Abstract
CD40 ligand (CD40L)/CD40 interactions play a central role in T-cell–dependent B-cell activation as previously shown by in vitro studies, the phenotype of CD40L knockout mice and the defective expression of CD40L in patients who have X-linked immunodeficiency with hyper-IgM. The distribution of CD40 in cells other than of myeloid and lymphoid lineages has suggested additional functions for this receptor/ligand couple. Here we show that CD40L stimulates myelopoiesis with a noticeable effect on megakaryocytopoiesis in cocultures of hematopoietic progenitor cells and bone marrow stromal cells. These results suggest a mechanism by which T-cell or platelet-associated or soluble CD40L may regulate myelopoiesis.
The interaction of CD40 ligand (CD40L), a type II transmembrane protein that is expressed on activated CD4+ T lymphocytes, with its cognate receptor CD40, constitutively present on B cells, is essential to the T-cell–dependent proliferation and differentiation of B cells.1-3 This is strikingly illustrated by the absence of germinal center, Ig isotype-switching, and B-cell memory in patients who have hyper-IgM syndrome, resulting from mutations in the CD40L gene and a subsequent lack of expression of functional CD40L at the surface of activated T cells.4-6 CD40 is also expressed on monocytes, macrophages, dendritic cells, epithelial cells, fibroblasts, and endothelial cells (ECs), suggesting that it has a broader function in vivo. For example, CD40 triggering on these cells stimulates the production of interleukin-6 (IL-6), IL-8, IL-1β, and tumor necrosis factor α (TNF-α) by monocytes,7 TNFα, IL-8, and macrophage-inhibitory protein-1α by dendritic cells,8,9Granulocyte-macrophage colony-stimulating factor (GM-CSF) by thymic epithelial cells,10 IL-6 by fibroblasts,11,12IL-6, GM-CSF, leukemia inhibitory factor (LIF), and IL-8 by ECs.13 14
As CD40L stimulates the release of cytokines such as GM-CSF, IL-6 or LIF, we asked the question whether CD40L could be a regulator of hematopoiesis through the regulation of hematopoietic growth factor (HGF) biosynthesis by cells in the bone marrow and its environment. In this study, we present evidence that CD40L indirectly stimulates myelopoiesis with a noticeable effect on megakaryocytopoiesis. The up-regulation of the production of flt3-ligand (FL) and thrombopoietin (TPO) are instrumental and essential to these actions.
Materials and methods
Reagents
The complementary DNA (cDNA) encoding the CD40L-CD8α protein (a kind gift of J. Banchereau, Schering Plough Laboratory for Immunological Research [Dardilly, France]) was obtained by Dr C. Van Kooten by fusing the cDNA encoding the extracellular domain of human CD40L in-frame with the cDNA encoding the extracellular part of mouse CD8α as previously described.15 COS cells (5 × 107) were transfected with 50 μg/mL of the expression plasmid, pME18S-CD40L-CD8α, using the diethylaminoethyl (DEAE)-dextran method. After 5 days of culture, supernatant from the COS cells was harvested, spun, filtered through a 0.2 μm-pore size filter, and concentrated by dialysis against high MW polyethylene glycol (MW 35000, Sigma, Saint Quentin Fallavier, France). This recombinant soluble CD40L-CD8α protein was functional as determined by its specific binding to CD40-transfected Jurkat cells, the binding revealed by staining with fluorescein isothiocyanate (FITC)-conjugated rat anti-mCD8α mAb (Boehringer Manheim, Manheim, Germany) and flow cytometric analysis. Concentration of CD40L (measured with a sCD40L enzyme-linked immunosorbent assay [ELISA] kit, Bender Medsystems, Coger, Paris) in the transfected COS cell supernatant ranged from 200 to 400 ng/mL. For cell stimulation, COS cell supernatants were used diluted 10-fold, therefore giving working concentrations ranging from 20 to 40 ng/mL. Blocking anti-CD40 mAb (Mab89) was produced in the Schering-Plough Laboratory for Immunological Research.16
Stromal cells
Endothelial cells from the umbilical cord.
Primary cultures of human umbilical vein endothelial cells (HUVECs) were obtained from freshly collected umbilical cords as originally described.17 No exogenous growth factors were added.
Human bone marrow endothelial cell line.
The transformed bone marrow endothelial cell (TrBMEC) line was derived from primary cultures of human bone marrow endothelial cells (HBMECs) by intranuclear injection into preconfluent cells of a DNA construct consisting of a portion of the human vimentin promotor gene driving the SV 40 early genes (T and t antigens).18 The cell line was maintained in IMDM containing 5% fetal calf serum (FCS), glutathione, penicillin, and streptomycin.
Long-term bone marrow cultures.
Long-term bone marrow cultures (LTBMCs) were established in 25 cm2 tissue culture flasks from light-density bone marrow cells. Low-density mononuclear cells were isolated by layering bone marrow over Ficoll-Hypaque (density 1.077 g/cm,3 Seromed, Biochrom KG, Berlin, Germany). Cells were grown in IMDM, supplemented with 12.5% FCS, 12.5% horse serum, penicillin, streptomycin, and 10−7 mol/L hydrocortisone (Sigma). Semiconfluent stromal cell layers were trypsinized, replated in 6-well plates, and grown until confluency. Cells were then washed to remove hydrocortisone and incubated in IMDM with penicillin and streptomycin.
Bone marrow-derived myofibroblastic cells.
Bone marrow-derived myofibroblasts were obtained as described.19
CD34+ cell isolation and cocultures with endothelial cells
CD34+ cells were isolated from cord blood or mobilized peripheral blood hematopoietic cells from patients undergoing peripheral blood stem cell transplantation as described.20Preparations giving 85% purity or more were used in the experiments. For cocultures, CD34+ hematopoietic progenitor cells were cultivated with a HUVEC monolayer in a contact or in a noncontact system with Transwell inserts (Costar, Cambridge, MA) essentially as described.20
Cytokine neutralization experiments
Neutralizing polyclonal antibodies directed against IL-1α, IL-1β, IL-6, IL-11, G-CSF, GM-CSF, and M-CSF were purchased from R&D systems (cat nos. AB-200-NA, AB-201-NA, AB-206-NA, AB-218-NA, AB-214-NA, AB-215-NA, and AB-216-NA, respectively). Neutralizing polyclonal anti-LIF antibody was a kind gift from V. Praloran, CHU Dupuytren, Limoges, France. In preliminary experiments, 5 μg/mL final concentration of each antibody were found to give an efficient neutralization of a combination of recombinant human IL-1α, IL-1β, IL-6, IL-11, G-CSF, GM-CSF, M-CSF, and LIF at concentrations of 50 ng/mL each.
Monoclonal anti-FL antibody (clone 40406.111, cat no. MAB308), neutralizing polyclonal anti-FL antibody (cat no. AF-308-NA), and neutralizing polyclonal anti-TPO antibody (cat no. AF-288-NA) were also purchased from R&D systems.
Immunofluorescence
Immunofluorescence for FL.
Stromal cells were left to adhere to FCS-coated coverslips. They were then fixed with 4% paraformaldehyde for 10 minutes, washed 3 times with phosphate-buffered saline (PBS), and blocked for 45 minutes with 2.5% FCS in PBS. Then, cells were incubated with the monoclonal anti-FL antibody for 30 minutes at room temperature, washed, and incubated with secondary FITC-labeled antimouse antibody (Sigma) for 30 minutes at room temperature. Control coverslips were incubated with combinations of control isotypic antibodies at the same protein concentrations. Coverslips were mounted with antifade medium (Vectashield, Biosys, Compiègne, France), sealed, and examined under an Olympus AX 70 microscope (Scop SA, Rungis, France).
Immunofluorescence for GPIIb/IIIa.
Immunofluorescence was carried out on cytospins after liquid cultures. Cells were fixed and processed as above. Staining was performed with FITC-labeled anti-CD41 (Coulter-Immunotech, Marseille, France).
Flow cytometry analysis
Cells were labeled with the indicated FITC- or phycoerythrin (PE)-labeled monoclonal antibodies or with the corresponding isotypic controls for 30 minutes at 4°C in phosphate-buffered saline–bovine serum albumin (PBS-BSA) 1% and analyzed on an EPICS XL flow cytometer (Coultronics, Paris, France). Cells were phenotyped with the following mAb: SJ1D1 (anti-CD13, Coulter/Immunotech, Marseille, France), Leu-M9 (anti-CD33, Becton/Dickinson); Leu-M3 (anti-CD14, Becton/Dickinson); Leu-12 (anti-CD19, Becton/Dickinson); 69(Plt-1) (anti-CD41, Coulter/Immunotech); SZ21 (anti-CD61, Coulter); and Bear1 (anti-CD11b, Coulter/Immunotech). Ten thousand events were stored in list mode and analyzed using System II software (Coultronics).
Western blotting
HUVECs or LTBMCs were cultured 48 hours with 10% supernatant of nontransfected COS cells or with 10% supernatant of CD40L-CD8α–transfected COS cells. The medium was then removed and replaced by RPMI with or without CD40L and the incubation continued for an additional 8 hours (to eliminate FCS that gives nonspecific signals on the blots). The conditioned medium was concentrated 30-fold by freeze-drying and analyzed by Western blotting according to standard procedures. Briefly, samples were subjected to 7.5% SDS-PAGE and electrotransferred to nylon membranes. Blots were developed using anti-FL or anti-TPO antibodies (R&D systems, cat no. AF-308-NA and cat no. AF-288-NA, respectively) and peroxydase-conjugated second antibody, followed by chemiluminescence detection (Amersham, Little Chalfont, UK). Recombinant human FL was a gift from Dr S. D. Lyman, Immunex Corp, Seattle, WA. Recombinant human TPO (carrier-free [cat no. 288-TP] [CF], as albumin gives nonspecific signal) was from R&D.
Cytokine measurements in culture medium
Cells were cultured for 48 hours with 10% supernatant of nontransfected COS cells or with 10% supernatant of CD40L-CD8α–transfected COS cells. The measurements of FL and TPO levels in culture supernatants were performed using ELISA kits purchased from R&D systems according to the manufacturer's instructions. According to the manufacturer, the minimum detectable dose of TPO is less than 15 pg/mL, the lowest TPO standard being 31.2 pg/mL.
Hematopoietic colony assay
Colony assays were performed using either the Methocult GF+ H4435 (StemCell Technologies Inc, Meylan, France [this assay detects granulocytic-monocytic, erythroid, and multilineage colonies]), following the manufacturer's recommendations, or as previously described.20 Colony assays for megakaryocyte progenitors were performed using MegaCult-C (StemCell Technologies), following the manufacturer's recommendations. Staining of megakaryocyte progenitors was performed using a monoclonal antibody to GP IIb (anti-CD41 from Coulter-Immunotech or EDU-3, a monoclonal antibody recognizing a complex-dependent epitope on GP IIb-IIIa21). Bound primary antibody was detected using a biotin-conjugated secondary antibody and avidin alkaline-phosphatase, following standard procedures. Cell nuclei were counterstained with Evans blue.
Statistics
Results were expressed as the mean ± SD. Significant differences between values obtained in each assay were determined when applicable using the Wilcoxon signed rank test. Differences were considered as significant when P < .05.
Results
CD40L enhances the proliferation and differentiation of CD34+ progenitor cells toward the myeloid lineage.
As illustrated in Figure 1, in cocultures of CD34+ progenitor cells with ECs, the addition of CD40L to the culture medium enhances the expansion of nucleated cells (Figure1A) and the expansion of clonogenic cells (Figure 1B).
Nucleated cells and colony-forming cell expansions in cocultures of CD34+ progenitors with HUVEC stimulated by CD40L.
CD34+ progenitors (20 000) isolated from cord blood were cultured with 10% supernatant of nontransfected COS cells (control), with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the absence or presence of anti-CD40 mAb at 5 μg/mL (CD40L + aCD40). Half of the medium was exchanged at day 6 with readdition or not of the antibody. At day 12, the expansion of the nucleated and of clonogenic cells was measured and cells plated for clonogenic assays. A and B: noncontact coculture. C and D: contact coculture. Results are expressed as the mean ± SD–fold cell expansion (A, C) or colony number (B, D) (for 105 cells), for 6 experiments (values significant for CD40L versus control [*] and CD40L + aCD40 versus CD40L [**]).
Nucleated cells and colony-forming cell expansions in cocultures of CD34+ progenitors with HUVEC stimulated by CD40L.
CD34+ progenitors (20 000) isolated from cord blood were cultured with 10% supernatant of nontransfected COS cells (control), with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the absence or presence of anti-CD40 mAb at 5 μg/mL (CD40L + aCD40). Half of the medium was exchanged at day 6 with readdition or not of the antibody. At day 12, the expansion of the nucleated and of clonogenic cells was measured and cells plated for clonogenic assays. A and B: noncontact coculture. C and D: contact coculture. Results are expressed as the mean ± SD–fold cell expansion (A, C) or colony number (B, D) (for 105 cells), for 6 experiments (values significant for CD40L versus control [*] and CD40L + aCD40 versus CD40L [**]).
Similar results were obtained in cocultures with a BMEC line or a HUVEC or LTBMC line (not shown). The expansions were qualitatively similar either in cocultures in which the progenitors are grown in contact with the stromal cells or in noncontact settings (Figure 1C and 1D). Similar results were obtained with CD34+ cells obtained from either cord blood or from G-CSF mobilized peripheral blood hematopoietic cells. The blocking effect of anti-CD40 mAb indicated the specificity of the action of CD40L.
Stimulation of megakaryocytopoiesis.
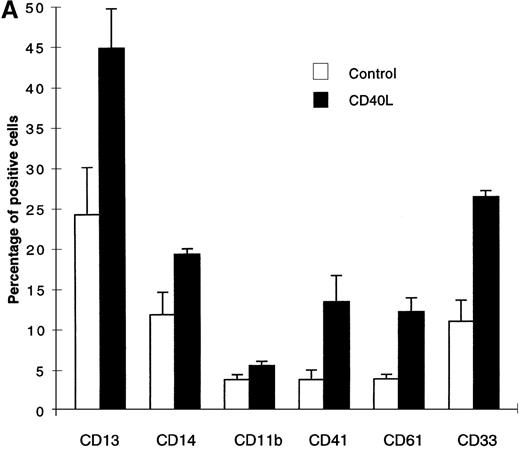
The expanded cell population in the presence of CD40L essentially belonged to the myeloid lineage (CD13, CD14, CD11b, CD33) and surprisingly, a significant number of cells expressing megakaryocytic markers CD41 and CD61 (Figure 2A) were also detected.
Cells expanded in the presence of CD40L.
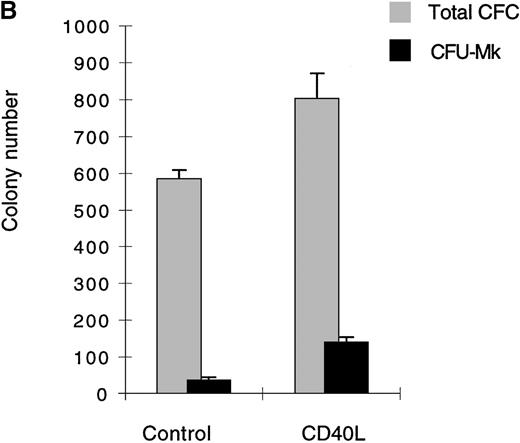
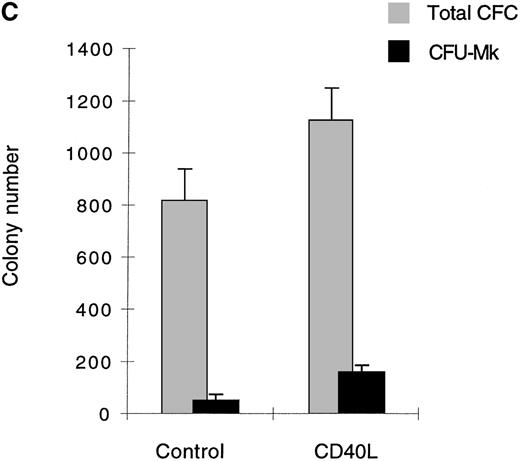
(A) Cells expanded in the presence of CD40L essentially belong to the myeloid and megakaryocytic lineages. Human CD34+progenitors were cultured 12 days in contact cocultures with ECs with 10% supernatant of nontransfected COS cells (control) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L). Results are expressed as the mean ± SD, of 4 experiments. (B, C) Megakaryocyte progenitors in the CD40L coculture system. Human CD34+progenitors were cultured 12 days in contact cocultures with HUVECs with 10% supernatant of nontransfected COS cells (control) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and assayed for colony-forming cells and colony-forming unit–megakaryocytes on a collagen-based system, followed by colony staining with an anti-GP IIb mAb (EDU-3) as described in “Materials and methods”. Center panel (B), noncontact cocultures; bottom panel (C), contact cocultures. Results are expressed as the mean ± SD colony number (for 105 cells), for 3 experiments.
Cells expanded in the presence of CD40L.
(A) Cells expanded in the presence of CD40L essentially belong to the myeloid and megakaryocytic lineages. Human CD34+progenitors were cultured 12 days in contact cocultures with ECs with 10% supernatant of nontransfected COS cells (control) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L). Results are expressed as the mean ± SD, of 4 experiments. (B, C) Megakaryocyte progenitors in the CD40L coculture system. Human CD34+progenitors were cultured 12 days in contact cocultures with HUVECs with 10% supernatant of nontransfected COS cells (control) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and assayed for colony-forming cells and colony-forming unit–megakaryocytes on a collagen-based system, followed by colony staining with an anti-GP IIb mAb (EDU-3) as described in “Materials and methods”. Center panel (B), noncontact cocultures; bottom panel (C), contact cocultures. Results are expressed as the mean ± SD colony number (for 105 cells), for 3 experiments.
No lymphoid cells were detected by specific staining of these cultures. In addition to phenotypic analysis, semisolid clonogenic assays showed an increased number of megakaryocytic colonies (average of 37.5 [for 590 colony-forming cells {CFC}] and 52.5 [for 810 CFC] CFU-Mk, in noncontact and contact cocultures, respectively, in the absence of CD40L versus 135 [for 800 CFC] and 162 [for 1120 CFC] CFU-Mk after stimulation with CD40L, an average of 3 experiments [Figures 2B and 2C]). The results were confirmed by immunofluorescence staining of cytospin preps with anti-CD41 mAb (not shown). Similar results were obtained in cocultures using LTBMCs as the feeder layer (not shown). Thus, the addition of soluble CD40L to cocultures stimulated the expansion of the myeloid lineage. It had a stimulatory effect on the megakaryocytic lineage.
Enhancement of clonogenic cell generation by CD40L is dependent on the augmentation of FL biosynthesis.
CD40L effects were indirectly mediated. First, we did not observe any significant direct effect of CD40L on the proliferation of CD34+ progenitors (not shown). Second, prestimulation of the EC monolayer for 48 hours with CD40L, followed by washing to remove CD40L before starting the coculture showed essentially the same results as those depicted in Figure 1. We therefore examined whether CD40L could modulate the expression of FL, a cytokine that has been shown to be of paramount importance in the proliferation of primitive hematopoietic cells.
CD40L treatment of ECs, LTBMCs, or bone marrow myofibroblasts enhanced expression of both the soluble form and the membrane-bound form of FL. Under basal conditions, LTBMCs and bone marrow myofibroblasts produced undetectable amounts of soluble FL, whereas CD40L induced production of soluble FL in these cells. In contrast, the ECs produced large amounts of soluble FL under basal conditions, production that was also enhanced with addition of CD40L to cocultures (Figure3A). These results were confirmed by Western blotting (Figure 3B, Western blotting analysis of HUVEC- and LTBMC-conditioned medium). ECs, LTBMCs, or bone marrow myofibroblasts expressed the membrane-bound form of FL, this expression being enhanced by treatment with CD40L (results illustrated in Figure 3C and D for ECs).
CD40L induces FL expression.
Stromal cells (either HUVECs, BMEC line [TrBMEC], LTBMCs, or bone marrow-derived fibroblasts [BMmyofibr]) were cultured 48 hours with medium alone (control) or 10% supernatant of nontransfected COS cells (COS) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and the resulting effect on the expression of FL studied. (A) Up-regulation of the production of the soluble form of FL. ELISA measurements showing the stimulation of the production of the soluble form of FL (average ± SD of 7 experiments). The production of soluble FL in LTBMCs or BMmyofibr is below the level of ELISA detection (15 pg/mL). No FL is detected in untransfected or transfected COS cell supernatants. Statistically significant results (*) are indicated. (B) Western blotting showing FL protein in HUVEC and LTBMC culture medium and increased production by CD40L. Track A, recombinant FL protein, track B and D, HUVECs and LTBMCs respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; track C and E, HUVECs and LTBMCs, respectively, cultured with 10% supernatant of CD40L-CD8α–transfected COS cells. (C) Up-regulation of membrane-bound FL expression by CD40L on ECs is shown by flow cytometry: darkgray, isotype control;gray, EC stimulated 48 hours with 10% supernatant of nontransfected COS cells, black, ECs stimulated 48 hours with 10% supernatant of CD40L-CD8α–transfected COS cells. (D, E) Indirect immunofluorescence showing up-regulation of FL by CD40L at the surface of ECs (top panel: ECs stimulated 48 hours with 10% supernatant of nontransfected COS cells; bottom panel: ECs stimulated with 10% supernatant of CD40L-CD8α–transfected COS cells).
CD40L induces FL expression.
Stromal cells (either HUVECs, BMEC line [TrBMEC], LTBMCs, or bone marrow-derived fibroblasts [BMmyofibr]) were cultured 48 hours with medium alone (control) or 10% supernatant of nontransfected COS cells (COS) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and the resulting effect on the expression of FL studied. (A) Up-regulation of the production of the soluble form of FL. ELISA measurements showing the stimulation of the production of the soluble form of FL (average ± SD of 7 experiments). The production of soluble FL in LTBMCs or BMmyofibr is below the level of ELISA detection (15 pg/mL). No FL is detected in untransfected or transfected COS cell supernatants. Statistically significant results (*) are indicated. (B) Western blotting showing FL protein in HUVEC and LTBMC culture medium and increased production by CD40L. Track A, recombinant FL protein, track B and D, HUVECs and LTBMCs respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; track C and E, HUVECs and LTBMCs, respectively, cultured with 10% supernatant of CD40L-CD8α–transfected COS cells. (C) Up-regulation of membrane-bound FL expression by CD40L on ECs is shown by flow cytometry: darkgray, isotype control;gray, EC stimulated 48 hours with 10% supernatant of nontransfected COS cells, black, ECs stimulated 48 hours with 10% supernatant of CD40L-CD8α–transfected COS cells. (D, E) Indirect immunofluorescence showing up-regulation of FL by CD40L at the surface of ECs (top panel: ECs stimulated 48 hours with 10% supernatant of nontransfected COS cells; bottom panel: ECs stimulated with 10% supernatant of CD40L-CD8α–transfected COS cells).
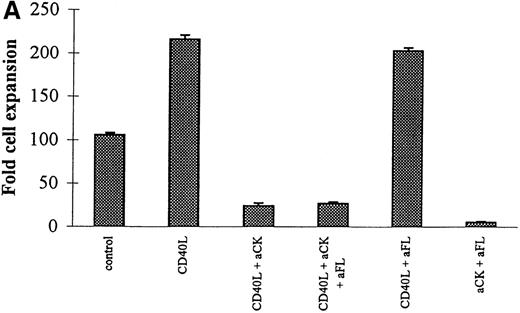
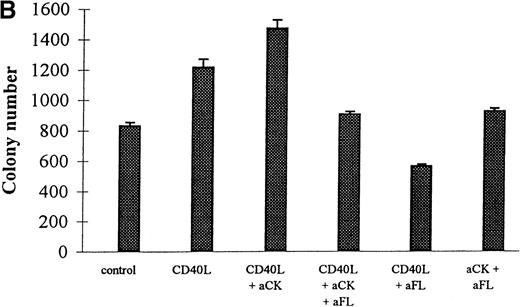
Neutralization of FL by specific antibody reduced the number of clonogenic cells in EC/CD34+ cocultures (Figure4 bottom panel) but did not modify the cell expansion (Figure 4 top panel). These experiments demonstrated the key role of FL in the control of the production of the clonogenic cells. Reduction to less than control levels was likely explained by the neutralization of the endogenous production of FL by EC. On the other hand, because it has been shown that CD40L up-regulates the production of other cytokines acting on the differentiation of committed hematopoietic progenitors, we also examined the results of neutralizing those cytokines in the CD40L coculture system. It turned out that this neutralization significantly reduced the capacity of the stromal cells—of all types tested—to support the expansion of hematopoietic progenitors (Figure 4 top panel) but significantly enhanced expansion of the clonogenic cells (Figure 4 bottom panel). In contrast, specific neutralization of FL had no further effect on cell expansion. Thus, CD40L indirectly modulated the pattern of cytokine production by stromal cells resulting in the expansion of both clonogenic and more mature hematopoietic cells.
Effects of CD40L on the proliferation and differentiation of hematopoietic progenitors: neutralization experiments.
CD34+ progenitors (10 000) were cocultured 12 days in contact with ECs with 10% supernatant of nontransfected COS cells (control) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the presence or absence of neutralizing antibodies. Half of the medium was exchanged at day 6 with readdition of the antibodies. At the end of the coculture, cell expansion was measured and cells were plated for colony assay. Colonies were scored at day 14. Results are expressed as the mean ± SD–fold cell expansion (top panel) or colony number (for 105 cells) measured (bottom panel) for 3 experiments (aCK: combination of neutralizing polyclonal anticytokine (IL-1α, IL-1β, IL-6, IL-11, G-CSF, GM-CSF, M-CSF, and LIF) antibodies; aFL: neutralizing polyclonal anti-FL antibody).
Effects of CD40L on the proliferation and differentiation of hematopoietic progenitors: neutralization experiments.
CD34+ progenitors (10 000) were cocultured 12 days in contact with ECs with 10% supernatant of nontransfected COS cells (control) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the presence or absence of neutralizing antibodies. Half of the medium was exchanged at day 6 with readdition of the antibodies. At the end of the coculture, cell expansion was measured and cells were plated for colony assay. Colonies were scored at day 14. Results are expressed as the mean ± SD–fold cell expansion (top panel) or colony number (for 105 cells) measured (bottom panel) for 3 experiments (aCK: combination of neutralizing polyclonal anticytokine (IL-1α, IL-1β, IL-6, IL-11, G-CSF, GM-CSF, M-CSF, and LIF) antibodies; aFL: neutralizing polyclonal anti-FL antibody).
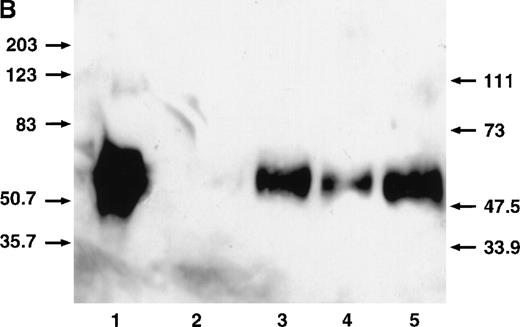
CD40L stimulates megakaryocytopoiesis by way of the induced production of TPO by stromal cells.
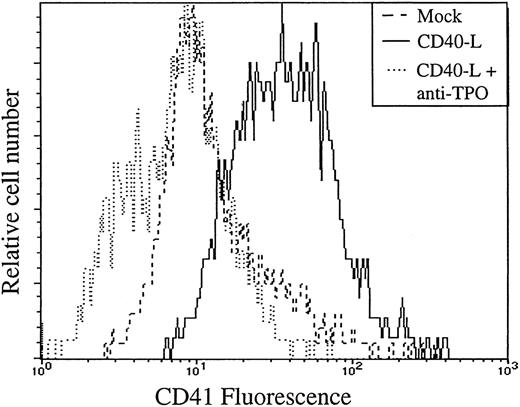
A finding of this study was that CD40L stimulated megakaryocytopoiesis. In an effort to understand the molecular basis for this megakaryocytopoiesis-promoting effect of CD40L, we measured the production of TPO by stromal cells. Under basal conditions, the concentrations of TPO were barely detectable, at the limit of ELISA detection and accuracy (15-30 pg/mL) in all cell types tested. However, CD40L induced production of TPO in all stromal cell types tested (Figure 5A), results confirmed by Western blotting that showed a moderate but consistent increase of TPO protein after stimulation (Figure 5B Western blot analysis of HUVEC- and LTBMC-conditioned medium). Neutralization of TPO in the CD40L coculture system resulted in the inhibition of the capacity of stromal cells, either ECs or LTBMCs to support the expansion of the megakaryocyte progenitors, as demonstrated by flow cytometry experiments (illustrated in Figure 6: in the presence of the anti-TPO antibody, there is a shift of the CD41 fluorescence to the level of the isotypic control), and colony assays that showed a complete absence of megakaryocytic colonies after neutralization of TPO (not shown). Neutralization of TPO did not affect cell expansion, the colony number was reduced by an average of 18% (3 experiments). Neutralization of FL did not affect the number of megakaryocytic colonies. This suggested that the increased production of TPO by stromal cells in the presence of CD40L was the main cause for the increased generation of megakaryocyte progenitors.
CD40L stimulates the production of TPO by stromal cells.
(A) ELISA on 48-hour-old conditioned medium in ECs, BMEC line LTBMCs, bone marrow-derived myofibroblasts cultured in the presence of 10% supernatant of nontransfected COS cell (left histogram), or with 10% supernatant of CD40L-CD8α–transfected COS cells (right histogram). No TPO was detected in untransfected or transfected COS cell supernatants. Results are expressed as the mean ± SD for 3 experiments. (B) Western blotting showing TPO protein in HUVEC- and LTBMC-culture medium and increased production by CD40L. 1, recombinant TPO protein, 2 and 4, LTBMCs and HUVECs, respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; 3 and 5, LTBMCs and HUVECs respectively, cultured with 10% supernatant of CD40L-CD8α– transfected COS cells. TPO was found migrating at an Mr of 58 to 68 kd. An additional species migrating at an Mr of approximately 90 kd (visible in track 1) can be seen on overloading and/or overexposure. We do not know the nature of this high-molecular weight species. Dotted line indicates the level of the lowest TPO standard (31.2 pg/mL).
CD40L stimulates the production of TPO by stromal cells.
(A) ELISA on 48-hour-old conditioned medium in ECs, BMEC line LTBMCs, bone marrow-derived myofibroblasts cultured in the presence of 10% supernatant of nontransfected COS cell (left histogram), or with 10% supernatant of CD40L-CD8α–transfected COS cells (right histogram). No TPO was detected in untransfected or transfected COS cell supernatants. Results are expressed as the mean ± SD for 3 experiments. (B) Western blotting showing TPO protein in HUVEC- and LTBMC-culture medium and increased production by CD40L. 1, recombinant TPO protein, 2 and 4, LTBMCs and HUVECs, respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; 3 and 5, LTBMCs and HUVECs respectively, cultured with 10% supernatant of CD40L-CD8α– transfected COS cells. TPO was found migrating at an Mr of 58 to 68 kd. An additional species migrating at an Mr of approximately 90 kd (visible in track 1) can be seen on overloading and/or overexposure. We do not know the nature of this high-molecular weight species. Dotted line indicates the level of the lowest TPO standard (31.2 pg/mL).
Neutralization of TPO impairs the capacity of stromal cells to support the generation of megakaryocyte progenitors.
CD34+ progenitors were cocultured 12 days with ECs with 10% supernatant of nontransfected COS cells (mock) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the presence (+) or not of a neutralizing anti-TPO antibody (anti-TPO). Cells were then assayed for the presence of CD41 megakaryocyte-specific lineage marker by flow cytometry.
Neutralization of TPO impairs the capacity of stromal cells to support the generation of megakaryocyte progenitors.
CD34+ progenitors were cocultured 12 days with ECs with 10% supernatant of nontransfected COS cells (mock) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the presence (+) or not of a neutralizing anti-TPO antibody (anti-TPO). Cells were then assayed for the presence of CD41 megakaryocyte-specific lineage marker by flow cytometry.
Discussion
In the CD40 coculture system, the proliferation and differentiation of the hematopoietic progenitor cells are dependent on the induction of cytokine biosynthesis by the stromal cells. Activation of ECs, fibroblasts, or epithelial cells through their CD40 antigen has been shown to result in the increased production of cytokines such as IL-6, IL-8, GM-CSF, G-CSF, or LIF.7-14 We extend those conclusions by demonstrating the role of CD40 signaling in the induction of FL and TPO biosynthesis.
FLs, either the soluble or the membrane-bound species, are expressed by bone marrow stromal cells in basal conditions,22,23 and we show that this expression is strongly reinforced by CD40L. The magnitude of the increased production of soluble FL varies from cell to cell, which may result from a cell-specific regulation of its proteolytic release.26 In the CD40 coculture system, the increased production of FL induced by CD40L appears to be responsible for the enhancement of clonogenic cell generation. This is in agreement with the key role of FL in the proliferation of primitive hematopoietic progenitors, either in a stroma-dependent system or in a stroma-free condition.24-35 The overall effect of CD40L stimulation could therefore be interpreted as the combined results of an increase in the number of clonogenic cells by FL and their loss due to their accelerated differentiation under the influence of differentiating cytokines. No lymphoid cells were produced in the CD40L coculture system. This is in agreement with data demonstrating an IL-3 requirement for the proliferation of B-cell precursors in the presence of CD40L.36 No IL-3 is produced in such coculture systems, either with ECs or LTBMCs.20
Our data show an unexpected role for CD40L in the control of megakaryocytopoiesis. TPO first thought to be of extramedullary origin has also been shown to be produced by bone marrow stromal cells,37 albeit at very low levels. The molecular basis of its regulation is poorly understood, in particular whether the rate of gene expression can vary in response to physiologic stimuli is not known.38-49 The current results indicate that CD40L may be a regulator of TPO expression. We do not know yet how CD40L increases the production of the TPO or FL proteins, whether it is via increased transcription of the gene, translational efficiency, or posttranslational mechanisms. As CD40L is present as a membrane-bound species on activated platelets,50 involvement of platelets may constitute the basis for a feedback mechanism regulating their production.
The data gathered in this study suggest that the couple CD40/CD40L may be involved in the control of hematopoiesis via modulation of the cytokine network in the bone marrow. As is the case for IL-1,51 CD40L could therefore be a blood-born mediator acting on the bone marrow stromal cells. It can be expected from these results that the action of CD40L on hematopoiesis would be to increase cell production of the granulocytic and megakaryocytic lineages in disease states such as septic or inflammatory conditions, conditions that are often accompanied by neutrophil leukocytosis or thrombocytosis. In this context, it has recently been observed that treatment with soluble CD40L increases granulocyte and platelet recovery after bone marrow grafting in mice.52 In agreement with these observations would be the general neutropenia described in patients who have hyper-IgM syndrome, resulting from mutations in theCD40L gene.53 CD40L could be brought to the bone marrow by way of the blood circulation as a soluble species or by circulating activated T lymphocytes or platelets. Concentrations of CD40L that were used in this study are approximately 10 to 20 times higher than concentrations that are susceptible to be attained in vivo in patients with inflammatory syndromes,54 and the in vitro activity of lower concentrations of CD40L deserves further investigation before extrapolation to in vivo systems can be made. Soluble CD40L can be released from the surface of activated T or B cells55,56 and the trimeric soluble form of CD40L has been shown to retain its biologic CD40 stimulating properties.57Alternatively, because activated T cells express CD40L, activation of CD40 on bone marrow stromal cells by activated T cells may represent a mechanism by which T cells themselves regulate the production of hematopoietic growth factors in the bone marrow and the release of blood cells.
Acknowledgment
J. Banchereau and Dr C. Van Kooten are gratefully acknowledged for the gift of the CD40L cDNA.
Supported by the Ligue Nationale contre le Cancer.
Reprints:Jean Ripoche, UMR 5540 CNRS, Université de Bordeaux 2, 146 rue Léo Saignat, 33076 Bordeaux, France; e-mail:jean.ripoche@umr5540.u-bordeaux2.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Nucleated cells and colony-forming cell expansions in cocultures of CD34+ progenitors with HUVEC stimulated by CD40L. / CD34+ progenitors (20 000) isolated from cord blood were cultured with 10% supernatant of nontransfected COS cells (control), with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the absence or presence of anti-CD40 mAb at 5 μg/mL (CD40L + aCD40). Half of the medium was exchanged at day 6 with readdition or not of the antibody. At day 12, the expansion of the nucleated and of clonogenic cells was measured and cells plated for clonogenic assays. A and B: noncontact coculture. C and D: contact coculture. Results are expressed as the mean ± SD–fold cell expansion (A, C) or colony number (B, D) (for 105 cells), for 6 experiments (values significant for CD40L versus control [*] and CD40L + aCD40 versus CD40L [**]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244001ax.jpeg?Expires=1769300447&Signature=xLc40BrGY1x7t35rXmD4NIShSaikbEOCTo-tHdecA4g3bsbN2he4xf3LxOAjT2aeXQqa9PDirrLr1xRThROwhP46vjZHJCQ1SHwnx16kf2v~od7sPRfDTuPfBa53t2OX89AHIbGafVSatoVJaq83nWExkKMCX5Z3XJA0BvJX2vAS7XUcqrzBzQ-qUmp1gDpo3yUs62uyxsPXXyDOaaifYR3K2vxTPTId4dDMsYhLKSmzjjLvAuv9GZsT6k7K0wNKnN-WRFXUPN4ABzq~cJbgID7cSA0OPfFX7GTnSPMeKUsv5vKHhcLyPZMxzwa3M-a~Ut~Mcr6eq8x5FGHBmbYRwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Nucleated cells and colony-forming cell expansions in cocultures of CD34+ progenitors with HUVEC stimulated by CD40L. / CD34+ progenitors (20 000) isolated from cord blood were cultured with 10% supernatant of nontransfected COS cells (control), with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the absence or presence of anti-CD40 mAb at 5 μg/mL (CD40L + aCD40). Half of the medium was exchanged at day 6 with readdition or not of the antibody. At day 12, the expansion of the nucleated and of clonogenic cells was measured and cells plated for clonogenic assays. A and B: noncontact coculture. C and D: contact coculture. Results are expressed as the mean ± SD–fold cell expansion (A, C) or colony number (B, D) (for 105 cells), for 6 experiments (values significant for CD40L versus control [*] and CD40L + aCD40 versus CD40L [**]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244001bx.jpeg?Expires=1769300447&Signature=f0GhARDaO0ohQ44QQ4ueDooJy3Go6s4fRCm8oNULUUtXZglhFUOErB52L6k~C-39QXmYJ9D4YaG8qmPfZbbo2Ar-64za8a1CCbxywSdvSdg-p5TkqEBRUFPrQXyKXRvhWUTTL2gA5zEYaWIjorzW44eha6H0gPOFVOLHaIwii21TPofW-VYHGmOVxYXWTJStlLBbtMjnDY7EQeSY95Z9yFGm~VdBitb9CxJGO5IfurK7dbVdEjFpCtOTxzXNtypC7aKkd3vHNDVoqUMGKqMyGK4r7OINne9CN5R0TadaUv5Oiv3bu0z1dI74J3k1bkrsHijk3ejs~YBkvmlsSCZdIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Nucleated cells and colony-forming cell expansions in cocultures of CD34+ progenitors with HUVEC stimulated by CD40L. / CD34+ progenitors (20 000) isolated from cord blood were cultured with 10% supernatant of nontransfected COS cells (control), with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the absence or presence of anti-CD40 mAb at 5 μg/mL (CD40L + aCD40). Half of the medium was exchanged at day 6 with readdition or not of the antibody. At day 12, the expansion of the nucleated and of clonogenic cells was measured and cells plated for clonogenic assays. A and B: noncontact coculture. C and D: contact coculture. Results are expressed as the mean ± SD–fold cell expansion (A, C) or colony number (B, D) (for 105 cells), for 6 experiments (values significant for CD40L versus control [*] and CD40L + aCD40 versus CD40L [**]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244001cx.jpeg?Expires=1769300447&Signature=wGMLl4yiE96s0A1TI36Gt903W-3hekV90g8F7p~uWbOtK34YSNP4lCeBFLf-yvPbhsgfQD-~F0yhxX8HggmU6VM0l6bZoOeSjrRKDKdprxU-P676v7yBrjTx0asN9ard81t09JvuzN9IV2hyoyxO4O7d-CpEeRaUCpFVfUS7kzOPy6S5-rAPmi7oV6xoVEpVEANqaaIeE3pqwsPKNsbuyRkCrfPZfThqAmnnpN3VkFI38JmnRCr-JEb2shdF~ZCAVFHcvgoFvNOWhx3h8bH-ldi3juV6XgFjpkjhecq1oliyRC5U5u0U9OwJiUriWnr0CwAoPPE39-GnYbyibqNCag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Nucleated cells and colony-forming cell expansions in cocultures of CD34+ progenitors with HUVEC stimulated by CD40L. / CD34+ progenitors (20 000) isolated from cord blood were cultured with 10% supernatant of nontransfected COS cells (control), with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) in the absence or presence of anti-CD40 mAb at 5 μg/mL (CD40L + aCD40). Half of the medium was exchanged at day 6 with readdition or not of the antibody. At day 12, the expansion of the nucleated and of clonogenic cells was measured and cells plated for clonogenic assays. A and B: noncontact coculture. C and D: contact coculture. Results are expressed as the mean ± SD–fold cell expansion (A, C) or colony number (B, D) (for 105 cells), for 6 experiments (values significant for CD40L versus control [*] and CD40L + aCD40 versus CD40L [**]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244001dx.jpeg?Expires=1769300447&Signature=mKaGdKY-AcOaw8QvilYmxHQXDct9O~ZWHRBFs7Avlk3R9cSZm~miHWF7bsXUsFAQYZQCfddH9mzp5c7zksrK0FJWPSOYQjh1dsfVo18nI4xSgBZfVONy1oca~vDhGE4BrEh1VQsno0aURTicmjSm5qWQPWP3FXgMPYGo9XhYjLrh-~7MYWdB6iWBi9dHLK4dkeybm0VTJHgBTCskZG7CyTft5kpkegbxD8zOjeuBg7PaSQDDRU7-hAVouYPNzZMq996mPK4jzc8B2U6uXfBkqPX8Spt0uWsAHu5aO~6Wfu~GI~WNmHsZAWOiZSOe7Drae-jJpG2olGWwY2DYHEZ1lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD40L induces FL expression. / Stromal cells (either HUVECs, BMEC line [TrBMEC], LTBMCs, or bone marrow-derived fibroblasts [BMmyofibr]) were cultured 48 hours with medium alone (control) or 10% supernatant of nontransfected COS cells (COS) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and the resulting effect on the expression of FL studied. (A) Up-regulation of the production of the soluble form of FL. ELISA measurements showing the stimulation of the production of the soluble form of FL (average ± SD of 7 experiments). The production of soluble FL in LTBMCs or BMmyofibr is below the level of ELISA detection (15 pg/mL). No FL is detected in untransfected or transfected COS cell supernatants. Statistically significant results (*) are indicated. (B) Western blotting showing FL protein in HUVEC and LTBMC culture medium and increased production by CD40L. Track A, recombinant FL protein, track B and D, HUVECs and LTBMCs respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; track C and E, HUVECs and LTBMCs, respectively, cultured with 10% supernatant of CD40L-CD8α–transfected COS cells. (C) Up-regulation of membrane-bound FL expression by CD40L on ECs is shown by flow cytometry: dark gray, isotype control;gray, EC stimulated 48 hours with 10% supernatant of nontransfected COS cells, black, ECs stimulated 48 hours with 10% supernatant of CD40L-CD8α–transfected COS cells. (D, E) Indirect immunofluorescence showing up-regulation of FL by CD40L at the surface of ECs (top panel: ECs stimulated 48 hours with 10% supernatant of nontransfected COS cells; bottom panel: ECs stimulated with 10% supernatant of CD40L-CD8α–transfected COS cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244003ax.jpeg?Expires=1769300447&Signature=0QU8Yrus~2xVhGgML01ZkWXqFvPSu3Gd6zb78swHGutIucqO0LB2p~tkNRLUm4L71NL6trPXizT9ZwCra7LkNcrXcKAPTUkQeDRKpm6f7TxbmNrFATUqBSnWSkXP5Vo0zvAPhKbZvHHBtBDsIpbESqcJgtM-4-HVAJEuWq9lCOUCLGrhJDmtICWor7SovxFG1gtmbzP7mdxcnw78Oni0KU1xc2cbQhRK207vn3YmVJpIhEHyZI2Q0bhFvGwvV5SQUSt7PxTgaTYph2inIckirsMlHiaxJiIbpprleAquLWraJOSn-aYT1GI0-eO4N4PhWGNaRkFozgV3VDXAb0-sDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD40L induces FL expression. / Stromal cells (either HUVECs, BMEC line [TrBMEC], LTBMCs, or bone marrow-derived fibroblasts [BMmyofibr]) were cultured 48 hours with medium alone (control) or 10% supernatant of nontransfected COS cells (COS) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and the resulting effect on the expression of FL studied. (A) Up-regulation of the production of the soluble form of FL. ELISA measurements showing the stimulation of the production of the soluble form of FL (average ± SD of 7 experiments). The production of soluble FL in LTBMCs or BMmyofibr is below the level of ELISA detection (15 pg/mL). No FL is detected in untransfected or transfected COS cell supernatants. Statistically significant results (*) are indicated. (B) Western blotting showing FL protein in HUVEC and LTBMC culture medium and increased production by CD40L. Track A, recombinant FL protein, track B and D, HUVECs and LTBMCs respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; track C and E, HUVECs and LTBMCs, respectively, cultured with 10% supernatant of CD40L-CD8α–transfected COS cells. (C) Up-regulation of membrane-bound FL expression by CD40L on ECs is shown by flow cytometry: dark gray, isotype control;gray, EC stimulated 48 hours with 10% supernatant of nontransfected COS cells, black, ECs stimulated 48 hours with 10% supernatant of CD40L-CD8α–transfected COS cells. (D, E) Indirect immunofluorescence showing up-regulation of FL by CD40L at the surface of ECs (top panel: ECs stimulated 48 hours with 10% supernatant of nontransfected COS cells; bottom panel: ECs stimulated with 10% supernatant of CD40L-CD8α–transfected COS cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244003bw.jpeg?Expires=1769300447&Signature=lAUTz5k1dbfQZB6PxTgnytNkZ0RiEbwRN2q83zJD5D9lrhC1hi6BcR41ApnQ0aZ4f1Jcw7Mrp9v4QoNGgMMSthUqDOqev1RSiu2rRpVEEXIDwKAA5nwMaW04Xo06cE0chm3IxEEQcXFbkgah9ojAgaT-dVzoul0czS-ZTqPdOG0YdZzeElrZQIW-jAmNQytCs22dqieozW5eQ2axLCKW~sIm7oYouc4mn2wOg5AqM6PXwuS8vmhFtRTc7TJeg11PlC7LbHdYT1EDDkxNySSfW0IQFMDfb~As7sFrTG0voxKOf9Zzl6ODoT9LK~b8s~0Tdb401oU-kuGz~~Ymxmi0LA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD40L induces FL expression. / Stromal cells (either HUVECs, BMEC line [TrBMEC], LTBMCs, or bone marrow-derived fibroblasts [BMmyofibr]) were cultured 48 hours with medium alone (control) or 10% supernatant of nontransfected COS cells (COS) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and the resulting effect on the expression of FL studied. (A) Up-regulation of the production of the soluble form of FL. ELISA measurements showing the stimulation of the production of the soluble form of FL (average ± SD of 7 experiments). The production of soluble FL in LTBMCs or BMmyofibr is below the level of ELISA detection (15 pg/mL). No FL is detected in untransfected or transfected COS cell supernatants. Statistically significant results (*) are indicated. (B) Western blotting showing FL protein in HUVEC and LTBMC culture medium and increased production by CD40L. Track A, recombinant FL protein, track B and D, HUVECs and LTBMCs respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; track C and E, HUVECs and LTBMCs, respectively, cultured with 10% supernatant of CD40L-CD8α–transfected COS cells. (C) Up-regulation of membrane-bound FL expression by CD40L on ECs is shown by flow cytometry: dark gray, isotype control;gray, EC stimulated 48 hours with 10% supernatant of nontransfected COS cells, black, ECs stimulated 48 hours with 10% supernatant of CD40L-CD8α–transfected COS cells. (D, E) Indirect immunofluorescence showing up-regulation of FL by CD40L at the surface of ECs (top panel: ECs stimulated 48 hours with 10% supernatant of nontransfected COS cells; bottom panel: ECs stimulated with 10% supernatant of CD40L-CD8α–transfected COS cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo01244003cx.jpeg?Expires=1769300447&Signature=0n1U-LCNgNt-3MlSnlOmMQOLjz9mqB0Yi6QC9jpiqz9c8Ha7IkBVOOg5Bvarm7XcsmXJLpa8~lVQDoYW9t3w1tRK6WNRDhkWyqKIY8OyCC0YZ17hn7tKDsQJ8AmjaU53UHSZLYkURE8AxStEEb8sEkACgEDhXoPkSVE8kaGv3t~tvPBRxNbKRFuX1DLToK961SJj8z5K8GkTIGKmRzS-SqF4l676lQb4eQAs1jerNjJVKGBOKpFGz7Afh6j8p3EBAo6Q8-P~TORx8nIkbiiGZ0Y0hprL4qn5h9GeWm0NbeBXY~hJ8RJ-TDURvo4Ov1wfvk~vUi30QzlemJidvY9SAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. CD40L induces FL expression. / Stromal cells (either HUVECs, BMEC line [TrBMEC], LTBMCs, or bone marrow-derived fibroblasts [BMmyofibr]) were cultured 48 hours with medium alone (control) or 10% supernatant of nontransfected COS cells (COS) or with 10% supernatant of CD40L-CD8α–transfected COS cells (CD40L) and the resulting effect on the expression of FL studied. (A) Up-regulation of the production of the soluble form of FL. ELISA measurements showing the stimulation of the production of the soluble form of FL (average ± SD of 7 experiments). The production of soluble FL in LTBMCs or BMmyofibr is below the level of ELISA detection (15 pg/mL). No FL is detected in untransfected or transfected COS cell supernatants. Statistically significant results (*) are indicated. (B) Western blotting showing FL protein in HUVEC and LTBMC culture medium and increased production by CD40L. Track A, recombinant FL protein, track B and D, HUVECs and LTBMCs respectively, cultured in the presence of 10% supernatant of nontransfected COS cells; track C and E, HUVECs and LTBMCs, respectively, cultured with 10% supernatant of CD40L-CD8α–transfected COS cells. (C) Up-regulation of membrane-bound FL expression by CD40L on ECs is shown by flow cytometry: dark gray, isotype control;gray, EC stimulated 48 hours with 10% supernatant of nontransfected COS cells, black, ECs stimulated 48 hours with 10% supernatant of CD40L-CD8α–transfected COS cells. (D, E) Indirect immunofluorescence showing up-regulation of FL by CD40L at the surface of ECs (top panel: ECs stimulated 48 hours with 10% supernatant of nontransfected COS cells; bottom panel: ECs stimulated with 10% supernatant of CD40L-CD8α–transfected COS cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3758/8/m_bloo0124403ez.jpeg?Expires=1769300447&Signature=KaF3TVgJXajTrPTQYHnTZ~kCF12iR-pEHgben63pPtY9j8Tow~4XCHuM9RaT1Aus2Fl6IW2KC7BXl1HwWk8J2qfq8kua9~OTFL-ZSamALoENOCZCWWixWeZjJp9AlP2mvPKsVJKaF3gIQa8Inf0fNb9SMJecH-FYwn8juOdqv9fPTCepFvpWr8USrE6UNfkpG~ZJ1J0XbAJTUeBQ84x5nSgRpwGUIwM7jYEZlFTyHrPP3UsYaf4I6xMSNNRjqZKC5NIc0oMEyGjUSwa9i7c3eM54TCQ6iS6huSkNIy4oXwzrsBrL8swsmiOovYMhovyut6LhIASs82DR773fhyA8eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal