Abstract
The limited efficacy of hematopoietic gene therapy can be improved by in vivo selection for transduced long-term repopulating cells (LTRC). We selected for G156A MGMT (▵MGMT) transduced LTRC present in 5 × 104 to 100 × 104 marrow cells infused into nonmyeloablated mice by the administration of O6-benzylguanine (BG) and BCNU every 3 to 4 weeks. To facilitate engraftment, mice were given a nonablative dose of BG and BCNU before infusion. Without selection, ▵MGMT was not detected in any hematopoietic colony-forming units (CFU) 24 to 30 weeks after infusion. After BG and BCNU, ▵MGMT+ CFU were frequently detected, and their proportions increased with each treatment cycle. After 2 to 3 cycles of BG and BCNU, many mice were stably reconstituted with 75% to 100% ▵MGMT+ CFU for at least 6 months, representing up to 940-fold enrichment. Thus, BG and BCNU stem cell toxicity allows ▵MGMT-transduced LTRC to repopulate the bone marrow. This degree of selection pressure in nonmyeloablated mice is far greater than that observed in previous drug-resistance gene transfer studies. These data support our approved clinical trial to select for drug-resistant, transduced hematopoietic cells, potentially decreasing cumulative drug-induced myelosuppression in patients with cancer. These data also suggest that ▵MGMT may be a potent, dominant, selectable marker for use in dual gene therapy.
Successful clinical gene transfer into hematopoietic cells requires the replacement of endogenous marrow with transduced cells. This necessitates either myeloablation and reconstitution with transplanted cells or the ability to select strongly for the transduced population. These strategies are limited by the low rate of transduction into long-term repopulating cells (LTRC) and, in a nonmyeloablative setting, infusion of small numbers of cells relative to a patient's total marrow, after which the infused cells must compete for engraftment. A number of hematopoietic gene therapy clinical trials have generated poor results, with genetic modification in less than 0.1% of cells.1-4 The use of a drug-resistance gene to enable selection for transduced hematopoietic stem cells (HSC) has been explored as a strategy to improve the outcome of these trials. Examples of drug-resistance gene transfer and selection include MDR-1,5,6 DHFR,7,8 and wtMGMT.9 In these studies, marrow from drug-treated mice yielded greater numbers of transduced, drug-resistant progenitors and increased tolerance to chemotherapeutic agents than did untreated controls. However, none of these studies have efficiently selected for LTRC without prior myeloablation.
Here, we report dramatic in vivo selection for LTRC transduced with a mutant form of MGMT (ΔMGMT) in nonmyeloablated mice. MGMT encodes the protein O6-alkylguanine–DNA–alkyltransferase (AGT), which repairs BCNU lesions on DNA by direct transfer onto its active site.10 The mutant AGT (δAGT) contains a single amino acid substitution from glycine to alanine at amino acid 156, which blocks inactivation by the AGT inhibitor, O6-benzylguanine (BG).11 BCNU forms a chloroethyl lesion on O6of guanine, which, if not repaired by AGT, is converted to a permanent, covalent, interstrand DNA cross-link between the modified guanine and the opposite strand cytosine in 8 to 12 hours.12 This distinguishes BCNU from other chemotherapeutic agents used to mediate in vivo selection; BCNU is a stem cell toxin because cross-links formed in quiescent early hematopoietic cells are cytotoxic regardless of the time lag between drug exposure and cell division. BG depletes wtAGT in bone marrow cells, sensitizing both early and late progenitors to BCNU.13 However, CD34+ cells that overexpress ΔMGMT efficiently repair DNA damage formed by BCNU even in the presence of BG and are selectively protected from cytotoxicity.14 In previous myeloablation/transplantation studies, we found that ΔMGMT-transduced stem cells were protected and enriched after BG and BCNU treatment.15 This led us to test whether ΔMGMT transduction and drug selection would allow small numbers of LTRC to repopulate the hematopoietic compartment of nonmyeloablated mice with drug-resistant transduced cells.
Materials and methods
MFG-▵MGMT vector and producer cells
The MGMT cDNA was cloned from VACO 6 colon cancer cells.16 VACO 6 MGMT cDNA differed slightly from the published sequence17 (accession code NM002412), encoding a Leu at codon 67 (Phe in published sequence) and a Phe at codon 73 (Leu in published sequence); the sequence diversity did not affect the activity or BG resistance of AGT. The VACO 6 sequence was confirmed in MGMT transgenic mice.16 The G156A substitution was introduced into MGMT as described elsewhere.14 The ΔMGMT cDNA was subcloned into the MFG retroviral vector (kindly provided by Dr P Robbins, University of Pittsburgh, Pittsburgh, PA), and GP + E86 and GP + envAm12 producer cell lines (kindly provided by Dr A. Bank, Columbia University, New York, NY) were established as described elsewhere.14 The titer of the retroviral producer cells was 5 × 105 infectious particles/mL.
Transduction protocol
Bone marrow progenitors were obtained from the femur and tibia of 6- to 8-week-old male C3H/HeNCrlBR mice (Charles River, Wilmington, MA) 48 hours after treatment with 150 mg/kg 5-fluorouracil (5-FU; Pharmacia, Kalamazoo, MI). The cells were transduced as previously described.15 The producers were rendered replication defective by treatment with 10 μg/mL of mitomycin C (Bedford Laboratories, Bedford, OH). Untransduced cells were exposed to the same conditions as were transduced cells. Transduced and untransduced cells were mixed at 1:4 and 1:20 ratios at a final concentration of 5 × 106 cells/mL and were used for transplantation. Then 3 × 106 cells from each mix were treated with 20 μmol/L BG and 0 to 40 μmol/L BCNU and were plated in methylcellulose.
Cell infusion and drug administration
Recipient 7- to 9-week-old male C3H/HeNCrlBR mice received 30 mg/kg BG and 10 mg/kg BCNU 48 hours before infusion with 1 × 106bone marrow cells by tail vein injection. Mice were kept in microisolator cages and given water supplemented with bacitracin and neomycin.
BG and BCNU were solubilized and injected intraperitoneally as previously described.15 BCNU and Sentry Grade Union Carbide PEG-400 were obtained from the Developmental Therapeutics Branch, National Cancer Institute (Bethesda, MD). BG was synthesized by Dr Robert Moschel at the Frederick Cancer Research Institute (Frederick, MD). Mice were treated with 30 mg/kg BG and 10 mg/kg BCNU or were left untreated every 3 to 4 weeks after infusion, and they were killed 24 to 30 weeks after infusion.
In vitro drug treatment and colony-forming unit assay
Bone marrow cells were incubated with 0 or 20 μmol/L BG for 1 hour followed by 0 to 80 μmol/L BCNU for 2 hours. Cells were washed free of drug and plated in triplicate in methylcellulose containing 100 ng/mL rSCF (Amgen, Thousand Oaks, CA), 100 U/mL mIL-3 (Genzyme, Cambridge, MA), 7.5 U/mL hEPO (Amgen), 40 μL pokeweed mitogen spleen cell-conditioned medium, and 0.1 mmol/L hemin and were cultured for 7 days at 37°C and 5% CO2. Colony-forming unit (CFU) colonies larger than 50 cells were enumerated.
Polymerase chain reaction for provirus detection
Polymerase chain reaction (PCR) analysis of genomic DNA was performed as previously described.15 Previously described proviral specific primers were used to amplify a 443-bp fragment, and mouse β-globin primers were used to amplify a 400-bp fragment.15 The PCR conditions were 40 cycles of 94°C for 45 seconds, 60°C for 45 seconds, and 72°C for 1.5 minutes. Positive and negative controls included genomic DNA from previously demonstrated ΔMGMT+ CFU and untransduced CFU, respectively, and a water control to ensure the lack of contaminating ΔMGMT sequences in reagents.
Blood analyses
Blood was obtained from the tail vein of mice and mixed with 0.5 mol/L EDTA to prevent clotting. Counts were performed using a Sysmex K-100 (Baxter, Deerfield, IL) and normalized for the blood-to-EDTA ratio.
FACS analysis for AGT expression
Spleens and thymi were minced into small pieces then grated through a fine mesh to generate single-cell suspension. Blood was obtained by cardiac puncture, and mononuclear cells were isolated by Ficoll–Hypaque separation. Cells were prepared for flow cytometry as previously described.15 Flow cytometry was performed using a Becton Dickinson FACScan, and 20,000 events were analyzed.
Results
Preinfusion treatment of mice with 30 mg/kg BG and 10 mg/kg BCNU is not myeloablative
Myelosuppression produced by BG and BCNU was measured in mice treated with 10 mg/kg BG 1 hour before 25 to 50 mg/kg BCNU or with 30 mg/kg BG before 10 to 50 mg/kg BCNU. The LD50 of the drug combination was 10 mg/kg BG plus 40 mg/kg BCNU or 30 mg/kg BG plus 30 mg/kg BCNU. In subsequent experiments, we administered 30 mg/kg BG and 10 mg/kg BCNU, which produced moderate myelosuppression. In mice killed 48 hours after treatment, hind limb marrow cellularity decreased 57% to 10.25 ± 2.5 × 106 cells in BG- and BCNU-treated mice (n = 4) compared with 23.8 ± 3.4 × 106 cells in controls (n = 3). The concentration of committed progenitors per 1 × 105 marrow cells decreased 77%, from 189 ± 34 to 43 ± 12; BFU-E were reduced by 77%, 161 ± 28 versus 36 ± 10; CFU-GM were reduced by 79%, 25 ± 6 versus 5.4 ± 1.9; and CFU-GEMM were reduced by 63%, 2.5 ± 1.9 versus 0.9 ± 0.9. Blood counts were significantly reduced (P < .05; n = 3) 7 days after drug treatment for WBC (5.0 ± 0.6 vs. 1.3 ± 0.4 [×103/μL]), RBC (3.8 ± 0.7 vs. 2.7 ± 0.5 [×106/μL]), and platelets (564 ± 297 vs. 169 ± 45 [×103/μL]), but they recovered by day 12. Nineteen additional mice received 30 mg/kg BG and 10 mg/kg BCNU; they were followed up for more than 6 months and had normal blood counts, indicating that this dose was myelosuppressive but not myeloablative.
Transduction efficiency to colony-forming units
An MFG–ΔMGMT retroviral vector was used to transduce 5-FU–enriched mouse bone marrow-derived progenitors.14After a 48-hour transduction, 75% (72 of 96) of CFU were ΔMGMT+ by PCR. Transduced cells were mixed with untransduced cells such that the transduced cell population constituted 5%, 25%, or 100% of the mixture. To assess the relative protection from BG and BCNU after ΔMGMT transduction, cells were treated with 20 μmol/L BG and increasing doses of BCNU. The BCNU IC50plus 20 μmol/L BG increased from 3 μmol/L to 19.5 μmol/L and 9.5 μmol/L in mixtures of 100% and 25% ΔMGMT-transduced cells, respectively (Figure1).
BG and BCNU resistance in transduced CFU.
After transduction, bone marrow was mixed with untransduced cells at final ratios of 1:4 and 1:20. The cells were treated with 20 μmol/L BG and 0 to 40 μmol/L BCNU then plated in methylcellulose, and CFU growth was scored. Error bars represent mean ± SD. ▪, ΔMGMT transduced; ⧫, 25% ΔMGMT transduced; •, 5% ΔMGMT transduced; ▴, untransduced.
BG and BCNU resistance in transduced CFU.
After transduction, bone marrow was mixed with untransduced cells at final ratios of 1:4 and 1:20. The cells were treated with 20 μmol/L BG and 0 to 40 μmol/L BCNU then plated in methylcellulose, and CFU growth was scored. Error bars represent mean ± SD. ▪, ΔMGMT transduced; ⧫, 25% ΔMGMT transduced; •, 5% ΔMGMT transduced; ▴, untransduced.
▵MGMT-transduced cell infusion and mouse survival after BG and BCNU treatment
Recipient mice were pretreated with 30 mg/kg BG and 10 mg/kg BCNU to induce myelosuppression and improve engraftment. Cohorts of mice were infused 48 hours later with 1 × 106 progenitors from the 5%, 25%, or 100% ΔMGMT-transduced cell mixtures, such that the number of cells infused from the transduced cell culture was 5, 25, or 100 ( × 104) cells (approximately 4, 20, or 75 [×104] transduced cells). Noninfused control mice did not receive ΔMGMT-transduced cells. Mice received 0, 1, 2, or 3 postinfusion cycles of BG and BCNU administered every 3 to 4 weeks. Only 1 of 6 control mice survived 4 cycles of BG and BCNU. In contrast, 66% of mice from the 5 × 104 cohort, 91% from the 25 × 104 cohort, and 100% from the 100 × 104 cohort survived 3 or 4 cycles of BG and BCNU treatment (P < .005; Table1), indicating a striking survival advantage for mice receiving ΔMGMT-transduced cells.
Mouse survival after BG and BCNU
| No. Cycles of BG and BCNU* . | No. ΔMGMT + Cells Infused (×104) . | |||
|---|---|---|---|---|
| 100 . | 25 . | 5 . | 0 . | |
| 1 | 20/20 | 26/26 | 33/33 | 19/19 |
| 2 | 17/17 | 22/23 | 27/30 | 13/15 |
| 3 | 13/13‡ | 16/17‡ | 15/23 | 6/10 |
| 4 | 6/6‡ | 5/6 | 7/10 | 1/6 |
| Rows 3, 4† | 62/751-153 | 7/16 | ||
| No. Cycles of BG and BCNU* . | No. ΔMGMT + Cells Infused (×104) . | |||
|---|---|---|---|---|
| 100 . | 25 . | 5 . | 0 . | |
| 1 | 20/20 | 26/26 | 33/33 | 19/19 |
| 2 | 17/17 | 22/23 | 27/30 | 13/15 |
| 3 | 13/13‡ | 16/17‡ | 15/23 | 6/10 |
| 4 | 6/6‡ | 5/6 | 7/10 | 1/6 |
| Rows 3, 4† | 62/751-153 | 7/16 | ||
Includes both preinfusion and postinfusion BG and BCNU.
Data from 100, 25, and 5 cohorts combined.
P < .05 vs. survival without ΔMGMT+ cell infusion.
P < .0001 vs. survival without ΔMGMT+ cell infusion.
Selection for transduced progenitors
Mice were killed 24 to 30 weeks after cell infusion to test for the presence of ΔMGMT-transduced LTRC at a time when infused, short-term, repopulating progenitors had been exhausted. There was no difference in mean bone marrow cellularity between drug-treated cohorts infused with ΔMGMT-transduced cells (30.7 × 106 ± 5.8 cells/mouse) and mice that did not receive postinfusion BG and BCNU (33.3 × 106 ± 3.9 cells/mouse). The proportion of CFU that contained the ΔMGMT proviral sequence was determined by proviral-specific PCR on individual colonies (Figure2). The ΔMGMT proviral sequence was undetectable in any of the 168 marrow-derived CFU tested from 9 mice that did not receive postinfusion BG and BCNU. PCR performed on whole bone marrow preparations from these mice also did not amplify the ΔMGMT sequence. We expected a limit of detection of 1%. Furthermore, there was no detectable δAGT expression in the bone marrow of these mice by Western blot. The inability to detect genetically modified cells suggests that transduced, infused progenitors are at a competitive disadvantage to endogenous stem cells in contributing to long-term hematopoiesis. This is consistent with previous reports suggesting that 5-FU–enriched, cytokine-stimulated cells engraft poorly to nonmyeloablated mice.18-20
In vivo selection for ▵MGMT-transduced CFU.
Representative PCR on genomic DNA obtained from individual bone marrow-derived CFU from mice in the 25 × 104 cohort given (a) 0 cycles (b) 1 cycle, (c) 2 cycles, or (d) 3 cycles of BG and BCNU; (e) mouse from 100 × 104 cohort given 3 cycles of BG and BCNU. +, positive control; −, negative control; H20, water control (no genomic DNA).
In vivo selection for ▵MGMT-transduced CFU.
Representative PCR on genomic DNA obtained from individual bone marrow-derived CFU from mice in the 25 × 104 cohort given (a) 0 cycles (b) 1 cycle, (c) 2 cycles, or (d) 3 cycles of BG and BCNU; (e) mouse from 100 × 104 cohort given 3 cycles of BG and BCNU. +, positive control; −, negative control; H20, water control (no genomic DNA).
In contrast, at least some CFU from each mouse in the 100 × 104 ΔMGMT+ (n = 9) and the 25 × 104 ΔMGMT+ (n = 9) cohorts and 9 of 10 of the mice in the 5 × 104ΔMGMT+ cohort treated with 2 or 3 cycles of BG and BCNU contained the ΔMGMT provirus. The proportion of CFU with provirus correlated with the number of cycles of BG and BCNU received and the number of ΔMGMT-transduced cells infused (Table2). Every CFU tested in 4 of 5 mice from the 100 × 104 cohort, which received 3 cycles of BG and BCNU, were ΔMGMT+, and 14 of 16 CFU were ΔMGMT+ in the fifth mouse. Furthermore, every CFU tested from 1 mouse from the 100 × 104 cohort treated twice and from 1 mouse from the 25 × 104 cohort treated with 3 cycles of BG and BCNU was ΔMGMT+, whereas 75% or more of the CFU from the other 6 mice in these cohorts contained ΔMGMT. One dose of BG and BCNU was sufficient to mediate selection for ΔMGMT-transduced LTRC at the 6-month time point because CFU from 3 of 4 mice from the 25 × 104 and the 100 × 104cohorts and 2 of 4 mice from the 5 × 104 cohort contained the ΔMGMT provirus. This implies that 1 cycle of drug exerted remarkable selection pressure, resulting in the survival of ΔMGMT+ LTRC among endogenous HSC 6 months after infusion and drug treatment.
Proportion of ▵MGMT+ CFU after drug treatment
| No. ΔMGMT+ Cells Infused . | No. Postinfusion Cycles of BG and BCNU . | % ΔMGMT+ CFU . | BCNU IC90 After 20 μmol/L BG (in μmol/L) . |
|---|---|---|---|
| 100 × 104 | 3 (n = 5) | 67/69 (97%) | 74.5 |
| 2 (n = 4) | 54/59 (92%) | 45 | |
| 1 (n = 4) | 13/64 (20%)* | 21 | |
| 0 (n = 3) | 0/61 (0%) | 7 | |
| 25 × 104 | 3 (n = 4) | 43/50 (86%) | 51 |
| 2 (n = 5) | 34/69 (49%) | 30 | |
| 1 (n = 4) | 8/56 (14%)* | 15 | |
| 0 (n = 3) | 0/55 (0%) | 6 | |
| 5 × 104 | 3 (n = 4) | 28/59 (47%) | 35 |
| 2 (n = 6) | 14/71 (20%)† | 14.5 | |
| 1 (n = 4) | 3/50 (6%)‡ | 13 | |
| 0 (n = 3) | 0/52 (0%) | 6 | |
| 0 (normal mouse) | 0 (n = 9) | N/A | 11 |
| No. ΔMGMT+ Cells Infused . | No. Postinfusion Cycles of BG and BCNU . | % ΔMGMT+ CFU . | BCNU IC90 After 20 μmol/L BG (in μmol/L) . |
|---|---|---|---|
| 100 × 104 | 3 (n = 5) | 67/69 (97%) | 74.5 |
| 2 (n = 4) | 54/59 (92%) | 45 | |
| 1 (n = 4) | 13/64 (20%)* | 21 | |
| 0 (n = 3) | 0/61 (0%) | 7 | |
| 25 × 104 | 3 (n = 4) | 43/50 (86%) | 51 |
| 2 (n = 5) | 34/69 (49%) | 30 | |
| 1 (n = 4) | 8/56 (14%)* | 15 | |
| 0 (n = 3) | 0/55 (0%) | 6 | |
| 5 × 104 | 3 (n = 4) | 28/59 (47%) | 35 |
| 2 (n = 6) | 14/71 (20%)† | 14.5 | |
| 1 (n = 4) | 3/50 (6%)‡ | 13 | |
| 0 (n = 3) | 0/52 (0%) | 6 | |
| 0 (normal mouse) | 0 (n = 9) | N/A | 11 |
In 1 mouse, 0 of 16 CFU tested were ΔMGMT+.
In 1 mouse, 0 of 9 CFU tested were ΔMGMT+.
In 2 mice, 0 of 15 and 0 of 12 CFU tested were ΔMGMT+.
Undetectable without selection, strong δAGT expression was observed in bone marrow, spleen, and blood mononuclear cells after BG and BCNU selection. Although not performed in these experiments, we have observed strong MFG-ΔMGMT expression in thymocytes after BG and BCNU.21 The proportion of cells overexpressing δAGT increased with each cycle of BG and BCNU, with overexpression in at least 48% of cells from all sources after 3 cycles (Figure3). Furthermore, bone marrow–derived CFU became increasingly resistant with each cycle of drug received (Figure4; Table 2). In contrast, CFU from mice that did not receive postinfusion BG and BCNU were as sensitive to BG and BCNU as untransduced controls. Therefore, ΔMGMT+cells had a distinct survival advantage after BG and BCNU treatment because of the expression of δAGT.
Flow cytometry on bone marrow, splenocytes and blood mononuclear cells from mice infused with 100 × 104▵MGMT-transduced cells after 1, 2, or 3 cycles of BG and BCNU.
Cells were fixed, permeabilized, and stained with the human AGT-specific monoclonal antibody mT3.1 and a PE-conjugated secondary antibody before analysis. There was no cross-reactivity with murine AGT. The proportion of cells overexpressing δAGT was determined by subtracting the histogram obtained from normal mouse tissue.
Flow cytometry on bone marrow, splenocytes and blood mononuclear cells from mice infused with 100 × 104▵MGMT-transduced cells after 1, 2, or 3 cycles of BG and BCNU.
Cells were fixed, permeabilized, and stained with the human AGT-specific monoclonal antibody mT3.1 and a PE-conjugated secondary antibody before analysis. There was no cross-reactivity with murine AGT. The proportion of cells overexpressing δAGT was determined by subtracting the histogram obtained from normal mouse tissue.
CFU become increasingly resistant to BG and BCNU after repetitive drug administration.
Bone marrow cells were treated with 20 μmol/L BG and 0 to 80 μmol/L BCNU, then plated in methylcellulose. CFU growth was scored, and resistance curves were generated. Error bars represent SD of mean percentage survival. ▪, 3 cycles of BG and BCNU; ▴, 2 cycles; •, 1 cycle; dashed line; open box, 0 cycles; dotted line, normal mice.
CFU become increasingly resistant to BG and BCNU after repetitive drug administration.
Bone marrow cells were treated with 20 μmol/L BG and 0 to 80 μmol/L BCNU, then plated in methylcellulose. CFU growth was scored, and resistance curves were generated. Error bars represent SD of mean percentage survival. ▪, 3 cycles of BG and BCNU; ▴, 2 cycles; •, 1 cycle; dashed line; open box, 0 cycles; dotted line, normal mice.
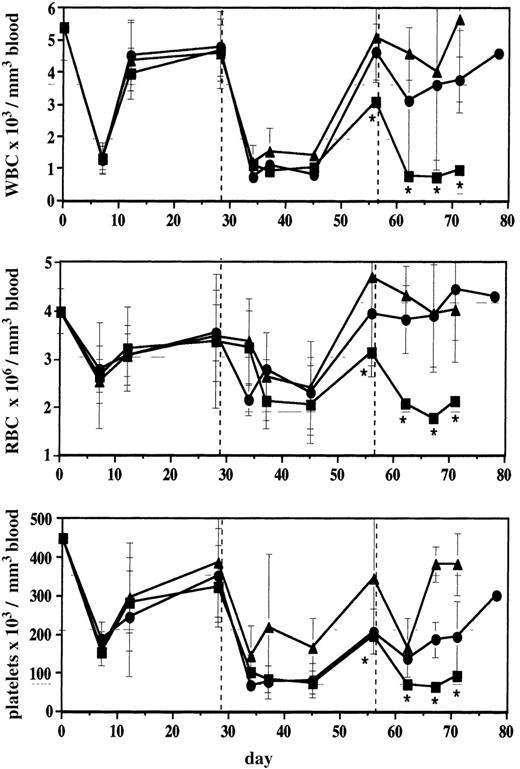
Mice infused with ▵MGMT-transduced cells are protected from myelosuppression
Blood cell counts were monitored in the 25 × 104and 100 × 104 cohorts and in noninfused controls during an 80-day period in which mice received 3 cycles of BG and BCNU at 4-week intervals (3 mice per group; Figure5). Mice from all 3 cohorts had a similar level of myelosuppression after the first postinfusion cycle of BG and BCNU, but after the second treatment, mice in the 100 × 104 cohort had higher WBC counts than control mice (P = .0522). After the third cycle of drug, mice infused with ΔMGMT-transduced cells had significantly higher counts than the control group (P < .05). Cumulative myelosuppression resulted in the death of 2 of 3 control mice after the second cycle, whereas all mice infused with ΔMGMT-transduced cells survived. In addition, mice receiving 2 or 3 doses of BG and BCNU were analyzed for the maintenance of blood counts 28 weeks after infusion. There was no significant decrease in WBC or RBC counts in drug-treated mice in any of the cohorts; however, platelet counts (× 103/μL) were significantly reduced (325 ± 85 in the 100 × 104 cohort, 362 ± 97 in the 25 × 104 cohort, and 271 ± 109 in the 5 × 104 cohort) compared with untreated controls (546 ± 35; P < .05). This reduction may have been caused by toxicity to untransduced early progenitors, insufficient numbers of transduced LTRC to maintain normal platelet counts, or poor proviral expression in the megakaryocyte lineage. These data demonstrate that enrichment for ΔMGMT-transduced hematopoietic cells by BG and BCNU protects mice from cumulative myelosuppression induced by this drug combination and that LTRC were protected by ΔMGMT cDNA transfer and expression.
Infusion of ▵MGMT-transduced cells protects mice from cumulative myelosuppression.
Blood counts from BG- and BCNU-treated mice were monitored for 70 to 80 days. The first treatment (dashed line) was performed 3 weeks after the infusion of bone marrow cells, and subsequent treatments were administered every 4 weeks. Each value represents the mean of 3 mice, except *, which was obtained from 1 surviving normal mouse. Error bars represent SD of mean. ▴, 100 × 104 cohort; •, 25 × 104 cohort; ▪, normal mice.
Infusion of ▵MGMT-transduced cells protects mice from cumulative myelosuppression.
Blood counts from BG- and BCNU-treated mice were monitored for 70 to 80 days. The first treatment (dashed line) was performed 3 weeks after the infusion of bone marrow cells, and subsequent treatments were administered every 4 weeks. Each value represents the mean of 3 mice, except *, which was obtained from 1 surviving normal mouse. Error bars represent SD of mean. ▴, 100 × 104 cohort; •, 25 × 104 cohort; ▪, normal mice.
No engraftment or selection was observed without the BG and BCNU preparatory regimen
Interestingly, mice not given a preparatory regimen of BG and BCNU 48 before cell infusion (n = 12) but given 2 cycles of BG and BCNU 3 and 7 weeks after infusion did not have any ΔMGMT+ CFU 6 months after infusion, despite an initial 70% transduction efficiency into infused CFU. The CFU of these mice were as sensitive to BG and BCNU as control mice (data not shown). Although Rao et al22have shown that prior ablation is not required for engraftment when at least 12.5 × 106 cells are infused, our data suggest that a niche may be required to allow engraftment of low numbers of cells. Without this niche, infused LTRC could not be sufficiently enriched by BG and BCNU. In contrast to mice that had not received BG and BCNU before infusion, mice given the preparatory regimen had a 57% reduction in total marrow cellularity at the time of infusion. This niche formed in the marrow appears to allow improved engraftment of the infused cells and subsequent BG and BCNU selection of LTRC.
Discussion
These data show that the infusion of a small number of ΔMGMT-transduced early hematopoietic progenitors into nonmyeloablated mice followed by treatment with BG and BCNU allows virtually complete, selective hematopoietic repopulation with ΔMGMT+ cells. This approach appears to offer an improvement over standard hematopoietic gene transfer methods, which require myeloablative preparation of the host to achieve similar levels of expression. BG and BCNU appear to exert strong selective pressure at the stem cell level because genetically modified cells are still observed 6 months after infusion, in some cases 5 months after the last drug treatment. Efficient selection at the stem cell level and long-term detection of genetically modified cells suggest applications of BG and BCNU selection for ΔMGMT+ cells in cancer therapy and dual gene transfer.
BG and BCNU selection for ΔMGMT+ LTRC is stronger than has been achieved in other in vivo selection studies. We defined the selection pressure as the fold-enrichment for CFU after BG and BCNU. Nonmyeloablated mice that received 100 × 104transduced progenitors (an estimated 6000 ΔMGMT+ CFU) and 2 cycles of BG and BCNU had nearly 100% ΔMGMT+ CFU (5.5 × 105 total ΔMGMT+ CFU) 4 months after the final drug treatment, a 92-fold enrichment for ΔMGMT+ CFU (Figure 6). After 3 cycles, the marrow remained nearly 100% ΔMGMT+,but δAGT expression increased, as shown in flow cytometry and drug-resistance data. Similarly, mice from the 25 × 104 cohort treated with 3 cycles of drug had nearly 100% ΔMGMT+ CFU, which calculates to 350-fold enrichment. Mice in the 5 × 104 cohort treated with 3 cycles of drug had approximately 50% ΔMGMT+ CFU, a 940-fold enrichment. Mice treated with only 1 cycle of BG and BCNU had approximately 20-, 56-, and 120-fold enrichment for ΔMGMT+ CFU in the 100, 25, and 5 × 104 cohorts, respectively. The strong selection pressure after 1 cycle of BG and BCNU might have resulted from BCNU-induced cross-links in unprotected HSC. These cross-links persist in quiescent HSC and become cytotoxic as they sequentially expand to support hematopoiesis. This results in an increasing proportion of ΔMGMT-transduced LTRC repopulating the marrow over time. Additional cycles of BG and BCNU increase HSC cross-links in unprotected cells and increases the demand on HSC to proliferate in response to myelosuppression. Ultimately, the selection pressure weakens because fewer BG- and BCNU-sensitive cells remain after each cycle.
BG and BCNU mediates strong selection pressure.
Fold enrichment for ΔMGMT+ CFU was calculated by dividing the total estimated number of ΔMGMT+ CFU at sacrifice by the number of ΔMGMT+ CFU infused. Within 1 × 105 bone marrow cells, we obtained approximately 200 CFU, which increased to 800 after 5-FU treatment. Because the CFU transduction efficiency was 75%, the total number of infused ΔMGMT+ CFU was 6000, 1500, and 300 in the 100, 25, and 5 × 104 cell populations, respectively. At sacrifice, we estimated 6 × 105 CFU present in 300 × 106 total mouse bone marrow cells. ▪, 5 × 104 cohort; ▴, 25 × 104cohort; •, 100 × 104 cohort.
BG and BCNU mediates strong selection pressure.
Fold enrichment for ΔMGMT+ CFU was calculated by dividing the total estimated number of ΔMGMT+ CFU at sacrifice by the number of ΔMGMT+ CFU infused. Within 1 × 105 bone marrow cells, we obtained approximately 200 CFU, which increased to 800 after 5-FU treatment. Because the CFU transduction efficiency was 75%, the total number of infused ΔMGMT+ CFU was 6000, 1500, and 300 in the 100, 25, and 5 × 104 cell populations, respectively. At sacrifice, we estimated 6 × 105 CFU present in 300 × 106 total mouse bone marrow cells. ▪, 5 × 104 cohort; ▴, 25 × 104cohort; •, 100 × 104 cohort.
In these studies, the number of ΔMGMT-transduced LTRC present in 5 × 104 5-FU enriched cells appears to be near the threshold level of LTRC necessary to partially repopulate a mouse with drug-resistant progenitors after drug selection. There are an estimated 25 LTRC in a population of 5 × 104 bone marrow cells after 5-FU treatment.23 Although the transduction efficiency into CFU was 75% in our experiments, relatively quiescent LTRC are expected to be transduced less efficiently by retroviral vectors.24 Assuming 1 ΔMGMT-transduced LTRC was sufficient to produce enough progeny cells to protect the mouse from myelosuppression, the variability after infusion of 25 LTRC implies that the transduction efficiency into LTRC was at least 4%. Southern blotting performed on the marrow of a mouse infused with 5 × 104 cells and given 2 cycles of BG and BCNU confirmed the low number of transduced LTRC. A dominant band and a possible second weaker band was observed, demonstrating that 1 or 2 transduced LTRC were actively involved in hematopoiesis 6 months after transplantation.
In contrast to BCNU, which facilitates strong selection by stem cell toxicity, other drugs commonly used to mediate in vivo selection for drug-resistance genes, such as paclitaxel for MDR1 selection and methotrexate for DHFR selection, are cytotoxic to cycling cells but not stem cells.25-27 Because the stem cell pool continues to participate in hematopoiesis, there is far less selection for transduced LTRC. Daily administration of these and similar drugs and cytokines can stimulate stem cells into cell cycle and may result in some HSC cytotoxicity.27 Repeated treatment has been used to mediate selection for L22Y DHFR-transduced LTRC by treatment with trimetrexate and the thymidine transport inhibitor, nitrobenzylmercaptopurineriboside (NBMPR). In a study by Allay et al,8 a 1:4 mix of transduced-to-untransduced hematopoietic progenitors was transplanted into lethally irradiated mice, and trimetrexate and NBMPR treatment resulted in a 4- to 6-fold enrichment for transduced cells. In contrast, our approach using nonmyeloablated mice permitted us to observe nearly 1000-fold enrichment over the course of drug-mediated selection, with persistent expression for at least 6 months.
Because we have demonstrated that selection for ΔMGMT-transduced LTRC in nonmyeloablated mice can completely repopulate the marrow and generate drug-resistant hematopoietic progeny, we have suggested that these concepts be tested in a clinical trial. The hypothesis is that the infusion of small numbers of transduced progenitor cells would allow emergence of drug-resistant marrow, resulting in amelioration of the cumulative myelosuppression previously observed in phase I trials with BG and BCNU.28,29 The poor transduction efficiency into early human LTRC may limit this approach. However, the current perspective that these cells are rarely transduced may be biased by the use of nonselectable genes. Further experimentation with in vivo selectable genes must be performed to resolve this issue. Based on the results presented in this report and on additional results from our laboratory,30 the United States Food and Drug Administration has recently approved a phase I trial of ΔMGMT gene transduction of CD34+ cells in patients undergoing sequential treatment with BG and BCNU.31 The end points of this trial include detection of transduced cells after each cycle of chemotherapy, detection of drug-resistant marrow CFU, monitoring for evidence of cumulative myelosuppression, and therapeutic response to the drug combination.
These data suggest that LTRC transduced with bicistronic vectors containing both ΔMGMT and a nonselectable therapeutic gene may be enriched by BG and BCNU. The success of this approach will be determined in experiments under way in our laboratory. Until techniques that improve transduction of stem cells are developed, in vivo selection for transduced LTRC would potentially ensure that sufficient numbers of cells are genetically modified and express protein levels necessary to modify a deficient phenotype. A drawback is the need to use cytotoxic drugs as selective agents, but this may be accomplished at doses low enough to avoid toxicity to nonhematopoietic tissues. If this is validated, in vivo selection using ΔMGMT should be considered a viable approach to improve the outcome of a number of hematopoietic gene therapy protocols.
Acknowledgments
We thank Keunmyoung Lee for creation of the MFG-ΔMGMT vector, Jane Reese for production of the high-titer producer clone, and James A. Allay for helpful discussions.
Supported by Public Health Service grants RO1CA73062, RO1ES06288, UO1CA75525, and P30CA43703.
Reprints:Stanton L. Gerson, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, BRB 3-West, Cleveland, OH 44106-4937; e-mail: slg5@cwru.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal