Abstract
In an attempt to develop efficient procedures of human hematopoietic gene therapy, retrovirally transduced CD34+ cord blood cells were transplanted into NOD/SCID mice to evaluate the repopulating potential of transduced grafts. Samples were prestimulated on Retronectin-coated dishes and infected with gibbon ape leukemia virus (GALV)-pseudotyped FMEV vectors encoding the enhanced green fluorescent protein (EGFP). Periodic analyses of bone marrow (BM) from transplanted recipients revealed a sustained engraftment of human hematopoietic cells expressing the EGFP transgene. On average, 33.6% of human CD45+ cells expressed the transgene 90 to120 days after transplantation. Moreover, 11.9% of total NOD/SCID BM consisted of human CD45+ cells expressing the EGFP transgene at this time. The transplantation of purified EGFP+ cells increased the proportion of CD45+ cells positive for EGFP expression to 57.7% at 90 to 120 days after transplantation. At this time, 18.9% and 4.3% of NOD/SCID BM consisted of CD45+/EGFP+ and CD34+/EGFP+ cells, respectively. Interestingly, the transplantation of EGFP− cells purified at 24 hours after infection also generated a significant engraftment of CD45+/EGFP+ and CD34+/EGFP+ cells, suggesting that a number of transduced repopulating cells did not express the transgene at that time. Molecular analysis of NOD/SCID BM confirmed the high levels of engraftment of human transduced cells deduced from FACS analysis. Finally, the analysis of the provirus insertion sites by conventional Southern blotting indicated that the human hematopoiesis in the NOD/SCID BM was predominantly oligoclonal.
Hematopoietic stem cells (HSCs) constitute an ideal target for human gene therapy because these very rare progenitors can repopulate the whole hematopoietic system of a recipient for his entire life. Given that current hematopoietic gene transfer strategies rely on the transplantation of grafts manipulated ex vivo, their success will require the optimization of HSC manipulation in 3 different aspects: (1) by developing ex vivo transduction protocols capable of maintaining and, ideally, expanding functional HSCs; (2) by increasing the HSC transduction efficacy of integrative vectors able to provide long-term expression in vivo; and (3) by improving cell selection procedures that may facilitate the preferential engraftment of transduced populations expressing the gene of interest.
At present, retroviral vectors constitute the most efficient tool for stably transducing HSCs. In fact, most of the goals mentioned above have already been achieved in mice transplanted with retrovirally transduced syngeneic hematopoietic cells.1,2 In this respect, although a progressive reduction of expression has been generally observed following transplantation of transduced bone marrow (BM) cells,3,4 relatively high levels of transgene expression have been reported in this experimental model.5-7
In larger experimental animals8,9 and in humans,10-12 success for stably transducing long-term repopulating cells has been limited. In fact, only very few studies have reported a significant level of gene transfer to the hematopoietic repopulating cells in these species.13,14 Additionally, the experimental assays used to investigate the functionality of transduced true human HSCs have been a limiting issue in the assessment of transduction efficiency of these cells. Although several authors reported efficient transduction into hematopoietic progenitors defined by in vitro assays, it is now well established that the functionality of the self-renewing HSCs can only be unambiguously demonstrated by evaluating the long-term repopulating ability of the samples using in vivo assays.15
Despite the disappointing results obtained in most of the hematopoietic gene therapy trials reported to date, recent developments entail a significant progress in the goal of transducing human HSCs.16-22 In this respect, new xenogenic transplantation models, mainly based on immunodeficient mice such as SCID,23 NOD/SCID,24 and bnx mice25have been developed to investigate the repopulating ability of human hematopoietic transduced grafts in vivo. New combinations of hematopoietic growth factors, capable of preserving or even expanding human hematopoietic precursors with in vivo repopulating ability have been reported.26,27 Molecules like fibronectin (FN) or FN-derived fragments such as CH-296 that can colocalize the hematopoietic target cells and the infective retroviral particles have been reported as efficient tools for enhancing the interaction between both elements, and thus, gene transfer efficiency.28Packaging cell lines generating pseudotyped retroviral vectors bearing envelopes of the gibbon ape leukemia virus (GALV)29 or the vesicular stomatitis virus G protein (VSV-G)30 also show an improved efficacy for transducing human hematopoietic progenitors.17,21 Finally, novel engineered retroviral promoters like the FMEV promoter (a hybrid containing sequences of the Friend mink cell focus-forming virus and the murine embryonic stem cell virus)31 and new marker genes such as those encoding the nerve growth factor receptor (NGFR)32 or the green fluorescent protein (GFP) and its derivatives have been generated,7 33 facilitating the rapid and reliable assessment of transgene expression in the target cells.
In this study, we transduced human cord blood samples with FMEV vectors encoding the enhanced GFP (EGFP) marker gene and investigated the kinetics of engraftment of retrovirally transduced cells in irradiated NOD/SCID recipients. In addition, we have studied by conventional Southern blotting the provirus copy number and the proviral insertion sites in human transduced cells repopulating the NOD/SCID recipients. By means of this molecular assay previously used for defining the HSC dynamics of the mouse,34 35 we have determined for the first time the clonal makeup of the human hematopoiesis engrafting NOD/SCID recipients. The relevance of our observations in the development of clinical protocols of stem cell gene therapy and the biology of human HSCs is discussed.
Materials and methods
Producer cell lines
The PG13 packaging cells (provided by A.D. Miller, Fred Hutchinson Cancer Research Center, Seattle, WA) were infected with 0.45 μm-filtered supernatants of the amphotropic PA317/EGFP1 producer cell line, which contains the SF-EGFP1 retroviral vector.33 One week later, EGFP expression was measured by FACS analysis in an EPICS ELITE-ESP (Coulter, Hialeah, FL). Single cells with an intermediate or high level of green fluorescence were sorted and seeded in 96-well cell culture plates by using an Autoclone device. The titer of infective virus generated by a total of 60 clones was tested by infecting HeLa cells with the corresponding infective supernatants, followed by FACS analysis of EGFP expression. The clone exhibiting the highest viral titer, termed PG13/EGFP7, was selected for further experiments. This clone grows normally, and viral titers of about 1 × 106 infectious particles/mL have been observed for more than 18 months.
Transduction of CD34+ cells
Cells were obtained from human umbilical cord blood after normal full-term deliveries, according to the protocol approved by the Ethical Committee of the Institut de Recerca Oncològica. CD34+ cells were purified by an immunomagnetic method (Minimacs, Miltenyi Biotec, Gladbach, Germany). Twenty-five cm2 canted neck tissue culture flasks (TPP, Trasadingen, Switzerland) were coated with recombinant CH-296 (Takara Shuzo, Otsu, Japan) at 4 μg/cm2. Purified CD34+ cells were stimulated in these culture flasks for 48 hours with Iscove's modified Dulbecco's medium (Gibco BRL, Grand Island, NY) supplemented with 12.5% defined horse serum (Biological Industries, Kibbutz Beit Haemek, Israel), 12.5% defined fetal calf serum (FCS) (Biological Industries), 2 mmol/L l-glutamine solution, 1 mmol/L sodium pyruvate solution, 10−3 mmol/L hydrocortisone, 10−4 mol/L β-mercaptoethanol, 50 IU/mL-50 μg/mL penicillin-streptomycin and with combinations of the following recombinant human growth factors: 10 ng/mL recombinant human megacaryocyte growth and development factor (rhMGDF), 100 ng/mL stem cell factor (rhSCF), 50 ng/mL flt-3 ligand (rhFL) (these 3 cytokines were kindly provided by Amgen, Thousand Oaks, CA). In some experiments, 100 ng/mL recombinant human interleukin-6 (rhIL-6) alone or in combination with 50 ng/mL rhIL-3 (both kindly provided by Novartis, Basel, Switzerland) were also included. Cells were incubated at a density of 0.5 to 1 × 105 cells/mL and maintained at 37°C in 5% CO2 in fully humidified incubators. For the infection, 90% of the medium was replaced daily with 0.45 μm-filtered fresh vector-containing supernatant and 4 μg/mL protamine sulfate, for 2 consecutive days. Cells were harvested 24 hours after the second transduction cycle.
Transplantation of NOD/SCID mice
NOD/LtSz-scid/scid (NOD/SCID) mice (deficient in Fc receptors, complement function, natural killer, B- and T-cell function) were used as recipients of the human hematopoietic cells. Mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were handled under sterile conditions and maintained in microisolators. Before transplantation, 6–8-week-old mice were total body irradiated with 2.5 Gy of x-rays (300 kV, 10 mA; Philips MG-324, Hamburg, Germany). Mice were transplanted either with unsorted cells obtained 24 hours after the second infection cycle or with purified EGFP+ or EGFP− cell fractions obtained by cell sorting in an EPICS Elite ESP cell sorter (Coulter).
Flow cytometry
Phenotype and EGFP expression analyses in transduced cells and in transplanted mice were conducted essentially as previously described.36 A minimum of 1 × 105 cells was incubated with 10% human AB serum in phosphate-buffered saline, and stained with phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody (mAb) (Anti-HPCA-2, Becton Dickinson, San Jose, CA) and allophycocyanin (APC)-conjugated anti-CD38 mAb (Clone HIT2, Caltag, Burlingame, CA) for 20 minutes at room temperature. Cells were washed and analyzed using an EPICS ELITE-ESP cytometer (Coulter) equipped with an argon ion laser tuned at 488 nm and a He-Ne laser tuned at 620 nm. Cells within a forward versus side scatter gate were analyzed for the expression of EGFP, CD34, and CD38 antigens. PE- and APC-conjugated mouse isotypic mAbs served as controls. CD34+/CD38low cells were defined as those cells expressing levels of CD34 above the isotypic control and levels of CD38 below the 5th percentile of APC fluorescence. For analyzing NOD/SCID recipients, hematopoietic samples were aspirated from the femoral BM at periodic intervals following transplantation, as previously described.36 At the end of the experiments, generally 90 to 120 days after transplantation, mice were killed and BM, peripheral blood, and spleen cells were analyzed by flow cytometry for the presence of human cells and EGFP expression. Aliquots containing 1 to 5 × 105 cells were stained with anti-human CD45-PECy5 mAb (Clone J33, Immunotech, Marseille, France) in combination with either antihuman-CD34-PE mAb, antihuman CD33-PE mAb (Anti-Leu-M9, Becton Dickinson), or antihuman CD19-PE (Anti-Leu-12, Becton Dickinson). Thereafter, red blood cells were lysed by adding 2.5 mL lysis solution (0.155 mol/L NH4Cl + 0.01 mol/L KHCO3 + 10−4 mol/L EDTA) and incubating at room temperature for 10 minutes. Cells were then washed in PBA (phosphate-buffered salt solution with 0.1% bovine serum albumin and 0.01% sodium azide), resuspended in PBA + 2 μg/mL propidium iodide (PI) added to the cells before the analysis, and analyzed by flow cytometry. In all instances, mAbs were titrated with reference cells and used at saturating concentrations. Cells labeled with conjugated nonspecific isotypic mAbs were used as controls. In addition, BM cells from nontransplanted NOD/SCID mice were also stained with the same antihuman mAbs. Listmode analysis was done by using the WinMDI free software (a kindly gift of Dr J Trotter, The Scrips Research Institute, La Jolla, CA).
Southern blotting of samples from NOD/SCID transplanted mice
Genomic DNA was extracted from BM or spleen cells as previously described.37 Briefly, 10 μg DNA was digested overnight with restriction enzymes that cut once within the provirus (BamHJ) or in both Long Terminal Reports (LTR) sequences (Asp718) (Figure 5). Digested DNA was electrophoresed on 0.8% agarose gels, transferred to nylon membranes, and hybridized with an EGFP probe obtained from the EcoRI/NotI fragment of the pEGFP-N1 plasmid (Clontech, Palo Alto, CA) to detect retroviral sequences. To determine the contribution of human cells on the analyzed tissues, membranes were stripped and rehybridized with a probe for the human CD4 gene38 (kindly provided by M.L. Toribio, CBM, Madrid, Spain). For densitometric analyses, samples were scanned and densitometered using the BIO-RAD FX Molecular Imager.
Marker rescue assay for helper virus production in the transplanted mice
The absence of helper viruses in infective supernatants and in BM samples from NOD/SCID recipients was confirmed by the marker rescue assay.39 A minimum of 3.5 × 105 fresh BM cells from each transplanted NOD/SCID mouse was incubated in supplemented Iscove's modified Dulbecco's medium (see preparation of medium for CD34+ cell transduction) in 6-well culture plates. After 24 hours of incubation, 1 mL of the supernatant sample was collected, filtered through 0.45-μm syringe filters, and added on HeLa cells seeded in 12-well culture plates in the presence of 4 μg/mL protamine sulfate. Plates were then centrifuged at 1800 rpm for 90 minutes and incubated for 48 hours at 37°C in 5% CO2. These cells were then trypsinized and analyzed for EGFP expression by flow cytometry. In no instance were detectable replication competent retroviruses deduced from this marker rescue assay (data not shown).
Statistics
Data are presented as the mean ± SE of the mean. The significance of differences between groups was determined by using the 2-tailed Student t test. The statistical analysis of the data was performed using the Stata Statistical Software, Release 5.0 (Stata Corporation, College Station, TX).
Results
Stable reconstitution of NOD/SCID mice with human cells expressing the EGFP transgene
In the first set of experiments, purified CD34+ human cord blood cells were transduced according to the gene transfer protocol described in Material and methods and then transplanted into NOD/SCID recipients for conducting in vivo studies of engraftment and transgene expression. Table 1 shows the phenotype of samples corresponding to 8 of 11 different experiments in which engraftment of human cells was observed at least at 1 time point after transplantation (Table 2).
Phenotype and EGFP expression in human cord blood cells used for transplantation into NOD/SCID mice
| Cord Blood Ref . | HGFs . | % CD34+ . | Fold Expansion (post vs preinfection) . | % EGFP+ in: . | ||||
|---|---|---|---|---|---|---|---|---|
| Preinfection . | Postinfection . | Total Cells . | CD34+ . | Total Cells . | CD34+ Cells . | CD34+/ CD38low Cells . | ||
| CB-4 | M/S/F/6/3 | 92.3 | 58.7 | 5.8x | 3.7x | 63.7 | 58.8 | 63.6 |
| CB-5 | M/S/F/6/3 | 92.1 | 88.1 | 0.8x | 0.8x | 72.1 | 71.2 | 35.6 |
| CB-9 | M/S/F/6/3 | 80.5 | 69.3 | 5.0x | 4.4x | 90.8 | 93.6 | 81.6 |
| CB-10 | M/S/F/6/3 | 94.0 | 74.5 | 3.4x | 2.7x | 92.1 | 93.3 | 86.7 |
| CB-14 | M/S/F/6/3 | 97.1 | 48.2 | 2.5x | 1.3x | 60.7 | 64.0 | 53.7 |
| CB-15 | M/S/F/6/3 | 94.2 | 46.7 | 2.2x | 1.1x | 68.4 | 77.2 | 59.4 |
| CB-16 | M/S/F | 79.6 | 64.2 | 1.0x | 0.9x | 32.0 | 37.8 | 34.5 |
| CB-17 | M/S/F | 86.4 | 65.1 | 2.1x | 1.6x | 30.5 | 33.3 | 30.4 |
| Mean ± SE | 89.5 ± 2.3 | 64.4 ± 4.8 | 2.8 ± 0.6 | 2.0 ± 0.5 | 63.8 ± 8.2 | 66.2 ± 8.0 | 55.7 ± 7.6 | |
| Cord Blood Ref . | HGFs . | % CD34+ . | Fold Expansion (post vs preinfection) . | % EGFP+ in: . | ||||
|---|---|---|---|---|---|---|---|---|
| Preinfection . | Postinfection . | Total Cells . | CD34+ . | Total Cells . | CD34+ Cells . | CD34+/ CD38low Cells . | ||
| CB-4 | M/S/F/6/3 | 92.3 | 58.7 | 5.8x | 3.7x | 63.7 | 58.8 | 63.6 |
| CB-5 | M/S/F/6/3 | 92.1 | 88.1 | 0.8x | 0.8x | 72.1 | 71.2 | 35.6 |
| CB-9 | M/S/F/6/3 | 80.5 | 69.3 | 5.0x | 4.4x | 90.8 | 93.6 | 81.6 |
| CB-10 | M/S/F/6/3 | 94.0 | 74.5 | 3.4x | 2.7x | 92.1 | 93.3 | 86.7 |
| CB-14 | M/S/F/6/3 | 97.1 | 48.2 | 2.5x | 1.3x | 60.7 | 64.0 | 53.7 |
| CB-15 | M/S/F/6/3 | 94.2 | 46.7 | 2.2x | 1.1x | 68.4 | 77.2 | 59.4 |
| CB-16 | M/S/F | 79.6 | 64.2 | 1.0x | 0.9x | 32.0 | 37.8 | 34.5 |
| CB-17 | M/S/F | 86.4 | 65.1 | 2.1x | 1.6x | 30.5 | 33.3 | 30.4 |
| Mean ± SE | 89.5 ± 2.3 | 64.4 ± 4.8 | 2.8 ± 0.6 | 2.0 ± 0.5 | 63.8 ± 8.2 | 66.2 ± 8.0 | 55.7 ± 7.6 | |
CD34+ cord blood cells were transduced as indicated in Materials and methods, and analyzed at the end of the last infection cycle before transplantation into NOD/SCID mice (NOD/SCID data are shown in Table 2).
HGFs indicates hematopoietic growth factors (3, IL-3; 6, IL-6; F, Flt3-ligand; M, megacaryocyte growth and development factor; S, stem cell factor).
Phenotype and EGFP expression of human CB cells engrafting the bone marrow of NOD/SCID mice
| Mouse Ref . | Graft Size ×106Cells . | % CD45+ . | % CD45+/EGFP+ . | % CD34+ . | % CD34+/EGFP+ . | % EGFP+ . | |
|---|---|---|---|---|---|---|---|
| In Total Bone Marrow . | In CD45+Cells . | In CD34+ Cells . | |||||
| 4M* | 7.1* | 23.4* | 9.2* | 8.3* | 1.7* | 39.5* | 20.0* |
| 5O | 1.9 | 9.5 | 8.8 | 2.3 | 2.0 | 92.6 | 87.5 |
| 9B† | 1.9† | 2.7† | 2.4† | 0.6† | 0.3† | 89.0† | 50.1† |
| 10C | 2.3 | 18.9 | 4.1 | 2.7 | 0.5 | 21.7 | 18.5 |
| 14B | 3.5 | 79.4 | 11.2 | 14.4 | 1.4 | 14.1 | 9.5 |
| 15A† | 1.5† | 83.6† | 56.6† | 32.9† | 16.8† | 67.7† | 51.0† |
| 15B | 1.5 | 83.7 | 33.5 | 13.8 | 5.9 | 40.0 | 43.1 |
| 16A | 2.8 | 30.5 | 16.7 | 3.4 | 0.9 | 54.8 | 26.5 |
| 16B | 2.8 | 83.0 | 3.0 | 12.5 | 1.1 | 3.6 | 8.8 |
| 17B | 2.3 | 66.1 | 5.7 | 10.1 | 0.6 | 8.6 | 6.0 |
| Mean ± SE | 2.4 ± 0.2‡ | 53.0 ± 12.2‡ | 11.9 ± 4.0‡ | 8.5 ± 2.1‡ | 1.9 ± 0.7‡ | 33.6 ± 12.0‡ | 28.6 ± 11.0‡ |
| Mouse Ref . | Graft Size ×106Cells . | % CD45+ . | % CD45+/EGFP+ . | % CD34+ . | % CD34+/EGFP+ . | % EGFP+ . | |
|---|---|---|---|---|---|---|---|
| In Total Bone Marrow . | In CD45+Cells . | In CD34+ Cells . | |||||
| 4M* | 7.1* | 23.4* | 9.2* | 8.3* | 1.7* | 39.5* | 20.0* |
| 5O | 1.9 | 9.5 | 8.8 | 2.3 | 2.0 | 92.6 | 87.5 |
| 9B† | 1.9† | 2.7† | 2.4† | 0.6† | 0.3† | 89.0† | 50.1† |
| 10C | 2.3 | 18.9 | 4.1 | 2.7 | 0.5 | 21.7 | 18.5 |
| 14B | 3.5 | 79.4 | 11.2 | 14.4 | 1.4 | 14.1 | 9.5 |
| 15A† | 1.5† | 83.6† | 56.6† | 32.9† | 16.8† | 67.7† | 51.0† |
| 15B | 1.5 | 83.7 | 33.5 | 13.8 | 5.9 | 40.0 | 43.1 |
| 16A | 2.8 | 30.5 | 16.7 | 3.4 | 0.9 | 54.8 | 26.5 |
| 16B | 2.8 | 83.0 | 3.0 | 12.5 | 1.1 | 3.6 | 8.8 |
| 17B | 2.3 | 66.1 | 5.7 | 10.1 | 0.6 | 8.6 | 6.0 |
| Mean ± SE | 2.4 ± 0.2‡ | 53.0 ± 12.2‡ | 11.9 ± 4.0‡ | 8.5 ± 2.1‡ | 1.9 ± 0.7‡ | 33.6 ± 12.0‡ | 28.6 ± 11.0‡ |
Data show analysis of individual recipients at 90 to 120 days after transplantation except for
(20 days) and for
(40 days).
Only data corresponding to 90 to 120 days after transplantation are considered for determinations of mean values. Numbers in the code of each recipient correspond to the origin of the cord blood graft in Table 1.
As deduced from Table 1, a decrease in the proportion of CD34+ cells was observed after the infection period, concomitant with an expansion in the total cell counts (2.8-fold) and in the absolute content of CD34+ cells (2-fold). The analysis of EGFP fluorescence in these samples showed a very high proportion of cells expressing the transgene, not only in the CD34+ population (66.2%) but also in the more immature CD34+/CD38low cell subset (mean 55.7%).
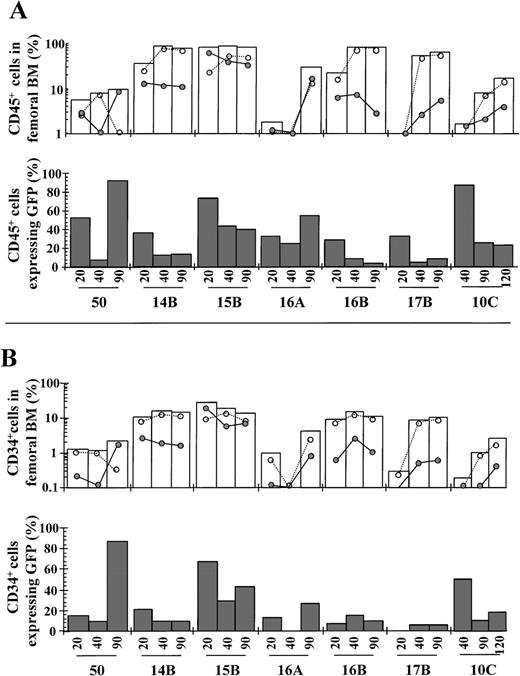
After transplantation of transduced cord blood samples into NOD/SCID mice, small marrow aspirates were periodically drawn to determine the kinetics of engraftment and the proportion of human cells expressing the transgene (Figure 1A and 1B, respectively). Individual data corresponding to the last BM sampling, and mean values corresponding to analysis performed at 90 to 120 days after transplantation, are shown in Table 2. Consistent with previous studies,40 a modest engraftment of CD45+ cells was observed in most animals at day 20 compared to that observed at days 90 to 120 after transplantation (empty bars in Figure 1A). At these latter periods, very high levels of engraftment of CD45+ cells were observed in the BM of most animals (average 53%). The kinetics of engraftment of human cells expressing the transgene (CD45+/EGFP+ cells; represented by filled points) and nonexpressing the transgene (CD45+/EGFP− cells; empty points) are also shown in Figure 1A. As deduced from this analysis, the engraftment kinetics of these 2 populations greatly varied among the different recipients, even in the case of recipients 16A and 16B, which were transplanted with similar aliquots of the same transduced cord blood sample. Despite this high level of variability, the overall proportion of CD45+/EGFP+ cells in the BM of recipients was essentially preserved throughout the observation period. When considering data obtained at 90 to 120 days after transplantation, an average of 11.9% of total NOD/SCID BM consisted of human CD45+ cells expressing the transgene (Table 2).
Kinetics of engraftment of transduced CD34+cord blood cells in NOD/SCID mice.
NOD/SCID mice were transplanted with unsorted transduced cells, as described in Materials and methods. At different times after transplantation, BM samples from NOD/SCID recipients were obtained and the rate of engraftment and proportion of EGFP fluorescent cells determined by FACS analysis. Panels A and B show, respectively, data deduced from human CD45+ and CD34+ analysis (Table 2 presents BM codes). White bars represent the kinetics of engraftment of human cells, either expressing (•) or not expressing (○) the EGFP transgene. Dark bars represent the proportion of human cells expressing EGFP. The phenotypic characteristics of the different grafts are shown in Table 1. Data corresponding to last analyses are shown in Table 2.
Kinetics of engraftment of transduced CD34+cord blood cells in NOD/SCID mice.
NOD/SCID mice were transplanted with unsorted transduced cells, as described in Materials and methods. At different times after transplantation, BM samples from NOD/SCID recipients were obtained and the rate of engraftment and proportion of EGFP fluorescent cells determined by FACS analysis. Panels A and B show, respectively, data deduced from human CD45+ and CD34+ analysis (Table 2 presents BM codes). White bars represent the kinetics of engraftment of human cells, either expressing (•) or not expressing (○) the EGFP transgene. Dark bars represent the proportion of human cells expressing EGFP. The phenotypic characteristics of the different grafts are shown in Table 1. Data corresponding to last analyses are shown in Table 2.
An overall reduction in the percentage of CD45+ cells expressing EGFP was observed from day 20 to day 40 after transplantation (filled bars in Figure 1A). However, these proportions were essentially maintained from day 40 to days 90 to 120 after transplantation. Moreover, in 2 animals (5O and 16A), the proportion of CD45+ cells expressing EGFP was higher at day 90 (93% and 55%, respectively) with respect to day 20 after transplantation (50% and 30%, respectively). On average about a third of the human CD45+ cells expressed the EGFP transgene at 90 to 120 days after transplantation (Table 2).
Analysis of engraftment was also conducted in the human CD34+ cell population present in recipient BM (Figure 1B). Essentially similar results to those observed in the CD45+analysis were obtained, because no evidence of generalized extinction of EGFP+ cells was observed in this cell population. Moreover, as deduced from our data, a high proportion of CD34+ cells expressed EGFP at 90 to120 days after transplantation (mean 28.6%; not significantly different from the proportion observed in the CD45+ population. Table 2).
Reconstitution of NOD-SCID mice transplanted with purified populations of EGFP+ and EGFP− cells
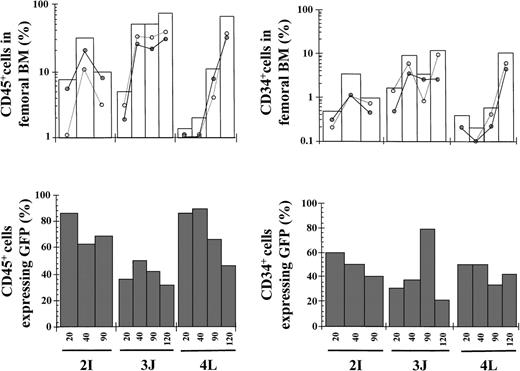
To investigate to what extent the engraftment of human EGFP expressing cells was increased by transplanting a purified population of EGFP+ cells, transduced grafts were subjected to cell sorting before the transplantation into NOD/SCID mice (95%-98% purity). As it was observed in mice transplanted with 2.4 × 106 unsorted cells, high levels of CD45+ and CD34+ cell engraftment were found in the BM of mice transplanted with 3.5 to 5.1 × 105purified EGFP+ cells (49.9% and 7.9% in total BM, respectively). Moreover, as much as 57.7% and 41.2% of the human CD45+ and CD34+ cells, respectively, expressed the EGFP transgene at 90 to 120 days after transplantation (Table3 and Figure2).
EGFP expression in transduced human cord blood cells before and after purification and transplantation into NOD/SCID mice
| Analysis of CB Grafts . | Analysis of NOD/SCID Recipients . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cord Blood (ref) . | % CD34+ Postinfection . | % EGFP+ Postinfection: . | % EGFP+ Postsorting . | Mouse (ref) . | Graft Size ×106 Cells . | CD45+CD45+/EGFP+CD34+CD34+/EGFP+% EGFP+ . | ||||||||
| Total Cells . | CD34+ . | CD34+/ CD38low . | Total Cells . | in total BM . | CD45+ . | CD34+ . | ||||||||
| CB-2 | 81.1 | 39.2 | 27.2 | 4.7 | (+) | 95.4 | 2I | 0.51 | 10.0 | 6.9 | 1.0 | 4.0 | 69.0 | 40.0 |
| (−) | 0.9 | 2G | 0.29 | 25.6 | 7.5 | 3.8 | 0.7 | 22.4 | 17.5 | |||||
| (−) | 0.9 | 2H | 0.29 | 89.4 | 16.8 | 12.9 | 2.2 | 18.8 | 17.3 | |||||
| CB-3 | 55.2 | 78.8 | 78.6 | N.D. | (+) | 98.1 | 3J | 0.42 | 72.9 | 22.8 | 12.0 | 2.5 | 31.3 | 20.7 |
| (−) | 1.03-151 | 3K3-151 | 0.093-150 | 0.73-151 | NE | NE | NE | NE | NE | |||||
| CB-43-150 | 58.7 | 63.7 | 58.6 | 63.6 | (+) | 96.2 | 4L | 0.35 | 66.9 | 30.9 | 10.7 | 4.5 | 46.3 | 42.3 |
| Mean ± SE | (+) | 96.5 | 0.43 ± 0.05 | 49.9 ± 20.4 | 20.2 ± 7.2 | 7.9 ± 3.5 | 3.7 ± 0.6 | 57.7 ± 11.2 | 41.2 ± 7.0 | |||||
| Mean | (−) | 0.9 | 0.29 | 57.5 | 12.2 | 8.3 | 1.5 | 20.6 | 17.4 | |||||
| Analysis of CB Grafts . | Analysis of NOD/SCID Recipients . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cord Blood (ref) . | % CD34+ Postinfection . | % EGFP+ Postinfection: . | % EGFP+ Postsorting . | Mouse (ref) . | Graft Size ×106 Cells . | CD45+CD45+/EGFP+CD34+CD34+/EGFP+% EGFP+ . | ||||||||
| Total Cells . | CD34+ . | CD34+/ CD38low . | Total Cells . | in total BM . | CD45+ . | CD34+ . | ||||||||
| CB-2 | 81.1 | 39.2 | 27.2 | 4.7 | (+) | 95.4 | 2I | 0.51 | 10.0 | 6.9 | 1.0 | 4.0 | 69.0 | 40.0 |
| (−) | 0.9 | 2G | 0.29 | 25.6 | 7.5 | 3.8 | 0.7 | 22.4 | 17.5 | |||||
| (−) | 0.9 | 2H | 0.29 | 89.4 | 16.8 | 12.9 | 2.2 | 18.8 | 17.3 | |||||
| CB-3 | 55.2 | 78.8 | 78.6 | N.D. | (+) | 98.1 | 3J | 0.42 | 72.9 | 22.8 | 12.0 | 2.5 | 31.3 | 20.7 |
| (−) | 1.03-151 | 3K3-151 | 0.093-150 | 0.73-151 | NE | NE | NE | NE | NE | |||||
| CB-43-150 | 58.7 | 63.7 | 58.6 | 63.6 | (+) | 96.2 | 4L | 0.35 | 66.9 | 30.9 | 10.7 | 4.5 | 46.3 | 42.3 |
| Mean ± SE | (+) | 96.5 | 0.43 ± 0.05 | 49.9 ± 20.4 | 20.2 ± 7.2 | 7.9 ± 3.5 | 3.7 ± 0.6 | 57.7 ± 11.2 | 41.2 ± 7.0 | |||||
| Mean | (−) | 0.9 | 0.29 | 57.5 | 12.2 | 8.3 | 1.5 | 20.6 | 17.4 | |||||
CD34+ cord blood cells were transduced, sorted on the basis of EGFP expression (see Materials and methods) and transplanted into NOD/SCID mice. Samples were analyzed at the end of the last infection cycle or at 90 to 120 days after transplantation of positive and negative fractions, except for
(40 days).
An aliquot of this CB was also transplanted into mice prior to the cell sorting process (see Tables 1 and 2). (+) positively and (−) negatively selected cells. NE indicates not evaluable; ND indicates not done. CB2 and CB3 samples were stimulated with MGDF/SCF/Flt3-ligand/IL-6. For CB4 stimulation IL-3 was also included.
Kinetics of engraftment of transduced purified EGFP+ cells in NOD/SCID mice.
Transduced cord blood samples underwent cell sorting, and cells positive for EGFP expression were transplanted into NOD/SCID mice. Symbols are as in Figure 1. The phenotypic characteristics of the different grafts and data corresponding to 120 days after transplantation are shown in Table 3.
Kinetics of engraftment of transduced purified EGFP+ cells in NOD/SCID mice.
Transduced cord blood samples underwent cell sorting, and cells positive for EGFP expression were transplanted into NOD/SCID mice. Symbols are as in Figure 1. The phenotypic characteristics of the different grafts and data corresponding to 120 days after transplantation are shown in Table 3.
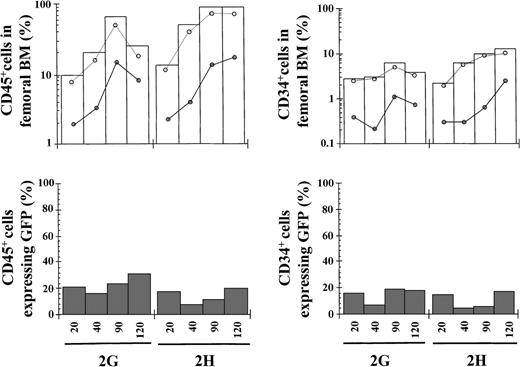
In 2 separate experiments, the cellular fraction corresponding to EGFP− cells was transplanted. Two animals receiving 2.9 × 105 cells engrafted in the long-term, whereas 1 recipient infused with only 9 × 104 cells did not engraft (Table 3 and empty bars in Figure3). Interestingly, a significant engraftment of CD45+/EGFP+ cells and CD34+/EGFP+ cells was also observed in these mice (filled points in Figure 3). In fact, 12.2% and 1.5% of total BM harvested at 120 days after transplantation consisted of human CD45+ and CD34+ cells expressing the transgene. Moreover, 20.6% and 17.4% of CD45+ and CD34+cells, respectively, were positive for EGFP expression at 120 days after transplantation (Table 3).
Kinetics of engraftment of transduced purified EGFP− cells in NOD/SCID mice.
Transduced cord blood samples underwent cell sorting and cells negative for EGFP expression were transplanted into NOD/SCID mice. Symbols are as in Figure 1. The phenotypic characteristics of the different grafts and data corresponding to 120 days after transplantation are shown in Table 3.
Kinetics of engraftment of transduced purified EGFP− cells in NOD/SCID mice.
Transduced cord blood samples underwent cell sorting and cells negative for EGFP expression were transplanted into NOD/SCID mice. Symbols are as in Figure 1. The phenotypic characteristics of the different grafts and data corresponding to 120 days after transplantation are shown in Table 3.
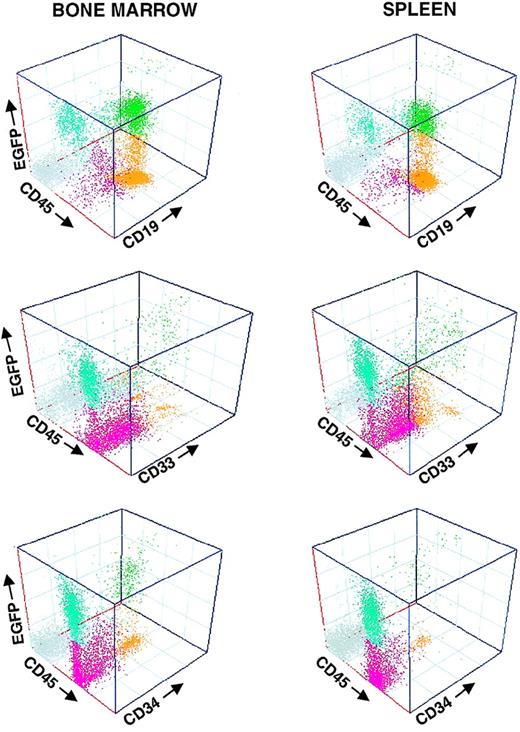
Multilineage expression of the EGFP transgene in vivo
To investigate whether transgene expression was restricted to particular hematopoietic lineages, EGFP fluorescence analysis of recipient hematopoietic tissues was coupled with analysis of lineage differentiation. In accordance with previous reports, human engraftments consisted predominantly of human B lymphocytes and myeloid cells. No human T lymphocytes, or minimal numbers of these cells, were found in the transplanted animals, either in the BM, spleen, thymus, or peripheral blood. Figure 4 shows the analysis of the BM and spleen of a representative NOD/SCID recipient at 90 days after transplantation (mouse 4L; Table 3 has further details). EGFP expression was clearly observed in the myeloid and the B-cell compartments, as well as in the CD34+ cell population. As expected, no EGFP+ cells were found in mouse populations negative for the human CD45 marker.
Multilineage engraftment of transduced cells in NOD/SCID mice.
Histograms represent the immunophenotyping of 1 recipient transplanted with sorted EGFP+ cells. Samples were analyzed at 120 days after transplantation. For further details see mouse 4L in Table 3 and Figure 2.
Genetic analysis of human transduced cells in NOD/SCID recipients
Because of the high levels of engraftment of human cells expressing the EGFP transgene, we aimed to correlate the data obtained by flow cytometry with the molecular information derived from Southern blot analysis.
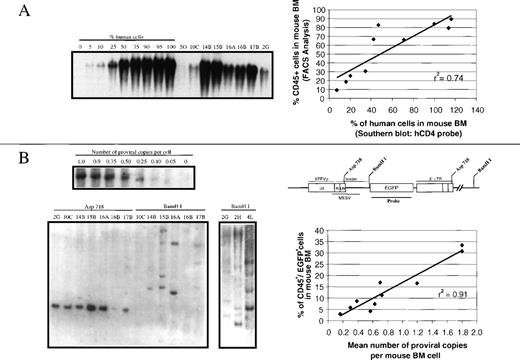
Estimations of human hematopoietic engraftment in BM samples were made on the basis of hybridizations of BM DNA with a human CD4 probe (see the representative analysis in Figure 5A). As expected, the values of human engraftment deduced from densitometric determinations of Southern blots correlated well (r2 = 0.74) with determinations of CD45+ cells by flow cytometry (Figure 5A).
Southern blot analysis of BM samples from mice transplanted with transduced cord blood cells.
Genomic DNA from BM cells harvested at 90 to 120 days after transplantation was digested with BamHJ or Asp718, and hybridized with probes for human CD4 and EGFP. (Panel A) Representative analysis of a human CD4 hybridization of BM DNA digested with BamHJ. Quantification of engraftment was done by densitometric analysis of test and control DNA samples prepared by mixing graded proportions of human and mouse DNA. Mouse 4L was transplanted with purified EGFP+ cells, and mice 2G and 2H with purified EGFP− cells. The correlation analysis of these data with respect to CD45+determinations (Tables 2 and 3) is shown in the plot. (Panel B) EGFP hybridizations of BM DNA samples digested with Asp 718 or BamHJ. A standard curve of the number of proviral copies per cell was constructed by mixing different proportions of DNA from a cell line bearing a single copy of EGFP with DNA from nontransduced cells. The correlation analysis of the estimated number of copies per BM cell with respect to determinations of the proportion of CD45+/EGFP+ cells in total BM (Tables 2 and 3) is shown in the plot. To investigate the clonal composition of the engrafted transduced human cells, samples were digested with BamHJ. Restriction sites within the EGFP1 vector are also shown.
Southern blot analysis of BM samples from mice transplanted with transduced cord blood cells.
Genomic DNA from BM cells harvested at 90 to 120 days after transplantation was digested with BamHJ or Asp718, and hybridized with probes for human CD4 and EGFP. (Panel A) Representative analysis of a human CD4 hybridization of BM DNA digested with BamHJ. Quantification of engraftment was done by densitometric analysis of test and control DNA samples prepared by mixing graded proportions of human and mouse DNA. Mouse 4L was transplanted with purified EGFP+ cells, and mice 2G and 2H with purified EGFP− cells. The correlation analysis of these data with respect to CD45+determinations (Tables 2 and 3) is shown in the plot. (Panel B) EGFP hybridizations of BM DNA samples digested with Asp 718 or BamHJ. A standard curve of the number of proviral copies per cell was constructed by mixing different proportions of DNA from a cell line bearing a single copy of EGFP with DNA from nontransduced cells. The correlation analysis of the estimated number of copies per BM cell with respect to determinations of the proportion of CD45+/EGFP+ cells in total BM (Tables 2 and 3) is shown in the plot. To investigate the clonal composition of the engrafted transduced human cells, samples were digested with BamHJ. Restriction sites within the EGFP1 vector are also shown.
Regarding hybridizations with the EGFP probe, samples digested with Asp 718 revealed the presence of a single band with the expected size of 2.5 kb (see the representative analysis in Figure 5B). Densitometric analysis of these bands indicated an average of 0.7 copies per BM cell in mice transplanted with unsorted transduced cells. A similar value was found in 2 animals receiving purified EGFP− cells (0.6 and 0.7 copies/cell in mice 2G and 2H), and 1.8 copies per cell were found in a mouse transplanted with purified EGFP+cells (4L). As shown in Figure 5B, a good correlation (r2 = 0.91) between the number of proviral copies per BM cell with respect to the proportion of human CD45+/EGFP+ cells in total BM was also observed.
Finally, to investigate the clonal makeup of transduced human hematopoiesis engrafting NOD/SCID mice, BM samples were digested with BamHJ (see the representative analysis in Figure 5B). At the time of analysis, the repopulation of human transduced cells was generally produced at the expense of 1 to 4 predominant repopulating clones, which accounted for 20% to 100% of the engraftment of human transduced cells. In addition, a variable number of less represented repopulating clones (up to 7) could be detected in some animals. This pattern of clonal repopulation was observed not only in mice transplanted with a low number of sorted cells (between 2.9 and 5.1 × 105 cells; see samples 4L, 2G, and 2H), but also in animals infused with larger (5-10 times) unsorted grafts (see samples 10C-17B), and regardless on the rates of engraftment of transduced cells.
Discussion
Long-term genetic modification of the human hematopoietic system relies on the efficient transduction of the self-renewing HSCs and the stable expression of the transgene in their progeny in vivo. Although gene transfer into human hematopoietic repopulating cells has traditionally been elusive, new tools and improved models of study allowed us to focus our investigation on the retroviral-mediated gene transfer into human hematopoietic progenitor cells with in vivo repopulating ability.
Analysis of EGFP expression in animals transplanted with unsorted transduced cells revealed an estimated rate of gene transfer into the NOD/SCID repopulating cells of 34%, assuming there are no differences between the repopulating ability of transduced and nontransduced precursors. Moreover, the high levels of engraftment observed in most animals implied that, on average, 11.9% of total NOD/SCID BM consisted of human cells expressing the transgene at 3 to 4 months after transplantation.
The use of retroviral vectors bearing the GALV envelope rather than the amphotropic envelope seems critical for achieving high efficiencies of retroviral gene transfer into very primitive human hematopoietic precursors. Expression of the receptor used by GALV pseudotyped vectors, Pit-1, has been reported to be higher in human CD34+ and CD34+/CD38− cells than the amphotropic receptor (Pit-2).41,42 Using the same retroviral vector that we used in these experiments, NOD/SCID mice transplanted with transduced cord blood samples expressed the EGFP transgene in a much higher proportion than mice transplanted with amphotropic vectors bearing the same transgene.17 In addition, the inclusion of the FN fragment CH-296 during the stimulation and the infection process may have been critical for the achievement of our results. In this respect, previous studies have shown the efficacy of this molecule not only for enhancing the retroviral gene transfer efficiency into human hematopoietic precursors,43 but also for preserving the regenerative capacity of these cells during the ex vivo manipulation of the graft.28 44
When considering the hematopoietic growth factors used for hematopoietic stimulation, no general consensus has been reached to date. Although FL was essential for preserving the ability of IL-3/IL-6/SCF-stimulated samples maintained in suspension cultures to repopulate bnx mice, this factor was not critical for transducing bnx-repopulating cells when a stromal or FN support was provided.18 44 It is controversial whether IL-3 induces excessive cell differentiation and subsequent loss of repopulating ability. In this respect, no evident differences were found in our animals when grafts were transduced with or without IL-3. Neither the inclusion of IL-6 in our basic combination of MGDF, SCF, and FL mediated significant improvements in the transduction efficiency or the repopulating ability of the cord blood samples. It is likely that these 3 early acting hematopoietic growth factors are sufficient for the efficient transduction and maintenance of the primitive human repopulating cells, at least when cultured on CH-296.
Significant levels of engraftment of human hematopoietic samples expressing a foreign transgene have been previously reported.16,17,19,21,22,25,45 However, our experiments in mice transplanted with unsorted cells show the highest levels of engraftment of human hematopoietic cells transduced with murine retroviral vectors reported in the literature to date. When our data are compared with those obtained after infection with lentiviral vectors capable of transducing quiescent human CD34+cells,46-48 no evident improvements in the gene transfer rates or engraftment of transduced cells are deduced from the data reported so far. In the study of Miyoshi et al,20 10% of the BM cells of NOD/SCID mice transplanted with human immunodeficiency vector-infected samples consisted of human cells expressing the EGFP transgene (data obtained at 56-154 days after transplantation). These results are comparable to those obtained in our mice transplanted with unsorted cells and below the values obtained in recipients receiving purified EGFP+ cells. Taken together these results suggest that, under adequate ex vivo manipulation procedures, HSC gene transfer protocols based on murine retroviral vectors may be as efficient as those involving short exposures to lentiviral vectors.
As deduced from the kinetics of EGFP expression in CD45+and CD34+ populations (Figure 1), reductions in the proportion of human cells expressing the transgene were generally more evident during the early stages of engraftment (before day 40 after transplantation). In some instances, however, marked increases in the proportion of human cells expressing the transgene were observed at longer times after transplantation. Differences in the transduction efficiency of the different subsets of repopulating cells, fluctuations in the proliferative expression of different repopulating clones, and silencing phenomena of the inserted proviruses are factors that may account for the kinetics of human EGFP+ cells that occurs in vivo. The relative contribution of each of these processes in the observed reductions of human cells expressing the transgene in vivo should be further studied in detail. However, the occurrence of trangene silencing in a fraction of the transduced cells can be inferred from our experiments with purified EGFP+ grafts. In these experiments, 96.5% pure EGFP+ samples were infused into these animals. However, on average, 42% of the human CD45+ cells and 59% of the human CD34+ cells from these animals did not express the transgene at 90 to 120 days after transplantation (Table 3). Although the presence of contaminating untransduced precursors in the purified grafts could account for these results, data shown in Table 3 do not support this hypothesis. In the case of mouse 3J, a total number of 420,000 purified EGFP+cells, including 230,000 CD34+/EGFP+ cells, and only 4400 CD34+/EGFP− cells were transplanted. As previously shown, even in the absence of competitive repopulating cells, either fresh or in vitro incubated cord blood samples with fewer than 10,000 CD34+ cells were not capable of engrafting the BM of irradiated NOD/SCID mice with CD45+values above 1%.40 Therefore, the presence of a human CD45+/EFGP− population representing as much as 50% of the BM from mouse 3J should be predominantly accounted by a transgene silencing phenomenon.
Also of significance from the cell sorting experiments is the striking observation that 10% to 30% of the human cells generated by purified EGFP− cells did express the transgene in vivo (Figure3). This observation indicates that a delayed expression of the marker gene is occurring in a significant proportion of transduced repopulating cells. Consistent with previous studies in which transgene expression was investigated in vitro,49 we observed optimal proportions of EGFP+ cells at 4 days after infection (data not shown). According to this information, protocols including in vitro selection procedures should carefully consider the advantages and disadvantages associated with prolonged incubations of transduced hematopoietic grafts.
The level of transgene expression in human myeloid, B cells and CD34+ cells observed in our animals not only implies an efficient transduction of primitive hematopoietic repopulating cells, but also a potent and stable activity of the promoter in about half the human population that engrafted recipient NOD/SCID mice. In FMEV vectors, the LTR of the spleen focus-forming virus (SFFV) is combined with the leader region of murine embryonic stem cell virus (MSCV) (Figure 5). When compared with the Moloney murine leukemia virus (MoMLV) enhancer, expression driven by the hybrid FMEV promoter is markedly enhanced not only in myeloerythroid cell lines but also in primary human hematopoietic progenitor cells.31
When considering our data from the Southern blots, it is worth mentioning the existence of a good correlation between the proportion of CD45+/EGFP+ cells in total BM with respect to the estimated number of proviral copies per BM cell (Figure 5B). Although our results suggest that transgene inactivation occurs in a number of engrafted cells, the above-mentioned correlation shows that limitations in the transduction efficiency or in the overall number of copies per target cell (or both) predominantly accounts for the low proportion of human EGFP-expressing cells observed in some animals (ie, 3.6%-8.8% in mice 16B, 17B; Table 2).
Regarding the clonal makeup of transplanted hematopoiesis, observations indicating that the hematopoiesis of transplanted recipients is monoclonal or oligoclonal were originally made in the murine hematopoietic system using the same approach followed in this study.2,35 More recently, using an inverse polymerase chain reaction (PCR) technique, Nolta et al50 showed the presence of T-cell and myeloid clones containing identical proviral integration sites, confirming that human precursors capable of differentiating into both lineages in bnx mice had been transduced with the retroviral vector. Using the inverse PCR approach, these authors also showed that mice transplanted with human cells transduced on stromal or FN support had an oligoclonal repopulation pattern. In particular, 1 to 6 marked clones accounted for the transduced human hematopoiesis observed in the bnx mice.45 The high and stable engraftments of human transduced cells in our NOD/SCID mice allowed us to confirm, by using Southern blot, a predominant oligoclonal reconstitution pattern of human hematopoiesis in a xenogenic transplantation model. In particular, our data indicate that a reduced number of repopulating clones, generally 1 to 4, accounted for most of the transduced human hematopoiesis that is detectable in the NOD/SCID mice at a specific time point. However, other repopulating clones with a lesser degree of contribution to human hematopoiesis are also observed in our studies. For the moment, the potential succession between these poorly represented clones and the predominant repopulating clones remains to be established.
In conclusion, our observations reveal an efficient transduction of human repopulating precursors capable of generating stable engraftment of cells expressing the transgene in vivo. We believe that procedures similar to that used in our study will facilitate the development of curative strategies of hematopoietic stem cell gene therapy for genetic and acquired diseases.
Acknowledgments
The authors wish to thank the Barcelona Cord Blood Bank for providing the cord blood samples and Gemma Capmany and Mercé Serravinyals for providing selected cord blood CD34+ cells. Also the authors wish to acknowledge the excellent technical assistance of S. Garcı́a and M.E. López for assistance in genetic analyses, I. Ormán in flow cytometry, and J. Martı́nez for carefully maintenance of the NOD/SCID mice. We are grateful to Ihor Lemischka and Anna Bigas for helpful discussions and critical reading of the manuscript.
Supported by grants from the Commission of the European Communities BMH-CT-983784; Fundación Ramón Areces; Comisión Interministerial de Ciencia y Tecnologı́a (SAF 96-0130 and 08.6/19/1997). T.P. is a recipient of a fellowship from CIRIT, Spain.
Reprints:Juan Bueren, Department of Molecular and Cellular Biology. CIEMAT, Madrid, Spain; e-mail: bueren@ciemat.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal