibodies.
Activated T cells induce synthesis of pro–IL-1β but not secretion of bioactive IL-1β in antigen-loaded dendritic cells
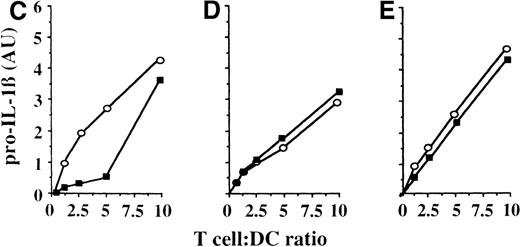
To investigate whether the pattern of synthesis and secretion of IL-1β by DCs is modulated in the course of specific immune responses to soluble antigens, DCs were pulsed with tetox for 18 hours and cocultured for 8 hours with autologous antitetox CD4+ T lymphocytes (proven antigen specific on the basis of their proliferative response to the relevant antigen5 27) at a T-cell:DC ratio of 10:1, and the presence of pro–IL-1β or mature IL-1β in DC lysates and supernatants was investigated. Although overnight incubation with tetox does not induce IL-1β expression (Figure 2A, lane 2), coculture of tetox-pulsed DCs with autologous antitetox CD4+ T lymphocytes results in the induction of pro–IL-1β synthesis, as indicated by the presence of the specific 35-kd pro–IL-1β band in DC lysates (lane 3). Of note, the same result is obtained after the challenge of tetox-loaded DCs with autologous CD4+ T cells generated against dph (lane 4), whereas no IL-1β synthesis is observed in the tetox-loaded DCs after coculture with autologous resting T cells (lane 5). These data suggested that the interaction between DCs and activated CD4+ T cells is sufficient to drive synthesis of IL-1β even in the absence of an antigen-specific contact. To further investigate this point, DCs pulsed with tetox were mixed with autologous antitetox or anti-dph CD4+ T cells at different T-cell:DC ratios (from 0.5:1 to 10:1) and the expression of IL-1β was investigated after 8 hours. Figure 2 (panels C to E) shows 3 experiments performed with cells obtained from 3 different donors. In 2 cases (panels D and E), no difference in the amount of pro–IL-1β induced by antigen-specific or nonspecific CD4+ T cells is observed, even at a low T-cell:DC ratio; in 1 donor (panel C), pro–IL-1β synthesis is induced by tetox-specific CD4+ T cells at a lower T-cell:DC ratio than by nonspecific T lymphocytes. In any case, no secretion of mature 17-kd IL-1β is detected, even at a high-specific CD4+ T-cell:DC ratio (Figure 2B); in agreement with this, no IL-1β bioactivity is found in supernatants from the different culture conditions (not shown).
Activated T cells induce synthesis of pro–IL-1β without secretion.
(A, B) DCs untreated (−, lane 1) or loaded with tetox for 16 hours (lanes 2 to 5) were incubated 8 hours alone (lanes 1 and 2) or in the presence of autologous antitetox T lymphocytes (+Ttetox, lane 3) or autologous antidermatophagoides T lymphocytes (+Tdph, lane 4) or with autologous resting T cells (+Tresting, lane 5) at a T-cell:DC ratio of 10:1. At the end of the incubation, cell lysates (A) and supernatants (B) were analyzed for their content in IL-1β by Western blotting. (C-E) Autologous specific (antitetox; ○) or aspecific (anti-dph; ▪) T lymphocytes were coincubated with tetox-loaded DCs for 8 hours at T-cell:DC ratios varying from 0.5:1 to 10:1. DC intracellular pro–IL-1β was analyzed by Western blotting and quantitated by densitometry.
Activated T cells induce synthesis of pro–IL-1β without secretion.
(A, B) DCs untreated (−, lane 1) or loaded with tetox for 16 hours (lanes 2 to 5) were incubated 8 hours alone (lanes 1 and 2) or in the presence of autologous antitetox T lymphocytes (+Ttetox, lane 3) or autologous antidermatophagoides T lymphocytes (+Tdph, lane 4) or with autologous resting T cells (+Tresting, lane 5) at a T-cell:DC ratio of 10:1. At the end of the incubation, cell lysates (A) and supernatants (B) were analyzed for their content in IL-1β by Western blotting. (C-E) Autologous specific (antitetox; ○) or aspecific (anti-dph; ▪) T lymphocytes were coincubated with tetox-loaded DCs for 8 hours at T-cell:DC ratios varying from 0.5:1 to 10:1. DC intracellular pro–IL-1β was analyzed by Western blotting and quantitated by densitometry.
Secretion of bioactive IL-1β by dendritic cells induced by interaction with allospecific T cells
We then analyzed the modulation of IL-1β synthesis and secretion by DCs on interaction with alloreactive T cells or with activated nonspecific T cells. Alloreactive T cells were derived from a primary parallel MLR and were shown to proliferate in response to the allogenic DCs used in the experiment but not to autologous DCs.5Nonspecific T cells were activated in an unrelated MLR, and thus were phenotypically and functionally comparable to alloreactive T cells, but displayed negligible proliferation to the autologous DCs used in the experiment.5 As shown in Figure3A, after 8 hours of incubation with alloreactive T cells, both induction of pro–IL-1β synthesis (panel A, lane 2) and secretion of the cytokine (panel B, lane 2) are observed. Notably, in addition to the precursor form, a 29-kd form and 3 to 4 discrete IL-1β bands with apparent molecular weights ranging from 12 to 22 kd, which display variable intensity in the different experiments, are detected in supernatants. Challenging DCs with nonspecific-activated T lymphocytes also leads to synthesis of pro–IL-1β (panel A, lane 3); however, differently from alloreactive T cells, nonspecific T lymphocytes induce secretion of only a small amount of pro–IL-1β and, in some donors, of the 29-kd band, with absence of the low-molecular weight bands (panel B, lane 3). When the same experiment is carried out on LPS-treated DCs, which constitutively produce and secrete pro–IL-1β (lane 4), similar results are obtained, although in some cases a stronger induction of pro–IL-1β synthesis and of IL-1β release by DCs on coculture with alloreactive T lymphocytes is observed (lane 5). In agreement with the results of the Western blot analyses, IL-1β secreted after contact of either untreated or LPS-treated DCs with allospecific T cells is biologically active, as shown by its property to induce ICAM1 expression on endothelial cells6 (Figure 3C). This ability, which is comparable to that of rIL-1β, is counteracted by IRAP,6 indicating that the ICAM1 inducing activity present in the supernatants is indeed IL-1. In contrast, IL-1 bioactivity is absent from supernatants of DCs, untreated or challenged with autologous T lymphocytes, even when the 29-kd band is present, indicating that an antigen-specific interaction is needed to get secretion of bioactive IL-1β.
Challenge of DCs with alloreactive, but not with nonspecific T lymphocytes, results in secretion of processed, bioactive IL-1β.
(A, B) DCs, untreated (lanes 1 to 3) or treated with LPS for the last 40 hours (lanes 4 to 6) were challenged with activated allospecific T lymphocytes (allo T, lanes 2 and 5) or autologous activated T lymphocytes (auto T, lanes 3 and 6) at a T-cell:DC ratio of 10:1. Cell lysates (panel A) and supernatants (sups, panel B) were analyzed as for their content in pro–IL-1β or IL-1β by Western blot. (C) Bioactivity of secreted IL-1β: 100 μL aliquots of supernatants from the different culture conditions was added to HUVEC and incubated at 37°C for 20 hours. At the end of the incubation, the ICAM1 molecules expressed by HUVEC were quantified by ELISA.
Challenge of DCs with alloreactive, but not with nonspecific T lymphocytes, results in secretion of processed, bioactive IL-1β.
(A, B) DCs, untreated (lanes 1 to 3) or treated with LPS for the last 40 hours (lanes 4 to 6) were challenged with activated allospecific T lymphocytes (allo T, lanes 2 and 5) or autologous activated T lymphocytes (auto T, lanes 3 and 6) at a T-cell:DC ratio of 10:1. Cell lysates (panel A) and supernatants (sups, panel B) were analyzed as for their content in pro–IL-1β or IL-1β by Western blot. (C) Bioactivity of secreted IL-1β: 100 μL aliquots of supernatants from the different culture conditions was added to HUVEC and incubated at 37°C for 20 hours. At the end of the incubation, the ICAM1 molecules expressed by HUVEC were quantified by ELISA.
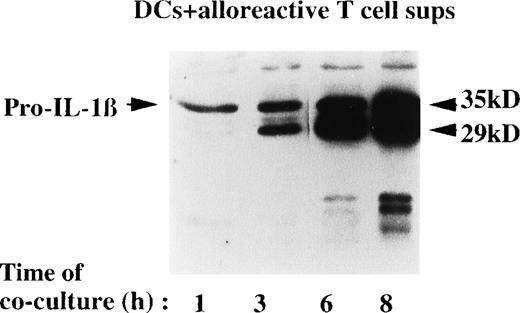
Kinetics analyses (Figure 4) revealed that, after incubation with allogenic T cells, both secretion of IL-1β and ICE activation increase with the time of coculture, as indicated by the appearance after 1 hour of the ICE-specific 29-kd band that increases thereafter. The appearance of the low-molecular weight bands is delayed, as they are easily detectable after 8 hours of coincubation.
Kinetics of IL-1β secretion after contact with allospecific T cells.
DCs were cocultured with alloreactive T lymphocytes for 1, 3, 6, or 8 hours at a T-cell:DC ratio of 10:1. At the end of each incubation time, the IL-1β present in the culture supernatants was analyzed by Western blotting.
Kinetics of IL-1β secretion after contact with allospecific T cells.
DCs were cocultured with alloreactive T lymphocytes for 1, 3, 6, or 8 hours at a T-cell:DC ratio of 10:1. At the end of each incubation time, the IL-1β present in the culture supernatants was analyzed by Western blotting.
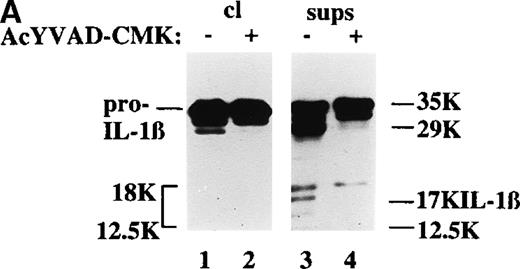
Although the 29-kd and the 17-kd bands are conceivably derived from ICE processing,6,17 20 it is unclear how the other low-molecular weight forms of secreted IL-1β are generated and whether they are biologically active. To clarify this point, the ICE inhibitor AcYVAD-CMK was added to the cultures and the pattern of secreted IL-1β as well as its biologic activity were investigated. As shown in Figure 5A, ICE inhibition results in the disappearance of both the 29-kd and 17-kd molecular forms; however, the IL-1β biologic activity of the supernatants, measured at different dilutions, is maintained (Figure 5B). These data indicate that, although ICE undergoes activation on DC-allospecific T-cell interaction, other convertase(s) are likely to operate on pro–IL-1β giving rise to biologically active IL-1β fragments.
ICE inhibition prevents the generation of ICE-specific bands but not the release of IL-1β bioactivity.
(A) Cell lysates (cl, lanes 1 and 2) or supernatants (sups, lanes 3 and 4) from DCs challenged for 8 hours with allogenic T cells at a T-cell:DC ratio of 10:1, in the absence (−, lanes 1 and 3) or presence (+, lanes 2 and 4) of 100 μmol/L AcYVAD-CMK, were analyzed as for their content in pro–IL-1β or IL-1β by Western blot. (B) Bioactivity of secreted IL-1β: aliquots of different dilutions (as indicated) of supernatants of DCs cultured 8 hours alone (○) with alloreactive T cells in the absence (▪) or presence (□) of 100 μmol/L AcYVAD-CMK were added to HUVEC and incubated at 37°C for 20 hours. At the end of the incubation, the ICAM1 molecules expressed by HUVEC were quantified by ELISA.
ICE inhibition prevents the generation of ICE-specific bands but not the release of IL-1β bioactivity.
(A) Cell lysates (cl, lanes 1 and 2) or supernatants (sups, lanes 3 and 4) from DCs challenged for 8 hours with allogenic T cells at a T-cell:DC ratio of 10:1, in the absence (−, lanes 1 and 3) or presence (+, lanes 2 and 4) of 100 μmol/L AcYVAD-CMK, were analyzed as for their content in pro–IL-1β or IL-1β by Western blot. (B) Bioactivity of secreted IL-1β: aliquots of different dilutions (as indicated) of supernatants of DCs cultured 8 hours alone (○) with alloreactive T cells in the absence (▪) or presence (□) of 100 μmol/L AcYVAD-CMK were added to HUVEC and incubated at 37°C for 20 hours. At the end of the incubation, the ICAM1 molecules expressed by HUVEC were quantified by ELISA.
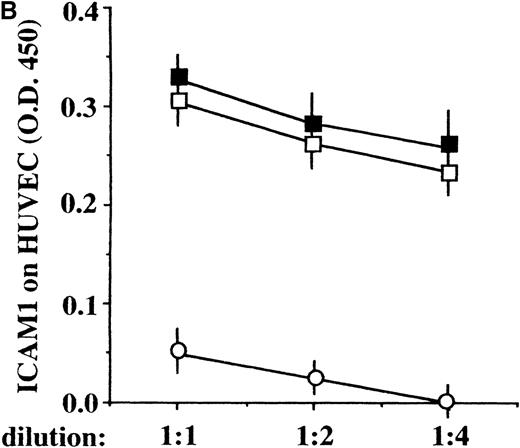
Role of CD4+ and CD8+ T lymphocytes in the modulation of IL-1β synthesis and secretion
The T-cell populations obtained from the different MLR were 50% to 55% CD4+ and 45% to 50% CD8+. To determine the relevant role of the 2 T-cell subsets in inducing synthesis and/or secretion of IL-1β by DCs, purified CD4+ or CD8+ T cells were obtained and cocultured with DCs as above. As shown in Figure 6A, CD4+ T lymphocytes (either alloreactive, lanes 1, or nonspecific, not shown) induce pro–IL-1β intracellular accumulation in immature DCs (upper panel) but not secretion of the processed forms (bottom panel). At variance, incubation with alloreactive CD8+ T cells induces neither synthesis nor secretion of IL-1β by immature DCs (lane 2). However, when DCs are pretreated with LPS, and hence they are producing pro–IL-1β (Figure1), interaction with CD8+ alloreactive T cells (lane 4) results in activation of ICE, as indicated by the appearance of the 29-kd IL-1β band, and secretion of small IL-1β molecular forms, with generation of bioactive IL-1β (Figure 6, panel B). Again, IL-1β bioactivity is not prevented by the ICE inhibitor Ac-YVAD-CMK (not shown), suggesting that additional protease(s), produced or activated by specific CD8+ T cells, are involved in IL-1β maturation. In contrast, activated CD4+ T cells do not affect processing and secretion of IL-1β by LPS-treated DCs (Figure6A, lane 3). It is of note that, as observed for the unfractionated autologous T cells (Figure 3), autologous CD8+ T lymphocytes, derived from an MLR against an unrelated stimulator, do not elicit processing and secretion of bioactive IL-1β by untreated or LPS-treated DCs (Figure 6A, lanes 5 and 6, and Figure 6B), supporting the hypothesis that antigen-specific interactions between CD8+ alloreactive T cells and DCs are needed for the generation of bioactive IL-1β.
Alloreactive CD8+ but not CD4+ T cells induce generation of bioactive IL-1β.
(A) DCs untreated (lanes 1, 2, and 5) or treated for the last 40 hours with LPS (lanes 3, 4, and 6) were challenged with alloreactive CD4+ (lanes 1 and 3) or CD8+ (lanes 2 and 4) T lymphocytes purified from the allospecific T-cell population, or with autologous CD8+ T lymphocytes purified from an MLR against an unrelated stimulator (Auto T cells, lanes 5 and 6) at a T-cell:DC ratio of 10:1. At the end of the coincubation cell lysates (cyt, upper panel) and supernatants (sec, bottom panel) were analyzed as for their content in IL-1β. (B) Bioactivity of secreted IL-1β: 100 μL aliquots of supernatants from the different culture conditions was added to HUVEC and incubated at 37°C for 20 hours. At the end of the incubation, the ICAM1 molecules expressed by HUVEC were quantified by ELISA.
Alloreactive CD8+ but not CD4+ T cells induce generation of bioactive IL-1β.
(A) DCs untreated (lanes 1, 2, and 5) or treated for the last 40 hours with LPS (lanes 3, 4, and 6) were challenged with alloreactive CD4+ (lanes 1 and 3) or CD8+ (lanes 2 and 4) T lymphocytes purified from the allospecific T-cell population, or with autologous CD8+ T lymphocytes purified from an MLR against an unrelated stimulator (Auto T cells, lanes 5 and 6) at a T-cell:DC ratio of 10:1. At the end of the coincubation cell lysates (cyt, upper panel) and supernatants (sec, bottom panel) were analyzed as for their content in IL-1β. (B) Bioactivity of secreted IL-1β: 100 μL aliquots of supernatants from the different culture conditions was added to HUVEC and incubated at 37°C for 20 hours. At the end of the incubation, the ICAM1 molecules expressed by HUVEC were quantified by ELISA.
CD40 engagement mediates IL-1β synthesis in dendritic cells
The observation that both specific and nonspecific activated CD4+ T cells are able to induce pro–IL-1β synthesis suggests that molecular structure(s) expressed by CD4+ T lymphocytes are responsible for this induction, even in the absence of an antigen-specific interaction. Triggering of CD40 has been reported to induce IL-1β expression in macrophages.30 As CD40L is present on activated CD4+ T cells and CD40-CD40L interaction has a central role in the immune response,31,32we investigated whether CD40 engagement results in pro–IL-1β synthesis also in DCs.
Figure 7A shows that culturing DCs with CD40L-transfected cells at a 1:1 ratio results in a time-dependent induction of intracellular pro–IL-1β. Secreted IL-1β is almost undetectable in supernatants and IL-1β biologic activity is absent (not shown). In turn, when activated T lymphocytes are pretreated with an anti-CD40L antibody and then cultured with DCs, the intracellular appearance of pro–IL-1β is prevented (Figure 7B). Thus, CD40 triggering is sufficient to induce synthesis of pro–IL-1β but not secretion of bioactive IL-1β.
CD40-CD40L interaction is responsible for the induction of IL-1β synthesis by DCs.
(A) DCs were incubated for different periods as indicated with CD40L transfected cells at a CD40L+ cell:DC ratio of 1:1. At the end of each incubation time, the intracellular content in pro–IL-1β was analyzed by Western blot and quantified by densitometry. (B) DCs were incubated for 8 hours alone (lane 1) or with alloreactive T cells (lanes 2 and 3), that were untreated (lane 2) or pretreated for 30 minutes with anti CD40L antibody, at T-cell:DC ratio 10:1. At the end of incubation the intracellular content in pro–IL-1β was analyzed by Western blot.
CD40-CD40L interaction is responsible for the induction of IL-1β synthesis by DCs.
(A) DCs were incubated for different periods as indicated with CD40L transfected cells at a CD40L+ cell:DC ratio of 1:1. At the end of each incubation time, the intracellular content in pro–IL-1β was analyzed by Western blot and quantified by densitometry. (B) DCs were incubated for 8 hours alone (lane 1) or with alloreactive T cells (lanes 2 and 3), that were untreated (lane 2) or pretreated for 30 minutes with anti CD40L antibody, at T-cell:DC ratio 10:1. At the end of incubation the intracellular content in pro–IL-1β was analyzed by Western blot.
Discussion
In this paper, we show that pro–IL-1β synthesis in DCs is induced by maturative/inflammatory stimuli, such as LPS, TNF-α, or by coculture with activated-specific or nonspecific CD4+ T lymphocytes. In all these conditions, however, DCs do not secrete biologically active IL-1β. In contrast, when pro–IL-1β synthesis is triggered by either one of these stimuli, the antigen-specific interaction with CD8+ T lymphocytes induces processing and secretion of mature bioactive IL-1β. The IL-1β synthesis driven by activated CD4+ T cells is mediated at least in part by CD40-CD40L binding. Thus, similar to what was reported for monocytes,30 the interaction between CD40L on activated CD4+ T cells and CD40 on DCs provides a molecular basis for the induction of pro–IL-1β synthesis. The observation that in one donor pro–IL-1β expression in DCs was triggered by specific T lymphocytes at a lower T-cell:DC ratio than by nonspecific T cells (see Figure 2C), may reflect an improved triggering of CD40 by CD40L, either quantitative or qualitative, favored by the antigen-specific binding; alternatively, other antigen-specific molecular interactions may contribute to the induction of pro–IL-1β synthesis.
The regulation of secretion of IL-1β by DCs differs from that by monocytes in 2 respects. First, in human monocytes a single stimulus given by bacterial products is sufficient to drive synthesis, processing and secretion of bioactive IL-1β.6,14,15 In contrast, DCs need at least 2 signals: one, represented by inflammatory stimuli or by CD40L-mediated contact with activated CD4+ T cells, induces intracellular accumulation of pro–IL-1β and is not antigen-specific; the other, provided by CD8+ T lymphocytes, is antigen specific and drives secretion of mature bioactive IL-1β. Second, whereas in monocytes processing and secretion are coupled,6,14 15 in DCs secretion of the precursor form may precede maturation of the cytokine.
These differences may reflect the different functions of the 2 cell types. Monocytes/macrophages, either resident or recruited, are mostly involved in inflammation and tissue reactions in natural immunity, in which IL-1β plays a major role.6 At variance, DCs are specialized in antigen presentation and in the initiation of the immune response1: in this context, it is possible that the function of IL-1β is that of amplifying the inflammatory component of the immune reaction, rather than to act directly on immunocompetent cells. This may be relevant in autoimmune diseases with high inflammatory component, such as rheumatoid arthritis.
The processing of the precursor pro–IL-1β molecules induced by CD8+ T cells is only partially mediated by ICE: indeed, in addition to the two 29-kd and 17-kd forms generated by ICE,6,16,17 other low-molecular weight forms are secreted on contact with allospecific T lymphocytes. Furthermore, although treatment with the ICE inhibitor Ac-YVAD-CMK prevents, as expected, the generation of the 29-kd and 17-kd forms, it inhibits neither the secretion of the additional low-molecular IL-1β forms nor, more importantly, the recovery of IL-1 bioactivity in the supernatants from the cocultures. These findings suggest that convertase(s) other than ICE are also involved in the maturation of IL-1β induced by alloreactive CD8+ T cells. In keeping with this, it has to be reminded that in ICE −/− mice, IL-1β activity is released after stimulation with some proinflammatory stimuli.20 Furthermore, it has been reported that granzyme A, a proteolytical enzyme released by cytotoxic T lymphocytes, is able to generate bioactive IL-1β.24 However, in our system, the release of a soluble enzyme does not seem sufficient, as the incubation of DCs with autologous T lymphocytes, derived from an MLR and hence phenotypically and functionally similar to alloreactive T lymphocytes, fails to induce the secretion of bioactive IL-1β. Thus, the generation and the release of biologically active IL-1β seem to require a cell-to-cell contact between DCs and alloreactive CD8+ T lymphocytes. The molecular mechanism underlying the alloreactive T-cell–mediated secretion of mature IL-1β remains to be elucidated. Understanding this mechanism could lead to the discovery of novel molecular targets, whereby control of IL-1β production during immune response may be effected.
Acknowledgments
We thank G. Angelini, A. Poggi, and R. Sitia for critical comments; P. Lane and Lofarma Allergeni for sharing their reagents; and the Blood Center of Gaslini Scientific Institute for providing buffy coats. The 3ZD monoclonal antibody was obtained from the NCI Biological Resources Branch, FCRDC.
Supported in part by grants from CNR (Target Project on Biotechnology), and Ministero Sanità (PF Oncology and special project AIDS). C.A. is the recipient of a fellowship from “Fondazione Levi-Montalcini.”
Reprints:Anna Rubartelli, National Institute for Cancer Research, Largo Rosanna Benzi, 10, 16132 Genova, Italy; e-mail:annarub@hp380.ist.unige.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal