Human herpesvirus-8 (HHV-8) genome encodes for genes homologous to human cellular genes such as interleukin-6 (IL-6), Cyclin-D, BCL-2, and IL-8 receptor (G-protein–coupled receptor [GCR]). We used reverse transcriptase-polymerase chain reaction to study the expression of these viral genes in lymphoproliferative disorders associated with HHV-8 infection. None of these genes was expressed in 1 case of benign, localized Castleman’s disease (CD), and only viral IL-6 and viral Cyclin-D were transcribed in 2 cases of benign lymphadenopathies with giant germinal center hyperplasia and increased vascularity. In contrast, all 4 genes were transcribed in 1 case of multicentric CD of plasma cell type with aggressive clinical course and in 1 primary effusion lymphoma cell line. Our study provides the evidence that various HHV-8 genes, homologous to cellular genes involved in control of proliferation and apoptosis, may be differently expressed in different lymphoid disorders in vivo.
IN 1996, WE REPORTED in BLOOD the detection of human herpesvirus-8 (HHV-8)/Kaposi’s sarcoma-associated herpesvirus (KSHV)-specific sequences in few cases of angioimmunoblastic lymphadenopathy with dysproteinemia and of reactive lymphadenopathies, characterized by giant germinal center hyperplasia and increased vascularity.1 We have continued to look for the presence of HHV-8 DNA sequences by polymerase chain reaction (PCR) in hundreds of cases of benign and malignant lymphoproliferations from Italy, but we identified only a few more cases, including 1 case of primary cerebral B-cell non-Hodgkin’s lymphoma,2 1 case of localized Castleman’s disease (CD) of hyalin vascular (HV) type,3 and 1 case of multicentric CD of plasma cell (PC) type, all occurring in human immunodeficiency virus (HIV)-negative patients, without Kaposi’s sarcoma (KS), confirming the rare occurrence of HHV-8 infection in pathologic lymphoid tissues.4
In the present study, we explored whether transcription of HHV-8 genes may occur in lymphoid tissues, in vivo. Recently, HHV-8 genome has been shown to contain a surprisingly high number of genes, encoding homologues to cytokines, oncoproteins, and cell cycle regulatory and signaling proteins that have apparently been acquired from the host cell.4 So far, the expression of such HHV-8 genes has been documented in KS biopsies and in few primary effusion lymphomas (PEL).4,5 The expression of viral interleukin-6 (vIL-6) has been also documented in few cases of HHV-8–positive MCD of PC type.5-7
Thus, we used reverse transcriptase-PCR (RT-PCR) to look for the expression of the HHV-8 genes homologous to human IL-6, cyclin-D, BCL-2, and IL-8 receptor (G-protein–coupled receptor [GCR])4 in 2 cases of benign lymphadenopathy with giant germinal center hyperplasia and increased vascularity1 and in the 2 cases of CD.
PATIENTS AND METHODS
Although the patient with localized CD of HV type had a benign clinical course and is still well, 5 years after diagnosis, without therapy,3 the patient with MCD of PC type suffered from a severe systemic disease, with massive lymphadenopathies and constitutional symptoms, resistant to treatment, and died 2 years after diagnosis from an opportunistic infection.
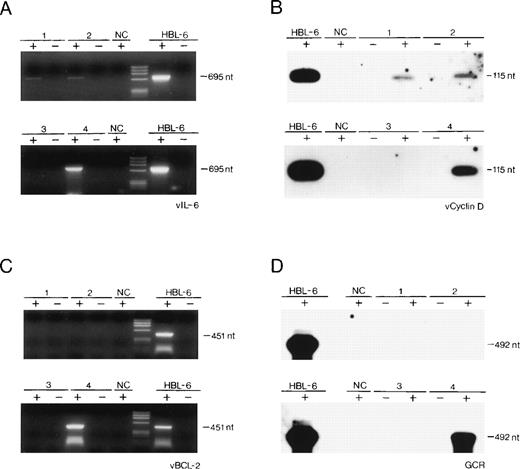
All of the tested samples harbored HHV-8 DNA sequences as detected by PCR, and the viral load was roughly similar to that found in 10 KS biopsies examined.1,3 Total RNA was also isolated from the HHV-8–infected PEL cell line, HBL-6 (kindly provided by Dr G. Gaidano, University of Eastern Piedmont, Novara, Italy), from the HHV-8–negative/Epstein-Barr virus (EBV)-positive Burkitt’s lymphoma cell line, Raji, from 10 HHV-8–negative lymph node biopsies.1 Expression of all 4 HHV-8 genes, namely vIL-6, v-cyclin-D, vBCL-2, and vGCR, was examined by standard RT-PCR technique performed on 1 μg RNA, after elimination of contaminating genomic DNA with RNase-free DNase, followed by phenol-chloroform extraction, as described elsewhere.8-10Positive results were evaluated after hybridization of the first amplified product with a specific internal oligonucleotide probe (v-cyclin-D and vGCR; Fig 1B and D) or after reamplification of the first amplified product and visualization on ethidium bromide (vIL-6 and vBCL-2; Fig 1A and C). Contamination by genomic DNA was excluded, because we failed to identify PCR products when using the DNase-treated RNA preparations in the absence of the RT reaction. As a standard, the actin gene was amplified from the same reverse transcribed samples (not shown).
(A through D) Detection by RT-PCR of the expression of cell-homologous genes of HHV-8 in 2 cases of reactive lymphadenopathy (lanes 1 and 2), in 1 case of localized CD of HV type (lane 3), in 1 case of multicentric CD of PC type (lane 4), and in 1 PEL cell line (HBL-6). NC, Raji cell line DNA. Negative controls included RT-PCR performed on RNA in the absence of reverse transcription reaction (−). nt, nucleotides; phiX174 HaeIII digested as molecular marker.
(A through D) Detection by RT-PCR of the expression of cell-homologous genes of HHV-8 in 2 cases of reactive lymphadenopathy (lanes 1 and 2), in 1 case of localized CD of HV type (lane 3), in 1 case of multicentric CD of PC type (lane 4), and in 1 PEL cell line (HBL-6). NC, Raji cell line DNA. Negative controls included RT-PCR performed on RNA in the absence of reverse transcription reaction (−). nt, nucleotides; phiX174 HaeIII digested as molecular marker.
RESULTS AND DISCUSSION
The results of the expression studies are summarized in Table 1 and in Fig 1. Expression of all 4 HHV-8 transcripts was undetectable in the HHV-8 DNA-negative samples (not shown; Fig 1).
Expression Studies of Cell-Homologous Genes of HHV-8 by RT-PCR in Lymphoproliferative Diseases
| Diagnosis . | vIL-6 . | v-Cyclin-D . | vBCL-2 . | vGCR . |
|---|---|---|---|---|
| Lymphoid tissues | ||||
| Reactive lymphadenopathy | Pos | Pos | Neg | Neg |
| Reactive lymphadenopathy | Pos | Pos | Neg | Neg |
| CD (multicentric, PC type) | Pos | Pos | Pos | Pos |
| CD (localized, HV type) | Neg | Neg | Neg | Neg |
| PEL cell line | ||||
| HBL-6 | Pos | Pos | Pos | Pos |
| Diagnosis . | vIL-6 . | v-Cyclin-D . | vBCL-2 . | vGCR . |
|---|---|---|---|---|
| Lymphoid tissues | ||||
| Reactive lymphadenopathy | Pos | Pos | Neg | Neg |
| Reactive lymphadenopathy | Pos | Pos | Neg | Neg |
| CD (multicentric, PC type) | Pos | Pos | Pos | Pos |
| CD (localized, HV type) | Neg | Neg | Neg | Neg |
| PEL cell line | ||||
| HBL-6 | Pos | Pos | Pos | Pos |
Our study reports, for the first time, that (1) HHV-8 genes, homologous to cell genes, may be transcribed in lymphoid tissues in vivo, out of the KS and acquired immunodeficiency syndrome (AIDS) settings4; (2) the expression of such viral genes is apparently different in the different lymphoproliferative diseases of our series, associated with HHV-8 infection, namely in benign (reactive lymphadenopathy and localized CD of HV type), atypical (multicentric CD [MCD] of PC type), and malignant (PEL) lymphoproliferative diseases. Our findings, although obtained on these few, rare cases, also suggest that the pattern of expression of HHV-8 genes homologous to cellular genes involved in cell proliferation (v-cyclin-D and vGCR) and apoptosis (vIL-6 and vBCL-2) may influence the nature of the lymphoproliferative process associated with this herpesviral infection.
Consistent with the transcription mapping of HHV-8 genome in the BC-1 lymphoma cell line using Northern blot analysis,11 our study may also suggest that, in the 2 cases of benign lymphadenopthies, the expression of v-cyclin-D and vIL-6 reflects a predominantly latent HHV-8 infection. Conversely, the expression of vGCR, documented in the case of MCD of PC type as well as in the HBL-6 cell line, suggests that HHV-8 is likely to replicate there, at least in a proportion of infected cells. Expression studies on a larger series of different lymphoproliferative diseases are necessary to understand if the differential expression of some HHV-8 genes is one of the possible mechanisms of HHV-8–induced lymphoproliferation in vivo.5 7
M.L. and P.B. contributed equally to this report.
Supported by a grant from Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.), Milano, Italy (M.L.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Giuseppe Torelli, MD, Department of Medical Sciences, Section of Hematology, University of Modena, Policlinico, Via del Pozzo 71, 41100, Modena, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal