Abstract
The deleterious effects of lipopolysaccharide (LPS) during endotoxic shock are associated with the secretion of tumor necrosis factor (TNF) and the production of nitric oxide (NO), both predominantly released by tissue macrophages. We analyzed the mechanism by which LPS induces apoptosis in bone marrow-derived macrophages (BMDM). LPS-induced apoptosis reached a plateau at about 6 hours of stimulation, whereas the production of NO by the inducible NO-synthase (iNOS) required between 12 and 24 hours. Furthermore, LPS-induced early apoptosis was only moderately reduced in the presence of an inhibitor of iNOS or when using macrophages from iNOS -/-mice. In contrast, early apoptosis was paralleled by the rapid secretion of TNF and was almost absent in macrophages from mice deficient for one (p55) or both (p55 and p75) TNF-receptors. During the late phase of apoptosis (12-24 hours) NO significantly contributed to the death of macrophages even in the absence of TNF-receptor signaling. NO-mediated cell death, but not apoptosis induced by TNF, correlated with the induction of p53 and Bax genes. Thus, LPS-induced apoptosis results from 2 independent mechanisms: first and predominantly, through the autocrine secretion of TNF- (early apoptotic events), and second, through the production of NO (late phase of apoptosis).
Mononuclear phagocytes represent a large family of cell types that includes tissue macrophages, Kupffer cells in the liver, Langerhans' cells in the epidermis, osteoclasts in the bone, microglia in the brain, and perhaps some of the interdigitating and follicular dendritic cells found in lymphoid organs.1,2 Macrophages exert key functions during the immune response. To perform most of these functions, macrophages must be activated.3 4 Thus, macrophages are able to kill bacteria, virus, or parasites directly; to secrete several immune regulators (tumor necrosis factor [TNF-α], interleukin (IL)1-β, IL6, etc); to process antigens and present them to T cells; and finally, to act as scavenger cells and to participate in tissue remodeling.
However, macrophages do not always play a positive role in the homeostasis of the immune system. Under some circumstances, macrophages have deleterious effects. This is the case of septic shock, which is a severe systemic inflammatory response triggered by the interaction of lipopolysaccharide (LPS) and some bacterial components with macrophages and other host cells.5,6 Although this interaction leads to the progressive release of a variety of proinflammatory cytokines, such as IL8, IL1-β, and IL6,7,8 experimental evidence points to nitric oxide (NO) and TNF as the primary mediators of the changes observed during septic shock.9-11 Central to the pathogenesis of endotoxic shock is the development of circulatory failure, characterized by hypotension, myocardial dysfunction, and tissue hypoxia that ultimately leads to multiorgan failure and death.11,12 Despite major advances in antimicrobial therapy and critical care, septic shock continues to have a mortality rate of 40%-70% and remains the leading cause of more than 100 000 deaths per year in the intensive care units of the United States alone.11 13
Although several reports14-16 suggest that excessive production of NO by the inducible NO synthase (iNOS) contributes to the circulatory failure during septic shock, the role of this enzyme in septic shock remains controversial. The use of iNOS -/-mice revealed the existence of both an iNOS-dependent and -independent pathway for LPS-induced hypotension.17-19 Deletion of the iNOS gene or blocking of the activity of iNOS resulted in either no protection,18,20 partial17,19 or total protection,21,22 or even led to detrimental effects23 24 during sepsis.
TNF affects the growth, differentiation, and function of many cell types, and it is a major mediator of inflammatory immune responses.10,25,26 TNF has also been suggested as a key mediator of the septic shock syndrome induced by either LPS or bacterial superantigens.27-29 The potent regulatory abilities of TNF-α are transduced by 2 distinct cell-surface receptors with 55 kd (Type I) and 75 kd (Type II) relative molecular weights.30 31
Most of the known cellular TNF-α responses have been attributed to the activation of p55 type I TNF-αR.32,33 In contrast, little is known about the function of p75 type II TNF-αR.34,35 Activation of the type I TNF-αR is necessary and sufficient for TNF-α–induced liver failure and hepatocyte apoptosis36 as well as for cytotoxicity and apoptosis in other cell types.37-39 Although p55 TNF-αR -/-mice seem to be resistant to endotoxic shock, they succumb to bacterial infections.40
Thus, LPS-dependent activation of macrophages, exposure to endogenous or exogenous NO, or treatment with TNF are enough to induce apoptosis in several cell types.41-43 Apoptosis has been involved in the ultimate multiorgan failure during septic shock. For this reason, we have analyzed the mechanisms involved in the LPS-induced apoptosis of macrophages, since these cells are mainly involved in the secretion of TNF and NO that plays a crucial role in the pathogenesis of endotoxic shock.
In this report we provide evidence that macrophage apoptosis induced by LPS is mediated by both NO and TNF production. However, each of these agents acts separately. TNF induces the early apoptotic events (3-6 hours), whereas iNOS-dependent apoptotic events occur later (12-24 hours). NO-induced apoptosis, but not TNF-α–dependent apoptosis, correlates with the induction of p53 and Bax.
Materials and methods
Reagents
LPS was obtained from Sigma Chemical (St Louis, MO). Recombinant murine TNF-α (rmTNF-α) was purchased from PrepoTech EC (London, UK). Recombinant murine interferon (IFN)-γ was kindly provided by Genentech (South San Francisco, CA). 4′6-diamidino-2-phenylindole (DAPI), (±)-S-nitroso-N-acetylpenicillamine (SNAP), andS-methylisothiourea sulfate (SMT) were all purchased from Calbiochem (La Jolla, CA). All other chemicals were of the highest available purity grade and were purchased from Sigma Chemical. Deionized water further purified with a Millipore Milli-Q system (Bedford, MA) was used.
Antibodies
For Western blot analysis, we used a rabbit antibody against mouse iNOS (M-19; Santa Cruz Biotechnology, Santa Cruz, CA), a sheep antimouse p53-PAN antibody (Boehringer Mannheim, Mannheim, Germany), and, as a control, a mouse antimouse β-actin antibody (Sigma Chemical). Peroxidase-conjugated antirabbit immunoglobulin G (IgG; Cappel, Turnhout, Belgium), anti-goat/sheep IgG (Boehringer), or antimouse IgG (Cappel) were used as secondary antibodies.
Plasmids and constructions
The plasmid corresponding to the rat iNOS full-length complementary DNA (cDNA) was kindly provided by Dr A. Felipe (University of Barcelona, Spain). Murine cDNA probes for TNF-α and Bax were kindly provided by Dr M. Nabholz (ISREC, Epalinges, Switzerland) and Dr R. Merino (University of Cantabria, Spain), respectively. As a control for RNA loading and transfer, we used an 18S rRNA transcript.44
Cell culture
Bone marrow-derived macrophages (BMDM) were isolated as previously described.45 Six-week-old BALB/C mice (Charles River Laboratories, Wilmington, MA) were killed by cervical dislocation, and both femurs were dissected free of adherent tissue. The ends of the bones were cut off and the marrow tissue was flushed by irrigation with media. The marrow plugs were dispersed by passing through a 25-gauge needle, and the cells were suspended by vigorous pipetting and washed by centrifugation. The cells were cultured in plastic tissue-culture dishes (150 mm) in 40 mL DMEM containing 20% FBS and 30% L-cell conditioned media as a source of macrophage colony-stimulating factor (M-CSF). Macrophages were obtained as a homogeneous population of adherent cells after 7 days of culture. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
BMDM from iNOS or TNF-αR knock-out (KO) mice and the corresponding controls were isolated under the same conditions. TNF-αRI KO mice46 and TNF-αRI/II double KO mice47 were kindly donated by Dr K. Matsushima from Kanazawa University, Japan; and Dr J. Peschon from University of Kentucky, respectively. The iNOS KO mice were kindly donated by Dr S. Mudgett17 and obtained as previously described.48
Analysis of DNA content with DAPI
Macrophages (106) previously subjected or not to LPS treatment were resuspended and fixed in ice-cold 70% ethanol. The cells were then washed in phosphate-buffered saline (PBS), resuspended in 0.2 mL of a solution containing 150 mmol/L NaCl, 80 mmol/L HCl, and 0.1% Triton X-100, and incubated at 0°-4°C for 10 minutes. Afterward, 1 mL of a solution containing 180 mmol/L Na2HPO4, 90 mmol/L citric acid, and 2 μg/mL DAPI, pH 7.4, was added to each sample. After incubating the cells at 4°C for 24 hours, their fluorescence was measured with an Epics Elite flow cytometer (Coulter, Hialeah, FL). For this analysis, we used an UV laser with an excitation beam of 25 mW at 333-364 nm and fluorescence was collected with a 525 nm band-pass filter. Cell doublets were gated out by comparing the pulse area versus the pulse width. A total of 12 000 cells were counted for each histogram, and cell-cycle distributions were analyzed with the Multicycle program (Phoenix Flow Systems, Inc; San Diego, CA).
In parallel experiments, cells stained with DAPI were mounted on a slide and visualized in a Zeiss fluorescent microscope. Pictures were taken, using a Kodak camera installed to the microscope. Under these conditions, condensed DAPI-stained chromatin was visualized in the nucleus of the apoptotic cells.
Analysis of apoptosis
DNA fragmentation due to internucleosomal cleavage was determined as described previously.49 Briefly, 3 × 106 macrophages were harvested and washed in ice-cold PBS. The cells were lysed in 0.5 mL of lysis buffer (50 mmol/L Tris-HCl, 10 mmol/L EDTA, 1% SDS, pH 8.0) for 16 hours at 4°C, and the lysates were centrifuged (15 000 × g) to separate high molecular weight DNA (pellet) from cleaved low-molecular-weight DNA (supernatant). The DNA supernatants were phenol-extracted twice and precipitated. The pellets were resuspended in Tris-EDTA buffer containing 250 μg/mL RNAse (Boehringer Mannheim). The samples were heated at 65°C for 10 minutes and subjected to electrophoresis in a 2% agarose gel containing ethidium bromide.
Low-molecular-weight apoptotic DNA, obtained as previously described, was also measured by an enzyme-linked immunosorbent assay (ELISA) technique (Cell Death Detection ELISA Plus; Boehringer Mannheim) that is directed against cytoplasmic histone-associated DNA fragments. Each point was performed in triplicate and the results were expressed as the mean ± SD.
Determination of NO production
NO production was estimated by measuring nitrate/nitrite in the cell culture media. Macrophages were cultured in DMEM without phenol-red (GIBCO Life Technologies, UK) to avoid interference with the Griess absorbance at 550 nm. Samples were stored at −80°C until assayed. Nitrate was converted to nitrite with Zea mays nitrate reductase (Calbiochem). Reduced samples were incubated with an equal volume of Griess reagent, and the absorbance at 550 nm was measured. The total nitrate/nitrite concentration was determined by comparison with a standard curve.
Protein extraction and Western blot analysis
Cells were washed twice in cold PBS and lysed on ice with lysis solution (1% Triton X-100, 10% glycerol, 50 mmol/L Hepes pH 7.5, 150 mmol/L NaCl, protease inhibitors). The protein concentration of the samples was determined with the Bio-Rad protein assay. The proteins from the cell lysates (100 μg) were boiled at 95°C in Laemmli SDS loading buffer, separated on 7.5% SDS-PAGE for the detection of iNOS or on 10% SDS-PAGE for p53 immunoblotting. Then, the proteins were electrotransferred to nitrocellulose membranes (Hybond-ECL; Amersham, Arlington Heights, IL). The membranes were blocked for at least 1 hour at room temperature in Tris buffered saline-0.1% Tween-20 (TBS-T) containing 5% nonfat dry milk and then incubated with TBS-T containing the primary antibody. For iNOS, p53, and β-actin immunoblotting, incubation was performed for 1 hour at room temperature. After 3 washes of 15 minutes each in TBS-T, the membranes were incubated with peroxidase-conjugated anti-goat/sheep (Boehringer), antirabbit, or antimouse IgG (Cappel) antibodies for 1 hour. After 3 washes of 15 minutes with TBS-T, ECL detection was performed (Amersham) and the membranes were exposed to x-ray films (Amersham). Quantitation of the blots was carried out by densitometric analysis.
Northern blot analysis
Total cellular RNA (20 μg), extracted with the acidic guanidinium thiocyanate-phenol-chloroform method,50 was separated in 1% agarose with 5 mmol/L 3-[N-morpholino]propanesulfonic acid (MOPS), pH 7.0/1 mol/L formaldehyde buffer. The RNA was transferred overnight to a GeneScreen nitrocellulose membrane (Life Science Products, Boston, MA) and fixed by UV irradiation (150 mJ). All probes were labeled with 32Pα-dCTP (Amersham) with the oligolabeling kit method (Pharmacia Biotech, Uppsala, Sweden). To check for differences in RNA loading, the expression of the 18S rRNA transcript was analyzed. After incubating the membranes for 18 hours at 65°C in hybridization solution (20% formamide, 5X Denhart's, 5X SSC, 10 mmol/L EDTA, 1% SDS, 25 mmol/L Na2HPO4, 25 mmol/L NaH2PO4, 0.2 mg/mL salmon sperm DNA, and 106 cpm/mL of 32P-labeled probe), they were exposed to Kodak X-AR films (Kodak, Rochester, NY). The bands of interest were quantified with a Molecular Analyst system (Bio-Rad Labs, Richmond, CA).
Determination of TNF- production
The secretion of TNF-α was measured with the use a commercial murine TNF-α ELISA kit (Quantikine M; R&D Systems, Minneapolis, MN); 105 cells were cultured in 24-well plates and stimulated with LPS. Supernatant samples were obtained at the indicated times and subjected to ELISA analysis.
Results
Induction of apoptosis by LPS
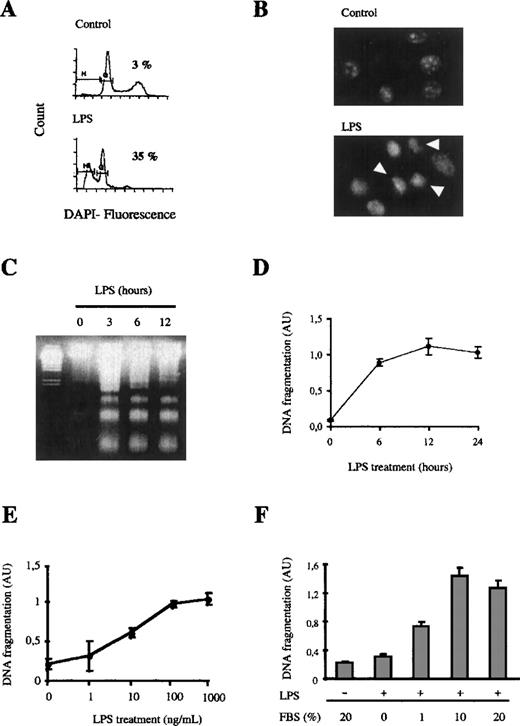
Bone marrow macrophages growing in the presence of M-CSF are unevenly distributed into the different phases of the cell cycle. On the activation with LPS, macrophages arrest at the G0/G1 phase of the cell cycle and die through the induction of apoptosis. This conclusion is supported by several observations: (1) the staining of the DNA with DAPI revealed that after 6 hours of LPS treatment, 35% of the cells had a subdiploid DNA content corresponding to that of apoptotic cells, in contrast with 3% of subdiploid cells in nontreated cell cultures (Figure1A); (2) macrophages treated with LPS and stained with DAPI showed condensed chromatin in the nucleus (Figure1B); and (3) electrophoresis on an agarose gel of the DNA obtained from macrophages treated with LPS showed the typical laddering observed after internucleosomal fragmentation of apoptotic DNA (Figure 1C). Therefore, all these results demonstrate that the treatment of bone marrow macrophages with LPS induces cell death by apoptosis. Moreover, apoptosis was quantified with the use of an ELISA kit that measures the presence of histone-associated DNA fragments. The induction of macrophage apoptosis by LPS was time- and dose-dependent (Figure 1D-E). The kinetics of induction of apoptosis was very fast and maximal induction was observed as soon as 3 hours after the start of LPS treatment. The levels of apoptosis did not further increase thereafter and up to 24 hours of stimulation.
LPS induces apoptosis in bone marrow macrophages.
(A and B) 106 macrophages were stimulated with 100 ng/mL of LPS for 6 hours. DNA was stained with DAPI, and induction of apoptosis was analyzed by cytometric analysis (A) or visualizing the cells in a fluorescence microscope (B). Apoptotic cells are marked with arrows. (C and D) LPS induces apoptosis in a time-dependent manner. Apoptotic DNA from macrophages treated with LPS (100 ng/mL) for the indicated times was analyzed by agarose gel electrophoresis (C) or by using an ELISA technique (D). (E) LPS induces apoptosis in a dose-dependent fashion; 105 macrophages were treated for 12 hours with the indicated concentrations of LPS. Apoptotic DNA was measured as in (D). (F) Apoptosis induced by LPS depends on the presence of FBS. The cells were treated with 100 ng/mL LPS for 12 hours in the presence of the indicated concentrations of FBS. Fragmentation of DNA was measured by ELISA. The ELISA experiments were performed in triplicate and represented as the mean value ± SD. These figures are representative of 4 independent experiments.
LPS induces apoptosis in bone marrow macrophages.
(A and B) 106 macrophages were stimulated with 100 ng/mL of LPS for 6 hours. DNA was stained with DAPI, and induction of apoptosis was analyzed by cytometric analysis (A) or visualizing the cells in a fluorescence microscope (B). Apoptotic cells are marked with arrows. (C and D) LPS induces apoptosis in a time-dependent manner. Apoptotic DNA from macrophages treated with LPS (100 ng/mL) for the indicated times was analyzed by agarose gel electrophoresis (C) or by using an ELISA technique (D). (E) LPS induces apoptosis in a dose-dependent fashion; 105 macrophages were treated for 12 hours with the indicated concentrations of LPS. Apoptotic DNA was measured as in (D). (F) Apoptosis induced by LPS depends on the presence of FBS. The cells were treated with 100 ng/mL LPS for 12 hours in the presence of the indicated concentrations of FBS. Fragmentation of DNA was measured by ELISA. The ELISA experiments were performed in triplicate and represented as the mean value ± SD. These figures are representative of 4 independent experiments.
Maximal induction of apoptosis was obtained at a concentration of 100 ng/mL of LPS (Figure 1E) and was serum-dependent (Figure 1F), which can be explained by the fact that the recognition of LPS by its high affinity receptor CD14 requires the previous association of LPS with the serum protein LBP (LPS-binding protein).51 52
Exogenous NO is toxic to macrophages, but iNOS-derived NO does not account to LPS-induced early apoptosis
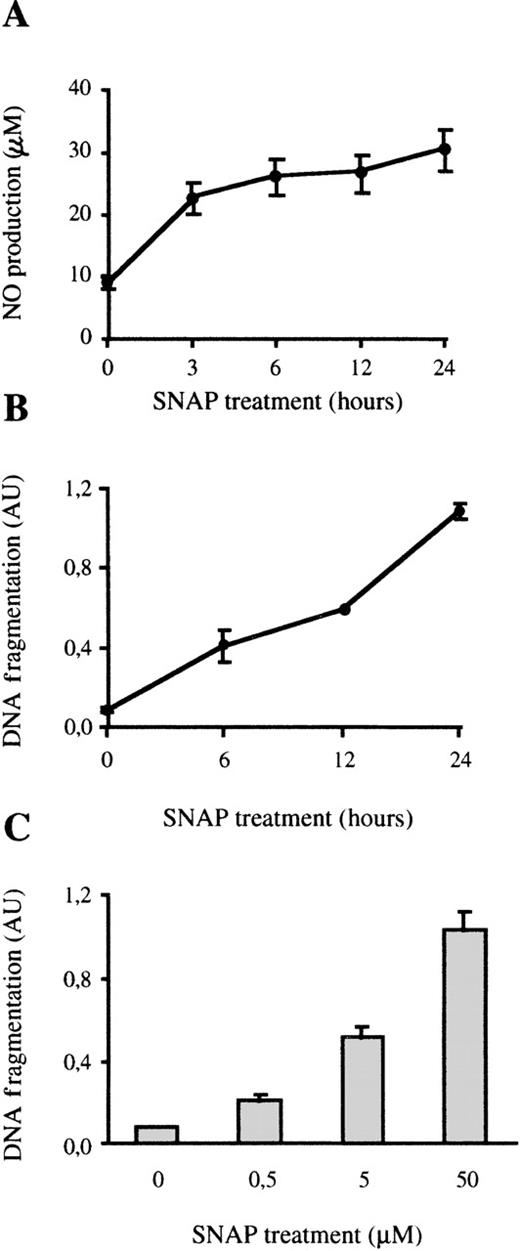
In several cell types, apoptosis induced by LPS has been linked to the cytotoxic effect of iNOS-derived NO.41,53,54 Because LPS induces the expression of iNOS in macrophages,41,42 we analyzed whether the induction of apoptosis in bone marrow microphages was also mediated by the generation of NO. The toxic effect of NO was tested by treating bone marrow macrophages with the NO-donor SNAP55 that spontaneously produces NO after being added to the culture (Figure 2A). SNAP induced apoptosis in macrophages in a time- and dose-dependent fashion as determined by measuring DNA fragmentation, by DNA laddering (Figure2B-C), or by cytometric analysis of DAPI-stained cells (data not shown). Therefore, as has been observed for other types of macrophages,41 42 exogenous NO is toxic for bone marrow macrophages.
Exogenous NO induces apoptosis in bone marrow macrophages.
(A) NO production by SNAP; 105 macrophages were treated with 50 μmol/L of SNAP for the indicated times and the production of NO was determined. (B) Time course of SNAP-induced apoptosis; 105 macrophages were treated with 50 μmol/L SNAP at the indicated times. Apoptosis was measured by ELISA. (C) The cells were treated for 24 hours with the indicated concentrations of SNAP, and apoptosis was detected as indicated above. Each experiment was performed in triplicate and the results of 1 representative of 2 independent experiments are represented as the mean value ± SD.
Exogenous NO induces apoptosis in bone marrow macrophages.
(A) NO production by SNAP; 105 macrophages were treated with 50 μmol/L of SNAP for the indicated times and the production of NO was determined. (B) Time course of SNAP-induced apoptosis; 105 macrophages were treated with 50 μmol/L SNAP at the indicated times. Apoptosis was measured by ELISA. (C) The cells were treated for 24 hours with the indicated concentrations of SNAP, and apoptosis was detected as indicated above. Each experiment was performed in triplicate and the results of 1 representative of 2 independent experiments are represented as the mean value ± SD.
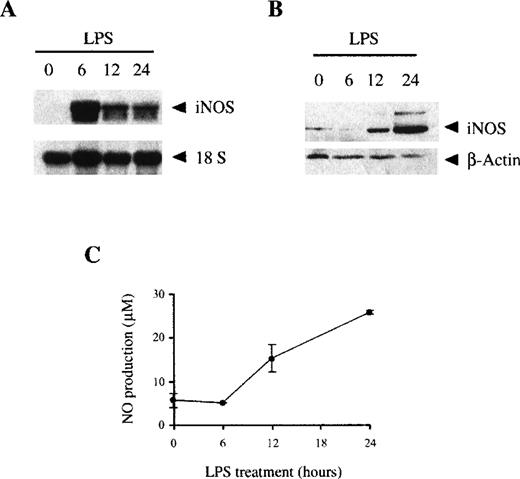
LPS induces the expression of iNOS in bone marrow macrophages. However, whereas mRNA expression was maximal after 6 hours of LPS treatment (Figure 3A), the expression of iNOS protein was a late event not observed until 12 hours of LPS treatment, reaching a maximum level after 24 hours (Figure 3B). The synthesis of iNOS protein correlated with NO production, which was not detected until 12-24 hours of LPS treatment (Figure 3C).
LPS induces the expression of iNOS and the production of NO.
The expression of iNOS induced by LPS was measured by Northern blot (A) or Western blot (B) as described in the “Materials and methods” section. (C) LPS induces the production of NO; 106 BMDM cultured in media without phenol-red were stimulated with 100 ng/mL LPS, and the supernatants were harvested at the indicated times. NO production was measured as the nitrite/nitrate levels. Each point was performed in triplicate and represented as the mean ± SD. These figures are representative of 3 independent experiments.
LPS induces the expression of iNOS and the production of NO.
The expression of iNOS induced by LPS was measured by Northern blot (A) or Western blot (B) as described in the “Materials and methods” section. (C) LPS induces the production of NO; 106 BMDM cultured in media without phenol-red were stimulated with 100 ng/mL LPS, and the supernatants were harvested at the indicated times. NO production was measured as the nitrite/nitrate levels. Each point was performed in triplicate and represented as the mean ± SD. These figures are representative of 3 independent experiments.
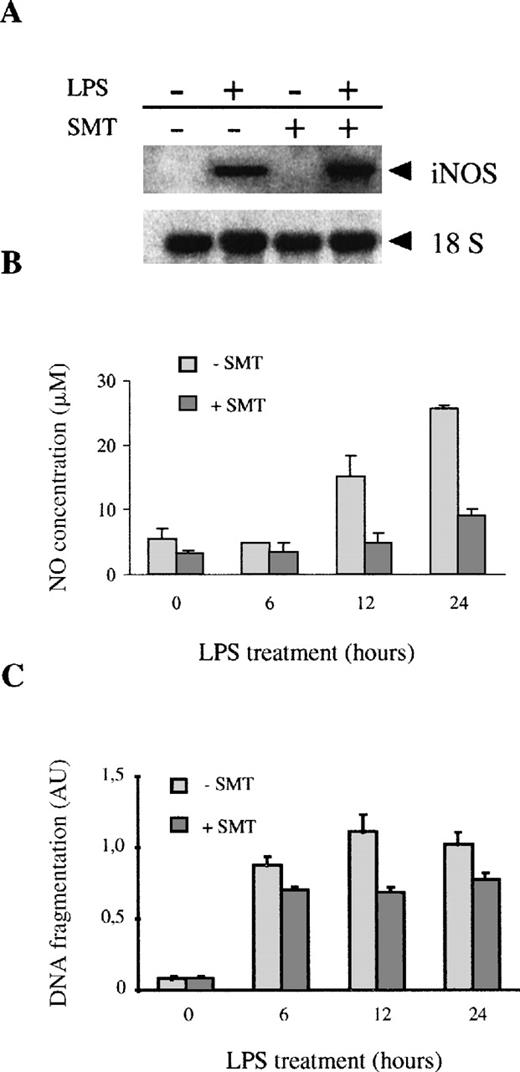
These results suggest that, although exogenous NO may induce macrophage apoptosis, the early apoptotic events induced by LPS (3-6 hours) are not related to the production of endogenous NO derived from iNOS. To further determine the role of endogenous NO in LPS-induced apoptosis, we blocked the LPS-induced production of NO by using the iNOS inhibitor SMT.56 57 SMT did not affect the induction of iNOS mRNA expression by LPS (Figure 4A). However, SMT totally blocked the NO production induced by LPS (Figure 4B). The treatment with SMT had a very weak effect on the LPS induction of apoptosis in macrophages (14% inhibition after 6 hours, 23% inhibition after 24 hours of LPS treatment) (Figure 4C). All this suggests that early macrophage apoptosis induced by LPS is not mediated by the production of endogenous NO.
Treatment of macrophages with SMT inhibits LPS-induced NO production but not apoptosis.
(A) The expression of iNOS was measured by Northern blot in macrophages treated with 100 ng/mL of LPS in the presence or absence of SMT, an iNOS inhibitor (20 μmol/L). (B) SMT inhibits LPS-induced NO production; 106 macrophages were treated with 100 ng/mL of LPS for the indicated times in the presence or absence of SMT (20 μmol/L). The production of NO was assessed by determination of the nitrate/nitrite levels. (C) SMT did not inhibit LPS-induced apoptosis. The cells were treated with 100 ng/mL of LPS for the indicated times in the presence or absence of SMT (20 μmol/L). Each experiment was performed in triplicate, and the results of 1 representative of 2 independent experiments are represented as the mean value ± SD.
Treatment of macrophages with SMT inhibits LPS-induced NO production but not apoptosis.
(A) The expression of iNOS was measured by Northern blot in macrophages treated with 100 ng/mL of LPS in the presence or absence of SMT, an iNOS inhibitor (20 μmol/L). (B) SMT inhibits LPS-induced NO production; 106 macrophages were treated with 100 ng/mL of LPS for the indicated times in the presence or absence of SMT (20 μmol/L). The production of NO was assessed by determination of the nitrate/nitrite levels. (C) SMT did not inhibit LPS-induced apoptosis. The cells were treated with 100 ng/mL of LPS for the indicated times in the presence or absence of SMT (20 μmol/L). Each experiment was performed in triplicate, and the results of 1 representative of 2 independent experiments are represented as the mean value ± SD.
Early apoptosis in LPS-stimulated macrophages is due to endogenous TNF-
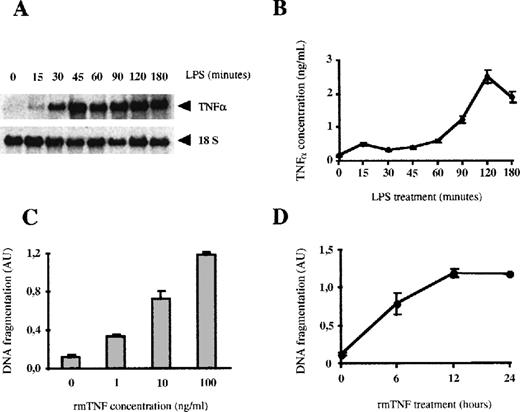
Because tissue macrophages are major producers of TNF-α,7 we analyzed the role of this cytokine in the LPS-induced apoptosis in macrophages. At 30 minutes after LPS stimulation, bone marrow macrophages already expressed high levels of TNF-α mRNA (Figure 5A). The protein levels of TNF-α increased very rapidly in the culture supernatants and reached a concentration of 2490 pg/mL after 2 hours. These levels of TNF-α are sufficient to induce apoptosis in BMDM, since doses between 1 and 10 ng/mL of rmTNF-α induced significant levels of apoptosis in these cells (Figure 5C). Moreover, the kinetics of induction of apoptosis by rmTNF-α is very similar to that triggered by LPS (Figure 5D), with a significant induction of apoptosis within the first 6 hours. Finally, the presence of the iNOS inhibitor SMT did not inhibit the apoptosis induced by TNF-α (data not shown), demonstrating that in bone marrow macrophages the apoptosis induced by TNF-α is not mediated through the production of NO.
LPS induces the autocrine secretion of TNF-, which produces apoptosis.
(A) LPS induces the mRNA expression of TNF-α. Total RNA (20 μg per lane) from macrophages was treated with 100 ng/mL of LPS for the indicated times was analyzed by Northern blotting. (B) LPS induces the secretion of TNF-α. The concentration of TNF-α in the culture supernatants was analyzed by ELISA. Each experiment was performed 3 times and represented as the mean value ± SD. (C) TNF-α induces apoptosis in bone marrow macrophages. Macrophages were stimulated for 12 hours with the indicated concentrations of rmTNF-α. Induction of apoptosis was measured by ELISA. (D) Time course of rmTNF-α–induced apoptosis. Macrophages were treated with rmTNF-α (100 ng/mL) for the indicated periods of time. Each experiment was performed in triplicate and represented as the mean ± SD, and 1 of 3 independent experiments is shown in this figure.
LPS induces the autocrine secretion of TNF-, which produces apoptosis.
(A) LPS induces the mRNA expression of TNF-α. Total RNA (20 μg per lane) from macrophages was treated with 100 ng/mL of LPS for the indicated times was analyzed by Northern blotting. (B) LPS induces the secretion of TNF-α. The concentration of TNF-α in the culture supernatants was analyzed by ELISA. Each experiment was performed 3 times and represented as the mean value ± SD. (C) TNF-α induces apoptosis in bone marrow macrophages. Macrophages were stimulated for 12 hours with the indicated concentrations of rmTNF-α. Induction of apoptosis was measured by ELISA. (D) Time course of rmTNF-α–induced apoptosis. Macrophages were treated with rmTNF-α (100 ng/mL) for the indicated periods of time. Each experiment was performed in triplicate and represented as the mean ± SD, and 1 of 3 independent experiments is shown in this figure.
So far we have shown that LPS induces TNF-α production and that TNF-α induces apoptosis in macrophages. LPS-induced secretion of TNF-α and apoptosis occurred almost simultaneously. Therefore, we wanted to determine the role of the autocrine production of TNF-α in the LPS-induced apoptosis of BMDM. For these experiments, we used macrophages from mice with both TNF-α receptors disrupted by genetic recombination (TNF-αR KO).46 47 The data presented were obtained from TNF-αRI/II double KO mice. Although not shown, most experiments were repeated with mice with the single type I TNF-α receptor disrupted, from which identical results were obtained.
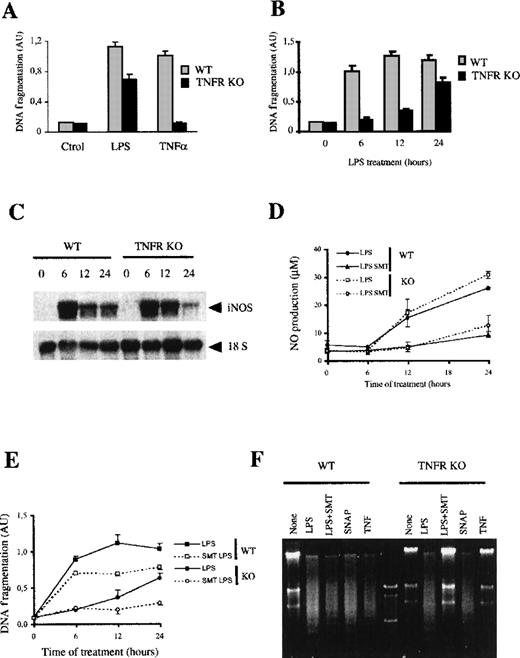
Macrophages from the TNF-αR KO mice did not undergo apoptosis on exposure to TNF-α (Figure 6A). Unlike exogenous TNF-α, LPS induced significant apoptosis in these macrophages (Figure 6A). After 24 hours of LPS treatment, induction of apoptosis in the TNF-αR KO macrophages was only 36% lower than that observed in the control mice. However, the time course of apoptosis induction was very different. LPS-induced apoptosis within the first 6 hours in macrophages from normal mice, whereas induction of apoptosis in the TNF-αR KO mice only started at 12 hours and increased up to 24 hours. These results suggest that in wild-type macrophages treated with LPS, the autocrine production of TNF-α is the major mediator of early apoptosis.
LPS-induced apoptosis in macrophages from TNF-R KO mice.
(A) LPS, but not TNF-α, induces apoptosis in macrophages from TNF-αR KO mice. Macrophages (105) from either wild-type or TNF-αR KO mice were treated for 24 hours with LPS (100 ng/mL) or TNF-α (100 ng/mL). DNA fragmentation was evaluated by measuring histone-associated DNA fragments by ELISA. (B) Time course of LPS-induced apoptosis in macrophages from TNF-αR KO mice. Cells from control and KO mice were treated with LPS (100 ng/mL) for the indicated periods of time. Apoptosis was determined as indicated previously. (C) Macrophages from TNF-αR KO mice express iNOS in response to LPS. Macrophages were treated with 100 ng/mL of LPS for the indicated times; 20 μg of total RNA per lane was analyzed by Northern blotting. (D) Production of NO in macrophages from TNF-αR KO mice. The production of NO was measured in cultures of macrophages from each group of mice stimulated with 100 ng/mL of LPS for the indicated times in the presence or absence of 20 μmol/L SMT. (E) SMT blocks LPS-induced apoptosis in macrophages from TNF-αR KO mice but not in control macrophages. Macrophages (105) from control and KO mice were treated with LPS (100 ng/mL) for the indicated periods of time in the presence or absence of SMT (20 μmol/L). Apoptosis was determined by ELISA. Each experiment was performed in triplicate and represented as the mean ± SD. (F) LPS-induced apoptosis in macrophages from TNF-αR KO mice is mediated by NO production. DNA fragmentation was analyzed in a 2% agarose gel electrophoresis. Macrophages of each group were treated with 100 ng/mL of LPS in the presence or absence of either 20 μmol/L SMT (iNOS inhibitor), 50 μmol/L SNAP (NO donor), or 100 ng/mL rmTNF-α.
LPS-induced apoptosis in macrophages from TNF-R KO mice.
(A) LPS, but not TNF-α, induces apoptosis in macrophages from TNF-αR KO mice. Macrophages (105) from either wild-type or TNF-αR KO mice were treated for 24 hours with LPS (100 ng/mL) or TNF-α (100 ng/mL). DNA fragmentation was evaluated by measuring histone-associated DNA fragments by ELISA. (B) Time course of LPS-induced apoptosis in macrophages from TNF-αR KO mice. Cells from control and KO mice were treated with LPS (100 ng/mL) for the indicated periods of time. Apoptosis was determined as indicated previously. (C) Macrophages from TNF-αR KO mice express iNOS in response to LPS. Macrophages were treated with 100 ng/mL of LPS for the indicated times; 20 μg of total RNA per lane was analyzed by Northern blotting. (D) Production of NO in macrophages from TNF-αR KO mice. The production of NO was measured in cultures of macrophages from each group of mice stimulated with 100 ng/mL of LPS for the indicated times in the presence or absence of 20 μmol/L SMT. (E) SMT blocks LPS-induced apoptosis in macrophages from TNF-αR KO mice but not in control macrophages. Macrophages (105) from control and KO mice were treated with LPS (100 ng/mL) for the indicated periods of time in the presence or absence of SMT (20 μmol/L). Apoptosis was determined by ELISA. Each experiment was performed in triplicate and represented as the mean ± SD. (F) LPS-induced apoptosis in macrophages from TNF-αR KO mice is mediated by NO production. DNA fragmentation was analyzed in a 2% agarose gel electrophoresis. Macrophages of each group were treated with 100 ng/mL of LPS in the presence or absence of either 20 μmol/L SMT (iNOS inhibitor), 50 μmol/L SNAP (NO donor), or 100 ng/mL rmTNF-α.
Apoptosis mediated by autocrine TNF- or endogenous NO is independent event in LPS-stimulated macrophages
To determine if the apoptosis mediated by the autocrine production of TNF-α is due to the production of NO, we studied the induction of iNOS and the secretion of NO in macrophages from TNF-αR KO mice. In these macrophages, LPS induced a pattern of expression of iNOS mRNA and NO production similar to that in control macrophages (Figure 6C-D). Furthermore, treatment with SMT also inhibited the production of NO to the same degree in both populations of macrophages (Figure 6D). These results suggest that in BMDM the autocrine secretion of TNF-α is not involved in the control of NO production in response to LPS. Moreover, the treatment with rmTNF-α did not induce NO secretion in control macrophages (data not shown).
The role of NO induction in late apoptosis was also tested by adding the iNOS inhibitor SMT. In the presence of this inhibitor, the LPS-induced apoptosis in macrophages from TNF-αR KO mice decreased 79% (Figure 6E). In control macrophages treated with LPS for 24 hours, SMT only resulted in a 23% reduction of apoptosis. These results were also confirmed by agarose electrophoresis of the apoptotic DNA (Figure6E). Therefore, these results suggest that TNF-α secretion by LPS plays a major role in the induction of early apoptosis, whereas NO production induced by LPS only induces the late apoptotic events. In control macrophages, LPS and TNF-α, induced the typical DNA laddering associated with apoptosis, which was not blocked by SMT. In contrast, LPS, but not TNF-α, induced DNA fragmentation in macrophages from TNF-αR KO mice. In this case, SMT totally blocked the effect of LPS, thus suggesting that the LPS-induced apoptosis of TNF-αR KO macrophages was mediated only through NO production.
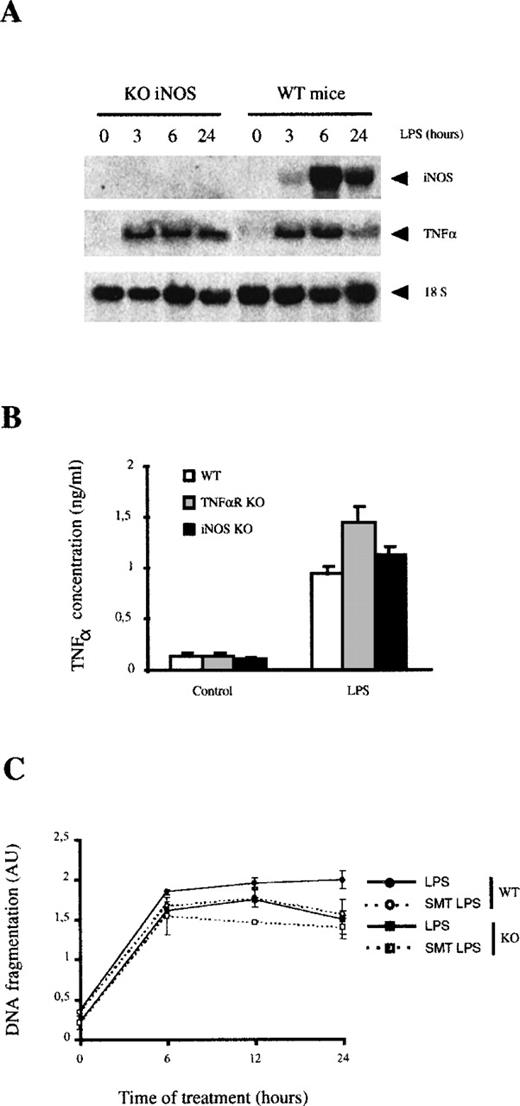
We also analyzed the apoptosis induced in macrophages from iNOS KO mice. After LPS stimulation, these cells expressed similar levels of TNF-α mRNA (Figure 7A) and secreted similar amounts of this cytokine than macrophages from wild-type or TNF-αR KO mice (Figure 7B). The apoptosis induced by LPS in macrophages from iNOS KO mice was almost identical to that observed in macrophages from control mice and followed similar kinetics (Figure7C). Only a slight reduction of apoptosis was observed after 24 hours of LPS treatment (26% reduction), at a time when NO-mediated apoptosis is significant in normal macrophages treated with LPS.
LPS-induced apoptosis in macrophages from iNOS KO mice.
(A) LPS induces TNF-α expression in iNOS KO macrophages. The expression of iNOS and TNF-α mRNA was analyzed by Northern blotting. (B) LPS induces TNF-α expression in iNOS KO macrophages. The secretion of TNF-α was analyzed by ELISA in macrophage cultures from each group of mice. Each experiment was performed 3 times and represented as the mean value ± SD. (C) LPS induced similar rates of apoptosis in macrophages from iNOS KO and in control macrophages. Macrophages (105) from control and KO mice were treated with LPS (100 ng/mL) for the indicated periods of time in the presence or absence of SMT (20 μmol/L). Apoptosis was determined by ELISA. Each experiment was performed in triplicate and represented as the mean ± SD.
LPS-induced apoptosis in macrophages from iNOS KO mice.
(A) LPS induces TNF-α expression in iNOS KO macrophages. The expression of iNOS and TNF-α mRNA was analyzed by Northern blotting. (B) LPS induces TNF-α expression in iNOS KO macrophages. The secretion of TNF-α was analyzed by ELISA in macrophage cultures from each group of mice. Each experiment was performed 3 times and represented as the mean value ± SD. (C) LPS induced similar rates of apoptosis in macrophages from iNOS KO and in control macrophages. Macrophages (105) from control and KO mice were treated with LPS (100 ng/mL) for the indicated periods of time in the presence or absence of SMT (20 μmol/L). Apoptosis was determined by ELISA. Each experiment was performed in triplicate and represented as the mean ± SD.
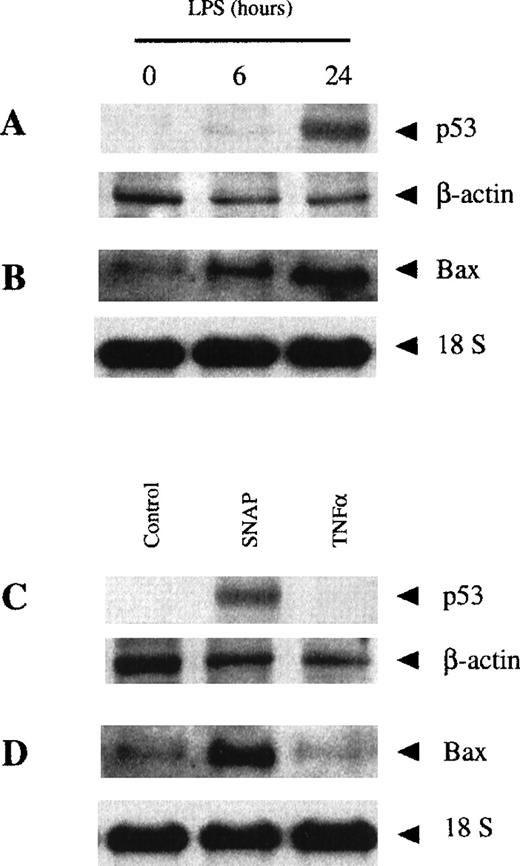
Finally, we were interested in determining the signaling mechanisms involved in the induction of macrophage apoptosis by either TNF-α or NO. Thus, we analyzed the expression of p53 and Bax, 2 genes involved in several apoptotic processes.58-61 The expression of p53 and Bax was detected after 24 hours of treatment with LPS, but not after 6 hours (Figure 8A-B). Therefore, the early apoptotic events (those that take place after 6 hours of treatment with LPS) occurred in the absence of p53 and Bax induction. However, the expression of these pro-apoptotic genes took place at the time at which macrophage apoptosis is dependent on NO production (24 hours of LPS stimulation). Therefore, we determined whether the expression of these genes could be induced by treatment with a NO donor or TNF-α in macrophages. Treatment with the NO donor SNAP, but not with recombinant TNF-α, induced the expression of p53 and Bax (Figure 8C-D). These results show that the early (TNF-α–dependent) and late (NO-dependent) mechanisms induced by LPS to produce apoptosis follow 2 different independent pathways.
NO-dependent, but not TNF-–dependent, LPS-induced apoptosis was due to p53 and Bax expression.
Macrophages from control mice were treated with LPS (100 ng/mL) for the indicated periods of time. Expression of p53 was analyzed by Western blotting (A), whereas expression of Bax mRNA was analyzed by Northern blotting (B). The expression of p53 (C) and Bax (D) was analyzed in macrophages treated with either 50 μmol/L SNAP or 100 ng/mL rmTNF-α for 24 hours. Northern and Western blotting were performed as described in the “Materials and methods” section.
NO-dependent, but not TNF-–dependent, LPS-induced apoptosis was due to p53 and Bax expression.
Macrophages from control mice were treated with LPS (100 ng/mL) for the indicated periods of time. Expression of p53 was analyzed by Western blotting (A), whereas expression of Bax mRNA was analyzed by Northern blotting (B). The expression of p53 (C) and Bax (D) was analyzed in macrophages treated with either 50 μmol/L SNAP or 100 ng/mL rmTNF-α for 24 hours. Northern and Western blotting were performed as described in the “Materials and methods” section.
Discussion
Endotoxic shock is a potentially lethal complication of systemic infection by gram-negative bacteria.5 6 The toxin responsible for the induction of endotoxic shock is the glycolipid LPS, the major component of the gram-negative bacterial wall. The release of LPS into the circulation activates a series of tissue responses that in their most severe forms lead to septic shock and death. Tissue macrophages play a major role in the generation of the endotoxic response. NO production and TNF-α secretion produced by these cells have been proposed as the primary mediators of this event.
Although an enhanced generation of NO by iNOS has been involved in the pathophysiology of septic shock,9,11 the inhibition of iNOS in rodent and human models for sepsis and the analysis of iNOS KO mice has produced conflicting results.17-24 Moreover, the involvement of TNF-α secretion in septic shock came from observations that antibodies or soluble receptors against TNF-α inhibited the deleterious actions of LPS during sepsis.27-29 However, the use of TNF-αR KO mice as an experimental model also gave contradictory results. Although mice deficient for the p55 kd TNF-αR seemed to be resistant to endotoxic shock, they still succumbed to bacterial infection.40
We have analyzed the role of both NO and TNF-α secretion in the LPS-induced apoptosis in BMDM. Our results demonstrate that LPS induces apoptosis in macrophages by 2 independent mechanisms: one is mediated by the autocrine production of TNF-α and the other is triggered by the production of NO. Although both mechanisms are involved in the apoptosis induced by LPS, they act independently, with different kinetics, and through separate pathways (Figure 8).
The cytotoxic effects of TNF-α in most cell types are only evident when RNA or protein synthesis are inhibited, suggesting that de novo RNA or protein synthesis protects cells from TNF-α cytotoxicity, probably by the induction of protective genes.62-64 In bone marrow macrophages (as is the case in other inflammatory cell types, including neutrophils and granulocytes or in endothelial cells and oligodendrocytes), TNF-α alone is sufficient to induce DNA fragmentation and cell death by apoptosis.43 65-67
The TNF-α signal is transduced by 2 distinct cell surface receptors, TNF-αRI and TNF-αRII.32,34 In this work we have reported the experiments performed with macrophages from the TNF-αRI/II double KO mice,47 but experiments using macrophages from the TNF-αRI KO mice46 produced identical results. Thus, this confirms that Type I p55 TNF-α receptor mediated the TNF-α-induced apoptosis in macrophages.33,36 37
TNF-α did not induce the expression of iNOS or NO production in macrophages.68 Macrophages from TNF-αR KO mice showed levels of LPS-induced expression of iNOS and NO production similar to those measured in control macrophages. In fact, recombinant TNF-α did not induce NO production in macrophages from control mice. Moreover, TNF-α-dependent apoptosis was not blocked by the iNOS inhibitor SMT or in macrophages from iNOS KO mice. Therefore, we conclude that the apoptosis induced by the autocrine production of TNF-α is independent of the production of NO. These results are in disagreement with other previous observations69 70 in which TNF-α has been associated to the production of NO.
Other studies71-73 have clarified the mechanism by which the 55 kDa TNF-α receptor signals toward the apoptotic response. This receptor contains a carboxy-terminal death-domain that appears to be required for the transmission of the apoptotic signal. Binding of TNF-α to the receptor triggers the formation of a multiprotein complex in which cytoplasmic proteins and the receptor interact through their respective death-domain motifs. On TNF-α stimulation, the receptor death domain binds to the death domain of a cytoplasmic protein called TRADD (TNF receptor I-associated death domain), which in turn binds to the death domain of another cytoplasmic protein, termed FADD/MORT-1. This protein also contains a death effector domain (DED) motif, which binds to the DED motif of ICE/Ced-3 protease FLICE/MACH-1 (Caspase 8). It has been suggested that activation of Caspase 8 initiates the activation of a cascade of caspases, which is the effector system for the apoptotic destruction of the cell. This model suggests that ligand binding to the TNF-α receptor activates the final death effector pathway apparently without any second messengers.
Besides, the LPS-induced apoptosis that is mediated by the production of NO occurs slowly and uses a different signaling pathway. In bone marrow macrophages, NO-dependent apoptosis correlated with the expression of p53 and Bax. Probably the DNA alterations induced by increasing levels of NO induced the expression of p53.61Moreover, the p53 expression induced by LPS in macrophages has been observed that could be blocked by iNOS inhibitors, such as arginine analogs.74 p53 regulates the transcription of the Bcl-2-related pro-apoptotic gene Bax.75 The expression of Bax is sufficient to produce the release of cytochrome C from the mitochondria76 and the activation of the mitochondrial apoptotic pathway,77 78 leading to apoptosis.
In summary, we have shown that LPS-induced apoptosis is mediated mostly through the autocrine production of TNF-α. However, when this pathway is inhibited, the apoptosis induced by LPS occurs through the induction of NO. The existence of 2 independent pathways activated by LPS may explain the inefficiency of several strategies directed against one of these mechanisms to prevent the deleterious effects of LPS during endotoxic shock. This finding underscores that salvage from ongoing septic shock may require the simultaneous interruption of more than one final pathway, each of them lethal for the host organism.
Acknowledgments
We thank Dr Antonio Felipe (University of Barcelona, Spain), Dr Ramon Merino (University of Cantabria, Santander, Spain), and Dr M. Nabholz (ISREC, Switzerland) for their gift of cDNAs used in this work. We are also grateful to Dr K. Matsushima (University of Kanazawa, Japan) and to Dr J. Peachon (University of Kentucky, USA) for the generous gift of the TNF-αR I and I/II double KO mice. We acknowledge the help received from Jaume Comas and Rosario González from the flow cytometry facility of the Serveis Cientı́fico Tècnics de la Universitat de Barcelona. We also thank Martı́ Cullell-Young for his editorial assistance.
Supported by grants from CICYT (SAF98-102 and PM 98/0200) to A.C. and by the Deutsche Forschungsgemeinschaft to C.B. (SFB 263, A5). J.X. and A.F.V. are recipients of a fellowship from the ComisióInterdepartamental de Recerca i Innovació Tecnològica (CIRIT). M.C. is recipient of a fellowship from Fundació August Pi i Sunyer.
J.X. and M.C. contributed equally to this work.
Reprints:Antonio Celada, Departament de Fisiologia, Facultat de Biologia, Av. Diagonal 645, 08028 Barcelona, Spain; e-mail:acelada@bio.ub.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal