Monocytoid B cells represent a morphologically conspicuous B-cell population that constantly occurs in Toxoplasma gondii-induced Piringer’s lymphadenopathy. Although widely believed to be closely related to splenic marginal zone B cells, neither this relationship, nor the B-cell differentiation stage of monocytoid B cells, nor their cellular precursors have been established. We have therefore examined monocytoid B cells for their expression of B-cell differentiation markers and the Ig isotypes at the RNA and protein level as well as for rearranged Ig heavy chain (H) genes and somatic mutations within the variable (V) region. The results obtained were compared with the corresponding features of other B-cell populations. The monocytoid B cells displayed immunophenotypical differences to all other B-cell populations. IgM and IgD expression was absent from most monocytoid B cells at the RNA and protein levels. Unrelated (polyclonal) Ig rearrangements were found in 85 of the 95 cells studied. Seventy-four percent of the rearranged VH genes were devoid of somatic mutations, whereas the remaining 26% carried a low number of somatic mutations. The majority of these showed no significant signs of antigen selection. This finding in conjunction with the predominantly unrelated Ig gene rearrangements indicates that most monocytoid B cells arise not by clonal proliferation but by transformation of polyclonal B cells. The B cells undergoing a monocytoid B-cell transformation are in the majority (74%) naive B cells, and only a minority are (26%) non–antigen-selected postgerminal center B cells. Thus, our data show that monocytoid B cells represent a distinct B-cell subpopulation.
MONOCYTOID B CELLS can be encountered in various reactive lymphadenopathies and some lymphomas, including Hodgkin’s disease,1-12 but occur most consistently in the form of cohesive sheets in Piringer’s lymphadenopathy usually caused by Toxoplasma gondii.13-17
Monocytoid B cells are characterized by an abundant pale cytoplasm, indented nuclei with inconspicuous nucleoli, and open chromatin.13,14 They are located in the marginal zones adjacent to the subcapsular and intermediary sinuses.14Because these features were regarded as characteristic of immature sinus histiocytes or blood monocytes, these cells were initially termed immature sinus histiocytes14 or monocytoid cells.15 When, thanks to the hybridoma technology, macrophage-specific and B-cell–specific marker molecules became available and background-free immunohistochemical staining methods were developed, it was found that monocytoid cells did not carry markers of the monocyte-macrophage system, but express B-cell antigens, suggesting their relationship to the B-cell lineage.18-21 Hence, 2 new designations were proposed: sinus B cells19 and monocytoid B cells.20 The latter term has prevailed.
The assignment of the monocytoid B cells to the B-cell system raised the question as to which B-cell subpopulation they are most closely related to. Although in some studies18,19 the monocytoid B cells could not be allocated to any of the established B-cell populations, the study by Burke and Sheibani22 suggested a close relationship to hairy cell leukemia cells and the study by Van den Oord et al23 and others21 suggested a close relationship to splenic marginal zone B cells. Whereas a relationship to hairy cell leukemia cells was not supported by other studies, the view that monocytoid B cells might represent the nodal counterpart of splenic marginal zone cells became widely accepted.23 This view had significant impact on the classification of primary nodal lymphomas growing in the marginal zone, in that all of them were lumped together under the term nodal marginal zone lymphoma, irrespective of whether they resembled immunophenotypically monocytoid B cells or splenic and Peyer’s patch marginal zone cells.24-29 The conclusion that monocytoid B cells and marginal zone cells represent the same B-cell subset was mainly reasoned by the claim that both cell populations resemble each other by a similar expression of IgM in the absence of IgD, a similar homing to marginal zones, and a similar VH gene mutation pattern. However, the close relationship was challenged by other studies19,21,30,31 demonstrating that most monocytoid B cells are, in contrast to splenic marginal zone cells, vastly or completely devoid of IgM and BCL-2. Further differences included the absence of intermingled T cells and the admixture of neutrophils in monocytoid B-cell areas,13,14 19 whereas the opposite finding was observed in marginal B-cell zones.
To clarify the B-cell differentiation stage of monocytoid B cells in relation to other B-cell subsets, to identify the B-cell population from which monocytoid B cells originate, and to determine whether monocytoid B cells arise polyclonally from many cells, oligoclonally from a few cells, or monoclonally from a single cell, we investigated the immunophenotype, the Ig isotype expression, and the rearrangement and VH gene mutation pattern of monocytoid B cells at the single-cell level. Our results show that monocytoid B cells are distinct from marginal zone cells of the spleen and mesenteric lymph nodes. They are heterogeneous in their differentiation stage in that the majority originate from unmutated naive B cells and a minority originate from mutated postgerminal center memory type B cells. Finally, monocytoid B cells are generated by the transformation of many cells, rather than by expansion of a few cells or a single cell.
MATERIALS AND METHODS
Tissue samples.
Eleven cases of Piringer’s lymphadenopathy from which paraffin blocks and snap-frozen material were available were taken from the files of the Institute of Pathology at the University Hospital Benjamin Franklin of the Free University of Berlin. All 11 cases were used for immunophenotypical analysis, 6 of them for in situ hybridization and 5 cases with the most prominent areas of monocytoid B cells were selected for the isolation of single cells. For the comparative analysis of mantle cells, germinal center cells, and marginal zone cells, 2 normal spleens and 2 to 4 mesenterial lymph nodes from 2 patients were included in our study.
Tissue processing and immunohistochemistry.
Immunohistochemical analysis of the monocytoid B cells was performed on formalin-fixed paraffin-embedded tissues. Paraffin sections were pretreated by heating in 0.01 mol/L citrate buffer, pH 6.0, for 2 minutes in a pressure cooker followed by cooling to room temperature and washing with Tris-buffered saline. Frozen sections, immunostained for single-cell isolation, were air-dried overnight and fixed in acetone for 10 minutes. For identification of the monocytoid B cells as well as B-cell subsets and T cells, antibodies directed against the following antigens were applied: CD20 (L26), DBA44, CD25 (Ber-Act 1), CD103 (Ber-Act 8), CD11c (KB90), BCL-2 (124), BCL-6 (594), IgM, IgD, IgA, IgE, CD3 (DAKO, Glostrup, Denmark), CD23 (1B12), CD10 (56C6), CD5 (4C7), CD4 (1F6; Novocastra, Newcastle-on-Tyne, UK), CD27 (1A4CD27; Beckman Coulter Inc, Fullerton, CA), Ki-67 (Mib-1; kindly provided by Prof J. Gerdes, Borstel Institute, Borstel, Germany), and CD45RA (Ki-B332; kindly donated by Prof M.R. Parwaresch, Kiel, Germany). The binding of antibodies was made visible either by the immuno-alkaline phosphatase-antiphosphatase (APAAP) technique or by the biotin-avidin-method, as previously described.33 34
In situ hybridization.
In situ hybridization for the detection of mRNA specific for IgM, IgD, and IgG was performed as previously described.35 In brief, after linearization of plasmids containing the IgD, M, and G heavy chain constant regions with appropriate restriction enzymes,35S-labeled run-off antisense and sense (control) transcripts were generated using T7 or SP6 RNA polymerases. Paraffin sections were pretreated by exposing them to 0.2 N HCl and 0.125 mg/mL pronase (Boehringer Mannheim, Mannheim, Germany), followed by acetylation with 0.1 triethanolamine, pH 8.0/0.25% (vol/vol) acetic anhydride and dehydration through graded ethanols. Slides were hybridized to 2 to 4 × 105 cpm of labeled probes overnight at 50°C. Washing and autoradiography was performed as described.36 Cells with a 4-fold signal intensity, as compared with background signal, were scored as positive. Hybridization with sense probes only showed homogeneously distributed low background signal. The probes for the Ig heavy chain constant regions were prepared from cDNA of peripheral blood lymphocytes or B-cell lines and cloned into the appropriate plasmids. The nucleotide sequence of the probes was confirmed by DNA sequencing.
Isolation of single cells.
Single cells, identified by their localization and their immunophenotype (ie, monocytoid B cells by their negativity for BCL-2 and CD21; mantle cells by their expression of IgD; germinal center cells by their positivity for BCL-6/CD10 and/or Ki-67; and splenic marginal zone cells by their IgM+/IgD−isotype profile), were isolated from frozen sections, as previously described.37 In brief, immunostained frozen tissue sections were covered with a buffer containing salmon-sperm DNA (1 mg/mL in phosphate-buffered saline [PBS]). Single cells were isolated from the surrounding tissue and transferred into a polymerase chain reaction (PCR) test tube by means of a manipulation capillary and reception capillary, which were fixed to a hydraulic micromanipulator. The whole isolation process was monitored by microscope. Isolated T cells and aliquots of the overlaying buffer drawn after each cell isolation process served as the negative controls.
Single-cell PCR.
PCR for the detection of IgH genes was performed as previously described.38-40 In brief, after proteinase K digestion of the isolated cells for 1 hour (1.0 mg/mL), a fully nested PCR using family-specific frame-work (FW) 1 primers for the first amplification and family-specific FW2 primers for reamplifications was used in conjunction with 2 nested primers for the joining region (JH). The first round of amplification was performed with 40 cycles consisting of 5 cycles of 15 seconds at 96°C, 30 seconds at 63°C, and 30 seconds at 72°C, followed by 35 cycles of 15 seconds at 96°C, 30 seconds at 57°C, and 30 seconds at 72°C in 2.5 mmol/L MgCl2, 200 μmol/L each dNTP, 300 ng of the FW primers (50 ng each), 100 ng of the JH primers, and 2 U Taq DNA polymerase (AmpliTaq; Perkin Elmer, Weiterstadt, Germany). The second round of amplification consisted of 35 cycles of 15 seconds at 96°C, 30 seconds at 63°C, and 30 seconds at 72°C. Six microliters of the reaction mixture was analyzed on an ethidiumbromide-stained polyacrylamide gel (PAGE, 6%). The visualized bands of the PCR products were isolated and directly sequenced in both directions by separate application of the PCR primers. The sequencing was performed with an automated fluorescence DNA sequencer (377A; Applied Biosystems, Weiterstadt, Germany) by using the Dye Deoxy Terminator method.
Sequence analysis.
The amplified VH rearrangements were analyzed for VH usage and for somatic mutations by comparison with germline VH-segments in the VBASE databank, and coding capacity was determined by translation of the sequences into amino acids. To exclude contaminations, all amplified sequences were also compared with our own and published data bank sequences.40 41
Antigen selection.
To determine the pattern of somatic mutations compatible with antigen selection, 2 methods were applied. First, the ratio of replacement to silent mutations (R/s) in the CDR2 and FW3 region was studied.42 A sequence was considered to be antigen selected when the R/s ratio in the CDR2 was higher than 2.9 and R/s ratio in the FW3 region was lower than 1.5. Second, only the R/s ratio of the somatic mutations in the FW3 region was considered. A sequence was regarded as being antigen selected when the R/s ratio was less than 1.6.43
RESULTS
Immunophenotype and admixed cells.
To update the phenotype of the monocytoid B cells and to determine their relationship to other B-cell populations, an immunophenotypical analysis was performed on 11 cases of Piringer’s lymphadenopathy, including new marker molecules (Tables 1 and 2 and Figs 1 and2). This investigation confirmed that monocytoid B cells differ from mantle B cells by a complete absence of BCL-2 (Fig 2b) and CD23 and a vast absence of sIgM and sIgD (Fig 1d and e). They can be distinguished from germinal center B cells by the absence of CD10, BCL-6, cytoplasmic Ig, and partial presence of DBA44, a hairy cell leukemia-associated molecule. From splenic and nodal marginal zone B cells, monocytoid B cells can be discriminated by a partial presence of DBA44, a complete presence of the Ki-B3 epitope of the CD45RA molecule, and additionally by a complete absence of BCL-2 and a nearly complete absence of sIgM. Different from all other B-cell subpopulations, the monocytoid B-cell zone was devoid of T cells and follicular dendritic cells (FDC) (Fig 2a and d), but contained a significant admixture of neutrophils. The proliferation rate of monocytoid B cells, varying from 10% to 25%, was significantly higher than that of mantle B cells (1% to 5%) as well as of splenic and nodal marginal zone B cells (5% to 10%), but considerably lower than that of germinal center B cells (50% to 100%), as demonstrated by the Ki-67 index (Fig 2c).
Immunophenotype and Cellular Admixtures of Monocytoid B Cells From 11 Cases of Piringer’s Lymphadenopathy in Comparison With Other B-Cell Populations
| Expression of . | Monocytoid B Cells* . | Mantle Cells* . | GC-B Cells* . | Memory-PB B Cells† . | Splenic MZB Cells‡ . | Nodal MZB Cells1-153 . |
|---|---|---|---|---|---|---|
| CD 20 | + | + | ++ | + | + | + |
| CD45RA | + | + | +/− | NA | − | − |
| DBA44 | 10-20% | 80% | − | NA | − | − |
| CD23 | − | +− | −/+ | − | − | − |
| CD10 | − | − | + | − | − | − |
| BCL-6 | − | − | + | − | − | − |
| BCL-2 | − | + | − | + | + | + |
| CD5 | − | −/+ | − | − | − | − |
| CD27 | − | − | − | + | NA | NA |
| CD25 | − | − | − | − | − | − |
| CD103 | − | − | − | − | − | − |
| CD11c | − | − | − | − | − | − |
| Ki-67 | 10-35% | 1-5% | 50-100% | NA | 5-10% | 5-10% |
| FDC | Absent | Present | Present | Absent | Absent | Absent |
| T Cells | Absent or very few | Some | Many | Some | Some | |
| Neutrophils | Many | Absent | Absent | Absent or very few | Absent |
| Expression of . | Monocytoid B Cells* . | Mantle Cells* . | GC-B Cells* . | Memory-PB B Cells† . | Splenic MZB Cells‡ . | Nodal MZB Cells1-153 . |
|---|---|---|---|---|---|---|
| CD 20 | + | + | ++ | + | + | + |
| CD45RA | + | + | +/− | NA | − | − |
| DBA44 | 10-20% | 80% | − | NA | − | − |
| CD23 | − | +− | −/+ | − | − | − |
| CD10 | − | − | + | − | − | − |
| BCL-6 | − | − | + | − | − | − |
| BCL-2 | − | + | − | + | + | + |
| CD5 | − | −/+ | − | − | − | − |
| CD27 | − | − | − | + | NA | NA |
| CD25 | − | − | − | − | − | − |
| CD103 | − | − | − | − | − | − |
| CD11c | − | − | − | − | − | − |
| Ki-67 | 10-35% | 1-5% | 50-100% | NA | 5-10% | 5-10% |
| FDC | Absent | Present | Present | Absent | Absent | Absent |
| T Cells | Absent or very few | Some | Many | Some | Some | |
| Neutrophils | Many | Absent | Absent | Absent or very few | Absent |
Abbreviations: GC, germinal center; PB, peripheral blood; MZB, marginal zone B; FDC, follicular dendritic cells; ++, strongly positive; +, positive; +/−, majority positive; −/+, minority positive; −, negative; NA, not analyzed.
Analyzed in 11 cases of Piringer’s lymphadenopathy.
Data from Klein et al43 and own unpublished findings.
Analyzed in 2 normal spleens with prominent marginal zones.
Analyzed in 2 to 5 mesenterial lymph nodes (with prominent marginal zones) from 2 patients.
Ig Isotypes at the Protein and RNA Level of Monocytoid B Cells From 11 Cases of Piringer’s Lymphadenopathy and Other B-Cell Populations
| Markers . | Monocytoid B Cells* . | Mantle Cell* . | GC-B Cells* . | Memory PB-B Cells IgM Only† . | Memory PB-B Cells IgG+† . | Splenic MZB Cells‡ . | Plasma Cells* . | Nodal MZB Cells2-153 . |
|---|---|---|---|---|---|---|---|---|
| Protein | ||||||||
| sIgM | −/+ | + | − | + | − | + | − | + |
| sIgD | −/+ | + | − | − | − | −/+ | − | − |
| sIgG | − | − | − | − | + | − | − | − |
| Cytoplasmic Ig IgG > IgA > IgD2-154 | − | − | + (many) | − | − | − | + | + (some) |
| RNA | ||||||||
| Low Igμ | −/+ | + | −/+ | + | − | NA | − | NA |
| Low Igδ | −/+ | + | − | NA | NA | NA | − | NA |
| Low Igγ | +/− | − | +/− | − | + | NA | − | NA |
| High Ig2-155 | − | − | + (many) | NA | NA | NA | + | NA |
| Markers . | Monocytoid B Cells* . | Mantle Cell* . | GC-B Cells* . | Memory PB-B Cells IgM Only† . | Memory PB-B Cells IgG+† . | Splenic MZB Cells‡ . | Plasma Cells* . | Nodal MZB Cells2-153 . |
|---|---|---|---|---|---|---|---|---|
| Protein | ||||||||
| sIgM | −/+ | + | − | + | − | + | − | + |
| sIgD | −/+ | + | − | − | − | −/+ | − | − |
| sIgG | − | − | − | − | + | − | − | − |
| Cytoplasmic Ig IgG > IgA > IgD2-154 | − | − | + (many) | − | − | − | + | + (some) |
| RNA | ||||||||
| Low Igμ | −/+ | + | −/+ | + | − | NA | − | NA |
| Low Igδ | −/+ | + | − | NA | NA | NA | − | NA |
| Low Igγ | +/− | − | +/− | − | + | NA | − | NA |
| High Ig2-155 | − | − | + (many) | NA | NA | NA | + | NA |
Same legend as in Table 1.
Analyzed in 5 cases of Piringer’s lymphadenopathy.
Analyzed in 2 normal spleens with prominent marginal zones.
Analyzed in 2 to 5 mesenterial lymph nodes (with prominent marginal zones) from 2 patients.
Cells with cytoplasmic Ig correspond to plasma cells and their precursors.
Cells with high Ig mRNA correspond to plasma cells and their precursors.
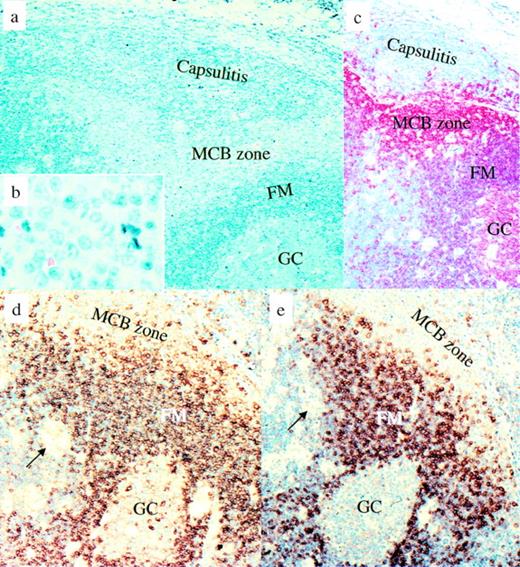
Piringer’s lymphadenopathy. Morphological and immunophenotypical features of monocytoid B cells in comparison with other B-cell populations. (a and b) Giemsa staining. (c) CD20 immunostaining: mantle cells and germinal center B cells are less intensively stained than the monocytoid B cells and display a slightly different red tone. (d) IgM immunostaining: monocytoid B cells are largely negative for IgM, which is different from the strongly IgM-positive follicle mantle cells. (e) IgD immunostaining. Only some monocytoid B cells are positive for IgD. Arrows show small clusters of epithelioid cells characteristic of Piringer’s lymphadenopathy. MCB, monocytoid B cells; FM, follicle mantle; GC, germinal center.
Piringer’s lymphadenopathy. Morphological and immunophenotypical features of monocytoid B cells in comparison with other B-cell populations. (a and b) Giemsa staining. (c) CD20 immunostaining: mantle cells and germinal center B cells are less intensively stained than the monocytoid B cells and display a slightly different red tone. (d) IgM immunostaining: monocytoid B cells are largely negative for IgM, which is different from the strongly IgM-positive follicle mantle cells. (e) IgD immunostaining. Only some monocytoid B cells are positive for IgD. Arrows show small clusters of epithelioid cells characteristic of Piringer’s lymphadenopathy. MCB, monocytoid B cells; FM, follicle mantle; GC, germinal center.
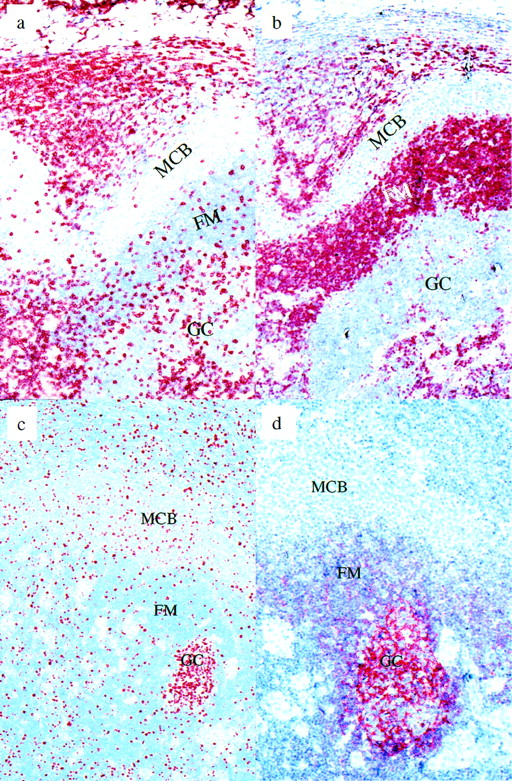
Piringer’s lymphadenopathy. (a) CD3 immunostaining: the monocytoid B-cell zone is nearly devoid of T cells, in contrast to the follicle mantle and germinal center. (b) BCL-2 immunostaining: monocytoid B cells are negative for BCL-2. (c) Ki-67 immunostaining: approximately 10% to 20% of the monocytoid B cells are in cell cycle. (d) CD21 immunostaining. Note the labeling of follicular dendritic cells within the germinal center and their absence from the monocytoid B-cell zone. MCB, monocytoid B cells; FM, follicle mantle; GC, germinal center.
Piringer’s lymphadenopathy. (a) CD3 immunostaining: the monocytoid B-cell zone is nearly devoid of T cells, in contrast to the follicle mantle and germinal center. (b) BCL-2 immunostaining: monocytoid B cells are negative for BCL-2. (c) Ki-67 immunostaining: approximately 10% to 20% of the monocytoid B cells are in cell cycle. (d) CD21 immunostaining. Note the labeling of follicular dendritic cells within the germinal center and their absence from the monocytoid B-cell zone. MCB, monocytoid B cells; FM, follicle mantle; GC, germinal center.
Ig isotype expression.
To check the validity of the Ig labeling results and whether the absence of Ig protein expression on most monocytoid B cells is due to a downregulation at the transcriptional or translational level, the expression of Ig mRNA (Igμ, δ, and γ) was studied and compared with the presence of the corresponding Ig protein (Table 2). Igμ and Igδ mRNA were detectable by in situ hybridization in approximately 20% of the monocytoid B cells, a finding in harmony with that obtained by immunohistochemistry for protein detection (Fig3a). An unexpected result was the finding of an expression of Igγ transcripts on the majority of monocytoid B cells in the absence of IgG protein molecules. The level of this RNA expression was low and corresponded with that of other B cells carrying surface membrane bound Ig (Table 2 and Fig 3b and c).
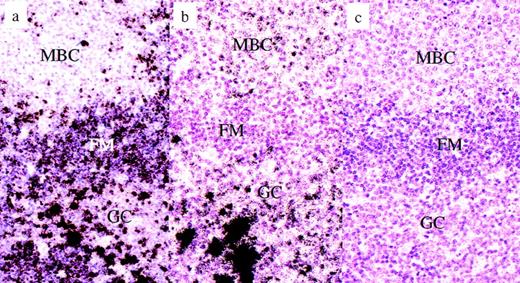
Piringer’s lymphadeno- pathy. Radioactive in situ hybridization for the detection of Ig heavy chain gene transcripts. (a) Igμ in situ hybridization, antisense: the vast majority of the monocytoid B cells are negative, whereas resting mantle B cells are clearly labeled. Some plasma B cells in the germinal center display extremely intensive labels. (b) Igγ in situ hybridization, antisense: most monocytoid B cells are weakly labeled, whereas mantle B cells are completely negative. Some germinal center B cells display a moderate labeling, whereas others are very intensely labeled (plasma cells). (c) Igγ in-situ hybridization, sense: negative control. No cells are labeled. MCB, monocytoid B cells; FM, follicle mantle; GC, germinal center.
Piringer’s lymphadeno- pathy. Radioactive in situ hybridization for the detection of Ig heavy chain gene transcripts. (a) Igμ in situ hybridization, antisense: the vast majority of the monocytoid B cells are negative, whereas resting mantle B cells are clearly labeled. Some plasma B cells in the germinal center display extremely intensive labels. (b) Igγ in situ hybridization, antisense: most monocytoid B cells are weakly labeled, whereas mantle B cells are completely negative. Some germinal center B cells display a moderate labeling, whereas others are very intensely labeled (plasma cells). (c) Igγ in-situ hybridization, sense: negative control. No cells are labeled. MCB, monocytoid B cells; FM, follicle mantle; GC, germinal center.
Ig gene rearrangements and VH mutation pattern.
Three hundred twenty-seven monocytoid B cells were isolated from the lymph node sections of 5 cases of Piringer’s lymphadenopathy. For comparison, single cells were isolated from mantle zones and germinal centers of the same and other cases, as well as from the marginal zone of 2 spleens, and were subjected to a single-copy PCR and sequence analysis for the demonstration of Ig heavy chain gene rearrangements and their mutation pattern. Ninety-five sequences (29%) were obtained from the 327 isolated monocytoid B cells, and 85 of these sequences (89%) were unique. In addition, 5 unrelated sequences occurred twice in 3 of the 5 cases of Piringer’s lymphadenopathy. The sequence comparison of the VH rearrangements with databank germline VH segments showed that 67 (74.4%) of the monocytoid B cells displayed unmutated VH genes ranging from 50% to 86.6% among the 5 cases investigated (Table 3). Twenty-three sequences (25.6%) harbored somatic VH mutations, with an average mutation frequency of 3.8% and ranging from 1 to 14 base substitutions (Table 3).
VH-Gene Rearrangements and Mutations in Monocytoid B Cells From 5 Cases of Piringer’s Lymphadenopathy
| Case No. . | Total No. of Isolated Cells . | IgR Unique/Identical . | IgR Without VH Mutations (%) . | IgR With VH Mutations (%) . | No. of VH Mutations/ Mutated Sequences . | ||
|---|---|---|---|---|---|---|---|
| Mean . | Percentage . | (range) . | |||||
| 1 | 105 | 24/1 | 16/24 (66%) | 8/24 (33%) | 7 | 4.6 | (1-14) |
| 2 | 53 | 16/2 | 13/16 (81.2%) | 3/16 (18.7%) | 2 | 1.3 | (1-4) |
| 3 | 90 | 21/0 | 18/21 (86%) | 3/21 (14.2%) | 6.6 | 4.3 | (1-14) |
| 4 | 31 | 14/0 | 7/14 (50%) | 7/14 (50%) | 5.5 | 3.7 | (5-7) |
| 5 | 48 | 15/2 | 13/15 (86.6%) | 2/15 (13.3%) | 5.6 | 3.8 | (4-9) |
| Total | 327 | 90/5 | 67/90 (74.4%) | 23/90 (25.6%) | 5.3 | 3.8 | (1-14) |
| Case No. . | Total No. of Isolated Cells . | IgR Unique/Identical . | IgR Without VH Mutations (%) . | IgR With VH Mutations (%) . | No. of VH Mutations/ Mutated Sequences . | ||
|---|---|---|---|---|---|---|---|
| Mean . | Percentage . | (range) . | |||||
| 1 | 105 | 24/1 | 16/24 (66%) | 8/24 (33%) | 7 | 4.6 | (1-14) |
| 2 | 53 | 16/2 | 13/16 (81.2%) | 3/16 (18.7%) | 2 | 1.3 | (1-4) |
| 3 | 90 | 21/0 | 18/21 (86%) | 3/21 (14.2%) | 6.6 | 4.3 | (1-14) |
| 4 | 31 | 14/0 | 7/14 (50%) | 7/14 (50%) | 5.5 | 3.7 | (5-7) |
| 5 | 48 | 15/2 | 13/15 (86.6%) | 2/15 (13.3%) | 5.6 | 3.8 | (4-9) |
| Total | 327 | 90/5 | 67/90 (74.4%) | 23/90 (25.6%) | 5.3 | 3.8 | (1-14) |
Abbreviations: IgR, Ig rearrangement; VH, variable region of the Ig heavy chain gene.
To check the validity of the results of our VH mutation analysis, 42 mantle zone B cells, 81 germinal center B cells, and 66 splenic marginal zone B cells were isolated and their VH rearrangements were amplified by PCR, resulting in 12, 25, and 21 amplificates, respectively. The sequence analysis of the PCR products showed that none of the VH gene rearrangements amplified from mantle zone B cells contained somatic mutations or a clonal relation. Germinal center B cells displayed mutated VH rearrangements in most instances that were partly clonally related with signs of ongoing mutations as evidenced by their intraclonal diversity. All VH gene rearrangements amplified from the isolated splenic marginal zone B cells were unique. The majority of them harbored somatic VH mutations (Table4).
Incidence and Percentage of VH Mutations in Monocytoid B Cells and Other B-Cell Populations
| Type of Cell . | Mutated Cells/ Analyzed Cells . | Percentage of VH Mutation . | References . |
|---|---|---|---|
| Monocytoid B cells | 23/90 (25.6%) | 3.8 | This study |
| Mantle B cells | 0/12 (0%) | 0 | This study |
| 0/9 (0%) | 0 | 46 | |
| Germinal center B cells | 23/25 (92%) | 4.454-150 | This study |
| 20/23 (87%) | 4.94-150 | 46 | |
| Splenic MZB cells IgM+/IgD− | 13/21 (62%) | 3.98 | This study |
| 15/16 (94%) | 4.34-151 | 48 | |
| PP MZB cells IgM+/IgD− | 18/23 (78%) | 5.9‡ | 51 |
| Type of Cell . | Mutated Cells/ Analyzed Cells . | Percentage of VH Mutation . | References . |
|---|---|---|---|
| Monocytoid B cells | 23/90 (25.6%) | 3.8 | This study |
| Mantle B cells | 0/12 (0%) | 0 | This study |
| 0/9 (0%) | 0 | 46 | |
| Germinal center B cells | 23/25 (92%) | 4.454-150 | This study |
| 20/23 (87%) | 4.94-150 | 46 | |
| Splenic MZB cells IgM+/IgD− | 13/21 (62%) | 3.98 | This study |
| 15/16 (94%) | 4.34-151 | 48 | |
| PP MZB cells IgM+/IgD− | 18/23 (78%) | 5.9‡ | 51 |
Abbreviations: MZB cells, marginal zone B cells; PP, Peyer’s patches; VH, variable region of the Ig heavy chain gene.
Displaying intraclonal diversity (ongoing mutations).
Only Ig rearrangements involving VH6 were considered for calculation by the investigators.
Only Ig rearrangements involving VH4.21 were considered for calculation by the investigators.
Our single-cell isolation technique was controlled by the analysis of 36 single T cells and 552 buffer samples that were drawn after each cell isolation. None of these controls gave rise to VH-specific PCR products, indicating that the isolated monocytoid B cells and control B cells were not contaminated by other cells or by their DNA.
Antigen-selection and VH-gene family usage.
Two methods were applied for the estimation of antigen selection in the mutated B cells. Both methods agreed in that the majority of the monocytoid B cells with VH mutations (88.8%; 16/18) lacked signs of antigen selection when the CDR2 and FW3 region were analyzed and 55.5% (10/18) lacked signs when only the FW3 proportion was drawn into consideration. Five sequences with somatic Ig mutations could not be used for the determination of antigen-selection due to their very low number of mutations. In contrast to the monocytoid B cells, most of the mutated splenic marginal zone cells showed signs of antigen selection.
Coding capacity.
Eighty-three of the 90 individual monocytoid B cells (93%) showed a functional VH rearrangement without stop codons or frame shift mutations. Four nonfunctional VH rearrangements occurred in the CDR3 proportion of the unmutated monocytoid B cells and consisted of 3 stop codons and 2 frame shifts, with 1 cell simultaneously carrying a stop codon in the FW3 region and a frame shift. The remaining nonfunctional VH rearrangement occurred in a mutated monocytoid B-cell and consisted of a deletion and 2 insertions in the FW3 region resulting in a frame shift.
DISCUSSION
In the present study, we have investigated monocytoid B cells to identify the precursor B cells of monocytoid B cells and to elucidate their relationship to other B-cell populations, including nodal and splenic marginal zone cells. For this purpose, we selected cases of Toxoplasma gondii-induced Piringer’s lymphadenopathy with prominent, and thus reliably identifiable, sheets of monocytoid B cells and analyzed their antigen profile, their Ig isotype expression at the RNA and protein levels, and their Ig gene rearrangement and VH gene mutation pattern. The results obtained were compared with the features of other B-cell populations with special reference to splenic marginal zone B cells and, when technically possible, with the features of nodal cells occurring in the marginal zone of mesenteric lymph nodes. Our immunohistochemical investigations confirmed the vast absence of IgM and the total absence of BCL-2 from monocytoid B cells and the presence of both molecules on marginal zone cells of the spleen and mesenteric lymph nodes. We could further show that the Ki-B3 epitope of the CD45RA molecule and the DBA44 molecule (characteristically associated with hairy cell leukemia cells) were totally absent from splenic and nodal marginal zone cells but were expressed either by all or by a proportion of monocytoid B cells, respectively. The proliferation rate was higher in monocytoid B cells than in splenic and nodal marginal zone cells. Our studies underscore the immunophenotypical differences between monocytoid B cells and all other B-cell subsets (Table 1).
Because the immunohistological demonstration of Ig is prone to artifacts due to high levels of soluble Ig in the serum,44we extended our studies to the expression of Ig mRNA. The in situ hybridization experiments showed, for Igμ and Igδ transcripts, a distribution similar to that of the corresponding proteins, indicating that the vast absence of IgM and IgD in monocytoid B cells is a true finding and that their expression is regulated at the transcriptional level. These findings confirm previous studies showing that monocytoid B cells are distinct from all known B-cell populations, including marginal zone cells of the spleen and mesenteric nodes, and dismiss the concept that monocytoid B cells represent the nodal equivalent of splenic marginal zone cells. An unexpected finding of our in situ hybridization studies was weak, but distinct signals for Igγ over the monocytoid B cells in the absence of IgG protein labeling. However, a similarly weak but distinct labeling for Igγ transcripts was found over most germinal center B cells, with the difference that, in the germinal center light zone, there were some very strong additional Igγ transcript signals. These strong signals could be allocated to germinal center cells being in the process of plasma cellular differentiation. This finding demonstrates the absence of plasma cellular differentiation from monocytoid B cells and thus adds to the differences between monocytoid B cells and germinal center cells. In conclusion, the present and previous phenotypical studies18,19 21 disclosed differences between monocytoid B cells and established B-cell populations and thus proved to be incapable of identifying the B-cell population that gives rise to monocytoid B cells.
We therefore turned our studies to the genetic level. This included the analysis of the rearrangement and mutation pattern of the VH genes in single monocytoid B cells isolated from 5 different cases. Ninety-five amplificates from 327 isolated monocytoid B cells could be obtained. The detection of VH rearrangements in the monocytoid B cells formally proved their B-cell nature. The finding that nearly all VH rearrangements (85/95 [89%]) of the monocytoid B cells were unique indicates that most, if not all, monocytoid B cells originate from many (polyclonal) B cells and not from a few cells or one B cell by oligoclonal or monoclonal expansion. This polyclonal origin of the monocytoid B cells is further substantiated by a VH family usage compatible with normal B cells excluding a derivation from B cells with a particular type of VH rearrangement.
The sequence analysis of the IgH rearrangements showed that most monocytoid B cells (95%) contained functional Ig genes. Of the monocytoid B cells, 74.4% were devoid of VH gene mutation and 25.6% carried somatic mutations at a frequency (3.8%) comparable to those of IgG- and IgA-positive peripheral blood memory B cells.43 45These findings indicate that the monocytoid B cells, despite their uniform morphological appearance, uniform antigen profile, and uniform homing, are not homogeneous but consist of a mixture of B cells, with the majority of them representing B cells at a pregerminal center stage of differentiation, like mantle cells, and a minority representing late B cells that have acquired somatic mutations during their passage through a germinal center, like memory B cells. However, most of the mutated monocytoid B cells lacked signs of antigen selection, drawing their derivation from normally selected memory B cells into doubt.
These findings are in partial conflict with a recent study29 reporting that splenic as well as nodal marginal zone B cells (designated as monocytoid B cells in the mentioned study) show somatic mutations and display clonal expansion. We therefore extended our genetic single-cell studies to splenic marginal zone B cells, mantle cells, and germinal center B cells. Unfortunately, marginal zone cells of mesenteric lymph nodes could not be included because of a lack of frozen tissue. In agreement with previous investigations,43,46,47 mantle cells did not show any clonal relationship or VH gene mutations, but clonally related VH rearrangements with ongoing somatic mutations were detectable in the germinal center cell population. Furthermore, our analysis of the single splenic marginal zone cells showed only unrelated (polyclonal) rearrangements with mostly antigen selected mutations, as shown in a previous study.48 The different results concerning the clonality and mutation pattern of the monocytoid B cells described by Tierens et al29 might have been caused by an erroneous comicrodissection of parts of the germinal centers leading to the appearance of dominant PCR products. This assumption is supported by the reported high frequency of crippling mutations (6 of 16 sequences [37.5%]), because disrupted VH genes to that extent are so far only reported for cells isolated from the inside but not from the outside of germinal centers.43 46
In conclusion, the absence of related rearrangements from most monocytoid B cells suggests that these cells arise by transformation of many polyclonal B cells, eg, unmutated mantle cells, and to a lesser extent of mutated immature (not fully antigen selected) memory B cells. It is tempting to speculate that this transformation might be induced by a B-cell superantigen expressed by Toxoplasma gondii. It has already been shown that Toxoplasma gondii possess superantigen or superantigen-like activity,49 50 which is effective on T cells and leads to a selective expansion of a certain T-cell subset. It could be that Toxoplasma gondii-expressed superantigens are also active on a subset of pregerminal center and postgerminal center B cells. The postulated superantigen(s) would induce a transformation of B cells of various differentiation stages (ie, unmutated and/or mutated B cells), including a change of their antigen profile and an isotype switch similar to the one seen in germinal centers that is associated with a downregulation of BCL-2, Ig, and CD23 and an upregulation of the proliferation. Furthermore, this superantigen could be responsible for the attraction of neutrophils into the monocytoid B-cell zone.
Taken together, our findings support the view that monocytoid B cells represent a separate B-cell subset that is distinct from all established B-cell subpopulations, including marginal zone cells of the spleen and mesenteric lymph nodes. They are not homogeneous, because 74.4% of monocytoid B cells appear to derive from unmutated naive pregerminal center B cells and 25.6% disclose VH mutations compatible with a derivation from postgerminal center B cells. The predominant expression of Igγ transcripts points towards an uncommon dissociation between isotype switch and somatic VH gene mutation, at least in the unmutated proportion of monocytoid B-cell, further stressing the distinctiveness of these cells.
ACKNOWLEDGMENT
The authors thank H. Lammert, E. Berg, H. Protz, D. Jahnke, and H.-H. Müller for their excellent technical assistance and L. Udvarhelyi for his help with the preparation of the manuscript.
Supported by a grant of the Deutsche Forschungsgemeinschaft (STE 318/5-2, STE 318/9-1).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Harald Stein, MD, Institute of Pathology, Benjamin Franklin University Hospital, Free University Berlin, Hindenburgdamm 30, 12200 Berlin, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal