Abstract
Graft rejection in allogeneic bone marrow transplantation (BMT) can occur when donor and recipient are mismatched at one or more major histocompatibility complex (MHC) loci. Donor T cells can prevent graft rejection, but may cause fatal graft-versus-host disease (GVHD). We tested whether irradiation of allogeneic donor lymphocytes would preserve their graft-facilitating activity while inhibiting their potential for GVHD. Infusions of irradiated allogeneic T cells did not cause GVHD in MHC-mismatched SJL → (SJL × C57BL6) F1, C57BL6 → B10.RIII, and C57BL6 → B10.BR mouse donor → recipient BMT pairs. The 60-day survival among MHC-mismatched transplant recipients increased from 2% (BM alone) to up to 75% among recipients of BM plus irradiated allogeneic splenocytes. Optimal results were obtained using 50 × 106 to 75 × 106 irradiated donor splenocytes administered in multiple injections from day −1 to day +1. Recipients of an equal number of nonirradiated MHC-mismatched donor splenocytes uniformly died of acute GVHD. The graft facilitating activity of the irradiated allogeneic splenocytes was mediated by donor T cells. Irradiation to 7.5 Gy increased nuclear NFκB in T cells and their allospecific cytotoxicity. Irradiated T cells survived up to 3 days in the BM of MHC-mismatched recipients without proliferation. Recipients of irradiated allogeneic splenocytes and allogeneic BM had stable donor-derived hematopoiesis without a significant representation of donor splenocytes in the T-cell compartment. Irradiated allogeneic T cells thus represent a form of cellular immunotherapy with time-limited biologic activity in vivo that can facilitate allogeneic BMT without causing GVHD.
GRAFT REJECTION AND graft-versus-host disease (GVHD) represent two potentially lethal immunological side effects of transferring hematopoietic cells between genetically nonidentical individuals.1-5 Graft rejection represents an immune response by host immune cells against donor stem cells. GVHD represents a complementary immune response by donor T cells against host cells and tissues. One approach to prevent fatal GVHD in allogeneic bone marrow transplantation (BMT) has been to remove immunocompetent T cells from the graft. Currently used methods of T-cell depletion include elutriation,6,7 absorption to lectin,8 application of specific monoclonal antibodies with complement or immunotoxin,9-11 or by specific selection of CD34+ donor stem cells.12 The potential to successfully transplant T-cell–depleted (TCD) allografts from antigenically mismatched donors without graft rejection or an increased rate of leukemia relapse would greatly extend the availability of allogeneic transplantation to patients without an antigenically matched sibling donor.13
One potential method of enhancing engraftment by allogeneic stem cells in transplantation is to add a population of donor immune cells that are incapable of causing GVHD, but retain graft-facilitating activity. Ionizing radiation has been shown to limit the capacity of lymphocytes to proliferate while preserving their cytotoxicity against tumor cells14 and their activity as veto cells against host immune cells.15 A human T-cell line (TALL-104) has been shown to be effective in eliminating clonogenic human leukemic cells when mixtures of irradiated T cells and leukemia cells were infused into human bone xenografts in immunodeficient severe combined immunodeficiency (SCID)-hu mice.16 In theory, irradiated donor lymphocytes could have all of the desirable short-term effects of donor T-cell infusions without causing GVHD.
We reasoned that the presence of irradiated donor leukocytes could counteract the ability of host cells to reject donor stem cells. Graft rejection of TCD allogeneic transplants appears to be mediated by a radioresistant host cell(s) that exerts a (transient) alloreactive cytotoxic effect against donor stem cells.2 17-19 We hypothesized that irradiated allogeneic donor T cells could facilitate BM engraftment by counteracting the host cytotoxic cells thereby allowing the survival of repopulating donor hematopoietic stem cells.
To test these hypotheses, we have used a model of graft failure for MHC mismatched allogeneic BMT in mice. Recipients of BM allografts and irradiated allogeneic spleen cells were successfully engrafted with and repopulated by T cells derived from the donor BM cells, with a minimal contribution from the spleen cell graft and without developing GVHD. These studies provide the preclinical basis for adoptive immunotherapy using multiple doses of irradiated donor lymphocytes in transplant recipients without producing cumulative toxicity.
MATERIALS AND METHODS
Animals.
SJL (H2s), (SJL × C57.BL6) F1 (H2s/b), B10.RIII (H2r), B10.BR (H2k), and CD45.1/CD45.2 congenic strains of C57.BL6 (H2b) mice aged 8 to 10 weeks were purchased from Charles River/Jackson Laboratories (Bar Harbor, ME). BA (Thy 1.1) mice on C57.BL6 (H2b) background were obtained from Dr Miriam Lieberman (Stanford University, Palo Alto, CA). CB17 scid/scid (Balb/c, H2d background) mice were purchased from the Emory University Animal Care Facility. Mice were given acidified sterile water and maintained in Micro isolator cages (Lab Products Inc, Maywood, NJ) at the Emory University Animal Care Facility. Experiments were performed in conformance with the Guide for the Care and Use of Laboratory Animals published by the National Academy Press, Washington, DC, 1996, and approval by the Emory University Institutional Animal Care and Use Committee (IACUC).
Donor cell preparations.
BM cells were harvested from mice by removing the femora and tibia and flushing the cells out of the BM shaft with sterile Hanks’ balanced salt solution (HBSS) containing 3% heat-inactivated fetal bovine serum (HBSS/FBS) using a 25-gauge needle. Splenocytes were harvested by perfusing the spleen with sterile HBSS/FBS.
Cytotoxicity assays.
Cytotoxicity assays were performed using the CytoTox 96 Assay (Promega Corp, Madison, WI) following the manufacturer’s protocol. Lysis of a fixed number of leukemia cells using a range of effector cell concentrations was calculated using the formula: % Cytotoxicity = 100 × (Experimental − Effector Spontaneous − Target Spontaneous)/(Target Maximum − Target Spontaneous). Separate determinations of the spontaneous release from effector cells were performed at each effector cell concentration.
T-cell depletion and enrichment of splenocytes.
Harvested spleen cells were suspended in phosphate-buffered saline (PBS) containing 5 mmol/L EDTA and 0.1% bovine serum albumin (BSA) and incubated with anti-FcR iii/ii antibody (Pharmingen, San Diego, CA) to block nonspecific antibody binding. Monoclonal antibodies were obtained from Pharmingen. The splenocytes were then incubated with saturating concentrations of either (1) biotinylated anti-CD3 antibody (positive selection) or (2) a combination of biotinylated anti–MAC-1, anti–Gr-1, and anti-C19 antibodies (negative depletion). After antibody staining, cells were washed once in HBSS/FBS, then resuspended with 125 μL Streptavidin Microbeads (Miltenyi Biotech Gmbh, Bergisch Gladbach, Germany) per 2.5 × 108cells. The T-cell and non–T-cell fractions were separated by immunomagnetic chromatography using the Vario MACS magnetic separation column (Miltenyi Biotech, Gmbh). Using either the method of positive selection or the method of negative depletion, the final purity of T cells was 75% to 85%. TCD splenocytes were the unbound cell fractions from the anti-CD3 column.
Irradiation and reconstitution.
Recipient mice were exposed to 10 Gy or 11 Gy of radiation from a137Cs source, delivered in 2 equal fractions 5 hours apart at a dose rate of 1.24 Gy/min. Lethally irradiated mice were maintained on oral aqueous antibiotics (1.1 mg/mL neomycin sulfate and 1,000 U/mL polymyxin sulfate) for 3 days before irradiation and for 4 weeks after BMT. Splenocytes were irradiated using a single fraction from the same137Cs source. All intravenous administrations were performed using a 25-gauge needle to inject 0.2 mL of a cell suspension prepared in HBSS/FBS into the retroorbital sinus into mice anesthetized with metofane or into a tail vein of recipient mice. BM transplant recipients were irradiated 1 to 2 days before BMT and received a single injection of BM or the appropriate mixture of BM and splenocytes on day 0. Recipients of allogeneic splenocytes received 1 to 3 injection(s) within the range of day −2 to day +1.
Analysis of hematopoietic engraftment of transplant recipients.
Mice were anesthetized with metofane and 0.2 mL peripheral blood was collected from the retroorbital venous sinus at 1 to 4 months after transplant. Red blood cells were depleted by 1G sedimentation using 3% Dextran T500 in HBSS followed by hypotonic saline lysis.20Fluorescence-activated cell sorting (FACS) analysis enumerated host and donor leukocytes and T cells using a variety of monoclonal antibodies specific for H2b and H2k MHC, as well as specific leukocyte markers (Thy 1.1, Thy 1.2, CD45.1, and CD45.2; Pharmingen). Propidium iodide was added at a concentration of 1 μg/mL and dead cells were electronically excluded.
Assessment of GVHD in transplant recipients.
All transplant recipients were evaluated for the presence of clinical GVHD as manifested by weight loss, alopecia and/or ruffled fur, diarrhea, and a decreased level of activity associated with a “hunched over” appearance. Necropsy was performed after euthanasia of moribund mice and of mice at predetermined time points. Hematoxylin-eosin–stained tissue sections of liver, gastrointestinal tract, and skin were examined microscopically for histologic evidence of GVHD and scored according to published criteria.21Abnormalities noted that were consistent with GVHD included single cell apoptosis in the epidermis (acute GVHD) and epithelial thickening and loss of adnexal structures of the skin (chronic GVHD). Cutaneous GVHD was graded on a scale of 0-1; gastrointestinal GVHD was graded on a scale of 0-2; and hepatic GVHD was graded on a scale of 0-3. An overall GVHD histologic score was calculated from the sum of individual scores for skin, intestine, and liver. Normal tissue, with no evidence of acute or chronic GVHD was given a score of 0. Severe GVHD, with apoptosis and/or epithelial thickening and adnexal loss of the skin, extensive sloughing of intestinal epithelium, and extensive hepatic parenchymal injury and inflammation, was given a (maximal) score of 6.
Nuclear extraction and electrophoretic mobility shift assay (EMSA).
The CD3+ enriched cell fractions from mouse splenocytes were selected with the Mini MACS system as described above and washed with PBS, then irradiated at 0, 7.5, or 30 Gy. After 6 hours incubation at 37°C, nuclear extracts were obtained according to the method of Schulze-Ostoff.22 Briefly, the cell pellets were lysed with HMK buffer A (1 mol/L EPES, 1 mol/L gCl2, 1 mol/LCl) containing 0.1% (wt/vol) Triton X at 4°C. Intact cell nuclei were collected by centrifugation, washed with HMK buffer, then lysed in 20 mmol/L HEPES, 25% glycerol, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA (HMK-NaCl) buffer. The protein content of the nuclear extracts supernatants was determined by Micro BCA Protein Assay. Constant amounts of nuclear protein were mixed with gel-shift binding buffer and32P-labeled oligonucleotide strands specific for NFκB (CD28RE, κB, AP-1, and Nf-interleukin-2 [IL-2]). The complex between the oligonucleotide and NFκB was revealed by autoradiography in an EMSA on a polyacrylamide gel in 0.5 × TBE buffer run at 100 V. Quantitative autoradiography was performed using the BETASCOPE 603 blot analyzer (Bio-Rad Model GS-700 Imaging Densitometer, Hercules, CA).23
Flow cytometric analysis of intracellular cytokine expression.
The effect of irradiation on the levels of intracytoplasmic IL-2, γ-interferon (IFN), tumor necrosis factor (TNF), and IL-4 protein in mouse T cells was measured by intracellular staining using flow cytometry with fluorescent-conjugated monoclonal antibodies to specific cytokines and T cells. Irradiated splenocytes were irradiated, incubated at 37°C for 4 to 20 hours, then treated with Brefeldin A (Sigma-Aldrich Corp, St Louis, MO) for an additional 4 hours before fixation, permeabilization, and flow cytometric analysis.24
Statistical analyses.
Survival differences between different groups were calculated with the Cox test comparing different survival curves in a pair-wise fashion. Differences between mean values were compared using the Student’st-test. Differences between the fraction of animals surviving at day 30 were determined using the Fisher’s test.25
RESULTS
Complete H2 disparity in mice is a major barrier to successful allogeneic BMT.
Mice transplanted with 0.5 × 106 BM cells from allogeneic MHC mismatched donors died of graft failure. Moribund animals had marked cytopenias of the peripheral blood (mean leukocyte counts of 0.2 ± 0.3 × 109/L, hematocrits of 5.3% ± 2.2%, and platelet counts of 19,000 ± 11,000). Less than 5% of peripheral blood and BM cells were donor-derived. In contrast, 80% to 90% of mice receiving an equal number of congenic MHC identical BM cells survived transplants (Table 1). Large doses (5 × 106 to 25 × 106 cells) of allogeneic C57.BL6 BM were needed to successfully engraft B10.BR and B10.RIII recipients (Table 1). Thus, the allogeneic barrier to transplantation using MHC mismatched donor-recipient pairs could be overcome by increasing the number of donor cells.26 27
The Immunologic Barrier to Allogeneic BMT Can Be Overcome by Large Numbers of Donor Cells
| BM Cells . | H2b → H2b . | H2r → H2r . | H2b → H2r . | H2b → H2k . | ||||
|---|---|---|---|---|---|---|---|---|
| 0.02 × 106 | 0% | n = 10 | ND | 0% | n = 5 | ND | ||
| 0.1 × 106 | 80% | n = 10 | ND | 0% | n = 5 | 0% | n = 5 | |
| 0.5 × 106 | 89% | n = 28 | 80% | n = 15 | 0% | n = 24 | 1% | n = 60 |
| 2.5 × 106 | ND | ND | ND | 60% | n = 10 | |||
| 5 × 106 | 100% | n = 10 | ND | 35% | n = 34 | 100% | n = 10 | |
| 25 × 106 | ND | ND | 100% | n = 5 | ND | |||
| BM Cells . | H2b → H2b . | H2r → H2r . | H2b → H2r . | H2b → H2k . | ||||
|---|---|---|---|---|---|---|---|---|
| 0.02 × 106 | 0% | n = 10 | ND | 0% | n = 5 | ND | ||
| 0.1 × 106 | 80% | n = 10 | ND | 0% | n = 5 | 0% | n = 5 | |
| 0.5 × 106 | 89% | n = 28 | 80% | n = 15 | 0% | n = 24 | 1% | n = 60 |
| 2.5 × 106 | ND | ND | ND | 60% | n = 10 | |||
| 5 × 106 | 100% | n = 10 | ND | 35% | n = 34 | 100% | n = 10 | |
| 25 × 106 | ND | ND | 100% | n = 5 | ND | |||
Unfractionated BM cells were transplanted from C57.BL6 (H2b) or B10.RIII (H2r) donor mice into C57.BL6, B10.RIII, or B10.BR (H2k) recipients after lethal irradiation with 10 Gy delivered in 2 doses. The percentage of mice surviving to day +60 posttransplant is shown.
Abbreviation: ND, not determined.
T cells lose proliferative capacity after exposure to greater than 5 Gy ionizing radiation, but survive transiently in vivo.
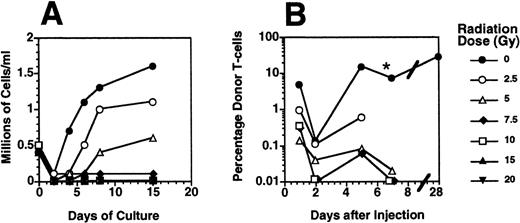
Mouse spleen cells were exposed to a single fraction of radiation between 0 Gy and 20 Gy and then cultured in the presence of anti-CD3 monoclonal antibody and IL-2. These culture conditions model an in vivo environment in which alloreactive T cells are stimulated in a MHC-mismatched recipient.28 Radiation doses of 7.5 Gy or more effectively prevented T-cell proliferation (Fig 1A). The effect of radiation on the proliferation of allogeneic T cells in vivo was determined by injecting groups of 2 to 3 Balb/c scid recipients with 15 × 106 allogeneic C57.BL6 splenocytes treated with 0 to 20 Gy irradiation. Aliquots of their peripheral blood were aliquots for the presence of donor H2b T cells on days 1, 2, 5, and 7 posttransplant. Nonirradiated Thy 1.1+, H2b donor T cells were easily detectable at each time point and increased in number as they proliferated in recipient SCID mice over 5 to 7 days, producing clinically evident acute GVHD by day 5 to 7 posttransplant. Mice that received allogeneic splenocytes irradiated to 2.5 Gy developed clinical GVHD 7 to 30 days after injection (Fig 1B). Mice that received allogeneic splenocytes irradiated at doses of 5 Gy and higher had declining numbers of detectable donor cells on 2, 5, and 7 days after injection and remained free of GVHD.29 30
The proliferative capacity of mouse T cells was inhibited by radiation doses of 5 Gy or greater. (A) Murine splenocytes were irradiated with a single dose between 0 and 20 Gy and then cultured in the presence of immobilized anti-CD3 monoclonal antibody in RPMI media containing 50 U/mL IL-2 and 10% vol/vol FBS. The numbers of viable T cells were counted after 2 to 15 days of culture. (B) Groups of 2 to 5 SCID mice (H2d) received intravenous injections of 15 × 106 splenocytes prepared from a CD45.1+C57.BL6 (H2b) donor and irradiated at the doses shown. One, 2, 5, and 7 days after injection, 200 μL peripheral blood were analyzed for the presence of donor-derived T cells by FACS using antibodies to CD45.1, CD3, and propidium iodide 1 μg/mL to exclude nonviable cells. The mean (n = 2 to 4) percentage of donor-derived CD3+, CD45.1+ cells among the nucleated blood cells at each time point is shown. *, Some of the mice that received nonirradiated allogeneic splenocytes were killed because of the development of GVHD at day 5 or day 7 postsplenocyte infusion.
The proliferative capacity of mouse T cells was inhibited by radiation doses of 5 Gy or greater. (A) Murine splenocytes were irradiated with a single dose between 0 and 20 Gy and then cultured in the presence of immobilized anti-CD3 monoclonal antibody in RPMI media containing 50 U/mL IL-2 and 10% vol/vol FBS. The numbers of viable T cells were counted after 2 to 15 days of culture. (B) Groups of 2 to 5 SCID mice (H2d) received intravenous injections of 15 × 106 splenocytes prepared from a CD45.1+C57.BL6 (H2b) donor and irradiated at the doses shown. One, 2, 5, and 7 days after injection, 200 μL peripheral blood were analyzed for the presence of donor-derived T cells by FACS using antibodies to CD45.1, CD3, and propidium iodide 1 μg/mL to exclude nonviable cells. The mean (n = 2 to 4) percentage of donor-derived CD3+, CD45.1+ cells among the nucleated blood cells at each time point is shown. *, Some of the mice that received nonirradiated allogeneic splenocytes were killed because of the development of GVHD at day 5 or day 7 postsplenocyte infusion.
The half-life of irradiated allogeneic T cells in vivo was estimated by measuring the survival of donor splenocytes in Balb/c scid mice (CD45.2, H2d). After sublethal irradiation, scidmice were injected with 25 × 106 of MHC-mismatched C57.BL6 splenocytes (Thy 1.2, CD45.1, H2b) irradiated to 0 Gy, 7.5 Gy, or 30 Gy and an equal number of nonirradiated C57.BL6 splenocytes (Thy 1.1, CD45.1, H2b). Two mice from each 3 groups of 6 scid were killed at 1, 4, and 36 hours. The frequency of CD45.1+ donor cells in the peripheral blood and BM of recipient mice was determined using flow cytometry. Overall, CD45.1+ donor cells were detected at frequencies between 0.1% and 1% for up to 36 hours (data not shown). Donor T cells irradiated to 7.5 Gy (Thy 1.2+) disappeared from the blood with a half-life of 7 hours and disappeared from the BM with a half-life of 83 hours. In contrast, nonirradiated Thy 1.1+donor T cells proliferated in the blood and BM during the same period. The overall frequency of irradiated (7.5 Gy) donor T cells in the BM 1 hour after injection was 0.004% (1 irradiated donor T cell per 25,000 host BM cells). The effect of a 30-Gy dose of radiation was to increase the rate of disappearance of the irradiated allogeneic T cells from both the blood and BM (data not shown).
Irradiated splenocytes retain alloreactive cytotoxicity in vitro.
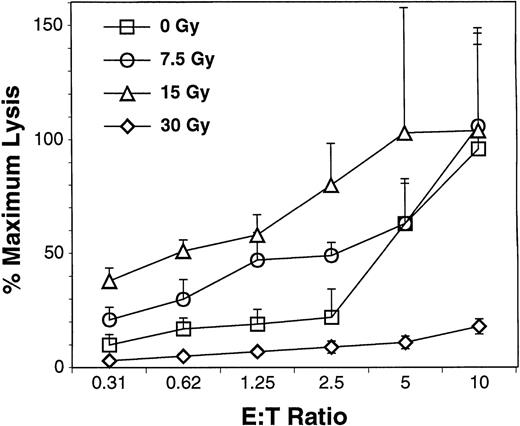
B10.BR splenocytes were cultured with allogeneic C1498 leukemia cells (derived from an H2b C57.BL6 mouse) for 14 days. Alloreactive T cells were then examined for cytotoxicity against C1498 leukemia cell targets 4 hours after exposure to 0 to 30 Gy irradiation. Figure 2 presents typical data from 1 of 3 experiments performed and is consistent with earlier reports of the effect of radiation on enhancing allospecific cytotoxicity after mixed lymphocyte culture.31 Higher doses of radiation (30 Gy) blunted the cytotoxic effect of the alloprimed T cells, and doses of 60 Gy eliminated their cytotoxic activity (data not shown).
Specific cytotoxicity of H2k effectors against H2b targets. CD8+ cells were selected from B10.BR splenocytes and lymph nodes by immunomagnetic beads. Cells were cultured in the presence of IL-2, IL-7, IL-12, and C1498 leukemia cells irradiated to 60 Gy (Boyer49). After 14 days of culture, the effector cells were irradiated between 0 and 30 Gy with a137Cs source. The irradiated and nonirradiated effector cells were incubated with nonirradiated C1498 target cells and specific cytotoxicity of the cells was measured with the CytoTox 96 Assay following the manufacturer’s protocol. The mean (±SD) of specific cytotoxicity for quadruplicate samples is shown for E:T ratios of 0.3:1 to 10:1.
Specific cytotoxicity of H2k effectors against H2b targets. CD8+ cells were selected from B10.BR splenocytes and lymph nodes by immunomagnetic beads. Cells were cultured in the presence of IL-2, IL-7, IL-12, and C1498 leukemia cells irradiated to 60 Gy (Boyer49). After 14 days of culture, the effector cells were irradiated between 0 and 30 Gy with a137Cs source. The irradiated and nonirradiated effector cells were incubated with nonirradiated C1498 target cells and specific cytotoxicity of the cells was measured with the CytoTox 96 Assay following the manufacturer’s protocol. The mean (±SD) of specific cytotoxicity for quadruplicate samples is shown for E:T ratios of 0.3:1 to 10:1.
Mouse T cells irradiated to 7.5 Gy have increased levels of NFκB, but their profile of IL-2, IL-4, γIFN, and TNF cytokine expression are not significantly changed.
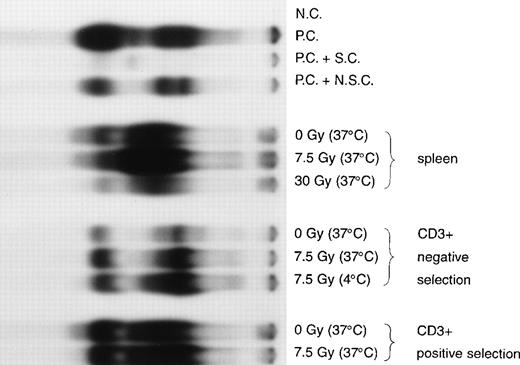
Irradiation to 7.5 Gy increased intranuclear NFκB in unfractionated splenocytes and caused a mean 4-fold increase in T cells that had been enriched using negative depletion of Mac-1/Gr-1/CD19+ cells (Fig 3). Maximal levels of NFκB in irradiated lymphocytes were seen 4 to 6 hours after radiation exposure (data not shown). Irradiated T cells incubated for 6 hours at either 4°C or 37°C had similar levels of intranuclear NFκB (Fig 3). T cells that were enriched from splenocytes by positive selection using an anti-CD3+ antibody had levels of NFκB that were significantly increased compared with T cells enriched by negative depletion and were only slightly increased by radiation (Fig 3). The activating effect of radiation was dose-dependent. A 30-Gy dose of irradiation did not increase intranuclear NFκB as much as irradiation to 7.5 Gy, probably due to increased rates of cell death at the higher radiation dose (data not shown). The temperature-independence of NFκB induction by radiation suggests that new protein synthesis may not be required for the activation of T cells by radiation. The temporal increase in intranuclear NFκB after radiation implies that an enzymatic or a nonenzymatic modification of existing nuclear or cytoplasmic proteins may be involved. Irradiation to 7.5 Gy did not change the pattern or magnitude of type 1 cytokine (IL-2, TNF, or γIFN) or type 2 cytokine (IL-4) expression among T cells from C57.BL6 mice in response to B10.BR allogeneic stimulators after incubation at 37°C for 6 to 24 hours (data not shown).
Enhanced NFκB in nuclear extracts of mouse T cells after exposure to ionizing radiation. T cells, enriched by either negative depletion or positive selection, or unfractionated splenocytes were irradiated at a dose rate of 5 Gy/min to 7.5 Gy or 30 Gy. Irradiated cells were cultured in RPMI + 10% FCS at 4°C or 37°C for 6 hours. Nuclear extracts were prepared and the relative level of nuclear NFκB determined by incubating 10 μg of nuclear protein with a 32P-labeled oligonucleotide containing NFκB binding sequence. The far left lane labeled “NC” contained water with no nuclear extract; lane “PC” contained a Hela cell nuclear extract (positive control for NFκB) incubated with the32P-labeled oligonucleotide alone; “PC + SC” contained a combination of the Hela cell nuclear extract, the32P-labeled oligonucleotide, and an excess of the unlabeled specific oligonucleotide competitor; and “PC + NSC” contained a combination of the Hela cell nuclear extract, the32P-labeled oligonucleotide, and an excess of an unlabeled nonspecific oligonucleotide. The next 3 lanes from the left contained nuclear extracts of unfractionated splenocytes irradiated at 0 Gy, 7.5 Gy, or 30 Gy then incubated at 37°C. The mean increase in the upper NFκB band of nonirradiated T cells compared with T cells irradiated to 7.5 Gy at 37°C was 4-fold (n = 5 EMSA from 2 separate isolations of T cells). Three lanes contained nuclear extracts of T cells enriched by negative selection irradiated at 0 Gy incubated at 37°C, 7.5 Gy incubated at 37°C, or 7.5 Gy incubated at 4°C. Two lanes on the right contained nuclear extracts of T cells enriched by positive selection irradiated at 0 Gy incubated at 37°C, or 7.5 Gy incubated at 37°C.
Enhanced NFκB in nuclear extracts of mouse T cells after exposure to ionizing radiation. T cells, enriched by either negative depletion or positive selection, or unfractionated splenocytes were irradiated at a dose rate of 5 Gy/min to 7.5 Gy or 30 Gy. Irradiated cells were cultured in RPMI + 10% FCS at 4°C or 37°C for 6 hours. Nuclear extracts were prepared and the relative level of nuclear NFκB determined by incubating 10 μg of nuclear protein with a 32P-labeled oligonucleotide containing NFκB binding sequence. The far left lane labeled “NC” contained water with no nuclear extract; lane “PC” contained a Hela cell nuclear extract (positive control for NFκB) incubated with the32P-labeled oligonucleotide alone; “PC + SC” contained a combination of the Hela cell nuclear extract, the32P-labeled oligonucleotide, and an excess of the unlabeled specific oligonucleotide competitor; and “PC + NSC” contained a combination of the Hela cell nuclear extract, the32P-labeled oligonucleotide, and an excess of an unlabeled nonspecific oligonucleotide. The next 3 lanes from the left contained nuclear extracts of unfractionated splenocytes irradiated at 0 Gy, 7.5 Gy, or 30 Gy then incubated at 37°C. The mean increase in the upper NFκB band of nonirradiated T cells compared with T cells irradiated to 7.5 Gy at 37°C was 4-fold (n = 5 EMSA from 2 separate isolations of T cells). Three lanes contained nuclear extracts of T cells enriched by negative selection irradiated at 0 Gy incubated at 37°C, 7.5 Gy incubated at 37°C, or 7.5 Gy incubated at 4°C. Two lanes on the right contained nuclear extracts of T cells enriched by positive selection irradiated at 0 Gy incubated at 37°C, or 7.5 Gy incubated at 37°C.
Splenocytes irradiated to 7.5 Gy have diminished GVHD potential in parental → F1 transplant model.
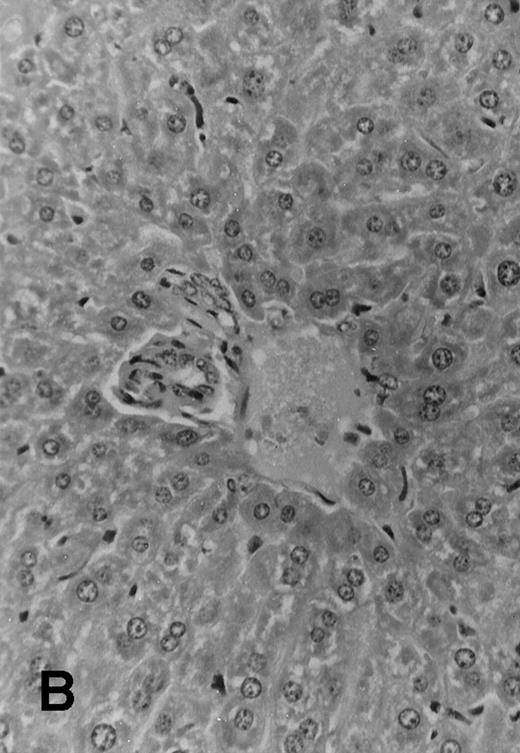
Five of 6 (83%) of (SJL × C57.BL6) F1 mice that received 1 × 106 TCD parental SJL BM cells survived to day +51 posttransplant. These mice appeared healthy and active, with a median weight of 35 ± 1.4 g. All 7 F1 mice that received TCD SJL BM and 30 × 106 irradiated (7.5 Gy) SJL splenocytes survived without evident clinical signs of GVHD. These mice appeared healthy and had a median weight of 35 ± 1.8 g. Seven of 8 (87.5%) F1 mice, which received a combination of SJL TCD BM and 30 × 106 nonirradiated SJL splenocytes, survived to day +51. However, these mice were wasted with a median weight of 24.4 ± 3.4 g and had patchy alopecia and diarrhea (Table 2). Necropsy of all surviving animals at day +51 showed lymphoid hypoplasia among recipients of nonirradiated allogeneic splenocytes. These animals had spleens with mean weights of 30 ± 20 mg compared with normal spleen weights among recipients of TCD BM alone (160 ± 30 mg, P < .0001) or TCD BM plus irradiated allogeneic splenocytes (150 ± 30 mg,P < .0001). Histology examination of samples of skin, liver, and intestine from transplanted mice showed variable degrees of lymphocytic infiltration of the liver, epithelial thickening, and autolysis and sloughing of intestinal epithelium. Evidence for acute GVHD was minimal among recipients of TCD BM alone (Fig 4A) or irradiated allogeneic lymphocytes (Fig 4B). Histologic examination of the liver from recipients of nonirradiated allogeneic splenocytes showed acute and chronic inflammation of the portal tract (Fig 4C) and focal hepatic parenchymal injury. The hepatic GVHD score (on a scale of 0 to 3) and overall GVHD score (on a scale of 0 to 6) for each animal was calculated according to the Materials and Methods section. Recipients of nonirradiated splenocytes had higher GVHD scores than the group that received irradiated splenocytes and the group that received TCD BM alone (Table 2).
Irradiated Allogeneic Splenocytes Do Not Produce GVHD When Transplanted Into MHC Mismatched Recipients
| . | BM Alone . | BM + Irradiated Splenocytes . | BM + Nonirradiated Splenocytes . |
|---|---|---|---|
| Mean weight (g) | 35 ± 1.4* | 35 ± 1.8* | 24.4 ± 3.4 |
| Mean spleen weight (mg) | 160 ± 30* | 150 ± 30* | 30 ± 20 |
| Mean histologic score for liver GVHD | 0.8 ± 0.4* | 0.1 ± 0.4* | 2.7 ± 0.5 |
| Mean histologic score for overall GVHD | 2 ± 0.7† | 0.7 ± 1* | 4.3 ± 1 |
| n = | 5 | 7 | 7 |
| . | BM Alone . | BM + Irradiated Splenocytes . | BM + Nonirradiated Splenocytes . |
|---|---|---|---|
| Mean weight (g) | 35 ± 1.4* | 35 ± 1.8* | 24.4 ± 3.4 |
| Mean spleen weight (mg) | 160 ± 30* | 150 ± 30* | 30 ± 20 |
| Mean histologic score for liver GVHD | 0.8 ± 0.4* | 0.1 ± 0.4* | 2.7 ± 0.5 |
| Mean histologic score for overall GVHD | 2 ± 0.7† | 0.7 ± 1* | 4.3 ± 1 |
| n = | 5 | 7 | 7 |
F1 mice (C57.BL6 × SJL) were irradiated to 10 Gy in 2 equal fractions and then transplanted with 1 × 106 SJL TCD BM cells on day 0. Two additional groups of mice received an equal number of BM plus 30 × 106 irradiated (7.5 Gy) SJL splenocytes or 30 × 106 nonirradiated SJL splenocytes on day 0. One mouse each died in the groups receiving BM alone or BM plus nonirradiated splenocytes before day +5. On day +51, all surviving mice were euthanized, weighed, and subjected to necropsy. Hematoxylin-eosin–stained sections of formalin fixed, paraffin embedded skin, intestinal tract epithelium, and liver were examined microscopically by an observer (JH) unaware of the treatment group. The mean body weight, spleen weight, and mean scores for the liver GVHD and overall GVHD for each treatment group are shown.
Differences between mean values compared with recipients of nonirradiated splenocytes are significant at a level of P < .0001.
Differences between mean values compared with recipients of nonirradiated splenocytes are significant at a level of P < .002.
Histology sections of liver from recipients of MHC mismatched TCD BM and allogeneic splenocytes. Sections of liver from (C57.BL6 × SJL) F1 mice that were transplanted on day 0 with 1 × 106 SJL TCD BM cells alone (A) or TCD BM in combination with 30 × 106 irradiated (7.5 Gy) splenocytes from SJL donors (B) or with 30 × 106 nonirradiated splenocytes (C). Mice were euthanized at day +51 and samples of liver tissue were fixed in 10% formalin, embedded in paraffin, and sections were stained with hematoxylin and eosin. Original magnification is ×165 in all 3 photomicrographs.
Histology sections of liver from recipients of MHC mismatched TCD BM and allogeneic splenocytes. Sections of liver from (C57.BL6 × SJL) F1 mice that were transplanted on day 0 with 1 × 106 SJL TCD BM cells alone (A) or TCD BM in combination with 30 × 106 irradiated (7.5 Gy) splenocytes from SJL donors (B) or with 30 × 106 nonirradiated splenocytes (C). Mice were euthanized at day +51 and samples of liver tissue were fixed in 10% formalin, embedded in paraffin, and sections were stained with hematoxylin and eosin. Original magnification is ×165 in all 3 photomicrographs.
Irradiated allogeneic splenocytes overcome graft failure in allogeneic BMT across the H2b → H2rmajor MHC barrier.
B10.RIII mice (n = 24) transplanted with 0.5 × 106C57.BL6 allogeneic BM cells alone had 0% survival at 30 days posttransplant. In contrast, recipients of an equal number of syngeneic B10.RIII BM cells had 80% 30-day survival (n = 15, Table 1). The addition of 5 × 106 irradiated (10 Gy) allogeneic C57.BL6 spleen cells increased survival among B10.RIII recipients of C57.BL6 BM to 20% (n = 5). Mice that received a total of 10 × 106 C57.BL6 spleen cells irradiated to 10 Gy had 41% survival at 30 days posttransplant (n = 17, P < .0001 compared with the survival of recipients of allogeneic BM cells alone). Greater than 85% of all blood leukocytes were donor-derived among B10.RIII mice that survived transplant with C57.BL6 allogeneic BM and irradiated splenocytes (data not shown).
The effects of the dose of irradiation and number of allogeneic splenocytes in facilitating H2b → H2ktransplants.
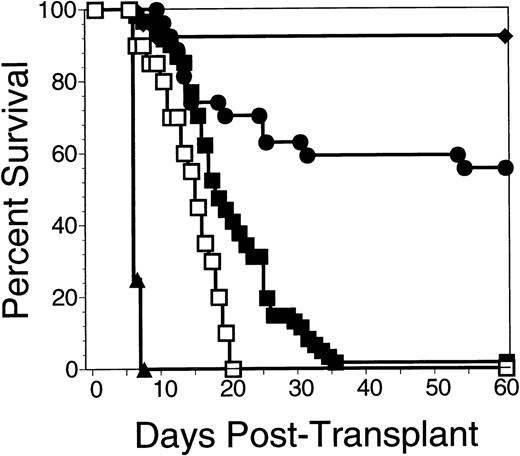
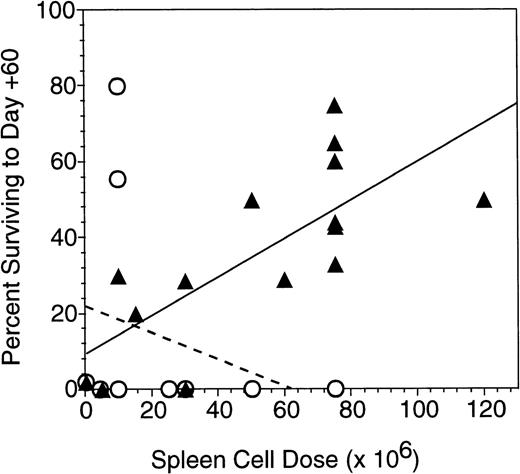
Experimental groups of 10 to 15 lethally irradiated B10.BR mice were transplanted with 0.5 × 106 C57.BL6 allogeneic BM cells on day 0 or BM in combination with 1 or more infusions of irradiated allogeneic spleen cells. Injection of a total of 10 × 106 allogeneic splenocytes irradiated to 5 Gy, 7.5 Gy, or 10 Gy produced similar effects on graft facilitation, with 60-day survival rates of 22%, 33%, and 30%, respectively. In contrast, all mice that received allogeneic BM plus 30 × 106irradiated (30 Gy) allogeneic splenocytes died of graft failure by day +60. Larger numbers of allogeneic splenocytes irradiated to 7.5 Gy were the most effective in promoting survival after lethal total body irradiation and transplantation with allogeneic bone marrow. As shown in Fig 5, recipients of allogeneic bone plus 75 × 106 irradiated allogeneic splenocytes had a 60-day survival posttransplant of 60% (28 mice from 3 experiments administering injections of irradiated splenocytes on day −1, 0, and +1). More recipients of irradiated splenocytes survived to 60 days than did recipients of BM alone (2% survival, n = 60, P< 1 × 10−6). In addition, more recipients of irradiated splenocytes survived to day 60 than did recipients of BM plus 75 × 106 nonirradiated allogeneic splenocytes (0% survival, n = 12, P < 1 × 10−6). Figure 6summarizes 13 different experiments in which 141 B10.BR mice received between 2.5 and 120 × 106 irradiated and nonirradiated allogeneic C57.BL6 splenocytes. The 60-day survival was roughly proportional to the cell dose of irradiated splenocytes, while recipients of more than 25 × 106 nonirradiated allogeneic splenocytes uniformly died of acute GVHD.
Survival of B10.BR mice transplanted with C57.BL6 BM cells was enhanced by irradiated C57.BL6 splenocytes. Data are pooled from 3 experiments in which groups of 8 to 10 B10.BR mice were irradiated to 10 Gy on day -2 and transplanted with 0.5 × 106 C57.BL6 allogeneic BM cells or 0.5 × 106syngeneic B10.BR BM cells on day 0. One group of 8 B10.BR mice received 3 injections of 25 × 106 nonirradiated C57.BL6 allogeneic splenocytes on day −1, day 0, and day +1 (▴), while a second group of 28 B10.BR mice received 3 injections of 25 × 106irradiated (7.5 Gy) allogeneic C57.BL6 spleen cells on day −1, day 0, and day +1 (•). A control group of 60 B10.BR mice received allogeneic BM cells alone (▪). Another control group of 20 B10.BR mice received irradiation without a transplant (□) and 26 B10.BR mice were transplanted with syngeneic H2k BM cells (⧫). Significant differences in survival using the Cox statistic were found between the group of mice receiving multiple infusions of irradiated allogeneic splenocytes and the group that received nonirradiated splenocytes (P = 3 × 10−6); the mice that received irradiated allogeneic splenocytes and mice that received allogeneic BM alone (P = 1 × 10−2); and the mice that received multiple infusions of nonirradiated allogeneic splenocytes and mice that received allogeneic BM alone (P= 3 × 10−7).
Survival of B10.BR mice transplanted with C57.BL6 BM cells was enhanced by irradiated C57.BL6 splenocytes. Data are pooled from 3 experiments in which groups of 8 to 10 B10.BR mice were irradiated to 10 Gy on day -2 and transplanted with 0.5 × 106 C57.BL6 allogeneic BM cells or 0.5 × 106syngeneic B10.BR BM cells on day 0. One group of 8 B10.BR mice received 3 injections of 25 × 106 nonirradiated C57.BL6 allogeneic splenocytes on day −1, day 0, and day +1 (▴), while a second group of 28 B10.BR mice received 3 injections of 25 × 106irradiated (7.5 Gy) allogeneic C57.BL6 spleen cells on day −1, day 0, and day +1 (•). A control group of 60 B10.BR mice received allogeneic BM cells alone (▪). Another control group of 20 B10.BR mice received irradiation without a transplant (□) and 26 B10.BR mice were transplanted with syngeneic H2k BM cells (⧫). Significant differences in survival using the Cox statistic were found between the group of mice receiving multiple infusions of irradiated allogeneic splenocytes and the group that received nonirradiated splenocytes (P = 3 × 10−6); the mice that received irradiated allogeneic splenocytes and mice that received allogeneic BM alone (P = 1 × 10−2); and the mice that received multiple infusions of nonirradiated allogeneic splenocytes and mice that received allogeneic BM alone (P= 3 × 10−7).
Sixty-day survival of B10.BR mice transplanted with allogeneic C57.BL6 BM and splenocytes. Recipient mice received 10 Gy irradiation in 2 equal fractions on day −2 or day −1 and transplanted with 0.5 × 106 BM cells from C57.BL6 donors on day 0. The 60-day survival of groups of 7 to 21 mice in 14 separate experiments receiving various numbers of irradiated (7.5 Gy) (▴) or nonirradiated (○) allogeneic splenocytes given in 1 to 3 injections on consecutive days around the day of BM transplant (day 0). Best-fit lines are shown for the survival of mice receiving different doses of irradiated allogeneic splenocytes (—) (r2 = .49) and unirradiated allogeneic lymphocytes (— — —).
Sixty-day survival of B10.BR mice transplanted with allogeneic C57.BL6 BM and splenocytes. Recipient mice received 10 Gy irradiation in 2 equal fractions on day −2 or day −1 and transplanted with 0.5 × 106 BM cells from C57.BL6 donors on day 0. The 60-day survival of groups of 7 to 21 mice in 14 separate experiments receiving various numbers of irradiated (7.5 Gy) (▴) or nonirradiated (○) allogeneic splenocytes given in 1 to 3 injections on consecutive days around the day of BM transplant (day 0). Best-fit lines are shown for the survival of mice receiving different doses of irradiated allogeneic splenocytes (—) (r2 = .49) and unirradiated allogeneic lymphocytes (— — —).
Irradiated allogeneic lymphocytes did not produce GVHD in the first week after transplantation into allogeneic recipients.
Three groups of B10.BR mice received either (1) 0.5 × 106 C57.BL6 allogeneic BM alone; (2) allogeneic BM plus 75 × 106 irradiated allogeneic lymphocytes; or (3) allogeneic BM plus 75 × 106 nonirradiated allogeneic lymphocytes. Mice were euthanized during the first week posttransplant, and necropsy was performed to determine whether acute GVHD had developed. Mice that received allogeneic marrow and nonirradiated allogeneic splenocytes had clinical and histological evidence of acute GVHD as evidenced by mean 15% loss of body weight by day +5 and a mean 24% weight loss by day +6 posttransplant. The peripheral blood on day +5 showed leukopenia (total leukocyte count of 0.9 ± 0.4 × 106 /L), but with relatively large numbers of donor T cells in the blood (14.4% of blood leukocytes). Histological examination of the livers of these recipients showed evidence of acute GVHD, with mean GVHD scores of 1 ± 1 on day +5 and 1.8 ± 0.5 on day +6. In contrast, mice which had received transplants containing allogeneic BM alone or BM plus irradiated allogeneic splenocytes had no significant weight loss and showed no histological evidence of GVHD (mean histology scores of 0). Pancytopenia was present (mean leukocyte counts of 0.3 ± 0.3 and 0.4 ± 0.3 × 106/L, respectively), but peripheral blood T cells derived from the irradiated donor splenocytes were not observed (<0.3% of leukocytes). Necropsies of moribund mice at 3 weeks posttransplant that had received allogeneic BM and irradiated allogeneic splenocytes showed marked pancytopenia and absence of donor-derived T cells in their peripheral blood.
Irradiated allogeneic splenocytes did not contribute significantly to donor-derived hematopoiesis in H2b → H2k transplants.
We used congenic strains of C57.BL6 mice expressing either Thy 1.1 versus Thy 1.2 or CD45.1 versus CD45.2 as BM and splenocyte donors. The origin of T cells in recipient mice was determined by the expression of the donor spleen or donor BM specific marker. Among the B10.BR mice that received injections of C57.BL6 splenocytes irradiated to 7.5 Gy, the overall mean (±standard deviation [SD]) fraction of peripheral blood cells that were donor-derived (H2b) was 49% ± 35% at day +30 to day +45. The mean frequency of donor-derived cells was relatively constant across multiple experiments involving different doses of irradiated splenocytes in combination with a fixed dose of allogeneic BM (Fig 7). Mixed chimerism was present in the T-cell compartment in all mice that received various doses of irradiated splenocytes and BM. The overall mean percentages of day +30 peripheral blood T cells in B10.BR transplant recipients that were derived from allogeneic donor BM was 25% ± 31%, while 11% ± 17% of peripheral blood T cells were derived from the irradiated allogeneic donor splenocytes. Significant numbers of blood T cells derived from the infusions of irradiated allogeneic splenocytes were only seen at the highest doses of splenocytes infused. Recipients of 75 × 106, or 120 × 106 irradiated allogeneic splenocytes had 9% ± 14% and 35% ± 22%, respectively, of peripheral blood T cells derived from the irradiated donor splenocytes at day +30. Interestingly, the fraction of T cells derived from the irradiated splenocytes declined to nearly nondetectable levels at 3 months posttransplant, even among recipients of the largest number of irradiated splenocytes. A typical flow cytometric plot of the blood of a recipient of the highest dose (120 × 106) of irradiated splenocytes analyzed at day +30 and day +112 is shown in Fig 8. In this animal, 88% of blood leukocytes were donor-derived at day +30 and 96% of blood leukocytes donor-derived by day +112 (Fig 8, upper panels). The T-cell compartment of the blood had mixed donor (11% of nucleated blood cells) and host-type T cells (8% of the blood cells) at day +30. By day +112, 27% of blood cells were donor-derived T cells and only 4% were of host type (Fig 8, upper panels). Thirty-five percent of donor-derived T cells were derived from the irradiated splenocytes at day +30, while spleen-derived T cells were undetectable at day +112 (Fig 8, lower panels).
Recipients of allogeneic BM and irradiated allogeneic splenocytes had mixed donor and host type hematopoiesis. The mean percentage of peripheral blood leukocytes expressing H2b(donor type; ▪) or H2k (host type; □) from B10.BR (H2k) mice surviving to day +30 after transplantation with C57.BL6 (H2b) BM and irradiated (7.5 Gy) splenocytes is shown. The conditions of the transplant are described in the legend to Fig 6. The error bars depict the SD of the mean.
Recipients of allogeneic BM and irradiated allogeneic splenocytes had mixed donor and host type hematopoiesis. The mean percentage of peripheral blood leukocytes expressing H2b(donor type; ▪) or H2k (host type; □) from B10.BR (H2k) mice surviving to day +30 after transplantation with C57.BL6 (H2b) BM and irradiated (7.5 Gy) splenocytes is shown. The conditions of the transplant are described in the legend to Fig 6. The error bars depict the SD of the mean.
Flow cytometric analysis of peripheral blood leukocytes from recipients of allogeneic TCD BM and irradiated allogeneic splenocytes. Peripheral blood was obtained from B10.BR recipients of 0.5 × 106 CD45.2+C57.BL6 TCD BM cells and 50 × 106 irradiated CD45.1+ C57.BL6 splenocytes. The conditions of the transplant are described in the legend to Fig 6. Upper panels: the total percentages of donor T cells (upper right quadrant), host-type T cells (lower right quadrant), and host non-T cells (lower left quadrant) and donor-type non-T cells (upper left quadrant) are shown for 2 representative animals bled on day +30 and day +112 posttransplant. Lower panels: the fraction of donor T cells derived from the irradiated splenocytes (CD45.1+, upper right quadrant) or from the donor BM (CD45.1−, lower right quadrant) are shown.
Flow cytometric analysis of peripheral blood leukocytes from recipients of allogeneic TCD BM and irradiated allogeneic splenocytes. Peripheral blood was obtained from B10.BR recipients of 0.5 × 106 CD45.2+C57.BL6 TCD BM cells and 50 × 106 irradiated CD45.1+ C57.BL6 splenocytes. The conditions of the transplant are described in the legend to Fig 6. Upper panels: the total percentages of donor T cells (upper right quadrant), host-type T cells (lower right quadrant), and host non-T cells (lower left quadrant) and donor-type non-T cells (upper left quadrant) are shown for 2 representative animals bled on day +30 and day +112 posttransplant. Lower panels: the fraction of donor T cells derived from the irradiated splenocytes (CD45.1+, upper right quadrant) or from the donor BM (CD45.1−, lower right quadrant) are shown.
Enhanced survival and donor hematopoiesis among recipients of irradiated allogeneic splenocytes requires donor-type T cells.
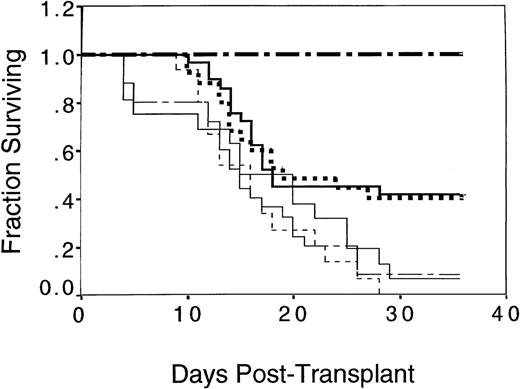
Splenocytes were fractionated into T-cell–enriched (TCE) or TCD subsets using magnetic bead separation and negative depletion with antibodies against either T-cell (CD3) or B-cell, monocyte, and granulocyte markers (CD19/Gr-1/MAC-1). The initial content of T cells in the spleen was 15% to 20%; the TCE fraction contained a mean of 90% to 98% T cells, while the TCD fractions contained 0.5% to 1.5% T cells. The TCD fraction was mainly B cells (78.8%). The content of natural killer (NK) 1.1+ cells were slightly reduced in both the TCD (2.85%) and TCE (2.02%) fractions compared with the frequency in unfractionated splenocytes (4.4%). The reduction of NK 1.1+ cells is consistent with the coexpression of CD3 and MAC-1 on minor populations of NK 1.1+ cells. Three experiments, involving 114 mice, were performed in which B10.BR mice were transplanted with mixtures of C57.BL6 allogeneic BM cells and TCD, TCE, or unfractionated C57.BL6 irradiated splenocytes. The number of T cells injected in the group that received TCE splenocytes was equivalent to the number of T cells present in 75 × 106 unfractionated spleen cells (15 to 20 × 106 T cells). Mice injected with TCD splenocytes received the same number of non-T cells as present in 75 × 106 unfractionated spleen cells (60 × 106 non-T cells). The survival of mice that received TCE irradiated splenocytes was equivalent to mice that received unfractionated irradiated splenocytes and superior to recipients of irradiated TCD splenocytes (Fig 9). Forty percent of the B10.BR recipients of allogeneic BM and unfractionated irradiated splenocytes, as well as 41% of the B10.BR recipients of TCE irradiated splenocytes, survived 40 days posttransplant. In contrast, only 7% or 8%, respectively, of the recipients of allogeneic BM or allogeneic BM and TCD splenocytes survived transplant (Fig 9, P= .006 comparing survival between recipients of TCD and TCE splenocytes).
Enhanced survival of B10.BR mice transplanted with C57.BL6 BM cells and irradiated C57.BL6 splenocytes depended on the presence of splenic T cells. Data are pooled from 3 experiments in which groups of 8 to 10 B10.BR mice were irradiated with 10 Gy on day −2 and transplanted with 0.5 × 106 C57.BL6 allogeneic BM cells and splenocytes or 0.5 × 106 syngeneic B10.BR BM cells on day 0. Allogeneic splenocytes were prepared daily from C57.BL6 mice, TCD, or TCE, as described in Materials and Methods, and irradiated to 7.5 Gy immediately before injection on days −1, 0, and +1. One group of 5 B10.BR mice received syngeneic B10.BR BM (▪▪ ▪); a second group of 25 B10.BR mice received a total of 75 × 106 C57.BL6 irradiated splenocytes with C57.BL6 BM (▪▪); a third group of 16 B10.BR mice received C57.BL6 BM alone (____); a fourth group of 25 B10.BR mice received a total of 60 × 106C57.BL6 TCD irradiated splenocytes with C57.BL6 BM (–— –); a fifth group of 29 B10.BR mice received a total of 15 × 106 C57.BL6 TCE irradiated splenocytes with C57.BL6 BM (▪▪▪); and a sixth group of 15 mice received a total of 75 × 106 nonirradiated C57.BL6 splenocytes with C57.BL6 BM (– – –). A significantly superior survival was found for both the groups of mice receiving multiple infusions of irradiated allogeneic splenocytes and the group receiving TCE irradiated allogeneic splenocytes compared with the group that received nonirradiated splenocytes (P < .01); the group that received TCD allogeneic splenocytes (P ≤ .01); and the group that received syngeneic BM (P < .05).
Enhanced survival of B10.BR mice transplanted with C57.BL6 BM cells and irradiated C57.BL6 splenocytes depended on the presence of splenic T cells. Data are pooled from 3 experiments in which groups of 8 to 10 B10.BR mice were irradiated with 10 Gy on day −2 and transplanted with 0.5 × 106 C57.BL6 allogeneic BM cells and splenocytes or 0.5 × 106 syngeneic B10.BR BM cells on day 0. Allogeneic splenocytes were prepared daily from C57.BL6 mice, TCD, or TCE, as described in Materials and Methods, and irradiated to 7.5 Gy immediately before injection on days −1, 0, and +1. One group of 5 B10.BR mice received syngeneic B10.BR BM (▪▪ ▪); a second group of 25 B10.BR mice received a total of 75 × 106 C57.BL6 irradiated splenocytes with C57.BL6 BM (▪▪); a third group of 16 B10.BR mice received C57.BL6 BM alone (____); a fourth group of 25 B10.BR mice received a total of 60 × 106C57.BL6 TCD irradiated splenocytes with C57.BL6 BM (–— –); a fifth group of 29 B10.BR mice received a total of 15 × 106 C57.BL6 TCE irradiated splenocytes with C57.BL6 BM (▪▪▪); and a sixth group of 15 mice received a total of 75 × 106 nonirradiated C57.BL6 splenocytes with C57.BL6 BM (– – –). A significantly superior survival was found for both the groups of mice receiving multiple infusions of irradiated allogeneic splenocytes and the group receiving TCE irradiated allogeneic splenocytes compared with the group that received nonirradiated splenocytes (P < .01); the group that received TCD allogeneic splenocytes (P ≤ .01); and the group that received syngeneic BM (P < .05).
In a second series of 2 experiments, recipient B10.BR mice were treated with a larger, more immunosuppressive dose of radiation (11 Gy), as well as a larger dose of C57.BL6 BM cells (1 × 106). These conditions permitted recipients of BM alone to survive and allowed us to evaluate the extent to which irradiated donor lymphocytes facilitate donor chimerism independently of their radioprotective effect. All of the recipients that received BM alone survived to day +60, with autologous hematopoietic recovery observed in 12 of 18 mice. In contrast, recipients of allogeneic BM plus 75 × 106 irradiated allogeneic splenocytes had 61% survival at 60 days posttransplant, with 82% of surviving mice showing greater than 90% donor-derived hematopoiesis (Table 3). Seven of 10 mice that received allogeneic BM plus TCD splenocytes survived to 60 days, but only 2 of 7 of these mice had donor hematopoiesis (Table 3). The median percentage (±SD) of donor cells among recipients of irradiated allogeneic splenocytes was 100% ± 42% compared with a median value of 5% ± 48% among recipients of allogeneic BM and TCD irradiated splenocytes (P = .04; Table 3). In addition, the median percentage of peripheral blood T cells that were derived from donor BM was 100% ± 42% among recipients of irradiated splenocytes (Table3). This value was significantly higher than the percentage of BM-derived T cells among recipients TCD irradiated splenocytes (13% ± 32%, P = .02, Table 3) and higher than recipients of BM alone (0% ± 47%, P = .02, Table 3). In these experiments, the contribution of the donor splenocytes to T-cell reconstitution was minimal (a median of 5% of blood T cells was derived from the irradiated donor splenocytes, Table 3).
Donor T Cells Mediate the Graft Facilitating Activity of Irradiated Allogeneic Splenocytes
| . | BM + Irrad Spleen . | BM + TCD Irrad Spleen . | BM Alone . | . | . |
|---|---|---|---|---|---|
| Fraction surviving | 11/18 | 7/10 | 18/18 | ||
| Fraction donor engrafted | 9/11 | 2/7 | 6/18 | ||
| PValues | |||||
| Irrad Sp v TCD Irrad Sp | Irrad Sp v BM | ||||
| % Donor cells | 100 ± 42 | 5 ± 48 | 0 ± 48 | P = .042 | P = .025 |
| T cells | |||||
| % Donor BM | 100 ± 42 | 13 ± 32 | 0 ± 47 | P = .021 | P = .023 |
| % Donor spleen | 0 ± 10 | 0 ± 0 | 0 ± 1 | ||
| % Host | 0 ± 40 | 88 ± 36 | 100 ± 47 | P = .030 | P = .018 |
| . | BM + Irrad Spleen . | BM + TCD Irrad Spleen . | BM Alone . | . | . |
|---|---|---|---|---|---|
| Fraction surviving | 11/18 | 7/10 | 18/18 | ||
| Fraction donor engrafted | 9/11 | 2/7 | 6/18 | ||
| PValues | |||||
| Irrad Sp v TCD Irrad Sp | Irrad Sp v BM | ||||
| % Donor cells | 100 ± 42 | 5 ± 48 | 0 ± 48 | P = .042 | P = .025 |
| T cells | |||||
| % Donor BM | 100 ± 42 | 13 ± 32 | 0 ± 47 | P = .021 | P = .023 |
| % Donor spleen | 0 ± 10 | 0 ± 0 | 0 ± 1 | ||
| % Host | 0 ± 40 | 88 ± 36 | 100 ± 47 | P = .030 | P = .018 |
B10.BR mice received 11 Gy irradiation on day −2 and were then transplanted with 1 × 106 BM cells from C57.BL6 mice, or a combination of BM and 75 × 106 irradiated (7.5 Gy) C57.BL6 splenocytes or BM and 60 × 106 TCD irradiated (7.5 Gy) C57.BL6 splenocytes. The C57.BL6 donor mice were all CD45.1+; BM and spleen donors varied according to their expression of Thy 1.1 (spleen) or Thy 1.2 (BM). The median percentages of peripheral blood cells that were donor-derived (CD45.1+) and the percentage of blood T cells that were derived from donor BM, donor spleen, or were of host origin were determined at day +60 to day +70 by flow cytometry.
Lymphocytes from recipients of irradiated allogeneic splenocytes are tolerant to both donor and host MHC types and do not produce GVHD when transferred to secondary BMT recipients.
The GVHD potential of lymphocytes from chimeric H2b→ H2k BM recipients of the highest dose (120 × 106) of irradiated splenocytes were compared with the GVHD potential of naive MHC mismatched H2b and H2k lymphocytes. Spleen cells from BM transplant recipients were serially transplanted into mice of both donor and host strains. B10.BR mice that had received C57.BL6 allogeneic BM and irradiated (7.5 Gy) C57.BL6 allogeneic splenocytes were killed at day +112. At this time, the mice were fully chimeric (>95% of blood cells were donor-derived). Splenocytes from the chimeric mice (>98% of T cells were derived from donor BM) were serially transplanted into lethally irradiated C57.BL6 or B10.BR mice in combination with BM that was syngeneic with the recipient strain. Splenocytes from the transplanted chimeric mice (that were predominantly H2b) did not cause clinically detectable GVHD or graft failure in animals of either H2b or H2k strains. Transplantation using naive MHC mismatched splenocytes lead to severe GVHD, which was fatal by day +30 in 3 of 4 recipients (Table 4). Flow cytometric analysis of blood, spleen, and BM from recipients of either tolerant or naive MHC mismatched splenocytes showed that MHC-mismatched T cells from recipients of irradiated splenocytes failed to proliferate in the secondary recipients. In contrast, transplanted naive MHC mismatched T cells proliferated and produced acute GVHD (Table 4).
Secondary Transfer of Splenocytes From B10.BR (H2k) Mice Transplanted With C57.BL6 Allogeneic BM and Irradiated (7.5 Gy) Splenocytes Into C57.BL6 and B10.BR Recipients
| Recipient Mice . | B10.BR (H2k) . | B10.BR (H2k) . | C57.BL6 (H2b) . | C57.BL6 (H2b) . |
|---|---|---|---|---|
| BM cells injected | B10.BR | B10.BR | C57.BL6 | C57.BL6 |
| Spleen cells injected | Naı̈ve C57.BL6 Spleen Transplant | Chimeric H2b/H2kSpleen Transplant | Naı̈ve B10.BR Spleen Transplant | Chimeric H2b/H2k Spleen Transplant |
| Clinical GVHD | +++ | None | ++ | None |
| Survival post-BMT | day 21, 21 | day >30, >30 | day 18, >30 | day >30, >30 |
| Weight at death | 18.7, 19 g | >26 g | 20 g | >26 g |
| % Donor H2b T cells in | ||||
| Peripheral blood | 23 | 7 | ND | ND |
| Spleen | 12 | 16 | ND | ND |
| BM | 17 | 3 | ND | ND |
| Recipient Mice . | B10.BR (H2k) . | B10.BR (H2k) . | C57.BL6 (H2b) . | C57.BL6 (H2b) . |
|---|---|---|---|---|
| BM cells injected | B10.BR | B10.BR | C57.BL6 | C57.BL6 |
| Spleen cells injected | Naı̈ve C57.BL6 Spleen Transplant | Chimeric H2b/H2kSpleen Transplant | Naı̈ve B10.BR Spleen Transplant | Chimeric H2b/H2k Spleen Transplant |
| Clinical GVHD | +++ | None | ++ | None |
| Survival post-BMT | day 21, 21 | day >30, >30 | day 18, >30 | day >30, >30 |
| Weight at death | 18.7, 19 g | >26 g | 20 g | >26 g |
| % Donor H2b T cells in | ||||
| Peripheral blood | 23 | 7 | ND | ND |
| Spleen | 12 | 16 | ND | ND |
| BM | 17 | 3 | ND | ND |
B10.BR or C57.BL6 mice were exposed to 10 Gy irradiation in 2 equal fractions and transplanted with 1 × 106 syngeneic BM cells and 25 × 106 splenocytes from naı̈ve B10.BR or C57.BL6 donors (as shown) or 25 × 106splenocytes obtained from B10.BR mice with mixed chimerism (H2b/H2k) that were killed 112 days after receiving 0.5 × 106 C57.BL6 BM cells and 120 × 106 irradiated (7.5 Gy) C57.BL6 splenocytes. Three of 4 recipients of naı̈ve allogeneic splenocytes died or were euthanized between day 18 and 21 with clinical evidence of acute GVHD including the appearance of ruffled fur, decreased motor activity, diarrhea, and “hunched over” posture. The remaining mice were killed at day +30. The peripheral blood, BM, and spleen of B10.BR mice surviving to day +30 were analyzed by flow cytometry for the presence of T cells derived from H2b donor splenocytes. Progeny of the irradiated splenocytes (genetically marked with CD45.1) were not detectable after transfer to secondary BMT recipients.
Abbreviation: ND, not detectable.
DISCUSSION
In this report, we have studied the ability of irradiated allogeneic leukocytes to facilitate engraftment of allogeneic stem cells without causing GVHD. In the H2b → H2k, and H2b → H2r BM transplant model systems, transplantation with 0.5 × 106allogeneic BM cells led to graft failure and death among more than 95% of recipient mice (Table 1 and Fig 5). The rare surviving mouse had pancytopenia and a predominance of host type T cells, implicating a population of relatively radioresistant host T cells in graft rejection. Doses of 5 Gy or greater inhibited the proliferative capacity of mouse T cells (Fig 1)31 and splenocytes irradiated to 7.5 Gy did not produce GVHD in MHC mismatched recipients (Table 2).30 Irradiated donor T cells could be detected in the BM of recipient SCID mice for more than 3 days. In addition, irradiated cytotoxic T-lymphocyte (CTL) effector cells had enhanced allospecific cytolytic activity compared with nonirradiated CTL effector cells (Fig 3).31 32 Based on these findings, we tested the effect of adding irradiated splenocytes to BM transplants across a major MHC barrier. The addition of allogeneic splenocytes irradiated between 5 Gy and 10 Gy facilitated engraftment by allogeneic BM cells without producing clinically evident GVHD, even when very large doses of allogeneic splenocytes were administered. The engraftment promoting effect of irradiated allogeneic lymphocytes was dependent on the dose of radiation used and the number of irradiated splenocytes infused (Fig 6). Multiple infusions of 25 × 106 irradiated splenocytes given around the day of transplantation were more effective than a single injection of 25 × 106 irradiated splenocytes given on day 0 (data not shown). The effect of irradiated splenocytes administered at the time of transplant was to favor donor-derived hematopoiesis rather than simply enhancing radioprotection. T cells present in the allogeneic splenocytes were necessary for this effect, but did not contribute directly to donor-derived hematopoiesis (Table 3).
The present study is in contrast to the two previous reports using animal models of BM transplantation that did not show clear evidence of the ability of irradiated allogeneic lymphocytes to facilitate engraftment. Gratwohl et al33 studied the role of irradiated allogeneic leukocytes in BMT using antigenically mismatched genetically unrelated donor-recipient pairs of rabbits transplanted with a combination of allogeneic BM and irradiated autologous BM. Deeg et al34 studied irradiated allogeneic lymphocytes in the outbred dog BMT model. In the study by Gratwohl, a series of 5 infusions of irradiated (15 Gy) donor lymphocytes from day 1 to day 10 did not prevent graft rejection (0 of 5 animals engrafted). The addition of cyclosporine to a combination of TCD allogeneic BM, irradiated autologous BM, and irradiated allogeneic buffy coat cells increased the rate of engraftment to 80%, but 40% of these recipients died of GVHD.33 In the report by Deeg, infusions of irradiated (20 Gy) donor lymphocytes from dog leukocyte antigens (DLA) mismatched BM donors did not prevent fatal graft failure.34The addition of viable donor lymphocytes to the allogeneic DLA mismatched BM graft resulted in an increased frequency of stable hematopoietic engraftment, but recipient dogs uniformly died of GVHD.35 In the dog model system, gamma irradiation (20 Gy) of whole blood products transfused before DLA-mismatched allogeneic BMT prevented sensitization to alloantigens, but did not induce allospecific tolerance.36
There is limited clinical experience using irradiated donor lymphocytes to treat human patients. Infusions of allogeneic donor lymphocytes irradiated to 15 to 20 Gy to allogeneic transplant recipients at high risk for graft rejection may reduce the risk of graft failure when given after BMT.8,37 38 In these studies, the contribution of the irradiated donor lymphocytes to hematopoietic engraftment or clinical GVHD was unclear. There was no way to distinguish the irradiated lymphocytes from the variable number of nonirradiated lymphocytes contained within the BM graft. There are two significant differences between the present study and earlier reports of using this strategy in animal transplant models and in patients. First, we used a lower dose of radiation to treat the allogeneic donor cells (7.5 Gyv 15 to 20 Gy). Second, we administered the irradiated allogeneic cells before and concomitant with the BM cells rather than as multiple infusions after the BM graft. In addition, we used donor strains that were congenically marked to determine whether donor T cells originated from the BM or the spleen cell transplant.
Irradiated splenocytes could potentially enhance engraftment by supplying sufficient radioresistant donor hematopoietic progenitor cells to achieve short-term hematopoietic engraftment and radioprotection. The irradiated hematopoietic progenitors would complement the limited number of stem cells in the allogeneic BM graft and allow animals to survive with autologous recovery. Studies by Till and McCulloch39 demonstrated that radiation doses of 7.5 Gy led to a 2.5 log reduction in the colony-forming unit (CFU) content of BM cells. However, H2b → H2k transplants using irradiated (7.5 Gy) allogeneic donor splenocytes alone did not result in hematopoietic engraftment of recipients (0 of 5 mice survived to day +30 (data not shown). CD45.1+ myeloid and monocytic progeny of irradiated allogeneic CD45.1 spleen cells were not detected in the blood of B10.BR recipients of C57.BL6 CD45.2 allogeneic BM and C57.BL6 CD45.1 irradiated spleen cells (data not shown). Therefore, the engraftment promoting effect of irradiated donor splenocytes was not due to an increased dose of radioresistant splenic hematopoietic stem cells.
The mechanisms for the graft facilitating activity of irradiated allogeneic lymphocytes may include the retention of cytotoxic or “veto” activity by irradiated donor cells that inhibit or eliminate residual host T cells.31,40 While radiation enhanced the cytotoxic activity of allostimulated CTL (Fig 2), a measurable effect of radiation on the allospecific cytolytic activity of noncultured splenocytes was not observed (data not shown). Thus, the data presented in Fig 2 demonstrate retention of biologic activity after irradiation, but do not directly demonstrate that graft facilitation of irradiated splenocytes is due to enhanced allospecific cytotoxicity. Second, a large number of allogeneic donor cells either overwhelm cytotoxic capacity of radioresistant host cells (“cold target inhibition”) or induce anergy among cytotoxic host cells and thereby permit the survival of allogeneic donor stem cells.41 Third, elaboration of cytokines by irradiated allogeneic lymphocytes could either inhibit graft rejection by host lymphocytes or promote the growth of allogeneic donor stem cells.26,33,42 43 While our study is potentially consistent with all 3 hypothetical mechanisms, the present data favor a central role for T cells in this process. The failure of TCD splenocytes to facilitate engraftment militates against a simple “cold target inhibition” mechanism.
The low frequency of allospecific T cells among unprimed splenocytes might argue against the role of antigen-specific T cells in enhancing engraftment after irradiation. Anti-H2d allospecific, cytolytic T cells are present at a frequency of 1/570 in C57.BL6 splenocytes.44 If anti-H2k allospecific CTL are present at a similar frequency, then 75 million donor splenocytes (circa 20 million T cells) would contain approximately 35,000 allospecific anti-H2k CTL or 1 million allospecific T cells/kg. Such a small number of allospecific lymphocytes might be inadequate to promote engraftment if they have lost the capacity for proliferation in vivo. However, new DNA synthesis is not needed for the generation of allospecific CTL,45 and an equivalent number of FACS-purified CD8+ BM “facilitating cells” have been shown to enhance engraftment of MHC mismatched c-kit+, Sca-1+, Lin− stem cells.46The critical time for engraftment of allogeneic stem cells across a major MHC barrier is likely in the first few days after BMT.47 Thus, even when nonirradiated allogeneic T cells are administered during the initial engraftment period, there is relatively little time for significant clonal expansion of allospecific T cells.
Preliminary data suggest that immunotherapy using irradiated donor lymphocytes have antileukemic activity in animal and human clinical model systems. Multiple infusions of irradiated allogeneic cells may be safely given to patients who have experienced relapse of leukemia or lymphoma after allogeneic BMT, with an antitumor response observed in 25% of patients (Waller et al48). Multiple infusions of irradiated allogeneic lymphocytes that retain short-term allospecific cytotoxicity and lack the potential for clonal expansion in vivo can be considered as a novel form of immunotherapy with defined pharmacokinetics. This method of immunotherapy might have clinical applications for facilitating TCD allogeneic hematopoietic stem cell transplants, as well as mediating a direct anticancer effect in transplant recipients.
ACKNOWLEDGMENT
We acknowledge Sylvia D. Ennis, Senior Research Project Coordinator for her editorial assistance with this manuscript and Richard Lopez for helpful discussions.
Supported in part by National Cancer Institute (NCI) Grant No. 1RO1CA74364 and the Leukemia Research Foundation (to E.K.W.). R.C. was supported by the Aplastic Anemia Foundation.
A.M.S., S.M., and T.W.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Edmund K. Waller, MD, PhD, Emory University School of Medicine, Division of Hematology and Oncology, Suite 1003, 1639 Pierce Dr, Atlanta, GA 30322; e-mail:ewaller@emory.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal