Arsenic compounds have recently been shown to induce high rates of complete remission in patients with acute promyelocytic leukemia (APL). One of these compounds, As2O3, induces apoptosis in APL cells via a mechanism independent of the retinoic acid pathway. To test the hypothesis that arsenic compounds may be effective against other forms of acute myelogenous leukemia (AML), we studied the membrane-permeable arsenic compound phenylarsine oxide (PAO). Because interleukin-1β (IL-1β) plays a key role in AML cell proliferation, we first tested the effect of PAO on OCIM2 and OCI/AML3 AML cell lines, both of which produce IL-1β and proliferate in response to it. We found that PAO inhibited the proliferation of both OCIM2 and OCI/AML3 cells in a dose-dependent fashion (0.01 to 0.1 μmol/L) and that IL-1β partially reversed this inhibitory effect. We then measured IL-1β levels in these cells by using an enzyme-linked immunosorbent assay and Western immunoblotting and found that PAO almost completely abolished the production of IL-1β in these AML cells, whereas it did not affect the production of IL-1 receptor antagonist. Because PAO inhibits activation of the transcription factor NF-κB and because NF-κB modulates an array of signals controlling cellular survival, proliferation, and cytokine production, we also studied the effect of PAO on NF-κB activation in AML cells and found that PAO suppressed the IL-1β–induced activation of NF-κB. Because inhibition of NF-κB may result in cellular apoptosis, we also tested whether PAO may induce apoptotic cell death in AML cells. We found that PAO induced apoptosis in OCIM2 cells through activation of the cystein protease caspase 3 and subsequent cleavage of its substrate, the DNA repair enzyme poly (ADP-ribose) polymerase. The PAO-induced apoptosis was caspase dependent, because it was completely blocked by the caspase inhibitor Z-DEVD-FMK. Finally, we tested the effect of PAO on fresh AML marrow cells from 7 patients with newly diagnosed AML and found that PAO suppressed AML colony-forming cell proliferation in a dose-dependent fashion. Taken together, our data showing that PAO is an effective in vitro inhibitor of AML cells suggest that this compound may have a role in future therapies for AML.
ACUTE MYELOGENOUS leukemia (AML) is a clonal hematologic malignancy characterized by abnormal proliferation of myeloid leukemia cells. Despite extensive clinical research with numerous combinations of cytotoxic agents, the overall prognosis of patients with AML remains poor (reviewed in Estey1). Thus, the search for more effective agents continues.
Arsenic compounds, such as arsenic trioxide (As2O3) and arsenic disulfide,2which are occasionally used in traditional Chinese medicine, were found to induce complete remission lasting for varying lengths of time in more than 70% of patients with acute promyelocytic leukemia (APL).3 In a recent study by Chen et al,4As2O3 induced apoptosis in APL cells. Because this apoptosis induction occurred independently of the retinoid pathway,4 we therefore hypothesized that arsenic compounds may effectively inhibit other forms of AML.
To test our hypothesis, we used phenylarsine oxide (PAO). This arsenic compound, which is structurally different from the arsenicals mentioned above, is a membrane-permeable molecule that inhibits phosphotyrosine phosphatase (PTPase).5 Inhibition of PTPase by agents such as PAO, in turn, inactivates the transcription factor NF-κB in various cell types, including hematopoietic cells.6 NF-κB modulates the effects of various transcription factors responsible for the proliferation of normal myeloid and leukemia cells,7and its activation induces the expression of various cytokines, including interleukin-1β (IL-1β; reviewed in Seibenlist et al8). IL-1β itself is a proinflammatory cytokine that plays a major role in stimulating the proliferation of AML cells.9-12 Thus, because PAO seemed to possess properties sufficient to inhibit AML cells, we studied its effects on AML cell lines and on fresh bone marrow (BM) cells from 7 patients with newly diagnosed AML.
Consequently, we found that PAO inhibited the proliferation of both AML cell lines and fresh AML marrow blast colony-forming cells and that PAO also inhibited the IL-1β–induced activation of NF-κB and activated caspase 3, thus inducing apoptotic cell death in AML cells.
MATERIALS AND METHODS
Cell lines.
The AML cell lines OCI/AML313 and OCIM214 were kindly provided by M.D. Minden (Ontario Cancer Institute, Toronto, Ontario, Canada). OCI/AML3 was established from an AML patient and OCIM2 from a patient with erythroleukemia. Both cell lines proliferate in the presence of culture medium and fetal calf serum (FCS) without exogenous growth factors. The leukemia cell lines HL60 and K562 were obtained from the American Type Culture Collection (ATCC; Rockville, MD). All cells were maintained in RPMI-1640 culture medium (GIBCO, Grand Island, NY) supplemented with 10% FCS (Flow Laboratories, McLean, VA).
Subjects.
BM aspirates were obtained from 7 AML patients with high marrow blast counts (see Table 1 for clinical data). All studies were performed with the patients’ informed consent and were approved by the Human Experimentation Committee of our institution.
Clinical Data on AML Patients
| Patient No. . | Age (yr)/ Sex . | Cytogenetic Abnormality . | FAB Category . | Hb (g/dL) . | WBC (×109/L) . | Platelets (×109/L) . | % Blasts . | % Blasts in BM . |
|---|---|---|---|---|---|---|---|---|
| 1 | 44/F | Misc | M2 | 6.3 | 129 | 87 | 96 | 51 |
| 2 | 78/M | −5,−7 | M2 | 10.3 | 14.5 | 26 | 74 | 77 |
| 3 | 67/F | Misc | M4 | 9.9 | 18.7 | 95 | 69 | 71 |
| 4 | 40/M | Dip,−Y | M2 | 8.0 | 2.9 | 34 | 61 | 73 |
| 5 | 69/F | t(15;17) | M2 | 8.2 | 6.7 | 53 | 88 | 65 |
| 6 | 64/F | Misc | M1 | 11.0 | 146.9 | 16 | 92 | 53 |
| 7 | 45/F | −5,−7 | M1 | 8.1 | 6.3 | 11 | 53 | 93 |
| Patient No. . | Age (yr)/ Sex . | Cytogenetic Abnormality . | FAB Category . | Hb (g/dL) . | WBC (×109/L) . | Platelets (×109/L) . | % Blasts . | % Blasts in BM . |
|---|---|---|---|---|---|---|---|---|
| 1 | 44/F | Misc | M2 | 6.3 | 129 | 87 | 96 | 51 |
| 2 | 78/M | −5,−7 | M2 | 10.3 | 14.5 | 26 | 74 | 77 |
| 3 | 67/F | Misc | M4 | 9.9 | 18.7 | 95 | 69 | 71 |
| 4 | 40/M | Dip,−Y | M2 | 8.0 | 2.9 | 34 | 61 | 73 |
| 5 | 69/F | t(15;17) | M2 | 8.2 | 6.7 | 53 | 88 | 65 |
| 6 | 64/F | Misc | M1 | 11.0 | 146.9 | 16 | 92 | 53 |
| 7 | 45/F | −5,−7 | M1 | 8.1 | 6.3 | 11 | 53 | 93 |
Abbreviations: FAB, French-American-British; Hb, hemoglobin; WBC, white blood cells; Misc, miscellaneous; Dip, Diploid.
Cell line clonogenic assay.
Clonogenic assays were performed as previously described.15Briefly, OCI/AML3, OCIM2, HL60, and K562 cells (2 to 4 × 104 cells/mL) were cultured in 0.8% methylcellulose (Fluka Chemical Corp, Ronkonkoma, NY), 10% FCS, and RPMI-1640 medium in the presence of PAO (Aldrich, Milwaukee, WI), which was dissolved in dimethyl sulfoxide (DMSO) at a concentration of less than 0.1%, with or without 10 ng/mL recombinant human (rh) IL-1β (molecular weight 17,500; Boehringer Mannheim Biochemicals, Indianapolis, IN). The culture mixture was placed in 35-mm Petri dishes (Nunc Inc, Naperville, IL) in duplicate or triplicate and maintained at 37°C with 5% CO2 in air in a humidified atmosphere. Colonies were counted after 7 days by using an inverted microscope. A colony was defined as a cluster of more than 40 cells.
Enzyme-linked immunosorbent assay (ELISA).
ELISAs were performed with IL-1β and IL-1 receptor antagonist (IL-1RA) ELISA kits (Cistron Biotechnology [Pine Brook, NJ] and Amersham Life Science [Arlington Heights, IL], respectively) as previously described.16 Cell lysates and standard dilutions of either IL-1β or IL-1RA were added to test wells in duplicate and incubated for 2 hours at 37°C. The test wells were then washed 3 times with phosphate-buffered saline (PBS), incubated with rabbit IL-1β antiserum for 2 hours, washed as previously described, and incubated for 30 minutes with goat antirabbit IgG conjugated to horseradish peroxidase. The test wells were vigorously washed, and a substrate (o-phenylenediamine dissolved in 3% hydrogen peroxide solution) and 4 N sulfuric acid were added. The color intensity was read within 15 minutes at a wavelength of 490 nm with a microplate autoreader (Model EL-309; Biotek, Winooski, VT). The average net optical densities (OD) of the standard IL-1β and IL-1RA concentrations were then plotted, and the amount of the corresponding cytokine in each sample was determined by interpolation from the standard curve.
Western immunoblotting for detection of IL-1β.
Cell lysates were assayed for protein concentration with the BCA Protein Assay Reagent kit (Pierce Chemical Co, Rockford, IL). Each set of paired samples was then adjusted to have the same protein concentration. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed by using a modification of the method of Laemmli.17 In brief, antigens were dissolved in Laemmli sample buffer at room temperature. Electrophoresis was conducted at a constant wattage (10 W) in running buffer cooled to 4°C. Stacking gels contained 4% (wt/vol) acrylamide, and separating gels contained 12% (wt/vol) acrylamide. Approximately 200 μL of sample protein was loaded into each of the appropriate lanes. Proteins separated by SDS-PAGE were then transferred to nitrocellulose membranes overnight at 30 V in a cooled (4°C) reservoir containing transfer buffer (25 mmol/L Tris, 192 mmol/L glycine, and 20% methanol, pH 8.3).18 Nitrocellulose membranes were then removed from the blot apparatus and placed in a Ponceau S staining solution (0.5% Ponceau S and 1% glacial acetic acid in H2O) for 5 minutes to verify the equal loading of protein in control and treated samples.19
After equal loading of protein was verified, the membranes were then rinsed for an additional 10 minutes and immunoscreened. In brief, the membranes were blocked in Blotto (5% dried milk dissolved in 50 mmol/L PBS) for at least 1 hour at room temperature. The membranes were then washed 3 times in PBS plus 0.5% Tween 20. Next, the membranes were incubated for 1 hour with polyclonal rabbit anti–IL-1β antibodies (Endogen Inc, Boston, MA) or with normal rabbit IgG (used as a control) diluted 1:200 in PBS containing 0.5% Tween 20. After incubation, the membranes were subjected to three 15-minute rinses in PBS containing 0.5% Tween 20. Bound antibody was detected with the ECL Western Blotting Detection System (Amersham Corp, Arlington Heights, IL). The membranes were incubated with antirabbit horseradish peroxidase-labeled antibody at a concentration of 1:2,000 in PBS plus 0.5% Tween 20 at room temperature for 1 hour. After this incubation, the membranes were washed in PBS containing 0.5% Tween 20, and bound antibody was detected according to the ECL protocol. The chemiluminescence of the membranes was detected by exposure to X-OMAT AR5 x-ray film (Kodak, Rochester, NY) in stainless steel cassettes (Sigma Chemical Co, St Louis, MO).
Electrophoretic mobility shift assay (EMSA) of NF-κB activation.
OCIM2 cells (2 × 104 cells/mL) were incubated in the presence of increasing concentrations of IL-1β at 37°C. Nuclear extracts were then prepared according to the method of Shreiber et al.20 Briefly, 2 × 106 cells were washed with cold PBS and suspended in a tube with 0.4 mL of lysis buffer (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol [DTT], 0.5 mmol/L phenylmethylsulfonyl fluoride [PMSF], 2.0 μg/mL leupeptin, 2.0 μg/mL aprotinin, and 0.5 mg/mL benzamidine). The tube was then placed on ice, where the cells were allowed to swell for 15 minutes. Afterward, 12.5 μL of 10% Nonidet P-40 was added. The tube was then vigorously vortexed for 10 seconds to homogenize its contents and then centrifuged for 30 seconds in a microcentrifuge. The nuclear pellet was resuspended in 25 μL of ice-cold nuclear extraction buffer (20 mmol/L HEPES, pH 7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 1 mmol/L PMSF, 2.0 μg/mL leupeptin, 2.0 μg/mL aprotinin, and 0.5 mg/mL benzamidine), and the tube was incubated on ice for 30 minutes with intermittent mixing. This nuclear extract (NE) was then centrifuged for 5 minutes in a microcentrifuge at 4°C, and the supernatant was either used immediately or stored at −70°C for later use. The protein content was measured by the method of Bradford.21
To determine NF-κB activation, EMSAs were performed as previously described.22 Briefly, 4 to 5 μg of NE was incubated with 16 fmol of 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotides from the human immunodeficiency virus-1 long terminal repeat (HIV-LTR; 5′-TTGTTACAAGGGACTT-TCCGCTGGGGGACTTTTCCAGGGAGGCGTGG-3′) in the presence of 1 to 2 μg of poly(dI-dC) in a binding buffer (25 mmol/L HEPES, pH 7.9, 0.5 mmol/L EDTA, 0.5 mmol/L DTT, 1% Nonidet P-40, 5% glycerol, and 50 mmol/L NaCl) for 20 minutes at 37°C (we used HIV-LTR–containing NF-κB binding sites in this assay because myeloid cells are targets for HIV). The DNA-protein complexes formed were separated from free oligonucleotides on a 4.5% or 7.5% native polyacrylamide gel using a buffer containing 50 mmol/L Tris, 200 mmol/L glycine, pH 8.5, and 1 mmol/L EDTA, after which the gel was dried. A mutated oligonucleotide probe was used to examine the specificity of NF-κB’s binding to the DNA. For supershift assays, NE was incubated with the antibodies for 15 minutes at room temperature before analyzing the NF-κB by EMSA. Radioactive bands were visualized with a Phosphor Imager (Molecular Dynamics, Sunnyvale, CA) using Imagequant software.
TdT-mediated dUTP nick-end labeling (TUNEL) assay for detection of apoptosis.
The apoptosis detection system Fluorescein (Promega, Madison, WI) was used to perform TUNEL assays.23 Briefly, 4% formaldehyde-treated cytospun cells were made permeable with 0.2% Triton-100 in PBS. After washing, slides were treated with equilibration buffer (supplied with kit) and then incubated with a TdT buffer (prepared according to the manufacturer’s instructions) for 60 minutes. The staining reaction was terminated by treating the slides with 2× SSC for 15 minutes. After washing, the slides were treated with an anti-fade solution and then mounted on slides with glass coverslips and rubber cement. The slides were analyzed using a fluorescence microscope.
Western immunoblotting for detection of caspase 3 and PARP proteins.
Cell lysates (from 5 × 105 cells) were used as described above. The following antibodies were used to detect the relevant proteins: monoclonal mouse antihuman CPP32 (Transduction Laboratories, Lexington, KY) to detect uncleaved caspase 3, polyclonal rabbit antihuman CPP32 (PharMingen, San Diego, CA) to detect cleaved caspase 3, and mouse antihuman PARP (Upstate Biotechnology, Lake Placid, NY) to detect PARP. Normal mouse IgG and normal rabbit serum were used as a control. To confirm detection of uncleaved caspase 3, Jurkat cells (ATCC) were used; to confirm detection of cleaved caspase 3 and PARP, 3T3 cells (ATCC) and HeLa cell (ATCC) nuclear extracts were used, respectively. Bound antibody was detected according to the ECL protocol (Amersham Life Science) as described above.
Adherent-cell fractionation.
Low-density BM mononuclear cells obtained by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) fractionation were incubated in plastic tissue-culture dishes or flasks (Falcon Plastics; Becton Dickinson, Oxnard, CA) with 10% FCS in α-medium (GIBCO). The fractionation procedure was repeated until no cells adhered to the tissue-culture dishes. Nonadherent cells harvested in this way contained less than 3% monocytes, as confirmed by the following techniques: (1) microscopic differential counting of at least 100 cells prepared with Wright’s stain and (2) nonspecific (α-naphthyl butyrate) esterase staining and immunocytochemical analysis with CD14 monoclonal antibodies (Becton Dickinson) to identify monocyte-promonocyte cells, as previously described.24 25
T-cell depletion.
T cells were depleted from the nonadherent fraction by negative immunomagnetic selection.26 In a modification of this technique, nonadherent BM cells were incubated with CD3 monoclonal antibodies (Becton Dickinson) at a concentration of 1 μg/106 cells in PBS with 0.25% FCS for 30 minutes at 4°C. The labeled cells were washed 3 times and then incubated with goat antimouse IgG-conjugated immunomagnetic beads (Advanced Magnetics, Cambridge, MA) at 4°C for 60 minutes in an end-over-end rotation at a 20:1 bead:cell ratio. Immunomagnetic bead-rosetted cells were removed with a magnetic particle concentrator (Advanced Magnetics), and unrosetted cells remaining in suspension were harvested by a Pasteur pipette. In some experiments, this procedure was repeated twice. The T-lymphocyte–depleted population contained less than 3% CD3 cells as assessed by an immunocytochemical technique performed on cytospun cells.24 25
AML blast colony assay.
A previously described method was used to assay AML blast colony formation.27,28 Briefly, 1 × 105 nonadherent T-cell–depleted BM cells were plated in 0.8% methylcellulose in α-medium supplemented with 10% FCS and 50 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Immunex Corp, Seattle, WA). PAO was dissolved in DMSO and added at the initiation of the cultures at concentrations ranging from 0.01 to 0.1 μmol/L in the absence or presence of 10 U/mL of IL-1β. The cultures were incubated in 35-mm Petri dishes in duplicate or triplicate for 7 days at 37°C in a humidified atmosphere of 5% CO2 in air. AML blast colonies were microscopically evaluated on day 7 of culture. A blast colony was defined as a cluster of 20 or more cells. Individual colonies were plucked, smeared on glass slides, and stained to confirm their leukemic cell composition. (That the AML blast colony assay identifies blasts rather than normal progenitors had been previously demonstrated by cytogenetic analysis of colonies obtained using this assay.29)
RESULTS
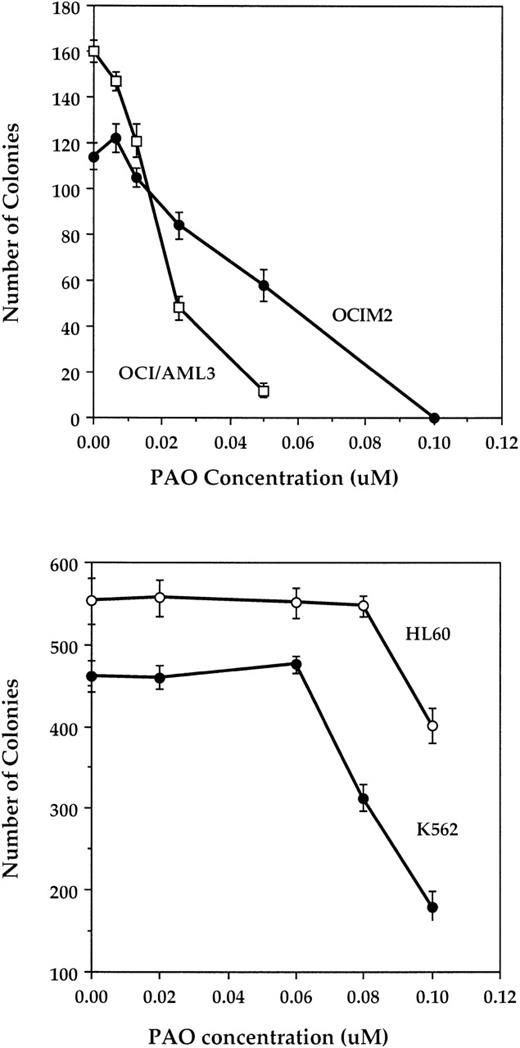
PAO inhibits leukemia cell line colony proliferation.
We began studying the effect of PAO on the proliferation of OCI/AML3 and the OCIM2 cell lines and found that PAO suppressed their colony-forming cell growth in a dose-dependent fashion at concentrations ranging from 0.01 to 0.1 μmol/L (Fig 1, upper panel). Although the OCI/AML3 cells were more sensitive to PAO than were OCIM2 cells, both cell lines were much more sensitive to PAO than either HL60 or K562 cells. Indeed, the growth of OCI/AML3 and OCIM2 cells was almost completely abolished by PAO at concentrations of 0.05 and 0.1 μmol/L, respectively, whereas HL60 and K562 cells were only partially inhibited (27% and 61%, respectively) by 0.1 μmol/L (Fig 1, lower panel). The DMSO that was used to dissolve PAO had no effect on the proliferation of either cell line.
Effect of PAO on AML cell line colony proliferation. The upper panel shows the effect of PAO on OCIM2 and OCI/AML3 cells, and the lower panel shows the effect on HL60 and K562 cells. Each data point represents the mean colony number ± SD in triplicate cultures.
Effect of PAO on AML cell line colony proliferation. The upper panel shows the effect of PAO on OCIM2 and OCI/AML3 cells, and the lower panel shows the effect on HL60 and K562 cells. Each data point represents the mean colony number ± SD in triplicate cultures.
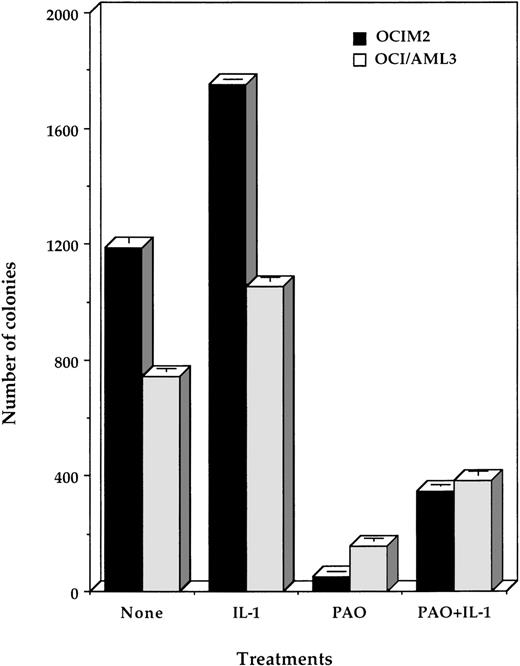
IL-1β partially reverses the inhibitory effect of PAO.
Because OCI/AML3 and OCIM2 cells, unlike HL60 and K562 cells, proliferate in response to IL-1β,12,30 we also tested whether IL-1β could affect the inhibitory effect of PAO. We found that 10 ng/mL IL-1β added at the initiation of culture partially reversed PAO’s suppressive effect (Fig 2). This is in keeping with previous studies in which we found that (1) OCI/AML3 and OCIM2 cells produce large quantities of IL-1β, which maximally stimulates their proliferation in an autocrine fashion; (2) the addition of exogenous IL-1β could not significantly stimulate their further growth; and (3) IL-1β antibodies could suppress their growth.12 30 Our current results therefore suggested that PAO might suppress IL-1β production by OCI/AML3 and OCIM2 cells.
Effect of PAO and IL-1β on OCIM2 and OCI/AML3 colony proliferation. Each data point represents the mean colony number ± SD in triplicate cultures. PAO was added to each culture at a final concentration of 0.08 μmol/L and IL-1β at 10 ng/mL.
Effect of PAO and IL-1β on OCIM2 and OCI/AML3 colony proliferation. Each data point represents the mean colony number ± SD in triplicate cultures. PAO was added to each culture at a final concentration of 0.08 μmol/L and IL-1β at 10 ng/mL.
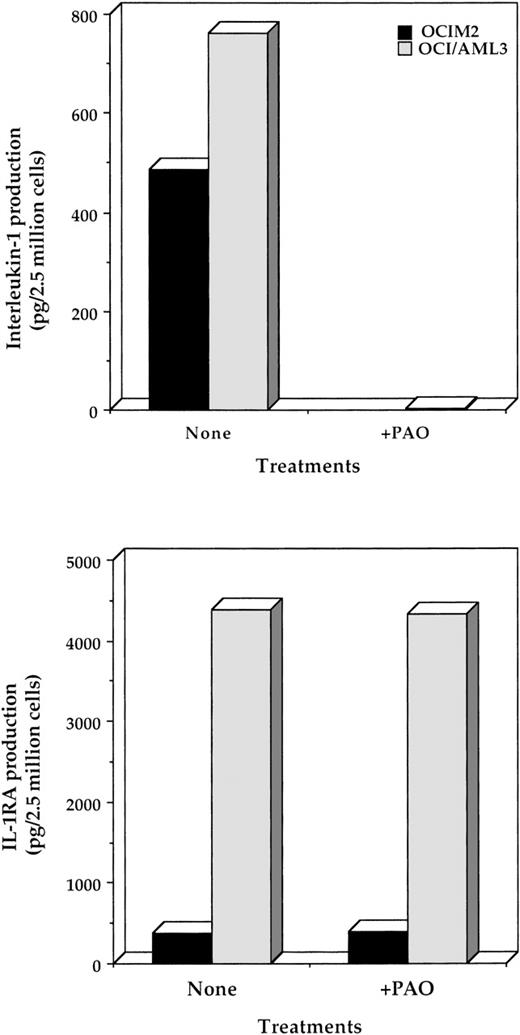
PAO inhibits IL-1β protein production.
In light of the results given above and published data indicating that PAO inhibits NF-κB,6 a binding site known to be present in the IL-1β promoter,31 32 we hypothesized that PAO inhibits IL-1β. To test this idea, we first incubated the cells with PAO and measured IL-1β protein levels in lysates of OCI/AML3 and OCIM2 cells. Using ELISAs, we found that 24 hours of incubation with 0.1 μmol/L PAO significantly reduced the production of IL-1β but not of IL-1RA protein by both cell types (Fig 3). These results indicated that the effect of PAO on IL-1β production is specific and does not result from a general suppression of protein synthesis. Because the ELISA detects both the uncleaved and cleaved forms of IL-1β, we also used Western immunoblotting to measure active (cleaved) IL-1β in OCIM2 line, whose morphology and origin do not resemble those of APL, and found that PAO significantly suppressed the production of mature IL-1β (Fig 4).
Effect of PAO on the production of IL-1β and IL-1RA by OCIM2 and OCI/AML3 cells. Cells were incubated in the presence or absence of 1.0 μmol/L PAO for 24 hours. The amount of IL-1β (upper panel) and IL-1RA (lower panel) produced by these cells was then assessed by ELISA as described in Materials and Methods.
Effect of PAO on the production of IL-1β and IL-1RA by OCIM2 and OCI/AML3 cells. Cells were incubated in the presence or absence of 1.0 μmol/L PAO for 24 hours. The amount of IL-1β (upper panel) and IL-1RA (lower panel) produced by these cells was then assessed by ELISA as described in Materials and Methods.
Effect of PAO on the production of mature IL-1β by OCIM2 cells. Cells were incubated in the presence and absence of 0.1 μmol/L PAO. The amount of mature IL-1β protein produced by these cells was then assessed by Western immunoblotting. The arrow points to the 17.5-kD mature IL-1β protein. Lane C shows control mature IL-1β protein, lane 1 shows protein from cells incubated in tissue culture media with DMSO, and lane 2 shows protein from cells incubated with PAO.
Effect of PAO on the production of mature IL-1β by OCIM2 cells. Cells were incubated in the presence and absence of 0.1 μmol/L PAO. The amount of mature IL-1β protein produced by these cells was then assessed by Western immunoblotting. The arrow points to the 17.5-kD mature IL-1β protein. Lane C shows control mature IL-1β protein, lane 1 shows protein from cells incubated in tissue culture media with DMSO, and lane 2 shows protein from cells incubated with PAO.
PAO inhibits IL-1β–induced NF-κB activation.
Because IL-1 is known to activate NF-κB,8 we sought to determine whether IL-1β activates NF-κB in OCIM2 cells. We did so by incubating the cells for 1 hour in the presence of increasing concentrations of IL-1β and then testing them for NF-κB activity by EMSA. As shown in Fig 5A, NF-κB activation increased with IL-1β dose, reaching a maximum at 10 ng/mL IL-1β. Next, we examined the effect of increasing concentrations of PAO on IL-1β–induced NF-κB activation. For this, OCIM2 cells were treated for 1 hour with 0.1, 0.3, and 1.0 μmol/L PAO and then for 1 more hour with the addition of 10 ng/mL of IL-1β to activate NF-κB. As shown in Fig 5B, PAO abolished the IL-1β–induced NF-κB activation in a dose-dependent manner, with maximum inhibition occurring at a PAO concentration of 1.0 μmol/L (the minor activation of NF-κB induced by 1.0 μmol/L of PAO was not significant by a quantitative analysis, as found in our previous study33).
(A) Activation of NF-κB by IL-1β. OCIM2 cells (2 × 106/mL) were incubated at 37°C with increasing concentrations of IL-1β for 1 hour. Nuclear extracts were then prepared and assayed for NF-κB as described above. (B) Inhibition of IL-1β–induced NF-κB activation by PAO. Cells were treated with PAO for 1 hour and then with IL-1β for 1 hour. Nuclear extracts were prepared and assayed for NF-κB activity as described in Materials and Methods.
(A) Activation of NF-κB by IL-1β. OCIM2 cells (2 × 106/mL) were incubated at 37°C with increasing concentrations of IL-1β for 1 hour. Nuclear extracts were then prepared and assayed for NF-κB as described above. (B) Inhibition of IL-1β–induced NF-κB activation by PAO. Cells were treated with PAO for 1 hour and then with IL-1β for 1 hour. Nuclear extracts were prepared and assayed for NF-κB activity as described in Materials and Methods.
The specificity of the NF-κB band in the EMSAs was demonstrated by its ability to compete with a cold oligo but not with an oligo containing a mutated NF-κB site. Thus, these results show that PAO blocked IL-1β–induced NF-κB activation.
PAO induces apoptosis in OCIM2 cells.
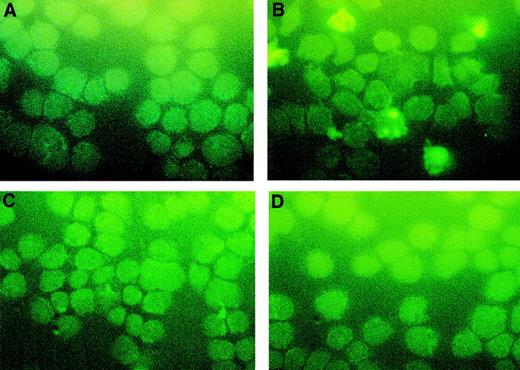
Because a lack of NF-κB activation may abolish cellular proliferation and lead to apoptotic cell death34,35 and the arsenic compound AS2O3 can induce apoptosis in APL cells,4 we hypothesized that PAO might have a similar effect on OCIM2 cells. To test this idea, OCIM2 cells at the peak of their growth were washed and then incubated in PBS in the presence and absence of 0.1 μmol/L PAO for 4, 6, and 8 hours. Using the TUNEL assay, we found that PAO induced apoptosis in these AML cells and that longer exposure to this compound increased the number of cells undergoing apoptotic cell death (Fig 6).
Induction of apoptosis by PAO. OCIM2 cells were incubated in the absence (A) and presence of PAO for 4 (B), 6 (C), and 8 (D) hours. Apoptotic cells appear yellow.
Induction of apoptosis by PAO. OCIM2 cells were incubated in the absence (A) and presence of PAO for 4 (B), 6 (C), and 8 (D) hours. Apoptotic cells appear yellow.
PAO induces apoptosis by cleaving caspase 3.
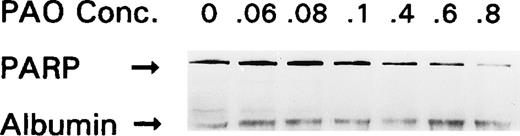
To determine the mechanism by which PAO induces apoptosis, OCIM2 cells were incubated in the absence and presence of 0.06, 0.08, 0.1, 0.4, 0.6, and 0.8 μmol/L of PAO for 4 hours and then harvested for Western immunoblot analysis as described above. As shown in Fig 7, PAO downregulated the expression of uncleaved PARP protein in a dose-dependent fashion. Because caspase activation seems to be an essential step in PARP cleavage and cellular apoptosis36-38 and because caspase 339-41appears to be involved in apoptosis induced in leukemia cells,42-44 we measured the levels of uncleaved and cleaved caspase 3 in OCIM2 AML cells. As shown in Fig 8, we found that the incubation of OCIM2 cells with 0.08 and 0.1 μmol/L of PAO upregulated the levels of the biologically active (cleaved) caspase 3 and the inactivated (cleaved) form of the DNA-repair enzyme PARP,36-38 thereby activating the apoptotic cascade. To further investigate whether caspase activation is essential for PAO-induced apoptosis, we incubated OCIM2 cells with 0.1 μmol/L of PAO with and without 50 μmol/L of the caspase inhibitor Z-DEVD-FMK.45 46 Using the TUNEL assay, we found that Z-DEVD-FMK completely blocked PAO-induced apoptosis (Fig 9).
Effect of PAO on PARP protein expression. OCIM2 cells were incubated in the absence and the presence of increasing concentrations of PAO. The results shown here were obtained after incubating OCIM2 cells for 4 hours.
Effect of PAO on PARP protein expression. OCIM2 cells were incubated in the absence and the presence of increasing concentrations of PAO. The results shown here were obtained after incubating OCIM2 cells for 4 hours.
Effect of PAO on caspase 3 and PARP cleavage. OCIM2 cells were incubated without PAO (lane C) and with it at a concentration of 0.08 μmol/L (lane 2) or 0.1 μmol/L (lane 1) for 8 hours. Cleavage of caspase 3 was already detected after 4 hours. Increments in the levels of cleaved caspase 3 (A) and cleaved PARP (B) are depicted.
Effect of PAO on caspase 3 and PARP cleavage. OCIM2 cells were incubated without PAO (lane C) and with it at a concentration of 0.08 μmol/L (lane 2) or 0.1 μmol/L (lane 1) for 8 hours. Cleavage of caspase 3 was already detected after 4 hours. Increments in the levels of cleaved caspase 3 (A) and cleaved PARP (B) are depicted.
Effect of Z-DEVD-FMK on PAO-induced apoptosis. OCIM2 cells were incubated for 6 hours without any drug (A), with 0.1 μmol/L of PAO (B), with 50 μmol/L of Z-DEVD-FMK (C), and with both PAO and Z-DEVD-FMK (D). Apoptotic cells appear yellow.
Effect of Z-DEVD-FMK on PAO-induced apoptosis. OCIM2 cells were incubated for 6 hours without any drug (A), with 0.1 μmol/L of PAO (B), with 50 μmol/L of Z-DEVD-FMK (C), and with both PAO and Z-DEVD-FMK (D). Apoptotic cells appear yellow.
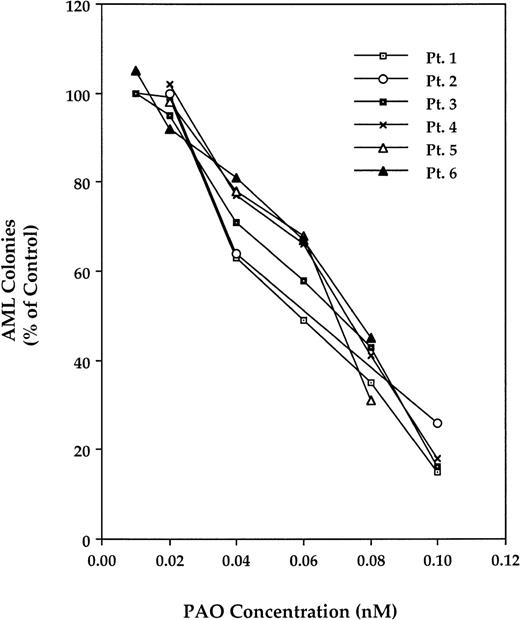
PAO inhibits fresh AML blast colony-forming cell proliferation.
We then studied the effect of PAO on the proliferation of fresh AML marrow blast colony-forming cells. For this, we used diagnostic BM cells from 7 AML patients whose clinical characteristics are depicted in Table 1. As shown in Fig 10, PAO inhibited AML blast colony-forming cell growth in a dose-dependent manner in all of the samples studied. Similar to its effect on AML cell lines, IL-1β, when added at the initiation of culture, partially reversed the inhibitory effect of PAO (Fig 11).
Effect of PAO on fresh AML blast colony-forming cells. After adherent cell fractionation of adherent cells and depletion of T lymphocytes, remaining cells were cultured in a clonogenic assay with PAO at concentrations ranging from 0.01 to 0.1 μmol/L. AML colonies are presented as the percentage of control (the mean number of colonies obtained in the absence of PAO). Data from patients no. 1 through 6 are depicted.
Effect of PAO on fresh AML blast colony-forming cells. After adherent cell fractionation of adherent cells and depletion of T lymphocytes, remaining cells were cultured in a clonogenic assay with PAO at concentrations ranging from 0.01 to 0.1 μmol/L. AML colonies are presented as the percentage of control (the mean number of colonies obtained in the absence of PAO). Data from patients no. 1 through 6 are depicted.
Effect of PAO and IL-1β on proliferation of AML colony-forming cells. Data from triplicate cultures of marrow samples from patient no. 6 (Expt I) and patient no. 7 (Expt II) are depicted. PAO (0.1 μmol/L) and IL-1β (10 ng) were added at the initiation of culture.
Effect of PAO and IL-1β on proliferation of AML colony-forming cells. Data from triplicate cultures of marrow samples from patient no. 6 (Expt I) and patient no. 7 (Expt II) are depicted. PAO (0.1 μmol/L) and IL-1β (10 ng) were added at the initiation of culture.
DISCUSSION
Throughout history, arsenic compounds have been useful therapeutic agents against many human ailments.47 The antileukemic properties of arsenic have been known since the mid 1800s.47 Arsenicals together with irradiation were the treatment of choice for chronic myelogenous leukemia until busulfan was introduced in 1953.48 However, in the late 20th century, research has concentrated on the toxic effects of arsenic compounds. Several investigators have shown a relationship between ingestion of or exposure to various arsenicals and the occurrence of lung,49 skin,50bladder,51 and hepatocellular52 cancer, and numerous studies have demonstrated that arsenic compounds are environmental carcinogens.53 Yet, recent reports from China show that As2O3 and arsenic disulfide can induce complete remission of APL2,3,54,55 via pathways different from those used by retinoids.4,56 These studies have prompted other investigators to explore the effects of various arsenicals on APL and other leukemias.57-59
The arsenical PAO is a membrane-permeable PTPase inhibitor that is active in hematopoietic cells.5,60 At high concentrations it causes nonspecific leakage in mitochondria. PAO also has been shown to inhibit early elevations in cytosolic calcium concentrations61 and to interfere with the insulin transduction pathway.62 Because PAO is also known to inhibit the activation of NF-κB6 in hematopoietic cells, we therefore sought to investigate its effects on AML.
NF-κB is a ubiquitous transcription factor and a major regulator of the immune system through its induction of expression of various inflammatory cytokines including IL-1β.32,33 NF-κB exists in the cytoplasm as a heterotrimeric complex with the inhibitor IκBα (reviewed in Seibenlist et al63). Within minutes of activation by inflammatory agents such as IL-1β, IκBα undergoes phosphorylation, ubiquitination, and proteolytic degradation, thus releasing the NF-κB p50-p65 complex for translocation from the cytoplasm to the nucleus. Whereas the activation of NF-κB induces cellular proliferation8 and protects cells from apoptotic cell death,35,64 its inhibition enhances spontaneous apoptosis34 or apoptosis induced by various stimuli such as irradiation or cytotoxic drugs.35
Several growth factors regulate hematopoietic cell survival by interfering with apoptotic signals.65-67 One of these is the cytokine IL-1β, a proinflammatory protein that has been implicated in early events in hematopoiesis. It induces the production of various cytokines and synergizes with several growth factors in stimulating hematopoietic progenitor multiplication.31,68In addition, as we and others have found, IL-1β plays an important role in AML cell proliferation (reviewed in Estrov et al68). Suppression of IL-1 production or inhibition of its interaction with the corresponding cellular receptors significantly inhibits AML progenitor cell growth.9-12,30,69 Furthermore, the activation of NF-κB appears to be an important step in the molecular events leading to IL-1β production8,31 32 and, as a result, also appears to stimulate leukemia cell proliferation.
In this light, we assumed that an effective NF-κB inhibitor such as PAO might either suppress the production of IL-1β, inhibit the direct NF-κB–mediated leukemia cell proliferation,7 or both. In a previous study70 we have already demonstrated that PAO can block the NF-κB–dependent expression of various adhesion molecules, thus suggesting that PAO inhibits the activity of NF-κB. Now, in our current study, we have found that PAO inhibits the proliferation of HL60 and K562 and, more significantly, of the IL-1–responsive OCI/AML3 and OCIM2 cells, that PAO suppressed the growth of the IL-1–responsive lines in a dose-dependent manner, and that IL-1β partially reverses this inhibitory effect. Together, these results suggest that at least part of the PAO-induced suppression observed in the present study was mediated through PAO’s inhibition of IL-1β production. Indeed, incubation of the OCI/AML3 and OCIM2 cell lines in the presence of PAO almost completely abolished the production of IL-1β but not IL-1RA protein. In addition, PAO significantly inhibited the IL-1β–induced NF-κB activity. Whereas IL-1β activated NF-κB in these cells, PAO suppressed it in a dose-dependent fashion. Thus, PAO inhibited both IL-1β production and the IL-1β–mediated activation of NF-κB, resulting in an additional reduction in the production of IL-1β.
Because NF-κB activation suppresses the signals for cell death and inhibition of NF-κB may result in apoptotic cell death,34,35 we tested the effect of PAO on the induction of apoptosis. We found that treatment of leukemia cells with PAO induced apoptotic cell death. Our results agree with those of Jimi et al,71 who recently found that inhibition of NF-κB by oligodeoxynucleotides to p65 and p50 abolished the IL-1–induced survival of osteoclasts.
Because most cell types require activation of a specific proteolytic cascade if apoptosis is to occur, we also wondered whether PAO might help activate that cascade in AML cells. In particular, we chose to study the effect of PAO on caspase 3. Caspase 3 is a key executioner of apoptosis39-41 whose activation downstream in the apoptotic cascade is essential for leukemia cell apoptosis.33,42,43Moreover, the activation of caspase 3 results in the cleavage of cellular substrates critical for cell survival, such as PARP and lamins, which, in turn, precipitates the morphological changes characteristic of apoptosis (reviewed in Cohen72). This approach of ours found support in the work of Zhang et al,73 who had already shown that arsenic trioxide downregulates the expression of bcl-2, an antiapoptotic protein known to inhibit the activation of caspase 3. As hoped, we found that PAO activated caspase 3 and consequently cleaved PARP. Interestingly, Barkett et al74 have recently reported that caspase 3 cleaves human Iκ-B-α in vitro at a conserved Asp-Ser sequence, thus creating a dominant inhibitor that prevents the activation of NF-κB and thereby adding another death signal.
Similar to its effect on AML cell lines and comparable to the effect of other IL-1 inhibitors,10-12 30 PAO suppressed AML progenitor proliferation and had its inhibitory effect partially reversed by IL-1β. These results indicate that the inhibition of IL-1β production is part of PAO’s inhibitory mechanism in AML cells.
Taken together, our data suggest that PAO, either through inhibition of NF-κB, suppression of IL-1β production, or both, may eliminate leukemia cells and so prove to be an effective agent in the treatment of AML.
ACKNOWLEDGMENT
The authors thank Jude Richard for editing the manuscript.
Supported in part by National Cancer Institute Grant No. PO1CA 55164 and by the Clayton Foundation for Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Zeev Estrov, MD, Department of Bioimmunotherapy, Box 302, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal