Abstract
Chemokines and their receptors are broadly expressed in different tissues and are involved in diverse biologic processes. Gene inactivation studies have shown that both stromal cell derived factor-1 (SDF-1) and chemokine receptor 4 (CXCR4) are essential for B lymphopoiesis. However, it is not yet clear by which mechanisms B lymphopoiesis is affected. In the present study, we have examined CXCR4 expression and function on primary B cells representing sequential stages of development (eg, pro-B, pre-B, immature, and mature B cells) in fetal and adult bone marrow. The expression of CXCR4 was observed to be sinusoidal. Expression was highest on pre-B cells, decreased as cells developed into immature B cells, and then increased again upon transition to the mature B-cell stage. The corresponding ligand SDF-1 was shown to trigger vigorous cell signaling and migration responses, which are restricted to early lineage B cells. The responsiveness to SDF-1 was markedly decreased for immature and mature B cells despite relatively high levels of CXCR4 expression. Thus, the diminished responsiveness to SDF-1 by more mature B cells was determined to be disproportionate to the level of CXCR4 expression. These findings raise the possibility that CXCR4 function is differentially controlled during B lymphopoiesis and may be relevant to the compartmentalization of B-cell precursors in the bone marrow.
CHEMOKINES ARE RESPONSIBLE for numerous activities in normal and pathologic states.1,2 They influence migration of lymphocytes through a gradient of chemokine concentrations and play a role in leukocyte adhesion and infiltration during inflammation.3-6 Some chemokines affect hematopoiesis and angiogenesis.7-11 Of these, the chemokine stromal cell derived factor-1 (SDF-1) and corresponding receptor chemokine receptor 4 (CXCR4) are essential for B lymphopoiesis and bone marrow myelopoiesis. Mice, in which either the gene for SDF-1 or CXCR4 has been inactivated, have reduced numbers of both pro-B (progenitor B cells) cells and (precursor B cells) pre-B cells in fetal liver and bone marrow.12-14
The mechanism(s) by which loss of SDF-1 or CXCR4 expression affects B lymphopoiesis is not clear. SDF-1 can induce chemotaxis of murine B-cell progenitors, thus suggesting that the SDF-1/CXCR4 axis may be important in directing the migration of B-cell progenitors to the appropriate bone marrow microenvironment.13,15,16Alternatively, SDF-1, which can stimulate proliferation of murine B-cell progenitors in vitro, also may trigger signaling pathways necessary for the retention and development of early lineage B cells in the bone marrow.17,18 In this regard, SDF-1 has been shown to induce calcium mobilization of murine progenitor B cells but, interestingly, not mature bone marrow B cells.15 In the current study, we wished to further examine for differential SDF-1 responsiveness relative to CXCR4 expression during B lymphopoiesis. In addition, the studies were performed on human bone marrow as differences in biologic responses might exist between human and mouse B lymphopoiesis.19
We examined primary human B cells representing sequential stages of development (eg, pro-B, pre-B, immature, and mature B cells) in bone marrow of the same individual. First, we found distinct CXCR4 expression patterns on bone marrow B-cell populations. Second, we showed that SDF-1 triggered marked biologic responses of early B lineage cells (eg, pro-B and pre-B), whereas the same responses of more mature B cells were diminished or absent. Interestingly, the diminished or lack of SDF-1 induced responsiveness by more mature B cells was disproportionate to the level of CXCR4 expression. These findings suggest that CXCR4 function may be regulated during B-cell development and, thus, influence the distinct anatomic location of B-cell progenitors in the bone marrow.
MATERIALS AND METHODS
Cells.
The following cell lines were obtained from ATCC: RS4:11 (CRL 1873) , REH (CRL 8286), HS-Sultan (CRL 1484). The JIM-1 cell line20was provided by Dr Jack W. Singer (Cell Therapeutics Inc, Seattle, WA), the 697 cell line21 was provided by Dr Max D. Cooper (Howard Hughes Medical Institute, Birmingham, AL), the RS11846 cell line22 was provided by Dr John Reed (La Jolla Cancer Institute, La Jolla, CA), the Blin-123 Nalm-16, the Nalm-6 cell lines24 were provided by Dr Tucker LeBien (University of Minnesota, Minneapolis, MN), the SU-DHL-6 cell line25was provided by Dr Alan Epstein (University of Southern California, Los Angeles, CA), and the 20A and 15A26 cell lines were established in our laboratory from patients with cold agglutinin disease.
Fetal bone marrow was obtained from 18- to 23-week-old fetuses. Adult heparinized bone marrow was obtained by iliac crest aspiration from healthy adult individuals. Umbilical cord blood cells were obtained from full-term deliveries without evidence of maternal or fetal infection. The isolated bone marrow cells were passed through a 70 μm filter to remove cellular debris and aggregates. Mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) gradient centrifugation (density 1.077 g/mL). Pelleted cells were collected and Ficoll (Pharmacia) was removed by washing the cells 3 times in 1X phosphate-buffered saline (PBS), pH 7.4. After the third wash, cells were resuspended in ice-cold staining buffer (1X PBS with 2% fetal bovine serum [FBS]). Fetal bone marrow cells were stored at 37°C in Iscove (GIBCO, Grand Island, NY), serum free medium for 24 hours before staining.
Antibodies.
Surface staining of bone marrow cells was performed with the following antibodies: fluorescein isothiocyanate (FITC)-labeled anti-κ Light chain (Sigma, St Louis, MO), FITC-labeled anti-λ Light chain (Pharmingen, San Diego, CA), phycoerythrin (PE)-labeled anti-CD19 (Becton Dickinson, San Jose, CA), Cy-Chrome-labeled anti-CD19 (Pharmingen, San Diego, CA), PECy5-labeled anti-CD34 (Immunotech, Westbrook, ME), PE-labeled anti-IgD (Pharmingen), PE-labeled anti-CXCR4 (clone 12G5) (Pharmingen). The secondary antibody was goat anti-mouse IgG-APC labeled (Accurate Chemical & Scientific Corporation, Westbury, NY). Nonconjugated mouse monoclonal antibody (MoAb) anti-CXCR4 (clone 12G5, kindly provided by Dr James Hoxie, University of Pennsylvania, Philadelphia, PA).
Cell sorting.
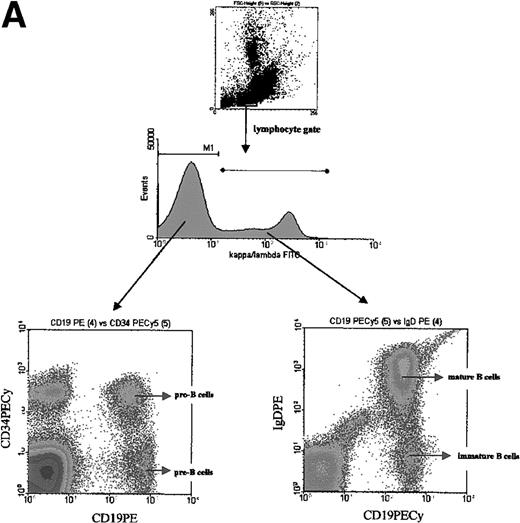
The Ficoll-Hypaque-purified bone marrow cells (Pharmacia) were suspended in ice-cold staining buffer (1X PBS, 2% FBS) and incubated for 30 min with the first step reagent. Cells were washed three times in staining buffer and incubated for 30 minutes with the second step reagents. Four stages of B-cell differentiation were isolated from bone marrow cells as previously described. The earliest B-cell population, designated here as pro-B cells, was κ−/λ−, CD19+, CD34+. The next developmental B-lineage populations were designated as pre-B cells, identified as κ−/λ−, CD19+, CD34−; followed by immature B cells identified as κ+/λ+, CD19+, IgD−; and mature B cells identified as κ+/λ+, CD19+, IgD+. CellQuest software (Becton Dickinson Immunocytometry Systems) was used to define gates. The gating strategy for the different B-cell subsets is shown on Fig 1A.
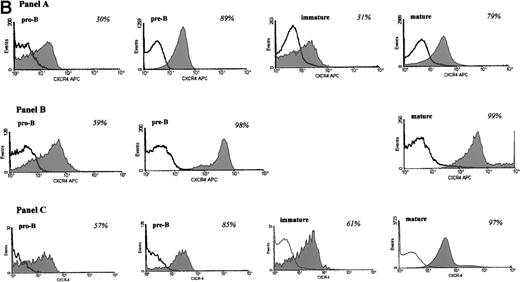
(A) Gating strategy for identifying different subsets of B cells from fetal, adult bone marrow, and umbilical cord blood. Cells were first gated based on forward and side scatter identifying the lymphocyte population. Staining for kappa and lambda light chains identified early (pro-B/pre-B), eg, κ−/λ−, and later (immature and mature B cells), eg, κ+/λ+, lineage B cells. Cells of the lymphocyte phenotype and negative for kappa/lambda immunoglobulin chains were further separated into CD19+, CD34+ pro-B cells and CD19+, CD34− pre-B cells. Cells positive for kappa/lambda immunoglobulin chains were further identified as immature B cells (CD19+, IgD−) and mature B cells (CD19+, IgD+). (B) CXCR4 expression during B-cell development in bone marrow. Expression of CXCR4 on B-cells from adult (A), fetal (B) bone marrow, and umbilical cord blood (C) is shown. Cells were incubated with anti-CXCR4 specific antibody and analyzed by flow cytometry. Triple gates were set on different B-cell subsets and the percentage of CXCR4 positive cells was determined. The threshold line for positive cells was based on the maximum staining of a matched isotypic antibody with irrelevant specificity used in the same concentration as the anti-CXCR4 antibody. The isotype control antibody binding was analyzed on the same triple gated B-cell subsets as for CXCR4 expression. Anti-CXCR4 labeled cells that were brighter than those stained with the isotype control antibody were defined as positive for CXCR4. The shaded part of the histogram represents cells stained with anti-CXCR4 antibody, and the black line represents staining with the isotype control. The numbers represent the percentage of CXCR4 positive cells. The results are representative of 5 experiments using adult bone marrow B cells and 3 experiments each of fetal bone marrow and umbilical cord blood B cells.
(A) Gating strategy for identifying different subsets of B cells from fetal, adult bone marrow, and umbilical cord blood. Cells were first gated based on forward and side scatter identifying the lymphocyte population. Staining for kappa and lambda light chains identified early (pro-B/pre-B), eg, κ−/λ−, and later (immature and mature B cells), eg, κ+/λ+, lineage B cells. Cells of the lymphocyte phenotype and negative for kappa/lambda immunoglobulin chains were further separated into CD19+, CD34+ pro-B cells and CD19+, CD34− pre-B cells. Cells positive for kappa/lambda immunoglobulin chains were further identified as immature B cells (CD19+, IgD−) and mature B cells (CD19+, IgD+). (B) CXCR4 expression during B-cell development in bone marrow. Expression of CXCR4 on B-cells from adult (A), fetal (B) bone marrow, and umbilical cord blood (C) is shown. Cells were incubated with anti-CXCR4 specific antibody and analyzed by flow cytometry. Triple gates were set on different B-cell subsets and the percentage of CXCR4 positive cells was determined. The threshold line for positive cells was based on the maximum staining of a matched isotypic antibody with irrelevant specificity used in the same concentration as the anti-CXCR4 antibody. The isotype control antibody binding was analyzed on the same triple gated B-cell subsets as for CXCR4 expression. Anti-CXCR4 labeled cells that were brighter than those stained with the isotype control antibody were defined as positive for CXCR4. The shaded part of the histogram represents cells stained with anti-CXCR4 antibody, and the black line represents staining with the isotype control. The numbers represent the percentage of CXCR4 positive cells. The results are representative of 5 experiments using adult bone marrow B cells and 3 experiments each of fetal bone marrow and umbilical cord blood B cells.
The sorting into highly purified populations was performed on a fluorescence-activated cell sorter (FACS) Vantage with Turbo Sort option (Becton Dickinson Immunocytometry Systems), with the purity over 98.5%. The sorter was aligned with glutaraldehyde fixed chicken red blood cells.
Cells used in chemotaxis and calcium flux assays were stored overnight at 37°C in Iscove Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO) (105 cells/mL), supplemented with 25% of artificial serum containing 1% delipidated, deionized, and charcoal-treated bovine serum albumin (BSA), 270 μg/mL iron saturated transferrin, insulin (20 μg/mL), and 2 mmol/l L-glutamine (all from Sigma, St Louis, MO).27
Cell surface staining.
Four color immunofluorescence analysis was used to examine the expression of chemokine receptors on the surfaces of different B-cell populations. Different subsets of B cells were identified as described above (Fig 1A). The fourth channel was used to define cell surface expression of CXCR4. Ten thousand events were acquired in the fourth channel after triple gating for B-cell subsets, as described above. The threshold line was based on the maximum staining of a matched isotypic antibody with irrelevant specificity (mouse IgG2a) used in the same concentration as the anti-CXCR4 antibody (1 μg/50 μL of staining buffer). The isotypic antibody binding was analyzed on the same triple gated B-cell subsets as for CXCR4 expression. The threshold line for defining CXCR4 positive and negative cells was set such that less than 1% of isotype positive cells was present to the right of the threshold line. Anti-CXCR4 labeled cells that were brighter than those stained with the isotypic antibody (present to the right of isotype control histogram) were defined as positive for CXCR4.
To detect internalization of CXCR4, cells were incubated for 30 minutes at 37°C with 125 nmol/L of SDF-1. After incubation, cells were put on ice to prevent resurfacing of the receptor, washed in ice-cold acid buffer (50 mmol/L glycine, 100 mmol/L NaCl, pH 3.0) to remove cell bound SDF-1, which could interfere with 12G5 binding to CXCR4, and then stained with anti-CXCR4 antibody.
The flow cytometer was calibrated with CaliBRITE-3 beads (Becton Dickinson Immunocytometry Systems) and FACSComp Software (Becton Dickinson Immunocytometry Systems). The data were analyzed with the WinMDI 2.7 software (kindly provided by Dr Joseph Trotter, Scripps Research Institute, La Jolla, San Diego, CA).
Quantitative FACS.
Quantitative FACS was performed by converting the mean fluorescence intensity of the cell subset into antibody-binding sites (ABS) by using a standardized microbeads kit (Sigma, St Louis, MO). This is a mixture of 5 microbead populations of uniform size, coated with goat anti-mouse antibodies that have different properties to bind mouse antibodies. Beads (105 per sample) were incubated with the same, saturating concentration of 12G5-PE labeled (Pharmingen) anti-CXCR4 antibody as the samples and were processed identically as the samples being quantified. The beads were then analyzed by using the same instrument settings as those for the sample cells. The binding capacities of the stained beads were then regressed against the corresponding geometric mean of each bead population and the mean fluorescence intensity of the CXCR4 on the cells was converted to ABS per cell by comparison with the regression curve generated. The mean fluorescence intensity of the isotype control (68-70 antibody binding sites) for each B-cell subset was also converted to ABS and subtracted from ABS values obtained with the experimental sample. The threshold of detection was 110 ABS per cell. Additional details on the relationship between ABS and mean fluorescence intensity values can be obtained from the manufacturer (Sigma).
Calcium signaling.
Sorted cells were stored for 12 to 18 hours before the experiment at 37°C in Iscove DMEM (GIBCO) (105 cells/mL), supplemented with 25% of artificial serum.27 Cells were suspended in 1 mL of 30°C Hanks’ balanced salt solution containing 1.3 mmol/L CaCl2, 0.5 mmol/L MgCl2, 1 μmol/L Indo-1AM (Molecular Probes, Eugene, OR), 0.01% F-127 Pluronic detergent (Molecular Probes), and incubated for 45 minutes at 30°C. Calcium mobilization was analyzed with Becton Dickinson FACStar Plus flow cytometer (Becton Dickinson Immunocytometry Systems), equipped with Time Zero Injection Module (Cytek, Fremont, CA) after stimulation with 100 ng/mL of SDF-1β (R&D Systems, Minneapolis, MN) or 2 μg/mL of anti-IgM MoAb (Sigma). Ultraviolet (UV) excitation wavelengths were 350 to 364 nm, emission 405 nm, and 510 nm. UV laser was aligned by using FluoresBRITE carboxyBB 6 μm microspheres (Poly Science, Niles, IL). Cells were incubated at 37°C for 5 minutes before analysis. The data were acquired with Lysis II software (Becton Dickinson Immunocytometry Systems) and analyzed with WinMDI 2.7 software.
Chemotaxis assay.
In the initial experiments, B-cell subsets were first sorted from total bone marrow and then placed into the upper compartment of the Transwell and allowed to migrate as described below. Sorted cells used in these experiments were stored for 12 to 18 hours before the experiment at 37°C in Iscove DMEM (GIBCO) (105 cells/mL) and supplemented with 25% of artificial serum.27
The ratio between the number of cells migrated in the presence of chemokine and the number of cells migrated in the presence of media alone was defined as the migration index.
In subsequent experiments, total bone marrow Ficoll-purified lymphocytes were put into the upper compartment (106 cells per well) of Transwell inserts (6.5 mm diameter, 5 μm filter pore size) (Costar, Cambridge, MA). The lower compartment contained 600 μL of serially diluted SDF-1β (R&D Systems) in 0.25% BSA, RPMI 1640. Cells were allowed to migrate for 2 hours at 37°C. Cells that passed through the membrane to the lower chamber were collected and stained with B-cell markers. Cells were counted by timed acquisition (600 seconds each sample) on FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems).
RESULTS
Expression of CXCR4 on different B-cell lines.
As a first measure for changes in CXCR4 expression during B-cell development, we analyzed different B-cell lines representing early and mature stages of B-cell development (Table1). Overall, the percentage of CXCR4 expressing cells was highest on B-cell lines representing early lineage (ie, pro-B/pre-B) B cells, although a much lower percentage was noted for mature B-cell lines derived from peripheral lymphomas.
Expression and Function of CXCR4 on Cell Lines Representing Different Stages of B-Cell Development
| B-cell Lines . | Percentage of Cells Expressing CXCR4 . | Chemotaxis Toward SDF-1 . | Calcium Mobilization After SDF-1 Stimulation . |
|---|---|---|---|
| Pro-B cells | |||
| RS 4-11 | 71.5 | ++ | − |
| Nalm 16 | 32.25 | ++ | + |
| Jim-1 | 99.8 | + | − |
| REH | 99.5 | + | + |
| Pre-B cells | |||
| 697 | 60.17 | ++ | + |
| Nalm-6 | 99.1 | ++ | + |
| Blin-1 | 98.9 | ND | ND |
| Mature B cells | |||
| 20A | 0.89 | − | − |
| 15A | 22.73 | − | − |
| RS11846 | 17.6 | + | − |
| HS-Sultan | 23.9 | + | − |
| SU-DHL-6 | 52.04 | + | ND |
| B-cell Lines . | Percentage of Cells Expressing CXCR4 . | Chemotaxis Toward SDF-1 . | Calcium Mobilization After SDF-1 Stimulation . |
|---|---|---|---|
| Pro-B cells | |||
| RS 4-11 | 71.5 | ++ | − |
| Nalm 16 | 32.25 | ++ | + |
| Jim-1 | 99.8 | + | − |
| REH | 99.5 | + | + |
| Pre-B cells | |||
| 697 | 60.17 | ++ | + |
| Nalm-6 | 99.1 | ++ | + |
| Blin-1 | 98.9 | ND | ND |
| Mature B cells | |||
| 20A | 0.89 | − | − |
| 15A | 22.73 | − | − |
| RS11846 | 17.6 | + | − |
| HS-Sultan | 23.9 | + | − |
| SU-DHL-6 | 52.04 | + | ND |
In the column representing chemotaxis toward SDF-1, (−) denotes a migration index less than 2, (+) denotes a migration index between 2 and 7, and (++) denotes a migration index more than 7. In the column representing calcium mobilization After SDF-1 Stimulation, (−) represents lack of detectable calcium mobilization and (+) represents the presence of detectable calcium mobilization.
Abbreviation: ND, not determined.
We also examined the function of CXCR4 on different cell lines. We found that as previously reported,15 CXCR4 mediated chemotaxis and calcium mobilization was generally high on pro-B and pre-B cell lines but low or absent on lymphoma derived B cell. However, some exceptions were noted. Certain pro-B cell lines were nonresponsive to SDF-1 (RS 4:11, JIM-1; see Table 1), as measured by calcium mobilization; some mature lymphoma derived cell lines did migrate to the SDF-1 ligand (RS11846, HS Sultan). Overall, the results of these studies were intriguing and suggested a correlation between CXCR4 function and developmental B-cell stage that warranted further investigation by using untransformed cells.
Expression of CXCR4 on subsets of primary B cells from adult bone marrow.
The expression of CXCR4 during B-cell development was examined on subsets of B lymphocytes from adult bone marrow (Fig 1A). As shown in Fig 1B, 30% of pro-B cells expressed CXCR4 (Panel A). Among pre-B cells, 89% of cells stained positive for CXCR4. At the next stage of B-cell development, the immature B-cell stage, the frequency of CXCR4 positive cells is decreased as compared with the pre-B cell population. Among mature B cells from bone marrow, the frequency of CXCR4 positive cells increased to 79%.
Expression of CXCR4 on subsets of B cells from fetal bone marrow and umbilical cord blood.
We were curious if CXCR4 expression differed during ontogeny. Therefore, we examined CXCR4 expression on different subsets of B cells from fetal bone marrow (Fig 1B, Panel B) and umbilical cord blood (Fig1B, Panel C). Similar to adult bone marrow, the percentage of cells expressing CXCR4 increases during the pro-B to pre-B cell transition (Fig 1B). Because of the small number of cells available for analysis, we were unable to determine CXCR4 expression on fetal immature B cells. However, the majority of mature B cells expressed CXCR4, which was also noted for mature B cells from adult bone marrow (Fig 1B). We noted that CXCR4 expression on pro-B cells from fetal bone marrow and umbilical cord blood B cells was higher than on pro-B cells from adult bone marrow. The reason for this difference in expression is currently not clear. However, CXCR4 expression on pre-B and mature B cells was similar during ontogeny.
CXCR4 mediated signal transduction on different B-cell subsets.
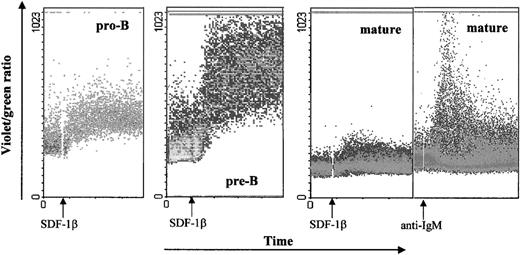
One of the events that characterize activation of the G-protein coupled receptors is mobilization of calcium from intracellular stores, leading to a transient rise in intracellular calcium concentration.1 28-30 We tested calcium mobilization after stimulation of B lineage cell subsets sorted from bone marrow with 100 nmol/L SDF-1β (Fig 2). Eighty-seven percent of pro-B and 81% of pre-B cells stimulated with SDF-1β showed a significant agonist-dependent calcium response, which appeared 15 to 20 seconds after stimulation and returned to base level after 2 to 3 minutes. The response by pro-B cells was considered particularly strong considering that on average the percentage of CXCR4 positive cells was lower than for pre-B and much lower than for mature B cells (see Fig 1B). In contrast, only 3% of mature B cells showed a calcium mobilization response to SDF-1β, despite their ability to respond to other stimuli (anti-IgM MoAb) (Fig 2).
SDF-1β induced calcium mobilization of B-cell subsets sorted from adult bone marrow. Sorted B cells were loaded with Indo-1AM and stimulated at the indicated time points (arrows) with the SDF-1 (100 nmol/L) or anti-IgM (2 μg/mL). The calcium mobilization is expressed as the violet/green ratio during the indicated time course. As a positive control, cells were stimulated with anti-IgM MoAb. The results are representative of 4 experiments using primary B cells from adult bone marrow. Two experiments were done on pro-B and pre-B cells from fetal bone marrow. In addition, data were confirmed by analysis of calcium mobilization of developmental stage specific B-cell lines: REH (pro-B), 697 (pre-B), Nalm-6 (pre-B) and 15A (mature).
SDF-1β induced calcium mobilization of B-cell subsets sorted from adult bone marrow. Sorted B cells were loaded with Indo-1AM and stimulated at the indicated time points (arrows) with the SDF-1 (100 nmol/L) or anti-IgM (2 μg/mL). The calcium mobilization is expressed as the violet/green ratio during the indicated time course. As a positive control, cells were stimulated with anti-IgM MoAb. The results are representative of 4 experiments using primary B cells from adult bone marrow. Two experiments were done on pro-B and pre-B cells from fetal bone marrow. In addition, data were confirmed by analysis of calcium mobilization of developmental stage specific B-cell lines: REH (pro-B), 697 (pre-B), Nalm-6 (pre-B) and 15A (mature).
Internalization of CXCR4 by exposure to SDF-1.
We also examined whether binding of SDF-1 would cause a similar degree of CXCR4 internalization on both early and late lineage B cells. CXCR4 was internalized on early and late B cells with a comparable decrease in expression, ie, an 86% decrease for early and an 87% decrease for late B-cell subsets (Fig 3).
SDF-1 induced internalization of CXCR4 from surface of early and mature B cells. Total bone marrow lymphocytes were incubated for 30 minutes at 37°C with 125 nmol/L of SDF-1 and placed on ice immediately after incubation to prevent resurfacing of the receptor. Cells were subsequently washed in ice-cold glycine buffer to remove cell bound SDF-1. Next, the cells were stained for CXCR4 expression in parallel with surface markers defining (A) early and (B) late lineage B cells (see Fig 1A). The early lineage B-cell population contained both pro-B and pre-B cells, whereas late lineage B cells contained immature and mature B cells. The shaded part of the histogram represents untreated cells; the gray line represents cells incubated with SDF-1; the dashed black line represents antibody isotype control. The results are representative of 3 experiments with primary B cells.
SDF-1 induced internalization of CXCR4 from surface of early and mature B cells. Total bone marrow lymphocytes were incubated for 30 minutes at 37°C with 125 nmol/L of SDF-1 and placed on ice immediately after incubation to prevent resurfacing of the receptor. Cells were subsequently washed in ice-cold glycine buffer to remove cell bound SDF-1. Next, the cells were stained for CXCR4 expression in parallel with surface markers defining (A) early and (B) late lineage B cells (see Fig 1A). The early lineage B-cell population contained both pro-B and pre-B cells, whereas late lineage B cells contained immature and mature B cells. The shaded part of the histogram represents untreated cells; the gray line represents cells incubated with SDF-1; the dashed black line represents antibody isotype control. The results are representative of 3 experiments with primary B cells.
Chemotactic responses of different B-cell subsets toward SDF-1β.
Chemotaxis was tested across a wide range of SDF-1β concentrations (0.125 nmol/L to 125 nmol/L), previously shown to mediate responses of T cells and macrophages.31,32 In the initial experiments (Fig 4, Panel A), bone marrow B cells were first sorted into purified populations (see Fig 1A) and then examined for migration to SDF-1, as previously reported for murine bone marrow B cells.15 Both pro-B and pre-B cells showed weak chemotactic responses to SDF-1. The maximal observed migration index (±1 SD) was 7.55 ± 4.17 for pro-B cells and 4.3 ± 3.7 for pre-B cells. Immature and mature B cells did not respond to SDF-1β; the highest migration indices (±1 SD) of 1.3 ± 0.42 and 1.4 ± 0.42 for immature and mature B cells, respectively, were not significantly different from controls (see Fig 4A).
Migration of different B-cell subsets from adult bone marrow to human SDF-1β. Chemotaxis of FACS-sorted B cells (A) or B cells from total bone marrow (B) in response to serial dilutions of SDF-1β (1 to 1,000 ng/mL) or medium alone is shown. In (B), early lineage B cells define a cell population that includes both pro-B (CD19+, κ−/λ−, CD34+) and pre-B (CD19+, κ−/λ−, CD34−) cells. Results are expressed as the migration index, which is defined as the number of cells migrated to the lower compartment in the presence of the chemokine divided by the number of cells that have migrated to the lower compartment in the presence of medium alone. A migration index 1 indicated the migration of 1.1% of total input cells and a migration index of 20 equaled 35% migration of total input cells. The results are representative of 4 experiments with primary B cells from total bone marrow and 2 with sorted bone marrow B cells.
Migration of different B-cell subsets from adult bone marrow to human SDF-1β. Chemotaxis of FACS-sorted B cells (A) or B cells from total bone marrow (B) in response to serial dilutions of SDF-1β (1 to 1,000 ng/mL) or medium alone is shown. In (B), early lineage B cells define a cell population that includes both pro-B (CD19+, κ−/λ−, CD34+) and pre-B (CD19+, κ−/λ−, CD34−) cells. Results are expressed as the migration index, which is defined as the number of cells migrated to the lower compartment in the presence of the chemokine divided by the number of cells that have migrated to the lower compartment in the presence of medium alone. A migration index 1 indicated the migration of 1.1% of total input cells and a migration index of 20 equaled 35% migration of total input cells. The results are representative of 4 experiments with primary B cells from total bone marrow and 2 with sorted bone marrow B cells.
Because of concern of cell stress caused by cell sorting, total bone marrow cells were loaded into Transwell and the migrated cells were then stained with appropriate markers to define B-cell subsets. Due to limitation in cell number, we defined the migrated B-cell subsets as either early lineage, eg, a cell population that included both pro-B and pre-B (CD19+, κ−/λ−, CD34+and CD19+, κ−/λ−, CD34−) (Fig 1A) cells, or late lineage, eg, immature (CD19+, κ+/λ+, IgD−) and mature B cells (CD19+, κ+/λ+, IgD+) (Fig 1A). In comparison to responses by other primary hematopoietic cells, the early lineage (pro-B and pre-B) cells showed a strong chemotactic response to SDF-1β.31-33 The maximal migration index (±1 SD) for early lineage B cells was 21.3 ± 3 at a SDF-1β concentration of 100 ng/mL (12.5 nmol/L) (see Fig 4B). Immature and mature B cells showed a much weaker chemotactic response toward SDF-1β with highest migration indices (±1 SD) of 5.4 ± 2 and 7.3 ± 2.2 for immature and mature B cells, respectively, at a comparable SDF-1β concentration of 100 to 1,000 ng/mL (see Fig 4B).
The chemotaxis data on sorted cells (panel A) are shown for comparison to previously reported data on sorted murine bone marrow B cells. Moreover, the data show the influence of mechanical stress conferred by high-speed cell sorting on the chemotaxis assay.
Comparison of CXCR4 expression and function between early and late lineage B cells.
For this comparison, we also calculated the number of anti-CXCR4 antibody binding sites per B cell to complement the CXCR4 expression analyses in Fig 1, which shows the percentage of anti-CXCR4 positive cells compared with the binding of an isotype control antibody. Due to a limitation in cell number described above, the migration responses to SDF-1 by early lineage B cells were calculated on a cell population that included both pro-B and pre-B cells. Therefore, as shown in Table2, we compared CXCR4 expression and function of early and late lineage B cells. The studies on early lineage B cells included both pro-B (CD19+, κ−/λ−, CD34+) and pre-B (CD19+, κ−/λ−, CD34−) cell populations and those on late lineage B cells included immature (CD19+, κ+/λ+, IgD−) and mature (CD19+, κ+/λ+, IgD+) B-cell populations (see Fig 1A). The number of CXCR4 molecules varied considerably between 3 different donors, with a range of 7,352 to 12,977 for early lineage (pro/pre) B cells and a range of 4,046 to 9,112 for late lineage (immature/mature) B cells (see Table 2).
The difference between the number of CXCR4 positive cells and anti-CXCR4 (12G5) antibody binding sites between early and late stages of B-cell development was not statistically significant (P > 2) (T-test to compare 2 independent sample means). The decrease in function between early and late lineage B cells was statistically significant as measured by calcium mobilization (P < .01) and chemotaxis (P < .05) (T-test to compare 2 independent sample means). Taken together, these data suggested that the decrease in SDF-1 responsiveness was disproportionate to changes in CXCR4 expression on developing human B-cell populations from bone marrow.
DISCUSSION
The importance of SDF-1 and CXCR4 in B lymphopoiesis has been shown in murine studies involving gene inactivation.12-14 However, the mechanisms by which these molecules affect B lymphopoiesis are not yet clear. In the current analysis of human B cells, we assessed primary bone marrow B cell and correlated CXCR4 expression and function at each developmental stage. We observed that unlike early lineage B cells that responded markedly to SDF-1, the late lineage B cells (eg, immature and mature) were poorly responsive to SDF-1 stimulation. For example, SDF-1 stimulation caused a calcium mobilization response of only 3% of mature B cells as compared with over 84% of early lineage B cells (Figs 2 and 5). In addition, the migration responses of immature/mature B cells were greatly diminished (more than 70%) compared with early lineage B cells (see Table 2 and Figs 4 and 5). Of note, a similar decrease in SDF-1 responsiveness has been observed in thymocyte subsets of different maturation stages.34
The reduction of SDF-1 responsiveness during B lymphopoiesis is disproportionate to the decrease in CXCR4 expression. The terms “CXCR4 positive cells” and “anti-CXCR4 (12G5) antibody binding sites per cell” are defined in Materials and Methods. Presence of calcium mobilization after stimulation describes the percentage of cells responding with calcium mobilization after SDF-1 stimulation. Migration of cells toward SDF-1 describes the ratio between the number of cells that migrated in the presence of chemokine and the number of cells that migrated in the presence of media alone. CXCR4 expression and function data of late lineage (immature and mature) B cells are normalized to the data of early (pro-B and pre-B) lineage B cells (defined as equal to 1). The data represent the mean of experiments shown in Figs 2 and 4 and Table 2.
The reduction of SDF-1 responsiveness during B lymphopoiesis is disproportionate to the decrease in CXCR4 expression. The terms “CXCR4 positive cells” and “anti-CXCR4 (12G5) antibody binding sites per cell” are defined in Materials and Methods. Presence of calcium mobilization after stimulation describes the percentage of cells responding with calcium mobilization after SDF-1 stimulation. Migration of cells toward SDF-1 describes the ratio between the number of cells that migrated in the presence of chemokine and the number of cells that migrated in the presence of media alone. CXCR4 expression and function data of late lineage (immature and mature) B cells are normalized to the data of early (pro-B and pre-B) lineage B cells (defined as equal to 1). The data represent the mean of experiments shown in Figs 2 and 4 and Table 2.
Comparison of Expression and Function of CXCR4 Between Early and Late Lineage B Cells
| . | Expression . | |
|---|---|---|
| Mean ± SD Percentage of CXCR4-Positive Cells (range) . | Mean No. ± SD of Anti-CXCR4 ABS (range) . | |
| Early lineage B cells | 87 ± 10 (80-99) | 9,608 ± 2,973 (7,352-12,977) |
| Late lineage B cells | 73 ± 17.04 (75-90) | 6,319 ± 2,572 (4,046-9,112) |
| Function | ||
| Percentage of Cells Responding With Calcium Mobilization After SDF-1β Stimulation | Migration of B Cells Toward SDF-1β (migration index) | |
| Early lineage B cells | 84 ± 3SD (pro-B + pre-B) | 21 ± 3SD |
| Late lineage B cells | 3 ± 1SD (mature B only) | 6.3 ± 2.1SD |
| . | Expression . | |
|---|---|---|
| Mean ± SD Percentage of CXCR4-Positive Cells (range) . | Mean No. ± SD of Anti-CXCR4 ABS (range) . | |
| Early lineage B cells | 87 ± 10 (80-99) | 9,608 ± 2,973 (7,352-12,977) |
| Late lineage B cells | 73 ± 17.04 (75-90) | 6,319 ± 2,572 (4,046-9,112) |
| Function | ||
| Percentage of Cells Responding With Calcium Mobilization After SDF-1β Stimulation | Migration of B Cells Toward SDF-1β (migration index) | |
| Early lineage B cells | 84 ± 3SD (pro-B + pre-B) | 21 ± 3SD |
| Late lineage B cells | 3 ± 1SD (mature B only) | 6.3 ± 2.1SD |
Flow cytometric studies were employed to determine within B-cell populations the percentage of CXCR4 positive cells, the mean number of anti-CXCR4 ABS and migration response to SDF-1. Analyses of early lineage B cells were performed on a cell population that included both pro-B (CD19+, κ−/λ−, CD34+) and pre-B (CD19+, κ−/λ−, CD34−) cells, whereas analyses of late lineage B cells were done on cell population that included both immature (CD19+, κ+/λ+, IgD−) and mature (CD19+, κ+/λ+, IgD+) B cells (see Fig 1A). The values representing the percentage of cells responding with calcium flux after SDF-1 stimulation were derived from experiments with sorted cells (Fig 3). The value given for early lineage B cells was obtained by combining the data from 4 experiments, each of pro-B and pre-B cells. The value given for late lineage B cells reflects the combined results of 4 experiments including sorted mature B cells only.
The magnitude of the response to SDF-1 could be related to either the frequency of CXCR4 positive cells or the number of CXCR4 receptor molecules expressed on B cells. In Table 2, early lineage B cells (pro-B and pre-B) are compared with late lineage B cells (immature/mature) from bone marrow. The percentage of CXCR4 positive cells among late lineage B cells decreased by ∼17%, and the number of 12G5 antibody binding sites on late B cells decreased by ∼35% in comparison with early lineage cells (see Figs 1B and 5 and Table 2). Nevertheless, mature B cells express similar or higher levels of CXCR4 compared with monocytes or T cells35 and these latter cell types show vigorous responses to SDF-1 as measured by calcium mobilization and chemotaxis.33,36-38 Moreover, when receptor signaling is measured as an average increase of calcium mobilization, a strong response is detectable on a cell population with as few as 11% of cells being CXCR4 positive.39 Therefore, the level of CXCR4 expression on mature B cells appears sufficient to generate an SDF-1 response as measured by chemotaxis or calcium mobilization.
We also assessed to what extent bound SDF-1 would lead to internalization of CXCR4 on early and late lineage B cells. As illustrated in Fig 3, CXCR4 was internalized by comparable levels on both early and late lineage B cells. At first, we considered the degree of CXCR4 internalized on mature cells to be unexpected in view of the poor/absent SDF-1 responsiveness measured by chemotaxis and calcium mobilization. However, internalization of CXCR4 has been shown to be uncoupled from G-protein-dependent intracellular signaling pathways.40-42 Rather internalization of CXCR4 by SDF-1 stimulation appears to be regulated by pathways, which do not lead to an increase in intracellular calcium.43 Thus, these data suggest that CXCR4 on both early and late lineage B cells can bind to SDF-1, which subsequently leads to internalization of the receptor. However, as discussed below there is a lack of correlation between CXCR4 expression and SDF-1 responsiveness as measured by chemotaxis and calcium mobilization.
When CXCR4 expression and function of mature B cells are normalized to early lineage B cells (Fig 5), the reduction in SDF-1 responsiveness during B lymphopoiesis is disproportionate to the decrease in CXCR4 expression. Based on these analyses, we speculate that CXCR4 function may be controlled during development by processes involving either RGS proteins, posttranslational modification and/or cross-desensitization of the chemokine receptor.38,44-50 With respect to posttranslational modification, for example, it may be possible that the tyrosine residues of CXCR4 on mature B cells are less sulfated compared with early lineage B cells, thus accounting for their poor response to SDF-1. As shown in vitro for T cells, cellular responses to chemokines may be influenced by their activation state.51However, we were unable to elicit a calcium mobilization response to SDF-1 on mature B cells from bone marrow, which had been activated in vitro with anti-IgM (data not shown). Nevertheless, it remains possible that the biologic cellular responses mediated by other chemokine receptors may be linked to B-cell activation state.
Clearly additional studies are needed to investigate the mechanisms underlying the lack of correlation between CXCR4 expression and SDF-1 responsiveness on developing bone marrow B cells. It is attractive to speculate, however, that the observed decrease in SDF-1 responsiveness during B-cell maturation may be related to the distinct anatomic location in the bone marrow of early and late lineage B lymphocytes. The earliest B-cell progenitors are located within or near the endosteum of the bone marrow. These cells, remaining in close contact with stromal reticular cells in the peripheral region of bone marrow, begin to differentiate into more mature B cells. During and after μ chain gene rearrangement, the B cells tend to move toward sinusoids along the processes of adventitial reticular cells, and after beginning to express sIgM, the immature B cells move into the sinusoid segment and are released into blood circulation.52,53SDF-1–mediated signaling triggers rapid adhesion of peripheral lymphocytes to ICAM-1 through activation of integrins.54Therefore, the SDF-1/CXCR4 axis may be important in retaining the early lineage B cells in the appropriate bone marrow compartment through activation of their adhesion molecules, eg, VLA-4, which binds to VCAM-1.55-59
ACKNOWLEDGMENT
We are grateful to Dr Robert Doms and members of his laboratory for helpful discussion during the course of the experiments described in this article. We thank Dr John G. Monroe for critical review of the manuscript and Dr Jonni Moore and Hank Pletcher for the assistance with flow cytometry.
Supported by Grant No. NIH P50 HL54516.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Leslie E. Silberstein, MD, Professor, Departments of Pathology and Laboratory Medicine and Medicine, University of Pennsylvania School of Medicine, 284 John Morgan Building, 36th and Hamilton Walk, Philadelphia, PA 19104-6082; e-mail:silbersl@mail.med.upenn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal