Abstract
CD4+ T helper 1 (Th1) cells and Th2 cells are distinguished based on the pattern of cytokines they are able to produce. Selectin ligands and chemokine receptors are differentially expressed in Th1 and Th2 cells, providing a basis for tissue-specific recruitment of helper T-cell subsets. However, the modes and mechanisms regulating tissue-specific localization of Th1 and Th2 cells are still largely unknown. Here, we show the preferential expression on Th1 cells of the integrin 6/β1, which is distinctly regulated by the Th1-inducing cytokines interleukin-12 (IL-12) and interferon-alfa (IFN-). The pattern of integrin 6/β1 regulation closely mirrors that of the chemokine receptor CCR1. Analysis of signal transducer and activator of transcription 4 (Stat4) activation by IL-12 and IFN- shows distinct signaling kinetics by these cytokines, correlating with the pattern of CCR1 and integrin 6/β1 expression. Unlike IFN-, the ability of IL-12 to generate prolonged intracellular signals appears to be critical for inducing integrin 6/β1 upregulation in Th1 cells. The expression and upregulation of CCR1 and 6/β1 integrin promotes the migration of Th1 cells. These findings suggest that the exquisite regulation of integrin 6/β1 and CCR1 may play an important role in tissue-specific localization of Th1 cells.
ANTIGENIC STIMULATION TRIGGERS differentiation of mature naive CD4+ T lymphocytes into effector/memory T-cell populations.1 Cytokines present at the initiation of the immune response direct the development of functionally distinct subsets of helper T cells, characterized by the production of discrete patterns of cytokines.2-4Interleukin-12 (IL-12) induces differentiation of type 1 T helper (Th1) cells,5 whereas IL-4 promotes Th2 cell development.6,7 IL-12 activates the signal transducer and activator of transcription (Stat) 4 in developing and differentiated Th1, but not in Th2 cells,8 whereas IL-4 induces Stat6 activation more efficiently in Th2 than Th1 cells.9 Studies on Stat4- and Stat6-deficient mice have shown that these transcription factors are essential for Th1 and Th2 cell differentiation, respectively.10-13 In humans, in addition to IL-12, type I interferons are able to activate Stat4 and induce Th1 cell development.14-17 By virtue of interferon-γ (IFN-γ) and lymphotoxin production, Th1 cells promote cell-mediated immune responses, leading to the eradication of intracellular pathogens.3 Cytokines produced by Th2 cells, such as IL-4 and IL-5, activate mast cells and eosinophils and enhance the production of IgE, promoting the development of allergic inflammation.18 19
A critical requisite for the maintenance of functionally polarized immune responses appears to be the acquisition of specific homing receptors that can ensure the localization of different types of effector T cells to distinct inflammatory sites.20,21 A multistep process mediated by the interplay of chemokines, chemokine receptors, and adhesion molecules, which involves rolling, firm adhesion, and diapedesis, regulates the homing of lymphocytes.22,23 The combinatorial and sequential involvement of a complex series of receptor/ligand interactions accomplishes the task of an appropriate distribution of functionally competent lymphocytes. Initial studies by Austrup et al demonstrated that the preferential expression of P- and E-selectin ligands on Th1 cells determines their selective recruitment to certain inflamed tissues.24,25 More recently, we and others have demonstrated differential expression and regulation of chemokine receptors in Th1 and Th2 cells.26-30 Despite the key role that integrins play in leukocyte recruitment and function,31 their expression and regulation in helper T-cell subsets has remained relatively unexplored.
Signals delivered by distinct cytokines may imprint developing helper T cells with specific homing patterns by setting the expression level of certain adhesion molecules and chemokine receptors.29,32Cytokines can further modulate chemokine receptor expression on differentiated helper T cells,33 34 perhaps to direct locomotion and/or retain extravasated effector T lymphocytes into specific microenvironments. In this study, we document the distinct abilities of IL-12 and IFN-α to regulate the expression of integrin α6/β1 and chemokine receptor CCR1. Our data indicate unique signaling kinetics by IL-12, which correlate with the pattern of regulation of integrin α6/β1 and CCR1. We also demonstrate that the upregulation of CCR1 and integrin α6/β1 expression by IL-12 can promote the migration of Th1 cells.
MATERIALS AND METHODS
Generation of polarized human helper T lymphocytes.
Human neonatal leukocytes were isolated from freshly collected, heparinized, neonatal blood by Ficoll-paque (Pharmacia Biotech AB, Uppsala, Sweden) density gradient centrifugation. Polarized helper T-cell lines were generated as previously described by stimulation with 2 μg/mL phytohemagglutinin (PHA; Wellcome, Beckenham, UK) in the presence of various combinations of cytokines and anticytokine antibodies.16 Th1 cells were generated by the addition of 5 ng/mL IL-12 (Hoffmann La Roche, Nutley, NJ) and 200 ng/mL neutralizing anti–IL-4 antibody (Pharmingen, San Diego, CA) with or without further addition of 103 U/mL IFN-α (Roferon A; Hoffman-La Roche AG, Basel, Switzerland). Th2 cells were generated by the addition of 10 ng/mL IL-4 (Pharmingen) and 2 μg/mL neutralizing anti–IL-12 antibodies, 17F7 and 20C2. The cells were cultured in complete medium (RPMI 1640; Sigma Chemical, St Louis, MO) supplemented with 5% FetalClone (Hyclone, Logan UT), 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 100 U/mL penicillin-streptomycin). On day 3, the cultures were washed and expanded in complete medium with addition of 100 U/mL IL-2 (Hoffman-La Roche). In selected experiments, CD4+ or CD8+ polarized T cells were purified by immunomagnetic negative selection using anti-CD8 or anti-CD4 monoclonal antibody (MoAb)-coated microbeads, following the directions provided by the manufacturer (Miltenyi Biotech, Bergisch Gladbach, Germany). The Lol p1-specific Th1 clone ET 3.22 and Th2 clone E 4.1, previously described,26 were restimulated with PHA and irradiated peripheral blood mononuclear cells, and cultured in a complete medium with 100 U/mL IL-2.
Stimulation conditions.
To analyze integrin α6 subunit and CCR1 expression by flow cytometry or Northern blotting, Th1 and Th2 cells were cultured at a density of 106/mL in complete medium and either left untreated or incubated with IL-12 (10 ng/mL) or IFN-α (103 U/mL) for the indicated times before harvesting and analysis. For the inhibition of IL-12–mediated signaling, 4 ng/mL of mouse p40 homodimer (p402) obtained as previously described35 and 10 μg/mL of anti–IL-12 receptor β1 2.4A1 MoAb were added together or at different times after the addition of 100 pg/mL of IL-12.
Cell surface staining.
Cells were washed in FACS buffer (50 mmol/L phosphate, 150 mmol/L NaCl, pH 7.4; 1% FetalClone; 0.05% sodium azide) and incubated with anti-huCD49f (α6 integrin subunit)-fluorescein isothiocyanate (FITC) MoAb or an isotype-matched control (Pharmingen) for 30 minutes on ice, washed, and analyzed by FACScan flow cytometry (Becton Dickinson, Mountain View, CA).
Northern Blot analysis.
The probe used for the detection of human integrin α6 mRNA was generated by polymerase chain reaction (PCR) from a cDNA template obtained from Th1 cells and primers designed to amplify a portion of the extracellular domain (upstream primer: 5′-CAA AGA TGT CTC GGG ATT CCT GC-3′; downstream primer: 5′-CAG CCT TCA ACT TGG ACA CTC GG-3′). The 390-bp PCR product was ligated into pCR 2.1 plasmid vector and transformed in INVαF′ One ShotEscherichia coli–competent cells, following the instructions provided by the manufacturer (TA cloning kit; Invitrogen, San Diego CA). The probe used for detection of CCR1 was obtained by a similar procedure, using primers that amplified the amino-terminal portion of the receptor (upstream primer: 5′-GTC AAT CGT CAG CAG GAT G-3′; downstream primer: 5′-ATG GAA ACT CCA AAC ACC AC-3′). The cDNA probes were purified on agarose gel followingEcoRI digestion, and 32P-labeled using a Random Primer labeling kit (Prime-It II kit; Stratagene, La Jolla, CA). Th1 cells were harvested at different times after stimulation and total RNA was extracted using TRIzol (Life Technologies, Grand Island, NY). Equal amounts of total RNA (10 μg/lane) were loaded on 1% agarose-formaldehyde gel. The specific mRNAs were detected by hybridization of nylon membranes with 32P-labeled probes for integrin α6, CCR1, and GAPDH, using the NorthernMax Kit (NorthernMax Kit; Ambion, Austin, TX). The filters were exposed to HyperFilm MP films (Amersham Life Science, Buckinghamshire, UK) between double intensifying screens (DuPont, San Diego, CA) at −70°C.
Whole cell extracts and gel shift assay.
For gel shift assays, Th1 and Th2 cells were left untreated or stimulated with IL-12 or IFN-α for the indicated times; 107 cells were harvested, washed, and lysed in 100 μL of ice-cold whole cell extraction buffer (20 mmol/L HEPES pH 7.9, 300 mmol/L NaCl, 10% glycerol, 0.5% NP-40, 1 mmol/L dithiothreitol [DTT], 0.1 mmol/L EDTA and EGTA, 10 μg/mL aprotinin, leupeptin, and NaF, 1 mmol/L PMSF and sodium orthovanadate). The lysates were incubated for 30 minutes on a shaker at 4°C; insoluble debris was removed by centrifugation (13,000 rpm, 4°C, 60 minutes) and stored at −80°C.
For gel shift analysis, a double-stranded oligonucleotide with the Stat4 binding sequence from the first intron of the human IFN-γ gene36 (5′-CGC GAA ATT TTA AGT GAA TTT TTT GAG TTT CTT TTA AAA TTT T-3′) was end-labeled with (γ-32P)adenosine triphosphate (ATP) using T4 polynucleotide kinase according to standard protocols. A 5-μg quantity of nuclear extracts was incubated with 0.1 to 0.5 ng of labeled probe (2 to 3 × 10 4 cpm) for 20 minutes at room temperature in 20 μL of buffer containing 10 mmol/L Tris pH 7.5, 2 mmol/L MgCl2, 25 mmol/L NaCl, 1 mmol/L DTT, 1 mmol/L EDTA, 5% glycerol, 0.3 mg/mL bovine serum albumin (BSA), and 2 μg of poly (dI-dC). For the inhibition assay, 1 μg of anti-Stat4 antibody (Santa Cruz Biotechnology) or control rabbit immunoglobulin was added to each nuclear extract 10 minutes before adding the probe. The reactions were analyzed by electrophoresis in a nondenaturing 5% polyacrylamide gel in 0.5X tris-borate/EDTA buffer (TBE). The gel was dried and exposed at −70°C for autoradiography. The radioactivity in specific protein-DNA complexes was quantified by exposure to a phosphorimager (GS-525; Bio-Rad Laboratories, Richmond, CA).
Analysis of intracellular calcium mobilization.
Th1 and Th2 cells were either left untreated or cytokine-stimulated for the indicated times. Fluo-3AM (Molecular Probes, Eugene, OR) loading was performed as previously described30 by incubating the cells (5 × 106/mL) in buffer A (Hanks’ balanced salt solution [HBSS] with 10 mmol/L HEPES) with 2 μmol/L Fluo-3AM at 37°C for 30 minutes. The incubation was prolonged for 30 minutes after addition of an equal volume of buffer B (HBSS with 10 mmol/L HEPES and 5% fetal calf serum [FCS]). Cells were washed twice in buffer B, resuspended at 2 × 106/mL and analyzed by FACS. Emissions at 525 and 613 nm were measured on a log scale before and after stimulation with the chemokines MIP-1α and IP-10 (R&D Systems, Minneapolis, MN), with acquisition of 3,000 events.
Transmigration assay.
For transmigration assay, 5 μm-pore polycarbonate filters of a 24-well Transwell chamber (Corning Costar, Cambridge MA) were coated on the upper and lower surfaces with extracellular matrix proteins or left uncoated. For the coating of filters, fibronectin or laminin (Life Technologies) were diluted in phosphate-buffered saline (PBS), and 50 μL of a 10-μg/mL solution were added on the upper surface of the filter and the filter was incubated for 2 hours at 37°C. After removal of the solution, the filter was turned upside down and the lower surface was similarly coated. Filters were stored at 4°C until use. Th1 and Th2 cells were washed, resuspended in serum-free RPMI, and labeled with green fluorescent PKH67 Cell Linker (Sigma) and red fluorescent PKH26 Cell Linker (Sigma), respectively. For the IL-12 treatment, Th1 cells were incubated 36 hours in complete medium with IL-2 (100 U/mL) and IL-12 (10 ng/mL) and subsequently washed and labeled as described earlier. Labeled Th1 and Th2 cells (1 to 2 × 106 each) were cultured together or separately in complete medium overnight. The cells were subsequently washed and resuspended in RPMI 1640 with 0.5% BSA. A 0.6-mL quantity of the same medium containing various concentration of SDF-1 or MIP-1α (R&D Systems) was added to the bottom chamber of the transwell, while 0.1 mL of cell suspension was added to the top chamber. Transwells were incubated for 3 hours at 37°C with 5% CO2. For blocking experiments, the cell suspension was incubated 15 minutes with anti-integrin α6 blocking GoH3 or rat IgG2a antibodies (each at 50 μg/mL) before addition to the top chamber. The number of migrated cells was evaluated as previously described.37 Briefly, after recovery from the lower compartment of the transwell, the number of transmigrated cells relative to the input was measured with a FACScan by 60 seconds acquisition at a flow rate of 60 μL/min.
RESULTS
Upregulation of integrin α6/β1 and chemokine receptor CCR1 by IL-12 in Th1 cells.
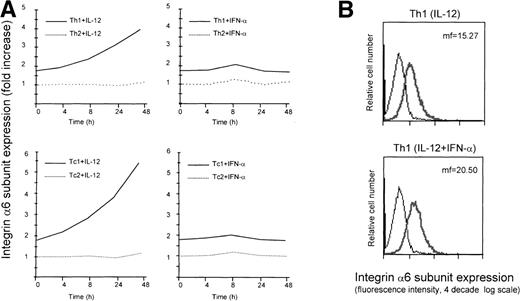
Differential expression of selectin ligands,24,25 as well as chemokine receptors,26-29 in helper T cell subsets has recently been reported. Furthermore, previous work has established an important role for IL-12 and type I interferons, which promote differentiation of Th1 cells,14,16 in controlling the expression of homing receptors in developing and effector T cells.32,38 Despite the fact that molecules belonging to the family of integrins represent key elements in the process of leukocyte recruitment,31 their role and regulation in Th1 and Th2 cells have remained poorly defined. To gain insights on the expression and modulation of integrins in helper T-cell subsets, we exploited a previously described system, which allows for the generation of functionally diverse human helper T-cell populations.16 Following this approach, we generated Th1 cells primed by the action of IL-12 and Th2 cells primed by the action of IL-4. We then analyzed the expression of several integrin subunits on these cells, and found that the laminin receptor integrin α6/β1 was expressed at a higher level on Th1 versus Th2 cells (Fig1A). In addition, Th1 cells that were generated by the combined action of the Th1-inducing cytokines IL-12 and IFN-α, exhibited a further increase in the expression of the integrin α6/β1 relative to the IL-12–primed Th1 cells (Fig 1B). On the basis of this observation, we asked whether IL-12 and IFN-α could modulate the expression of integrin α6/β1 in established human Th1 and Th2 cells. To investigate this possibility, differentiated, 7- to 10-day-old Th1 or Th2 cells were stimulated with IL-12 or IFN-α and cell surface expression of the integrin α6/β1 evaluated at different times. This analysis showed that IL-12 induced a marked and sustained upregulation of the integrin α6/β1 on the surface of Th1, but not Th2 cells. In contrast, IFN-α had only a minor and transient effect even on Th1 cells (Fig 1A). The analysis of similarly polarized CD8+ type 1 cytotoxic T cells (Tc1) and Tc2 cell populations confirmed a selective and sustained upregulation of the integrin α6/β1 in response to IL-12, but not IFN-α, in Tc1 cells (Fig 1A). Notably, the degree of upregulation of the integrin α6/β1 was greater in Tc1 cells (≈3-fold increase) than in Th1 cells (≈2-fold increase). Expression and modulation of the integrin α6/β1 were also analyzed on established antigen-specific human Th1 and Th2 type T-cell clones. This analysis confirmed the preferential expression and selective IL-12–mediated upregulation of the integrin α6/β1 in Th1, but not Th2 clones (Fig 1C). Despite the differences in the expression level of additional integrins observed between Th1 and Th2 cells (B. Clissi, D. D’Ambrosio, R. Pardi, manuscript in preparation), we found that IL-12 uniquely modulated the expression of integrin α6/β1.
Regulation of cell surface expression of the integrin 6/β1 in Th1 and Tc1 cells. (A) Cord blood–derived type 1 (solid lines) or type 2 (dotted lines) polarized cell lines were cultured in a medium supplemented with IL-2 and stimulated with IL-12 (left panels) or IFN- (right panels) as described in Materials and Methods. At different times after stimulation with the cytokines, the cells were harvested, washed, and stained for surface expression of the integrin 6/β1. The mean fluorescence intensity measured for untreated Th2 cells was given the arbitrary value of 1 and all other values were expressed as fold increase over this value. Surface expression of the integrin 6/β1 was analyzed on CD4+ Th1 and Th2 cells (upper panels), and CD8+ Tc1 and Tc2 cells (lower panels). One representative experiment of 3 performed is shown. (B) Th1 cells were generated by the action of either IL-12 or IL-12 + IFN-. Cells were stained with FITC-conjugated anti-CD49f MoAb (thick lines) or with an isotype matched control (thin lines) and cell surface expression of integrin 6/β1 was analyzed by FACS. One representative experiment of 5 is shown. (C) ET 3.22 Th1 clone (upper panel) and E 4.1 Th2 clone (lower panel) were cultured in a medium supplemented with IL-2 and either left untreated (n.t.) or stimulated with IL-12 for 24 hours. Cells were stained with FITC-conjugated anti-CD49f MoAb (black lines) or with an isotype-matched control (grey lines). One experiment of 3 performed is shown.
Regulation of cell surface expression of the integrin 6/β1 in Th1 and Tc1 cells. (A) Cord blood–derived type 1 (solid lines) or type 2 (dotted lines) polarized cell lines were cultured in a medium supplemented with IL-2 and stimulated with IL-12 (left panels) or IFN- (right panels) as described in Materials and Methods. At different times after stimulation with the cytokines, the cells were harvested, washed, and stained for surface expression of the integrin 6/β1. The mean fluorescence intensity measured for untreated Th2 cells was given the arbitrary value of 1 and all other values were expressed as fold increase over this value. Surface expression of the integrin 6/β1 was analyzed on CD4+ Th1 and Th2 cells (upper panels), and CD8+ Tc1 and Tc2 cells (lower panels). One representative experiment of 3 performed is shown. (B) Th1 cells were generated by the action of either IL-12 or IL-12 + IFN-. Cells were stained with FITC-conjugated anti-CD49f MoAb (thick lines) or with an isotype matched control (thin lines) and cell surface expression of integrin 6/β1 was analyzed by FACS. One representative experiment of 5 is shown. (C) ET 3.22 Th1 clone (upper panel) and E 4.1 Th2 clone (lower panel) were cultured in a medium supplemented with IL-2 and either left untreated (n.t.) or stimulated with IL-12 for 24 hours. Cells were stained with FITC-conjugated anti-CD49f MoAb (black lines) or with an isotype-matched control (grey lines). One experiment of 3 performed is shown.
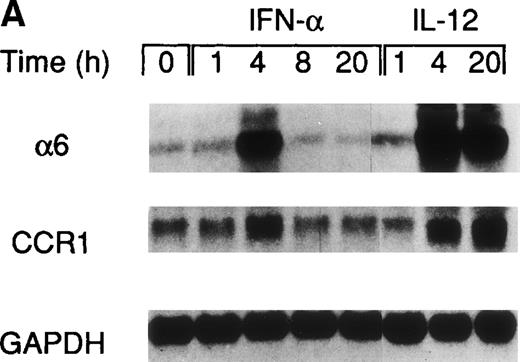
A recent study by Rogge et al, aimed at the identification of the set of genes regulated by IL-12 in Th1 cells by the use of complementary DNA microarrays (L. Rogge, E. Bianchi, M. Biffi, et al, manuscript in preparation), confirmed the selective upregulation of integrin α6/β1 and indicated that, among several chemokine receptors, CCR1 was similarly upregulated by IL-12. Taken together, these observations suggested that IL-12 may act as a selective modulator of the expression of a particular set of homing receptors in Th1 cells and that the expression of CCR1 and integrin α6/β1 may be subject to a similar regulation. Thus, to further explore the regulation of these receptors, we measured mRNA expression of integrin α6 subunit and CCR1 upon stimulation of Th1 cells with either IL-12 or IFN-α. This analysis showed that the expression of the integrin α6 mRNA was enhanced as early as 4 hours after stimulation by either of the 2 cytokines and continued to increase steadily after stimulation with IL-12, whereas it returned to basal levels within 8 hours from the time of stimulation with IFN-α (Fig 2A). The analysis of CCR1 mRNA expression showed a pattern of regulation virtually identical to that of the integrin α6 (Fig 2A), suggesting a common mode of regulation for both CCR1 and integrin α6. It should be noted that the enhancement of integrin α6 mRNA induced by IFN-α was consistently higher than upregulation at the cell surface level (compare Figs 1A and2A). Several factors, such as the half-life of the integrin α6 and/or the requirement for pairing with the integrin β1 subunit, could explain the differences observed between mRNA and cell surface expression levels. To evaluate the functional cell surface expression of CCR1, we measured the intracellular calcium mobilization in response to the CCR1 ligand chemokine MIP-1α after culturing Th1 and Th2 cells with IL-12 or IFN-α for 24 hours. Th1 cells treated with IL-12, but not IFN-α exhibited enhanced responsiveness to MIP-1α (Fig 2B), while responsiveness to IP-10 (CXCR3 ligand) remained unchanged (data not shown). In contrast, the responsiveness of Th2 cells to MIP-1α was not significantly modulated by either IL-12 or IFN-α (Fig 2B).
Regulation of integrin 6 subunit and CCR1 by IL-12 and IFN- in Th1 cells. Th1 cells were cultured in medium supplemented with IL-2 and stimulated with IL-12 or IFN- as described in Materials and Methods. At different times after stimulation with the cytokines, the cells were harvested, washed, and total RNA extracted. 10 μg of total RNA purified from untreated or cytokine-stimulated Th1 cells were subsequently used for Northern blot analysis. Filters were hybridized with 32P-labeled probes for integrin 6, CCR1, and GAPDH. One representative experiment of 2 performed is shown. (B) 24 hours after the addition of the cytokines, Th1 and Th2 cells were harvested, washed, and loaded with Fluo-3AM. Untreated cells (dotted lines), IFN-–treated cells (dashed lines), or IL-12–treated cells (solid lines) were analyzed by FACS before and after addition of MIP-1. Time of addition of MIP-1 is indicated by the arrow. The response is expressed as fold increase of mean fluorescence intensity at time 0 for emissions at 525 nm. One representative experiment of 5 performed is shown.
Regulation of integrin 6 subunit and CCR1 by IL-12 and IFN- in Th1 cells. Th1 cells were cultured in medium supplemented with IL-2 and stimulated with IL-12 or IFN- as described in Materials and Methods. At different times after stimulation with the cytokines, the cells were harvested, washed, and total RNA extracted. 10 μg of total RNA purified from untreated or cytokine-stimulated Th1 cells were subsequently used for Northern blot analysis. Filters were hybridized with 32P-labeled probes for integrin 6, CCR1, and GAPDH. One representative experiment of 2 performed is shown. (B) 24 hours after the addition of the cytokines, Th1 and Th2 cells were harvested, washed, and loaded with Fluo-3AM. Untreated cells (dotted lines), IFN-–treated cells (dashed lines), or IL-12–treated cells (solid lines) were analyzed by FACS before and after addition of MIP-1. Time of addition of MIP-1 is indicated by the arrow. The response is expressed as fold increase of mean fluorescence intensity at time 0 for emissions at 525 nm. One representative experiment of 5 performed is shown.
Correlation between the kinetics of IL-12– and IFN-α–mediated signaling and the regulation of integrin α6 subunit expression in Th1 cells.
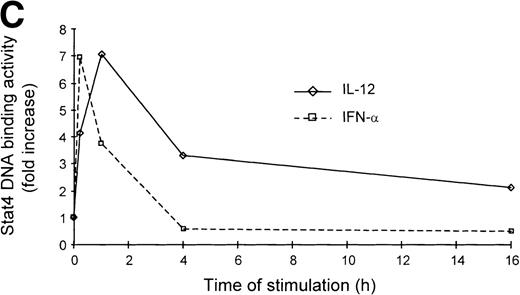
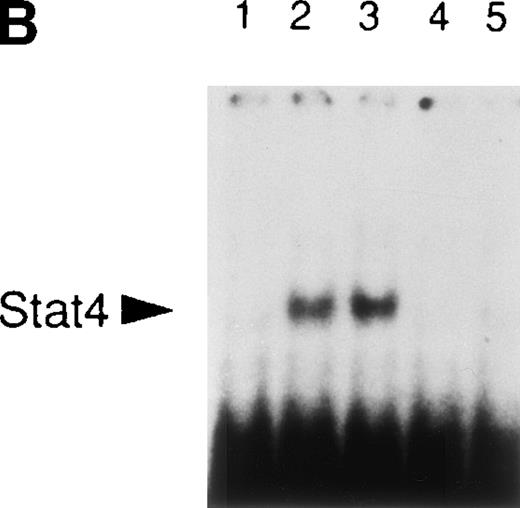
It is well established that IL-12 induces tyrosine phosphorylation of Stat4 in Th1 but not in Th2 cells.8,16 A recent report by Rogge et al documented that IFN-α shares with IL-12 the ability to activate Stat4 and to induce Th1 cell-development.17However, our findings of differential modulation of CCR1 and integrin α6/β1 by IL-12 and IFN-α suggested important qualitative differences in signal strength or duration between these cytokines. Thus, given the fact that Stat4 is the putative transcriptional activator responsible for the regulation of gene expression shared by IL-12 and IFN-α, we investigated the kinetics of Stat4 activation in response to both cytokines. Analysis of specific Stat4 DNA binding activity in whole cell extracts obtained from Th1 cells showed that Stat4 activation in response to IL-12 peaked at 1 hour and slowly declined, but was still detectable after 16 hours (Fig3A and C). In contrast, Stat4 activation by IFN-α peaked as early as 15 minutes and returned to baseline within 4 hours after addition of the cytokine in both Th1 and Th2 cells (Fig 3A and C). Consistent with our recent report,17 IL-12 activated Stat4 in Th1 but not Th2 cells, whereas IFN-α was able to activate Stat4 in both helper T-cell subsets (Fig 3A and B). The identity of the specific Stat4:DNA complexes was verified by supershifting with anti-Stat4 antibodies (Fig 3B). Based on these data, it is conceivable that the failure of IFN-α to stably upregulate CCR1 and integrin α6 subunit may depend, at least in part, on its inability to signal for a sufficient period of time.
Different kinetics of Stat4 activation in response to IL-12 and IFN- in Th1 cells. Th1 and Th2 cells were cultured in a medium supplemented with IL-2 and stimulated with IL-12 or IFN- and cells were harvested, washed, and lysed as described in Materials and Methods. Five micrograms of whole cell extracts were incubated with a32P-labeled oligonucleotide with the sequence of a Stat4 DNA-binding site. Migration of specific Stat4: DNA complexes is indicated by the arrow. (A) Kinetics of inducible Stat4 DNA-binding activity upon stimulation of Th1 and Th2 cells with either IL-12 or IFN- for 0.25, 1, 4, and 16 hours. (B) Inducible Stat4 DNA-binding activity in Th1 (lanes 1-3) and Th2 cells (lanes 4-6) before (lanes 1 and 4) or 30 minutes after stimulation with IL-12 (lanes 2 and 5) or IFN- (lanes 3 and 6). Nuclear extracts from IL-12–stimulated Th1 cells were incubated with anti-Stat4 (lane 7) or control (lane 8) rabbit polyclonal antibodies. (C) A densitometric analysis of the kinetics of Stat4 DNA-binding activity induced by IL-12 or IFN- in Th1 cells from the experiment shown in (A).
Different kinetics of Stat4 activation in response to IL-12 and IFN- in Th1 cells. Th1 and Th2 cells were cultured in a medium supplemented with IL-2 and stimulated with IL-12 or IFN- and cells were harvested, washed, and lysed as described in Materials and Methods. Five micrograms of whole cell extracts were incubated with a32P-labeled oligonucleotide with the sequence of a Stat4 DNA-binding site. Migration of specific Stat4: DNA complexes is indicated by the arrow. (A) Kinetics of inducible Stat4 DNA-binding activity upon stimulation of Th1 and Th2 cells with either IL-12 or IFN- for 0.25, 1, 4, and 16 hours. (B) Inducible Stat4 DNA-binding activity in Th1 (lanes 1-3) and Th2 cells (lanes 4-6) before (lanes 1 and 4) or 30 minutes after stimulation with IL-12 (lanes 2 and 5) or IFN- (lanes 3 and 6). Nuclear extracts from IL-12–stimulated Th1 cells were incubated with anti-Stat4 (lane 7) or control (lane 8) rabbit polyclonal antibodies. (C) A densitometric analysis of the kinetics of Stat4 DNA-binding activity induced by IL-12 or IFN- in Th1 cells from the experiment shown in (A).
To test whether sustained signaling by IL-12 is necessary to induce a significant upregulation of integrin α6/β1 expression, we stimulated Th1 cells with IL-12, and IL-12–mediated signaling was interrupted at different times by the addition of anti–IL-12 receptor β1 MoAb in conjunction with soluble p40 homodimer. The combination of IL-12 p40 homodimer and anti–IL-12 receptor β1 MoAb efficiently competed for the binding of IL-12 with its receptor35 and rapidly blocked IL-12–mediated Stat4 activation (Fig4B). Addition of this inhibitory cocktail at the time of addition of IL-12 completely blocked integrin α6/β1 upregulation measured after 48 hours (Fig 4A). The inhibitory effect was still optimal when the inhibitory cocktail was added up to 4 hours following the initial stimulation with IL-12. In contrast, when IL-12 p40 homodimer and anti–IL-12 receptor β1 MoAb were added 8 hours or even 16 hours after the addition of IL-12, only a partial decrease of IL-12–mediated integrin α6/β1 upregulation could be observed (Fig4A). These data strongly suggest that the ability of IL-12 to generate long-lasting intracellular signals is critical for a significant upregulation of integrin α6/β1 expression in Th1 cells.
Requirement of prolonged IL-12–mediated signaling for integrin 6/β1 upregulation in Th1 cells. (A) Th1 cells were cultured in medium supplemented with IL-2 and stimulated with IL-12 as before. At 0, 1, 2, 4, 8, and 16 hours after stimulation with IL-12, a combination of anti–IL-12R β1 MoAb and mouse p40 homodimer were added to the culture as described in Materials and Methods. After 48 hours from the initial stimulation, the cells were harvested, washed, and stained for surface expression of integrin 6/β1. The expression of integrin 6/β1 measured after 48 hours of stimulation with IL-12 (black bar) was taken as 100% of expression. The experiment shown is representative of 2 performed. (B) Stat4 DNA-binding activity in Th1 cells before (lane 1), or after 30 minutes (lane 2) and 60 minutes (lanes 3-5) of stimulation with IL-12 (lanes 2-5). A combination of anti–IL-12R β1 MoAb and mouse p40 homodimer was added either at the time of addition of IL-12 (lane 4) or 30 minutes after (lane 5). Migration of specific Stat4:DNA complexes is indicated by the arrow.
Requirement of prolonged IL-12–mediated signaling for integrin 6/β1 upregulation in Th1 cells. (A) Th1 cells were cultured in medium supplemented with IL-2 and stimulated with IL-12 as before. At 0, 1, 2, 4, 8, and 16 hours after stimulation with IL-12, a combination of anti–IL-12R β1 MoAb and mouse p40 homodimer were added to the culture as described in Materials and Methods. After 48 hours from the initial stimulation, the cells were harvested, washed, and stained for surface expression of integrin 6/β1. The expression of integrin 6/β1 measured after 48 hours of stimulation with IL-12 (black bar) was taken as 100% of expression. The experiment shown is representative of 2 performed. (B) Stat4 DNA-binding activity in Th1 cells before (lane 1), or after 30 minutes (lane 2) and 60 minutes (lanes 3-5) of stimulation with IL-12 (lanes 2-5). A combination of anti–IL-12R β1 MoAb and mouse p40 homodimer was added either at the time of addition of IL-12 (lane 4) or 30 minutes after (lane 5). Migration of specific Stat4:DNA complexes is indicated by the arrow.
Upregulation of integrin α6/β1 and CCR1 promotes the migration of Th1 cells.
Given the pattern of regulation of integrin α6/β1 and CCR1 observed in Th1 cells, we next investigated the functional consequence of these findings. We first analyzed the migratory behavior of Th1 and Th2 cells on laminin, which is the ligand for the integrin α6/β1. For this purpose, we measured transmigration of Th1 and Th2 cells through transwell filters that were either coated with laminin or with fibronectin or were left uncoated. Th1 and Th2 cells were labeled with fluorescent dyes and the percentage of cells that had migrated to the bottom chamber of the transwell was evaluated by FACS analysis. This analysis showed that Th1 and Th2 cells migrated similarly on fibronectin-coated and uncoated filters, whereas Th1 cells migrated 2-fold more efficiently than Th2 cells on laminin-coated filters (Fig5A). Addition of blocking antibodies to the integrin α6 subunit dramatically inhibited the migration of both Th1 and Th2 cells on laminin, but had no significant effect on the migration of either subset on fibronectin (Fig 5B). The fact that the antibodies to the integrin α6 subunit also inhibited the migration of Th2 cells is not surprising, since lower but detectable levels of integrin α6/β1 were expressed on these cells (Fig 1). The preferential migration of Th1 cells on laminin was observed regardless of whether Th1 and Th2 cells were cultured together or separately (data not shown), indicating that the increased migration of Th1 cells is an intrinsic property of this cell subset mediated by the engagement of the integrin α6/β1. We next wished to determine the functional consequence of the IL-12–mediated upregulation of integrin α6/β1 and CCR1. Th1 cells were either stimulated with IL-12 or left untreated and their migration in response to MIP-1α (CCR1 ligand) or SDF-1 (CXCR4 ligand) was analyzed (Fig 6). To optimally evaluate the effect of IL-12, we express the results of these experiments as the ratio of the migrated IL-12 treated versus untreated Th1 cells (Fig 6). Spontaneous migration of IL-12–treated Th1 cells was enhanced by laminin but not fibronectin (ratio equal to 1 on fibronectin; 1.25 on laminin; Fig 6). IL-12–treated Th1 cells exhibited enhanced chemotactic responsiveness to MIP-1α on fibronectin at all doses of chemokine (ratio equal to 1.2 to 1.3), but no further significant enhancement of migration was detected on laminin (ratio equal to 1.2 to 1.3; Fig 6). In contrast, IL-12–treated Th1 cells exhibited diminished responsiveness to SDF-1 on both fibronectin and laminin (Fig 6). Indeed, in agreement with recently published results,39 40 IL-12 downregulated the expression of the SDF-1 receptor CXCR4 on Th1 cells (data not shown). Thus, consistent with its proinflammatory function, IL-12 increases the responsiveness to inflammatory chemokines such as MIP-1α and Rantes, while it reduces the responsiveness to the constitutive chemokine SDF-1 by opposite effects on chemokine receptor expression. Overall, these results show that the IL-12 mediated upregulation of integrin α6/β1 and CCR1 enhances the migration of Th1 cells. However, it should be pointed out that we could not detect an additive effect resulting from the engagement of both CCR1 and integrin α6/β1 by their respective ligands. Thus, it appears that in our system the upregulation of either of these receptors may accomplish maximal enhancement of migration. Nevertheless, it remains conceivable that in conditions of suboptimal engagement of integrin α6/β1 and CCR1, as it might occur in vivo, these receptors could functionally cooperate to achieve tissue-specific localization of Th1 cells.
Integrin 6/β1 binding to laminin promotes the preferential migration of Th1 cells. (A) Mixed fluorescent-labeled Th1 (□) and Th2 (▪) cells were added to triplicate uncoated (n.c.), fibronectin- (FN), or laminin- (LM) coated transwell filters. (B) Mixed fluorescent-labeled Th1 (□) and Th2 (▪) cells were incubated with anti-integrin 6 blocking GoH3 antibody (anti-6) or an isotype-matched control (con.Ig) 15 minutes before the assay and then were added to triplicate FN- or LM-coated transwell filters.
Integrin 6/β1 binding to laminin promotes the preferential migration of Th1 cells. (A) Mixed fluorescent-labeled Th1 (□) and Th2 (▪) cells were added to triplicate uncoated (n.c.), fibronectin- (FN), or laminin- (LM) coated transwell filters. (B) Mixed fluorescent-labeled Th1 (□) and Th2 (▪) cells were incubated with anti-integrin 6 blocking GoH3 antibody (anti-6) or an isotype-matched control (con.Ig) 15 minutes before the assay and then were added to triplicate FN- or LM-coated transwell filters.
Upregulation of integrin 6/β1 and CCR1 by IL-12 promotes the migration of Th1 cells. IL-12–treated or untreated fluorescent-labeled Th1 cells were added to triplicate transwell filters that had been coated with fibronectin or laminin. Indicated concentration of chemokines SDF-1 and MIP-1 was added to the lower compartment of the transwell. The percentage of input cells that migrated to the bottom of the transwell after 3 hours of incubation was determined by flow cytometric analysis as described for Fig 5. Data are expressed as the ratio of migrated IL-12–treated versus untreated Th1 cells. The dashed horizontal line indicates the ratio equal to 1, which would result from an equivalent migration of IL-12–treated versus untreated Th1 cells. The experiments shown are representative of 5 performed.
Upregulation of integrin 6/β1 and CCR1 by IL-12 promotes the migration of Th1 cells. IL-12–treated or untreated fluorescent-labeled Th1 cells were added to triplicate transwell filters that had been coated with fibronectin or laminin. Indicated concentration of chemokines SDF-1 and MIP-1 was added to the lower compartment of the transwell. The percentage of input cells that migrated to the bottom of the transwell after 3 hours of incubation was determined by flow cytometric analysis as described for Fig 5. Data are expressed as the ratio of migrated IL-12–treated versus untreated Th1 cells. The dashed horizontal line indicates the ratio equal to 1, which would result from an equivalent migration of IL-12–treated versus untreated Th1 cells. The experiments shown are representative of 5 performed.
DISCUSSION
As postulated in the multistep model of leukocyte extravasation,22,23 the combinatorial involvement of different sets of homing receptors appears to regulate tissue-specific recruitment of functionally distinct subsets of helper T cells. In this model, functional programs and homing patterns of helper T cells should be tightly coupled and coregulated. In analogy with the acquisition of cytokine-production profiles,41 it will have to be determined whether the expression of specific homing receptors is achieved through a stochastic process involving epigenetic control of gene expression. Multiple cytokines regulate, with distinct features, the differentiation and the effector functions of helper T cells. For instance, IL-12 and IFN-α share the ability to promote differentiation of human Th1 cells,14,17 but IL-12 may play a unique role in enhancing the effector functions of differentiated Th1 cells.4,42 Responsiveness to IL-12 but not to IFN-α is lost in Th2 cells,17 suggesting that these two cytokines may regulate distinct functions in differentiated helper T cells. Here, we identify a unique pattern of regulation of the integrin α6/β1 and the chemokine receptor CCR1 by the distinct nonoverlapping action of IL-12 and IFN-α. Integrin α6/β1 was found to be preferentially expressed on Th1 cells and its expression enhanced by priming of Th1 cells in the presence of IFN-α. Interestingly, Sallusto et al have shown a similar modulation of the chemokine receptor CCR1.29 These data indicate that IFN-α cooperates with IL-12 to prime developing Th1 cells for high expression of CCR1 and integrin α6/β1. Although the basis of this cooperation are not fully defined, IFN-α can act directly on T cells to activate Stat4 and increase the expression of IL-12 receptor β2 chain, thus enhancing IL-12 responsiveness.17,43 The enhancement of IL-12 responsiveness could provide a mechanism by which IFN-α cooperates with IL-12 to increase the expression of CCR1 and integrin α6/β1 in developing Th1 cells. Notably, IL-12 and IFN-α have different effects on already differentiated Th1 cells. Expression of both integrin α6/β1 and CCR1 were significantly and lastingly enhanced by IL-12 but not IFN-α, indicating divergent effects of the two cytokines on the regulation of these receptors. The pattern of integrin α6/β1 and CCR1 upregulation in Th1 cells correlated with the different kinetics of Stat4 activation by IL-12 and IFN-α. Sustained signaling by IL-12 appeared to be necessary for upregulating integrin α6/β1 on Th1 cells. Thus, IFN-α may fail to markedly and lastingly upregulate expression of integrin α6/β1 and CCR1 in established Th1, as well as Th2, cells by virtue of its short-lived signal transduction. However, it cannot be ruled out that the transient effect of IFN-α on integrin α6/β1 and CCR1 expression may still, to some extent, influence the migratory behavior of differentiated helper T cells. Because IFN-α can signal and activate Stat4 in both Th1 and Th2 cells, it is possible that the requirement of sustained signaling for increased expression of integrin α6/β1 and CCR1 may be a key feature to prevent upregulation by IFN-α of certain Th1 cell-associated homing programs in Th2 cells. Although IFN-α shares with IL-12 the ability to activate Stat4 in human T cells, it can also activate Stat1, Stat2, and Stat3,44,45 generating different intracellular signaling complexes. IFN-α is a potent and rapid inducer of antiviral responses44-48 and it has been shown to upregulate the IL-12 receptor β2 chain43 and IFN-γ production by Th1 cells.17 Thus, it is feasible that the short-lived and unique Stats-containing signaling complexes induced by IFN-α may be sufficient to induce the expression of a set of Th1-associated genes44,48 but not others, such as integrin α6/β1 on differentiated Th1 cells. Our findings suggest that, in addition to the recently documented remodeling of chromatin structure,49 signal strength and duration may contribute to ensure T helper cell-specific gene expression.
Integrin α6 is widely expressed and pairs with the β1 or β4 integrins to form receptors for laminins in a variety of cell types.50 Laminins are a critical component of basal membranes and an important constituent of tissue architecture.51,52 Thus, integrin α6-mediated interaction with laminins may play a critical role in the ability of cells to extravasate and migrate. Disregulated expression and function of the α6 integrins appear to provide a crucial contribution to the invasive behavior of certain types of tumors.53,54 In leukocytes, the major laminin receptor is composed of α6 and β1 integrins .55 In our system, migration of Th1 cells on laminin coated filters was completely inhibited by blocking antibodies against integrins α6 and β1 (Fig 5 and data not shown), indicating that the integrin α6/β1 is indeed the major laminin receptor expressed on these cells. Given the fact that integrin β1 can dimerize with various α integrins, the selective upregulation of the integrin α6 subunit may ensure the marked increase in integrin α6/β1 expression relative to the other β1 integrins. Recent work documented a specific function for the integrin α6 subunit in the emigration of Langerhans cells from the epidermis upon their maturation, a process that requires crossing of the basal membrane.56 In addition, a recent study suggested that the integrin α6A splice variant expressed on T cells may play an important role in their migratory capacity.57 Our findings showing that integrin α6/β1 interaction with laminin promotes the preferential migration of Th1 cells, and the exquisite regulation of this receptor in helper T cell subsets, suggest the novel concept that the integrin α6/β1 may provide an important contribution in the tissue-specific homing of Th1 cells.
It is noteworthy that in certain chronic inflammatory conditions, typically attributed to disregulated Th1 cell-mediated immune responses, abnormal deposition of laminin and basal membrane thickening are common findings.58,59 Hence, Th1 cells by virtue of their preferential expression of the integrin α6/β1 laminin receptor may possess a high capacity to invade and localize within this type of tissue. Moreover, it is possible that the selective upregulation of both CCR1 and integrin α6/β1 induced by the IL-12 produced locally by macrophages may contribute to the localization and/or retention of Th1 cells into those inflammatory sites. Indeed, it has been shown that migration of T helper cells to tumor sites was augmented in mice treated with IL-12.60
Several studies have shown that the engagement of integrins by cell-associated or extracellular matrix ligands may enhance proliferation and/or cytokine production by leukocytes.31,61 Notably, a previous study reported that the production of Th1-type cytokines by mononuclear cells obtained from the synovial fluid of patients affected by rheumatoid arthritis was markedly upregulated by culturing the cells on laminin,62suggesting that the engagement of the laminin receptor may enhance Th1 cell-specific effector functions. The regulation of integrin α6/β1 and CCR1 expression suggests that these receptors may cooperate in promoting the migration and regulate the homing of Th1 cells. The selective modulation of these receptors illustrates an additional level of control for tissue-specific homing of helper T-cell subsets.
ACKNOWLEDGMENT
We thank Paola Vigano’ for cord blood samples, Maurice Gately for anti–IL-12R β1 MoAb and mouse p40 homodimer, Rosmarie Lang for technical assistance, and Paola Panina-Bordignon, Henry Hess, and Luciano Adorini for helpful discussions and comments.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Daniele D’Ambrosio, MD, PhD, Roche Milano Ricerche, Via Olgettina 58, I-20132 Milan, Italy; e-mail:daniele.dambrosio@roche.com.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal