Abstract
To explore the activation patterns of signal transducer and activator of transcription 3 (Stat3) in acute myeloid leukemia (AML), we examined whether the phosphorylation of tyrosine705 (Tyr705) and serine727 (Ser727) residues was abnormally regulated in cells from patients with AML. In 5 of 20 (25%) patients with AML, Stat3 was constitutively phosphorylated on Tyr705 and Ser727, which were not further up-regulated by treatment with IL-6. Furthermore, Stat3 was constitutively bound to the IRE response element in these cells as determined by electrophoretic mobility shift assay, and stimulation with IL-6 did not result in increased DNA binding. Interestingly, AML cells with constitutive Stat3 activation also secreted high levels of IL-6 protein. Treating these AML cells with anti-IL-6 resulted in restored IL-6–inducible Stat3 phosphorylation on both Tyr705 and Ser727 with low or undetectable basal phosphorylation levels in unstimulated cells. In contrast, treatment with anti-IL-1 did not result in altered Stat3 phosphorylation patterns. The constitutive IL-6 expression was associated with elevated levels of suppressor of cytokine signaling-1 (SOCS-1) and SOCS-3 mRNA expression, which were not down-regulated by anti-IL-6. These data indicate that the constitutive Stat3 activation in the investigated AML blasts is caused by high IL-6 secretion levels, thus stimulating the Jak/Stat pathway in an autocrine manner, a paracrine manner, or both.
Acute myeloid leukemia (AML) is characterized by an accumulation of immature blasts in the bone marrow, resulting in a disturbed production of normal hematopoietic cells.1Although little is known about the precise mechanisms of pathogenesis at the molecular level of this disease, AML is often associated with chromosomal translocations and inversions, affecting gene expression in ways that lead to defects in normal programs of cell proliferation, differentiation, and survival.2-5 The most frequent targets of chromosomal translocations are transcription factors that result in the recombination of normally unrelated sequences from different chromosomes into hybrid genes that encode fusion products with altered function.2,4 However, the chromosomal translocations and inversions found in patients with AML are highly divergent, and the precise molecular defects in AML still have to be elucidated.2
IL-6 is a pleiotropic cytokine that can be constitutively expressed in AML cells.6 It initiates its action by binding to its receptor, composed of 2 subunits: an 80-kd IL-6 binding protein and a 130-kd transmembrane signal-transducing component (gp130).7-9 The gp130 receptor protein is also used by other members of the IL-6 cytokine family, including IL-11, oncostatin M, leukemia inhibitory factor, and ciliary neurotrophic factor.10-12 Activation of IL-6 signal transduction involves gp130 dimerization, ligand-dependent tyrosine phosphorylation of the gp130-associated protein–tyrosine kinases Jak1, Jak2, and Tyk2, and tyrosine phosphorylation of signal transducer and activator of transcription 3 (Stat3).13 Tyrosine phosphorylation of Stat3 occurs at a single-residue tyrosine residue (Tyr705) located in a conserved SH2 domain, allowing Stat dimerization and transcription activation.13 In addition to tyrosine phosphorylation, Stat3 is serine phosphorylated at a single residue (Ser727) in response to IL-6 and to other extracellular factors, including interferon-γ (IFN-γ) and epidermal growth factor.14,15 Recently, it was demonstrated that maximal Stat3 transactivation requires phosphorylation on a unique serine residue, Ser727, and that IL-6 induces Ser727 phosphorylation of Stat3 by a signal transduction cascade involving Vav, Rac-1, MEKK, and SEK.16
Recently, a family of cytokine-inducible inhibitors of signaling was identified that down-regulates the Jak/Stat signaling pathway.17-19 The proteins in this family, including cytokine-inducible SH-2–containing protein and SOCS/Jak-binding protein/Stat-induced Stat inhibitor proteins, are proteins containing SH2 domains that interact with Jak, thus preventing the activation of Stat.17-19 Specifically, SOCS-1 and SOCS-3 are implicated in the down-regulation of the IL-6–induced activation of Stat3.20-23 Moreover, SOCS-1 and SOCS-3 can quickly be up-regulated by IL-6.18,19 21
However, the activation of Stat has not only been implicated in gp130 receptor downstream signaling, it may be caused by oncogene activation. Abnormal activation of Stat1, Stat3, Stat5, and Stat6 has been demonstrated in cells transformed by Src, Abl, and various other oncoproteins and tumor viruses.24-32 In addition, in acute leukemia a spontaneous activation of Stat has been observed. Constitutive DNA binding of Stat1 and Stat5 was found in acute lymphocytic leukemia, whereas constitutive DNA binding and tyrosine phosphorylation of Stat1, Stat3, and Stat5 were detected in several patients with AML.25,33-35 Furthermore, constitutive Stat1 and Stat3 serine phosphorylation has been found in some patients with AML and many patients with chronic lymphocytic leukemia.36 Although it has not been demonstrated that constitutive Stat3 activation is contributive in the development of leukemias, the consistent finding of abnormal Stat3 activation in these cells suggests that Stat might fulfill a role in the ongoing process of transformation. Recently, it was demonstrated that Stat3 plays a key role in G1- to S-phase cell-cycle transition through the up-regulation of cyclins D2, D3, A, and cdc25A and the concomitant down-regulation of p21 and p27.37 Thus, constitutive Stat3 activation might lead to a growth advantage of the malignant counterpart.
Here, we report that constitutive activation of Stat3 is observed in 25% of the investigated patients with AML, caused by autocrine secretion of IL-6, thus leading to continuous activation of the Jak/Stat pathway. The high expression levels of IL-6 protein are also associated with the increased expression of SOCS-1 and SOCS-3 mRNA. Blocking the action of secreted IL-6 by treatment with anti-IL-6 leads to a loss of constitutive Stat3 phosphorylation and normal IL-6–induced Stat3 activation patterns.
Materials and methods
Patient population and isolation of acute myeloid leukemia cells
Peripheral blood cells from 20 patients with AML were studied after informed consent was obtained. Patients with AML were defined according to the classification of the FAB committee as M1 to M6 (Table1).38 AML blasts were isolated by density-gradient centrifugation, as described.39 Cells were cryopreserved in aliquots of 20 to 50 × 106cells/mL in 10% dimethyl sulfoxide (Sigma, St. Louis, MO) and 10% FCS (Hyclone, Logan, UT) using a method of controlled freezing and storage in liquid nitrogen. After thawing, T-lymphocytes were depleted by 2-aminoethylisothioronium bromide-treated sheep red blood cell rosetting. The cell population consisted of more than 98% AML blasts as determined by May–Grünwald–Giemsa staining. Fluorescence-activated cell sorting analysis showed less than 1% CD3-positive cells (Becton Dickinson, Sunnyvale, CA).
FAB classifications, phosphorylation patterns, and IL-6 secretion levels of patients with AML
| Patient . | FAB . | Constitutive Tyr705 and Ser727 phosphorylation . | IL-6 secretion (pg/mL) . |
|---|---|---|---|
| 1 | M2 | + | 5924 |
| 2 | M4 | − | 0 |
| 3 | M0 | − | 323 |
| 4 | M4 | − | 0 |
| 5 | M4 | − | 0 |
| 6 | M2 | − | 0 |
| 7 | M1 | − | 0 |
| 8 | M4 | − | 0 |
| 9 | M5A | − | 0 |
| 10 | M2 | − | 0 |
| 11 | M5A | + | 23667 |
| 12 | M1 | − | 0 |
| 13 | M1 | − | 0 |
| 14 | M5 | + | 4058 |
| 15 | M4 | − | 0 |
| 16 | M2 | − | 0 |
| 17 | M2 | − | 0 |
| 18 | M0 | + | 7040 |
| 19 | M2 | + | 946 |
| 20 | M2 | − | 208 |
| Patient . | FAB . | Constitutive Tyr705 and Ser727 phosphorylation . | IL-6 secretion (pg/mL) . |
|---|---|---|---|
| 1 | M2 | + | 5924 |
| 2 | M4 | − | 0 |
| 3 | M0 | − | 323 |
| 4 | M4 | − | 0 |
| 5 | M4 | − | 0 |
| 6 | M2 | − | 0 |
| 7 | M1 | − | 0 |
| 8 | M4 | − | 0 |
| 9 | M5A | − | 0 |
| 10 | M2 | − | 0 |
| 11 | M5A | + | 23667 |
| 12 | M1 | − | 0 |
| 13 | M1 | − | 0 |
| 14 | M5 | + | 4058 |
| 15 | M4 | − | 0 |
| 16 | M2 | − | 0 |
| 17 | M2 | − | 0 |
| 18 | M0 | + | 7040 |
| 19 | M2 | + | 946 |
| 20 | M2 | − | 208 |
Cell culture, reagents, and antibodies
HepG2 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS). AML blasts were cultured at 37°C at a density of 2 × 106/mL in RPMI 1640 media (Flow, Rockville, MD) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FCS. For reverse transcription–polymerase chain reaction (RT–PCR) analysis, cells from patient 11 with AML were cultured for 24 hours in RPMI 1640 and the supernatant was collected, and this was used to culture cells from patient 7 with AML, as indicated in the text. Cells were stimulated with 10 ng/mL human recombinant IL-6 (a generous gift from Dr S. C. Clark, Genetics Institute, Cambridge, MA) or 10 ng/mL IFN-γ (Endogen, Woburn, MA). Antibodies against Stat3 (C-20 and K-15; Santa Cruz Technology, Santa Cruz, CA) were used in dilutions of 1:4000. Antibodies against Stat3 (Tyr705) and Stat3 (Ser727) were obtained from New England BioLabs and were used in a 1:1000 dilution. Anti-IL-6 was a gift from L. van Aarden (CLB, Amsterdam, The Netherlands) and was used in a dilution of 1:1000, which was shown to inhibit the biologic activity of more than 100 ng/mL IL-6 (data not shown). Anti-IL-1α was a gift from S. Gillis (Immunex, Seattle, WA), and anti-IL-1β was purchased from R&D Systems (Minneapolis, MN). Both antibodies were used in a dilution of 1:250.
Western blotting
Cells were plated in 12-well culture plates, stimulated, washed, and collected in phosphate-buffered saline. Cells were lysed in Laemmli sample buffer and boiled for 5 minutes before separation on 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels. The proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Etten-Leur, The Netherlands) in Tris–glycine buffer at 100 mA for 1.5 hours using an electroblotter (Pharmacia, Woerden, The Netherlands). Membranes were blocked with phosphate-buffered saline containing 5% nonfat milk before incubation with antibodies. Binding of each antibody was detected by HRP labeled pig-anti-rabbit secondary antibodies using enhanced chemiluminescence (ECL) according to the manufacturer's recommendations (Amersham, Roosendaal, The Netherlands). Western blots were quantified using Image-Pro (Media Cybernetics, Silver Spring, MD).
Electrophoretic mobility shift assay
Nuclear extracts were prepared from 107 cells, as described previously, according to the rapid Dignam method.40 A double-stranded synthetic oligo comprising the IL-6RE of the ICAM-1 promoter (upper strand: 5′-CGCGTAGCTTAGGTTTCCGGGAAAGCACG-3′) was32P-labeled by filling in the 5′-protruding ends with α32P-dATP and Klenow enzyme. Five micrograms nuclear extract was incubated with 20 000 cpm-labeled probe for 20 minutes at 26°C, and gel retardation analysis was performed on native 4% polyacrylamide gels in 0.5 × TBE. In supershift experiments, 1 μL anti-Stat3 antibodies (C-20 supershift reagent; Santa Cruz Technologies) or anti-Stat1 antibodies (Transduction Laboratories, Lexington, KY) were added.
RNA extraction and RT–PCR
For RT–PCR, total RNA was isolated from 107 cells using Trizol according to the manufacturer's recommendations (GIBCO Life Technologies). Three micrograms RNA per sample was reverse transcribed with M–MuLV reverse transcriptase (Boehringer Mannheim, Almere, The Netherlands). For PCR, 2 μL cDNA was amplified using β-2–globulin primers (forward: 5′-CCAGCAGAGAATGGAAAGTC-3′; reverse: 5′-GATGCTGCTTACATGTCTCG), SOCS-1 primers (forward: 5′-CACGCACTTCCGCACATTCC-3′; reverse: 5′-TCCAGCAGCTCGAAGAGGCA-3′), or SOCS-3 primers (forward: 5′-TCACCCACAGCAAGTTTCCCGC-3′; reverse: 5′-GTTGACGGTCTTCCGACAGAGATGC-3′) in a total volume of 50 μL using 2 U Taq polymerase (Boehringer Mannheim). After 25 cycles, 15-μL aliquots were run on 1.5% agarose gels.
IL-6 secretion
Then 2 × 106 cells were plated in 1 mL RPMI 1640 containing 10% FCS and treated with anti-IL-6 antibodies as indicated. After 24 hours, cell-free supernatants were obtained by centrifugation of the suspension. IL-6 protein levels were measured using the commercially available enzyme-linked immunosorbent assay according to the manufacturer's recommendations (CLB, Amsterdam, The Netherlands)
Statistics
For IL-6 secretion, experiments were performed in triplicate, and differences between groups were tested for significance using the 2-tailed t test. P < .05 was considered significant.
Results
Constitutive and non-IL-6–inducible Stat3, Tyr705, and Ser727 phosphorylation and DNA binding in AML cells
To assess the phosphorylation status of Stat3 in AML cells, blasts of 20 untreated patients were cultured in RPMI 1640 containing 10% FCS and either were left unstimulated or were stimulated with 10 ng/mL IL-6 for 15 minutes. Total cell extracts were subjected to Western blot analysis, and Stat3 was visualized using specific antibodies against phosphorylated Stat3 on Tyr705 and Ser727. Of 20 patients under investigation, 5 (25%) showed constitutive Stat3 tyrosine and serine phosphorylation, which was not further up-regulated by stimulation with IL-6 (Figure 1; patients 1, 11, 14, 18, 19). Fifteen patients showed normal transient IL-6–induced Stat3 phosphorylation patterns with similar kinetics as in HepG2 cells (Figure 1). Interestingly, the expression levels of Stat3 varied strongly among the AML samples. In addition, there was a strong variation in IL-6–induced Stat3 phosphorylation levels; in patients with AML with constitutive Stat3 activation, the degree of phosphorylation was relatively low (Figure 1).
Constitutive and non-IL-6–inducible Stat3 phosphorylation patterns are observed in 25% of the investigated patients with AML.
AML cells and HepG2 cells were plated in 12-well culture plates, either unstimulated or stimulated with 10 ng/mL IL-6 for 15 minutes (AML) or for 5 to 60 minutes (HepG2), and equal amounts of total cell lysates were subjected to SDS–PAGE followed by Western blot analysis. Phosphorylated Stat3 was visualized using phospho-specific antibodies recognizing Tyr705- and Ser727-phosphorylated Stat3. As a control, equal amounts of total cell lysates were Western blotted using antibodies against Stat3 (K-15).
Constitutive and non-IL-6–inducible Stat3 phosphorylation patterns are observed in 25% of the investigated patients with AML.
AML cells and HepG2 cells were plated in 12-well culture plates, either unstimulated or stimulated with 10 ng/mL IL-6 for 15 minutes (AML) or for 5 to 60 minutes (HepG2), and equal amounts of total cell lysates were subjected to SDS–PAGE followed by Western blot analysis. Phosphorylated Stat3 was visualized using phospho-specific antibodies recognizing Tyr705- and Ser727-phosphorylated Stat3. As a control, equal amounts of total cell lysates were Western blotted using antibodies against Stat3 (K-15).
Nuclear extracts were isolated from 3 AML samples (Figure2; patients 17, 18, 19), and Stat3 DNA binding was studied on the IRE from the ICAM-1 promoter. The AML cells characterized by IL-6– induced Stat3 Tyr705 phosphorylation also demonstrated IL-6– induced Stat3 DNA binding. Supershift analysis demonstrated that the complexes bound to the IRE in response to 10 ng/mL IL-6 were composed of Stat3 but not of Stat1. In contrast, stimulation with 10 ng/mL IFN-γ strongly induced Stat1 DNA binding. Constitutive Stat3 Tyr705 correlated well with constitutive DNA binding, which could not be further up-regulated by IL-6. Finally, Western blotting experiments using antibodies recognizing Tyr701– phosphorylated Stat1 demonstrated no constitutive or IL-6–induced Stat1 phosphorylation (data not shown). Taken together, these data demonstrated that approximately 25% of the patients with AML were characterized by a constitutive activation of Stat3 not inducible by IL-6.
Electrophoretic mobility shift assay of AML samples.
AML cells (patients 17, 18, 19) were either unstimulated or stimulated with 10 ng/mL IL-6 or IFN-γ as indicated. Nuclear extracts were isolated and analyzed for Stat3 binding activity by electrophoretic mobility shift assay using a 32P-labeled IRE probe from the human ICAM promoter. Supershift assays were performed using antibodies against Stat1 and Stat3, and in competition experiments a 100-fold molar excess of IRE or an aspecific probe was used.
Electrophoretic mobility shift assay of AML samples.
AML cells (patients 17, 18, 19) were either unstimulated or stimulated with 10 ng/mL IL-6 or IFN-γ as indicated. Nuclear extracts were isolated and analyzed for Stat3 binding activity by electrophoretic mobility shift assay using a 32P-labeled IRE probe from the human ICAM promoter. Supershift assays were performed using antibodies against Stat1 and Stat3, and in competition experiments a 100-fold molar excess of IRE or an aspecific probe was used.
Constitutive Stat3 activation is correlated with high IL-6 secretion levels
To further investigate the nature of the constitutive Stat3 activation in AML cells, IL-6 protein levels were determined in cell-free supernatants after 24 hours of culture. Patients characterized by constitutive Stat3 phosphorylation and DNA binding demonstrated high levels of spontaneous IL-6 protein secretion (946 to 23 667 pg/mL), whereas AML cells with IL-6 inducible Stat3 activation showed low or no detectable IL-6 protein levels (Table 1).
Treatment of AML cells with anti-IL-6, but not anti-IL-1, restores Stat3 inducibility by IL-6
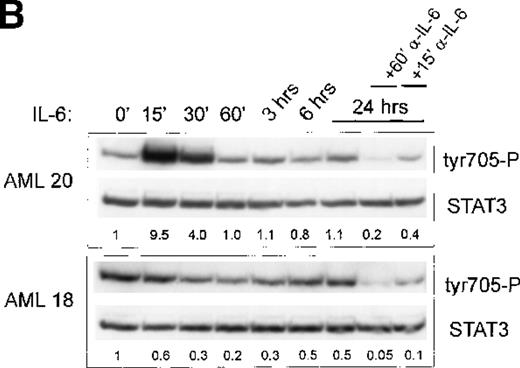
Because AML cells characterized by constitutive Stat3 activation also spontaneously secreted high levels of IL-6 protein into the medium, we questioned whether IL-6 might be responsible for the constitutive Stat3 activation. Subsequently, AML cells (patients 1, 11, 14, 18, 19) were cultured for 24 hours in the absence and presence of anti-IL-6 and were washed; this was followed by IL-6 stimulation for 15 minutes. Pretreatment with anti-IL-6 resulted in inhibition of the basal Tyr705 and Ser727 phosphorylation of Stat3. A representative experiment is shown in Figure 3A. In addition, Stat3 Tyr705 and Ser727 phosphorylations could now be induced by treatment with IL-6 to 18.4-fold and 6.8-fold induction, respectively.
Treatment with anti-IL-6 but not anti-IL-1 blocks constitutive Stat3 phosphorylation and restores IL-6 inducibility.
(A) AML cells (patient 19) were cultured in 12-well plates and pretreated with either anti-IL-6 (1:1000) or left untreated. After 24 hours, cells were washed and were left unstimulated or were stimulated with 10 ng/mL IL-6 for 15 minutes. (B) AML cells (patients 18, 20) were cultured in 12-well plates and stimulated with 10 ng/mL IL-6 for several time periods as indicated. After 24 hours, cells were treated with anti-IL-6 (1:1000) as indicated for 15 or 60 minutes. Equal amounts of total cell lysates were subjected to SDS–PAGE, followed by Western blot analysis as described in A. (C) AML cells (patient 18) cultured in 12-well plates were either pretreated with anti-IL-1 (1:250) overnight or left untreated. After 24 hours, cells were washed and stimulated with 10 ng/mL IL-6 for 15 minutes as indicated. Equal amounts of total cell lysates were Western blotted as described in A.
Treatment with anti-IL-6 but not anti-IL-1 blocks constitutive Stat3 phosphorylation and restores IL-6 inducibility.
(A) AML cells (patient 19) were cultured in 12-well plates and pretreated with either anti-IL-6 (1:1000) or left untreated. After 24 hours, cells were washed and were left unstimulated or were stimulated with 10 ng/mL IL-6 for 15 minutes. (B) AML cells (patients 18, 20) were cultured in 12-well plates and stimulated with 10 ng/mL IL-6 for several time periods as indicated. After 24 hours, cells were treated with anti-IL-6 (1:1000) as indicated for 15 or 60 minutes. Equal amounts of total cell lysates were subjected to SDS–PAGE, followed by Western blot analysis as described in A. (C) AML cells (patient 18) cultured in 12-well plates were either pretreated with anti-IL-1 (1:250) overnight or left untreated. After 24 hours, cells were washed and stimulated with 10 ng/mL IL-6 for 15 minutes as indicated. Equal amounts of total cell lysates were Western blotted as described in A.
To study further the kinetics of Stat3 Tyr705 phosphorylation with longer stimulation of IL-6, in 2 patients AML cells with and without constitutive Stat3 activation were treated with IL-6 for varying time periods from 15 minutes to 24 hours (Figure3B). IL-6 induced a quick and strong up-regulation of Stat3 Tyr705 phosphorylation in patient 20, whose levels returned to low basal levels after 60 minutes. In patient 18, IL-6 did not induce Stat3 Tyr705 phosphorylation within 24 hours. Furthermore, the time-course of the action of anti-IL-6 was investigated in both patients after 24 hours of stimulation with IL-6. As depicted in Figure 3B, 15-minute treatment with anti-IL-6 already reduced Tyr705 phosphorylation, which was completely reduced to low basal levels after 60-minute treatment with anti-IL-6. These data indicate that constitutive Stat3 phosphorylation can quickly be down-regulated by treating the cells with anti-IL-6.
Previously, it was demonstrated that IL-1 is an important cytokine involved in IL-6 production by AML cells.1,41 42Thus, the constitutive secretion of IL-6 may be caused by a spontaneous secretion of IL-1. To investigate this possibility, AML cells (n = 3 patients) with spontaneous phosphorylation of Stat3 were cultured for 24 hours in the presence or absence of anti-IL-1. A typical example is depicted in Figure 3C. Pretreatment with anti-IL-1 for 24 hours did not reduce the spontaneous tyrosine phosphorylation nor restore the IL-6 inducibility of Stat3 phosphorylation, indicating that IL-1 is not responsible for the spontaneous IL-6 protein secretion in the investigated AML cells.
Constitutive Stat3 activation and high IL-6 protein secretion levels correlate with high-expression levels of the Stat inhibitors SOCS-1 and SOCS-3
SOCS proteins represent a family of negative regulators of cytokine signaling that probably switch off the cytokine signal by binding to Jak proteins through SH2 domains, thereby inhibiting the activation of Stat.17-19 To investigate the IL-6–induced up-regulation of SOCS-1 and SOCS-3 in AML cells, we prepared cDNA of unstimulated or IL-6–stimulated cells and performed RT–PCR using specific primers for SOCS-1 and SOCS-3. SOCS-1 and SOCS-3 mRNA levels were low in unstimulated AML cells that were characterized by IL-6–inducible Stat3 phosphorylation and were quickly up-regulated by IL-6 (Figure 4, lanes 4 and 5). In contrast, AML cells characterized by high IL-6 secretion levels and constitutive Stat3 phosphorylation patterns showed high basal levels of SOCS-1 and SOCS-3, whereas exogenous added IL-6 did not further enhance the expression (Figure 4, lanes 1 and 2). Similar results were obtained in RT–PCR analyses for 2 other patients with AML with constitutive Stat3 activation (patients 1 and 14; data not shown). Surprisingly, treatment of these AML cells with anti-IL-6 for 2 hours did not result in reduced SOCS-1 and SOCS-3 mRNA levels (Figure 4, lane 3), suggesting that other cytokines able to induce the expression of SOCS-1 and SOCS-3 were secreted by these AML cells. To underscore this possibility, the cell-free supernatant of patient 11 with a constitutive Stat3 activation was collected and added to that of patient 7 without a constitutive Stat3 activation. As demonstrated in Figure 4, culturing the AML cells of patient 7 in this supernatant resulted in high and non-IL-6–inducible levels of SOCS-1 and SOCS-3 expression (Figure 4; lanes 7 to 9), even when it was depleted of IL-6 by applying saturating amounts of anti-IL-6 to the supernatant (Figure 4, lanes 10 to 12). Taken together, these data indicate that in AML cells characterized by high IL-6 secretion levels and constitutive Stat3 phosphorylation, SOCS-1 and SOCS-3 expression is disturbed.
High lL-6 secretion levels correlate with high SOCS1 and SOCS3 expression levels.
AML cells (patients 11, 7) were cultured in 12-well plates and stimulated for 1 hour with IL-6 (lanes 2, 5, 8, 11) or with anti-IL-6 for 2 hours (lanes 3, 6, 9, 12). In lanes 1 to 6, cells were cultured in RPMI 1640 for 24 hours before stimulation. In lanes 7 to 9, cells from patient 7 were cultured in the AML supernatant from patient 11. In lanes 10 to 12, cells from patient 7 were cultured in the AML supernatant from patient 11, which was depleted of IL-6 by adding anti-IL-6. Total RNA was isolated, 3 μg RNA was reverse transcribed with M–MuLV reverse transcriptase, and cDNA was used in a PCR reaction using specific primers for SOCS-1, SOCS-3 or β2-μglobulin as a control.
High lL-6 secretion levels correlate with high SOCS1 and SOCS3 expression levels.
AML cells (patients 11, 7) were cultured in 12-well plates and stimulated for 1 hour with IL-6 (lanes 2, 5, 8, 11) or with anti-IL-6 for 2 hours (lanes 3, 6, 9, 12). In lanes 1 to 6, cells were cultured in RPMI 1640 for 24 hours before stimulation. In lanes 7 to 9, cells from patient 7 were cultured in the AML supernatant from patient 11. In lanes 10 to 12, cells from patient 7 were cultured in the AML supernatant from patient 11, which was depleted of IL-6 by adding anti-IL-6. Total RNA was isolated, 3 μg RNA was reverse transcribed with M–MuLV reverse transcriptase, and cDNA was used in a PCR reaction using specific primers for SOCS-1, SOCS-3 or β2-μglobulin as a control.
Discussion
Although the exact function of IL-6–induced Stat3 signaling in hematopoietic cells is not well defined, it has been suggested that IL-6 plays an important role in the proliferation and survival of early hematopoietic progenitor cells.43,44 In addition, IL-6 signaling results in gene expression patterns important for lineage-restricted differentiation along the myeloid and lymphoid lineages.45-48 Particularly, Stat3 activation has been implicated in macrophage differentiation.49 However, the effects of IL-6 on the growth and survival of AML cells are variable. In most patients, growth-supportive effects of IL-6 are described especially in conjunction with additional cytokines, whereas in some patients a growth-inhibitory effect is observed.50-54Constitutive Stat3 activation has been demonstrated in AML and is described in 15% to 20% of patients.33-36 In the current study, it is demonstrated that in 25% of the patients with AML, constitutive phosphorylation of Tyr705 and Ser727 is observed that is not further inducible by IL-6. It is also demonstrated that constitutive Stat3 phosphorylation is related to the autocrine secretion of IL-6 and that a constitutive non-IL-6– inducible Stat3 activation pattern in AML is correlated with increased expression levels of SOCS1- and SOCS-3.
IL-6–induced Stat3 transactivation involves phosphorylation of Tyr705 and Ser727 residues. Tyrosine phosphorylation allows Stat dimerization, translocation to the nucleus, and binding to target gene promoters. Although it is still unclear how Stat3 Ser727 phosphorylation is linked to transcriptional activation, it has been clearly demonstrated in many cellular settings that this residue is essential for maximal Stat3 transcriptional potential.14,15 It has been speculated that Stat3 Ser727 phosphorylation allows binding of specific cofactors, thus coupling the RNA polymerase II machinery to Stat transcription factors. In blast cells of patients with AML, we found a constitutive Stat3 DNA binding and phosphorylation on both Tyr705 and Ser727 in approximately 25% of the patients, demonstrating that Stat3 is not only constitutively bound to target gene promoters, it also constitutively initiates gene transcription. Recently, it has been demonstrated that Stat3 plays a key role in G1- to S-phase cell-cycle transition through the up-regulation of cyclins D2, D3, A, and cdc25A and the concomitant down-regulation of p21 and p27.37 Thus, the constitutive Stat3 activation might affect proliferation and cell survival, leading to a growth advantage over normal cells. Indeed, constitutive Stat3 activation leads to higher cell survival rates and to lower susceptibility for cytostatic agents (Schuringa J.J., unpublished observations). Similar results have been described in patients with multiple myeloma whereby the constitutive Stat3 activation is associated with an up-regulation of BcL–xL and the concomitant prevention of apoptosis,55 though the cause of the constitutive Stat3 activation might be different for AML cells. Furthermore, Bromberg et al56 demonstrated that a constitutively active Stat3 mutant, in which 2 cysteine residues within the C-terminal loop of the SH2 domain are substituted, induces cellular transformation and strongly up-regulates the expression of cyclin D1, BcL–xL, and c-myc. These data suggest that constitutive Stat3 activation may contribute by multiple mechanisms to the malignant phenotype of cells.
Strikingly, in the investigated AML cells, the constitutive Stat3 phosphorylation was caused by the autocrine secretion of IL-6 because treatment of anti-IL-6 resulted in a restored IL-6 inducibility of Stat3 Tyr705 and Ser727 phosphorylation. Therefore, we conclude that gp130 downstream signaling is normal in the investigated AML cells and that the constitutive Stat3 activation is not linked to oncogene activation, as has been described in cells transformed by Src, Abl, and various other oncoproteins and tumor viruses.24 The cause of the constitutive expression of IL-6 in the selected number of patients with AML was not elucidated, but it seems not to have been caused by IL-1. Previous studies have demonstrated that spontaneous IL-6 secretion was linked to NF-κB DNA binding that could not be blocked by anti-IL-1, suggesting an aberrant function or triggering of the IκB kinases or related proteins.6,57,58 59
The negative feedback loop of SOCS-1 and SOCS-3 expression, which is normally quickly up-regulated by IL-6, was constitutively activated in the patients with AML with constitutive Stat3 phosphorylation. SOCS expression was relatively high, and IL-6 did not further up-regulate SOCS mRNA levels. In line with the elevated SOCS-1 and SOCS-3 expression, the levels of Stat3 phosphorylation were lower in these patients than the IL-6–induced phosphorylation levels in AML cells without a constitutive Stat3 activation. Surprisingly, treating constitutive AML with anti-IL-6 did not result in reduced SOCS-1 and SOCS-3 mRNA levels, whereas Stat3 Tyr705 and Ser727 phosphorylation levels were reduced to basal levels. The discrepancy between both findings seems to be related to the fact that additional cytokines also regulate SOCS expression. It has been demonstrated that, among IL-6, many other cytokines and growth factors can up-regulate the expression of SOCS-1 and SOCS-3, including leukemia inhibitory factor, IL-4, IFN-γ, and granulocyte–colony-stimulating factor.18,19 21 However, it is intriguing that after treatment with anti-IL-6, the IL-6–induced Stat3 phosphorylation was totally restored despite the persistent expression of SOCS-1 and SOCS-3. These findings suggest that the functional activity of the negative feedback loop is not only determined by the degree of expression but may also depend on post-translational modification.
In conclusion, the data demonstrate that the autocrine and paracrine secretion of IL-6 by AML cells causes the constitutive activation of Stat3, which may have important consequences for the growth and survival characteristics of AML cells.
Supported by grant RUG 96-1217 from the Dutch Cancer Foundation.
Reprints:E. Vellenga, Department of Hematology, University Hospital Groningen, Hanzeplein 1, 9713 GZ, Postbus 30001 9700 RB, Groningen, The Netherlands; e-mail: e.vellenga@int.azg.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal