Peripheral blood T cells in patients with paroxysmal nocturnal hemoglobinuria (PNH) comprise a mixture of residual normal and glycosylphosphatidylinositol (GPI)-deficient PNH cells. Using multicolor flow cytometry, we demonstrated significant differences between the proportions of naive and memory cells within these populations. PNH T cells comprise mainly naive cells (CD45RA+CD45R0−), whereas normal T cells in the same patients were predominantly memory (CD45RA−CD45R0+) cells. Functional analyses showed that GPI-deficient CD45RA+ T cells can convert to a CD45R0+ phenotype. We present data from a PNH patient in remission for 20 years who still had significant numbers of GPI-deficient T cells; these showed a normal distribution of naive and memory components. The predominantly naive phenotype of GPI-deficient T cells seen in PNH patients with active disease likely reflects the phenotype of recent normal thymic emigrants. In patients where hematopoiesis was predominantly derived from the PNH stem cell, absolute numbers of both naive PNH CD4+ cells and CD8+ cells show an inverse correlation with patient age, implying this age-related decline in T-cell production is secondary to a decrease in thymic activity rather than a stem cell defect.
PAROXYSMAL NOCTURNAL hemoglobinuria (PNH) is an acquired pluripotent hematopoietic stem cell (HSC) disorder in which a somatic mutation of the X-linked PIG-A gene results in a partial or absolute deficiency of all proteins linked to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor.1-6PNH is closely associated with bone marrow failure syndromes and many patients have severe bone marrow aplasia, pancytopenia, and little residual normal hematopoiesis.7,8 After onset of the disease and failure of normal hematopoiesis, the vast majority of new blood cell formation in PNH patients is clonally derived from the PNH stem cell. Although the major emphasis of published work has been on abnormalities of erythroid and myeloid cells, we and others have shown that GPI-deficient lymphocyte populations are present in a high proportion of patients with PNH.9-11 Peripheral blood T cells in most PNH patients comprise an admixture of residual normal T cells and T cells belonging to the PNH clone. These studies not only confirm that PNH is a true pluripotent HSC disorder, but also open up the possibility of using GPI deficiency as a surrogate biological marker to study T lymphopoiesis. PNH lymphocytes can be identified by lack of expression of GPI-anchored proteins such as CD48, CD52, CD58, or CD59. What effect, if any, this deficiency has on lymphocyte function in vivo or in vitro has not been extensively investigated, although previous studies have described regulatory roles for CD48 and CD59 in T-cell activation and an in vitro growth advantage for PNH T cells compared with normal T cells.10,12-15 In vivo activation of normal T cells by foreign antigen induces upregulation in expression of class II major histocompatability complex (MHC) antigens (HLA-DR) and cytokine receptors such as CD25. If the stimulus is from a previously unencountered antigen, then memory T-cell formation occurs as an integral part of the immune response. The acquisition of T-cell memory is associated with a switch in CD45R isoform expression from a CD45RA+R0− naive phenotype to CD45RA−R0+ primed or memory cell phenotype.16-19 As this is a dynamic process and occurs throughout the life span of an individual, there are always detectable HLA-DR+ T cells and long-lived memory (CD45R0+) T cells in adult peripheral blood.
In this present study, we examined in detail the differential expression of CD45R and HLA-DR determinants by normal and GPI-deficient T-cell populations in patients with PNH. In addition, the absence of GPI-linked antigens from PNH T cells provided a novel marker with which to examine the contribution of thymic-dependant and independent pathways to the maintenance of T-cell numbers and the relationship to patient age.
MATERIALS AND METHODS
Patients studied.
Peripheral blood samples from a series of 28 adult patients with PNH and 1 patient known to have had a spontaneous remission of PNH 20 years previously, were screened for the presence of GPI-deficient T-cell clones using established protocols.9 Informed consent was obtained from individual patients before specimen collection. The initial diagnosis of PNH was made by demonstrating the absence of GPI-linked proteins from red blood cell and/or granulocyte cell membrane by flow cytometry or by a positive Ham test.20 21 PNH T-cell clones were detected in 22 of 29 cases. The patient group comprised 9 males and 13 females with an age range of 23 to 74 years. Further analyses of these 22 patients undertaken for the purposes of this study included determination of the absolute numbers of T cells and T-cell subsets by flow cytometry as detailed below. Full blood counts including absolute numbers of lymphocytes were performed using a Sysmex K1000 cell counter (Sysmex, Milton Keynes, UK). In vitro functional studies were performed on 2 patients.
Separation of mononuclear cells.
Mononuclear cells were fractionated from 10 mL volumes of EDTA anticoagulated peripheral blood by conventional density sedimentation with Lymphoprep (Nycomed, Oslo, Norway). Cells collected from the plasma/lymphoprep interface were washed twice with 10 mL volumes of FACSFlow (Becton Dickinson, San Jose, CA) supplemented with 0.5% bovine serum albumin (FACSFlow/BSA; Sigma Chemicals, St Louis, MO) and the white blood cell count adjusted to 50 × 109/L for subsequent immunophenotyping studies.
Lymphocyte subsets.
An initial whole blood lysis screen was performed to assess the proportions and absolute numbers of T cells and T-cell subsets. Briefly, 30 μL volume of a prestandardized combination of CD4/CD8/CD3 monoclonal antibodies (fluorescein isothiocyanate [FITC]/phycoerythrin [PE]/PE:Cy5) was pipetted into a 12 × 75 mm polystyrene tube (Becton Dickinson). One hundred microliters of EDTA anticoagulated whole blood was then added, mixed, and incubated in the dark at ambient temperature for 20 minutes, punctuated by gentle mixing every 5 minutes. A 2-mL volume of a 1/10 dilution of fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson) was added to the tube, gently mixed, and incubated for a further 10 minutes in the dark. The tubes were then centrifuged at 300g for 90 seconds, the supernatant discarded, and the white blood cell pellet resuspended in a 2-mL volume of FACSFlow/BSA. The cells were again pelleted and washed with a further 2 mL volume of FACSFlow/BSA. After this final wash stage, the cells were resuspended in a 300-μL volume of a 1/10 dilution of CellFIX (Becton Dickinson) and incubated for a minimum of 5 minutes before flow cytometry analysis. The cells were then analyzed using a FACScan flow cytometer (Becton Dickinson) and Lysis II software (Becton Dickinson) collecting a minimum of 1 × 104 gated lymphocyte events identified by low forward scatter (FSC) and low side scatter (SSC) characteristics. Absolute numbers of T cells, T-helper, and T-cytotoxic cells were calculated using the Sysmex lymphocyte count multiplied by the percentage of lymphocytes expressing CD3, CD3+CD4+, and CD3+CD8+determinants divided by 100, respectively. Additional analyses of CD4 versus CD8 dot plots for CD3 gated lymphocytes showed that the percentage of dual positive (CD4+CD8+) was less than 1% in all cases.
Immunophenotyping and flow cytometry of lymphocytes by whole blood lysis.
A total of 100-μL volumes of whole blood were stained as described above with 30-μL volumes of the following combinations of monoclonal antibodies (FITC/PE/PE:Cy5): CD48/CD45RA/CD3, HLA-DR/CD48/CD3; CD48/CD45RA/CD4, CD48/CD45RA/CD8. The cells were analyzed by flow cytometry using a FACScan flow cytometer with Lysis II software. A minimum of 5 × 103 gated events were collected on specific lymphocyte populations identified by low side scatter characteristics and either CD3, CD4, or CD8 positivity (summarized in Fig 1). Data was stored as list mode files for subsequent analysis. Quadrant statistical analyses of dot plots of CD48 versus either CD45RA or HLA-DR were undertaken, and the proportions of normal and PNH lymphocytes expressing CD45RA or HLA-DR calculated. Absolute numbers of GPI-deficient naive T helper and T-cytotoxic lymphocytes were calculated by mutiplying the absolute numbers of CD4+ or CD8+ lymphocytes by the percentage of CD48−CD45RA+R0− cells within each fraction, divided by 100.
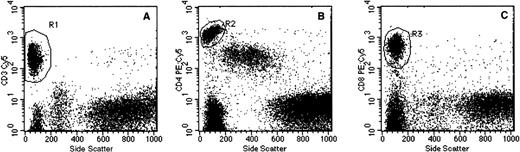
Flow cytometry gating procedures. Representative dot plot examples of (A) side scatter (SSC) versus CD3, (B) SSC versus CD4, and (C) SSC versus CD8 used for identification of T cells, T-helper cells, and T cytotoxic cells, respectively. Specimens were prepared by whole blood lysis as detailed in Materials and Methods. The individual gating regions (R1, R2, and R3) denote the live gates used for data acquisition.
Flow cytometry gating procedures. Representative dot plot examples of (A) side scatter (SSC) versus CD3, (B) SSC versus CD4, and (C) SSC versus CD8 used for identification of T cells, T-helper cells, and T cytotoxic cells, respectively. Specimens were prepared by whole blood lysis as detailed in Materials and Methods. The individual gating regions (R1, R2, and R3) denote the live gates used for data acquisition.
Immunophenotyping and flow cytometry of mononuclear cells.
A total of 10 μL volumes of mononuclear cells were stained in U-shaped microtiter plate wells with the following three-color combinations of monoclonal antibodies (FITC/PE/PE:Cy5): CD59/CD45R0/CD4, and CD59/CD45R0/CD8. The cell/antibody combinations were mixed, incubated at room temperature for 30 minutes, and then centrifuged at 400g for 15 seconds to pellet the cells. After removal of excess antibody and washing twice with 150 μL volumes of FACSFlow/BSA, the cells were resuspended to 300 μL in FACSFlow before flow cytometry studies. Cells were then analyzed by flow cytometry using a FACScan flow cytometer with Lysis II software. A minimum of 2 × 104 gated lymphocytes were collected on the basis of low side scatter characteristics and either CD4 or CD8 positivity. The proportions of normal and PNH lymphocytes expressing CD45R0 were derived from quadrant statistical analysis of CD59 versus CD45R0 dot plots.
Functional studies/cell culture anti-CD3 solid phase stimulation of T cells.
To examine the ability of both normal and PNH T cells to convert from a CD45RA+ phenotype to a CD45R0+ phenotype, peripheral blood lymphocytes were stimulated in vitro with anti-CD3 using a method described by Frolova et al.22 Briefly, 20-mL volumes of peripheral blood were collected from 2 patients with PNH (PNH 042 and 043) into sterile sodium heparin anticoagulant (Becton Dickinson Vacutainer Systems, Rutherford, NJ). Mononuclear cells were isolated under sterile conditions using density gradient sedimentation as described above. The mononuclear cells were washed twice with 10-mL volumes of sterile RPMI-1640 media (GIBCO, Paisley, UK) containing 5% fetal calf serum (GIBCO), penicillin (100 U/mL), and streptomycin (100 μg/mL). The final cell count was adjusted to 1 × 109/L for cell culture studies. Anti-CD3 solid phase 50-mL cell culture flasks (Nalge Nunc International, Roskilde, Denmark) were prepared in advance by coating with 10-mL volumes of anti CD3 (OKT3-American Type Culture Collection [ATCC]) at a concentration of 10 μg/mL and incubated overnight at 4°C. The flasks were then washed 5 times with 10-mL volumes of sterile phosphate-buffered saline (pH 7.2). Mononuclear cells were cultured in a humidified 5% CO2 atmosphere at 37°C. After 5 days in culture, cells were harvested and stained with the following combinations of monoclonal antibodies for flow cytometry CD45R0/CD48/CD4 and CD45R0/CD48/CD8 as described above. A minimum of 1 × 104 gated lymphocytes were collected on the basis of side scatter characteristics and either CD4 or CD8 positivity. The proportions of normal and PNH lymphocytes expressing CD45R0 were derived from quadrant statistical analysis of CD48 versus CD45R0 dot plots and compared with the values obtained for cells before stimulation.
Monoclonal antibodies.
The sources and specificities of all monoclonal antibodies used for immunophenotyping were as follows: CD3 (OKT3 FITC, PE and PE:Cy5), CD8 (OKT8 FITC, PE and PE:Cy5), and HLA-DR (L243 FITC and PE) from American Type Culture Collection (ATCC), Rockville, MD; CD4 (QS4120 FITC, PE and PE/Cy5) and CD45R0 (UCHL1 FITC and PE) from University College and Middlesex School of Medicine, London, UK; CD45RA (2H4 PE) Coulter-Immunotech, Luton Bedfordshire, UK; CD48 (MEM102 FITC and PE) and CD59 (MEM43 FITC) from Cymbus Biotechnology Limited, Chandler’s Ford, Hampshire, UK.
RESULTS
The clinical, hematological, and laboratory features of the 22 patients examined in the study are summarized in Table 1. Nine patients had hypoplastic PNH, 11 hemolytic PNH, and a single patient, who originally presented with hemolytic PNH, was nonhemolytic with only 0.5% GPI negative red blood cells and a normal hemoglobin. GPI-deficient red blood cells and neutrophils were found in all cases, except patient PNH 030, who had a spontaneous remission 20 years previously. The median size of the PNH granulocyte clone was 91.6%, this was taken as a reliable indicator that the majority of hematopoiesis was clonally derived from the PNH stem cell. With the exception of 1 patient (detailed below), no one was currently receiving immunosuppressive therapy. Patient PNH007 was given a single course of cyclosporin A in 1990 and PNH012 was unresponsive to a single course of antilymphocyte globulin given in 1988. Patient PNH043 is currently receiving cyclosporin A.
Clinical and Laboratory Features of Patients Studied
| Patient ID . | Age (yrs)/ Sex . | Clinical Features* . | GPI-Deficient Clone (%)† . | CD4 Count/ (109/L)‡ . | CD8 Count/ (109/L)‡ . | ||
|---|---|---|---|---|---|---|---|
| Granulocytes . | Red Blood Cells . | T Cells . | |||||
| PNH003 | 39/M | Hypoplastic | 5.9 | 2.2 | 0.2 | 0.39 | 0.90 |
| PNH007 | 29/F | Hemolytic | 96.2 | 52.5 | 30.4 | 0.45 | 0.63 |
| PNH008 | 29/F | Was hemolytic1-153 | 1.4 | 0.5 | 21.8 | 0.74 | 1.09 |
| PNH012 | 33/F | Hypoplastic | 44.3 | 49.8 | 24.2 | 0.61 | 0.64 |
| PNH019 | 45/M | Hemolytic | 81.7 | 32.0 | 9.9 | 0.99 | 0.64 |
| PNH020 | 25/M | Hypoplastic | 95.4 | 73.8 | 16.8 | 0.64 | 0.31 |
| PNH022 | 44/F | Hemolytic | 93.0 | 20.9 | 8.7 | 0.33 | 0.20 |
| PNH027 | 74/M | Hemolytic | 99.6 | 89.6 | 0.3 | 0.32 | 0.08 |
| PNH030 | 66/F | Spontaneous remission 20 years ago | <0.1 | <0.1 | 9.4 | 0.86 | 0.66 |
| PNH033 | 34/F | Hemolytic | 99.5 | 79.5 | 21.4 | 0.34 | 0.18 |
| PNH034 | 53/F | Hemolytic | 73.8 | 32.8 | 2.1 | 0.74 | 0.37 |
| PNH042 | 55/M | Hypoplastic | 94.9 | 48.8 | 0.6 | 0.47 | 0.39 |
| PNH043 | 43/M | Hypoplastic | 96.8 | 30.7 | 5.0 | 0.24 | 0.40 |
| PNH047 | 37/F | Hemolytic | 44.1 | 12.4 | 0.5 | 0.33 | 0.56 |
| PNH055 | 43/F | Hypoplastic | 76.3 | 5.4 | 5.5 | 1.07 | 0.63 |
| PNH058 | 25/F | Hemolytic | 95.3 | 57.3 | 6.8 | 0.94 | 0.34 |
| PNH063 | 37/F | Hypoplastic | 5.9 | 9.1 | 8.1 | 0.27 | 0.23 |
| PNH072 | 36/M | Hemolytic | 96.4 | 31.3 | 16.5 | 0.44 | 0.44 |
| PNH079 | 54/F | Hypoplastic | 95.9 | 84.9 | 17.8 | 0.40 | 0.21 |
| PNH087 | 24/M | Hypoplastic | 76.5 | 60.0 | 2.9 | 0.64 | 0.69 |
| PNH090 | 36/F | Hemolytic | 91.6 | 76.6 | 12.3 | 0.73 | 0.46 |
| PNH091 | 23/M | Hemolytic | 71.4 | 41.5 | 13.6 | 0.18 | 0.16 |
| Patient ID . | Age (yrs)/ Sex . | Clinical Features* . | GPI-Deficient Clone (%)† . | CD4 Count/ (109/L)‡ . | CD8 Count/ (109/L)‡ . | ||
|---|---|---|---|---|---|---|---|
| Granulocytes . | Red Blood Cells . | T Cells . | |||||
| PNH003 | 39/M | Hypoplastic | 5.9 | 2.2 | 0.2 | 0.39 | 0.90 |
| PNH007 | 29/F | Hemolytic | 96.2 | 52.5 | 30.4 | 0.45 | 0.63 |
| PNH008 | 29/F | Was hemolytic1-153 | 1.4 | 0.5 | 21.8 | 0.74 | 1.09 |
| PNH012 | 33/F | Hypoplastic | 44.3 | 49.8 | 24.2 | 0.61 | 0.64 |
| PNH019 | 45/M | Hemolytic | 81.7 | 32.0 | 9.9 | 0.99 | 0.64 |
| PNH020 | 25/M | Hypoplastic | 95.4 | 73.8 | 16.8 | 0.64 | 0.31 |
| PNH022 | 44/F | Hemolytic | 93.0 | 20.9 | 8.7 | 0.33 | 0.20 |
| PNH027 | 74/M | Hemolytic | 99.6 | 89.6 | 0.3 | 0.32 | 0.08 |
| PNH030 | 66/F | Spontaneous remission 20 years ago | <0.1 | <0.1 | 9.4 | 0.86 | 0.66 |
| PNH033 | 34/F | Hemolytic | 99.5 | 79.5 | 21.4 | 0.34 | 0.18 |
| PNH034 | 53/F | Hemolytic | 73.8 | 32.8 | 2.1 | 0.74 | 0.37 |
| PNH042 | 55/M | Hypoplastic | 94.9 | 48.8 | 0.6 | 0.47 | 0.39 |
| PNH043 | 43/M | Hypoplastic | 96.8 | 30.7 | 5.0 | 0.24 | 0.40 |
| PNH047 | 37/F | Hemolytic | 44.1 | 12.4 | 0.5 | 0.33 | 0.56 |
| PNH055 | 43/F | Hypoplastic | 76.3 | 5.4 | 5.5 | 1.07 | 0.63 |
| PNH058 | 25/F | Hemolytic | 95.3 | 57.3 | 6.8 | 0.94 | 0.34 |
| PNH063 | 37/F | Hypoplastic | 5.9 | 9.1 | 8.1 | 0.27 | 0.23 |
| PNH072 | 36/M | Hemolytic | 96.4 | 31.3 | 16.5 | 0.44 | 0.44 |
| PNH079 | 54/F | Hypoplastic | 95.9 | 84.9 | 17.8 | 0.40 | 0.21 |
| PNH087 | 24/M | Hypoplastic | 76.5 | 60.0 | 2.9 | 0.64 | 0.69 |
| PNH090 | 36/F | Hemolytic | 91.6 | 76.6 | 12.3 | 0.73 | 0.46 |
| PNH091 | 23/M | Hemolytic | 71.4 | 41.5 | 13.6 | 0.18 | 0.16 |
Hypoplastic patients defined by an absence of macroscopic hemoglobinuria.
GPI-deficient clones as measured by flow cytometry. Clone size includes both partially deficient (type II) and completely deficient (type III) populations.
References ranges: CD4 lymphocytes 0.53 to 1.75 × 109/L; CD8 lymphocytes 0.30 to 1.02 × 109/L.
Patient previously hemolytic with no hemolytic attacks since 1992 (spontaneous recovery).
PNH T-cell clones.
Of the 21 patients studied with active disease, the median proportion of PNH T cells was 8.7% (range, 0.2% to 30.4%). One case (PNH063) showed a population of partially GPI-deficient T cells comprising 8.1% of total.
HLA-DR expression by CD48+ and CD48−subpopulations of T cells.
To study the expression of HLA-DR by PNH (CD48−) T cells compared with the same patient’s residual normal (CD48+) T cells, three-color flow cytometry studies were performed on 13 patients with previously identified PNH T-cell clones. Results from these studies (Table 2) showed absent or reduced levels of HLA-DR expression within the PNH component when compared with patient constituent normal T cells. These differences in HLA-DR expression were statistically significant (pairedt-test: t = 5.64; degrees of freedom [df], 12; P < .001). Representative two-color dot plots of CD48 versus HLA-DR for CD3 gated lymphocytes (Fig 2A and B) clearly illustrate the differences in HLA-DR expression by PNH T cells compared with constituent normal T cells. Furthermore, in 7 of 13 (54%) cases analyzed, the normal T-cell component comprised a significantly higher proportion of HLA-DR expressing cells than would be expected for normal healthy controls suggesting an ongoing immune response. Interestingly, the patient with partially GPI-deficient T cells showed similar results to the patients with a total GPI deficiency (Fig 2C).
Comparative Expression of CD45RA and HLADR by CD48+ (Normal) and CD48− (GPI-Deficient) Subpopulations of T Cells in Patients With PNH
| Patient ID . | T Cells (CD3+)* . | |||
|---|---|---|---|---|
| CD48+ CD45RA+ % . | CD48− CD45RA+ % . | CD48+ HLADR+ % . | CD48− HLADR+ % . | |
| PNH003 | 56 | 74 | 39 | 6 |
| PNH007 | 65 | 94 | 29 | 16 |
| PNH008 | 37 | 85 | 12 | 3 |
| PNH012 | 48 | 87 | 32 | 3 |
| PNH019 | 25 | 75 | ND | ND |
| PNH022 | 37 | 82 | 24 | 5 |
| PNH034 | 44 | 83 | 12 | 6 |
| PNH043 | 57 | 89 | 23 | 4 |
| PNH055 | 71 | 95 | 11 | 4 |
| PNH063 | 20 | 62 | 23 | 17 |
| PNH072 | 56 | 93 | ND | ND |
| PNH079 | 42 | 72 | 23 | 13 |
| PNH087 | 62 | 94 | 16 | 4 |
| PNH090 | 61 | 91 | 5 | 1 |
| PNH091 | 20 | 62 | 17 | 2 |
| Normal range† | ND | NA | <20 | NA |
| Patient ID . | T Cells (CD3+)* . | |||
|---|---|---|---|---|
| CD48+ CD45RA+ % . | CD48− CD45RA+ % . | CD48+ HLADR+ % . | CD48− HLADR+ % . | |
| PNH003 | 56 | 74 | 39 | 6 |
| PNH007 | 65 | 94 | 29 | 16 |
| PNH008 | 37 | 85 | 12 | 3 |
| PNH012 | 48 | 87 | 32 | 3 |
| PNH019 | 25 | 75 | ND | ND |
| PNH022 | 37 | 82 | 24 | 5 |
| PNH034 | 44 | 83 | 12 | 6 |
| PNH043 | 57 | 89 | 23 | 4 |
| PNH055 | 71 | 95 | 11 | 4 |
| PNH063 | 20 | 62 | 23 | 17 |
| PNH072 | 56 | 93 | ND | ND |
| PNH079 | 42 | 72 | 23 | 13 |
| PNH087 | 62 | 94 | 16 | 4 |
| PNH090 | 61 | 91 | 5 | 1 |
| PNH091 | 20 | 62 | 17 | 2 |
| Normal range† | ND | NA | <20 | NA |
As determined by three-color flow cytometry using CD48/CD45RA/CD3 and HLA-DR/CD48/CD3 combinations of monoclonal antibodies as described in Materials and Methods section. Values in the table are the percentage of either CD48+ or CD48− cells expressing either CD45RA or HLA-DR determinants.
Abbreviations: ND, not determined; NA, not applicable.
T cells identified on the basis of strong CD3 positivity and low SSC characteristics. Statistical comparisons were made using a pairedt test: Statistically significant differences in expression of CD45RA and HLA-DR were shown between CD48+ and CD48− lymphocytes.
Normal range for HLA-DR expression by T cells derived from analysis of 27 normal adult controls.
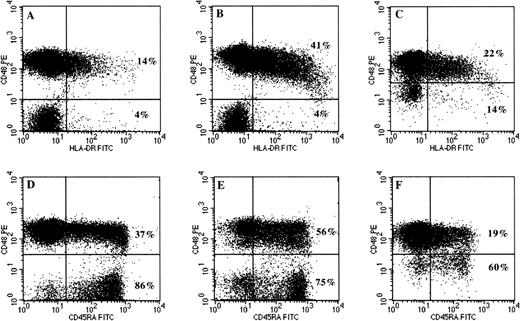
Analysis of HLA-DR and CD45RA expression on normal (CD48+) and PNH (CD48−) components of peripheral blood T cells by three-color flow cytometry. Initial gating of T cells was based on low SSC characteristics and CD3 positivity as described in Materials and Methods and in Fig 1. The upper two-color dot plots (A through C) show the difference in HLA-DR expression between CD48+ and CD48− T cells in 3 representative patients with PNH. The percentage of normal and PNH T cells expressing HLA-DR are shown in the upper and lower quadrants, respectively. Not only is HLA-DR expression absent on GPI negative T cells (plots A and B), but also on T cells with partial GPI deficiency (plot C). The lower dot plots (D through F) show the differences in CD45RA expression by normal (CD48+) and PNH (CD48−) T cells. The proportion of naive T cells is much higher within the GPI-deficient compartment, independent of whether there is complete (plots D and E) or partial deficiency (plot F) of GPI-anchored proteins. The normal T-cell components have a predominantly memory (CD45RA−) phenotype.
Analysis of HLA-DR and CD45RA expression on normal (CD48+) and PNH (CD48−) components of peripheral blood T cells by three-color flow cytometry. Initial gating of T cells was based on low SSC characteristics and CD3 positivity as described in Materials and Methods and in Fig 1. The upper two-color dot plots (A through C) show the difference in HLA-DR expression between CD48+ and CD48− T cells in 3 representative patients with PNH. The percentage of normal and PNH T cells expressing HLA-DR are shown in the upper and lower quadrants, respectively. Not only is HLA-DR expression absent on GPI negative T cells (plots A and B), but also on T cells with partial GPI deficiency (plot C). The lower dot plots (D through F) show the differences in CD45RA expression by normal (CD48+) and PNH (CD48−) T cells. The proportion of naive T cells is much higher within the GPI-deficient compartment, independent of whether there is complete (plots D and E) or partial deficiency (plot F) of GPI-anchored proteins. The normal T-cell components have a predominantly memory (CD45RA−) phenotype.
To determine whether increased HLA-DR expression by T cells was associated with hemolytic or aplastic forms of the disease, statistical analysis of the data was undertaken. No significant relationship was found (Mann-Whitney; z = −1.10; P = .2701). The possibility that the percentage of T cells that were HLA-DR+ may inversely correlate with CD4 count as a reflection of peripheral expansion as a result of T-cell depletion was also examined. No correlations were found (Spearman’s (rs) −0.125; P = .577).
CD45RA expression by CD48+ and CD48−subpopulations of T cells.
To compare the expression of CD45RA by PNH T cells with patients’ residual normal T cells, three-color flow cytometry studies were performed on 15 patients with PNH T-cell clones. Results from these studies (Table 2) showed a much higher proportion of CD45RA+ (naive) T cells within the PNH component when compared with the residual normal T cells. These differences were highly statistically significant (paired t test: t = 15.42; df 14; P < .001). Typical two-color dot plots of CD48 versus CD45RA for CD3 gated lymphocytes (Fig 2D through F) clearly illustrate the differences in CD45RA expression by completely and partially deficient PNH T cells compared with residual normal T cells.
CD45RA expression by CD48+ and CD48−subpopulations of CD4+ and CD8+ T-cell subsets.
Having shown clear differences in CD45RA expression between normal and PNH T cells, the analysis was extended to examine expression of CD45RA by normal and PNH components of CD4+ and CD8+lymphocyte subsets. CD4+ lymphocytes from 18 patients with PNH T-cell clones were studied by three-color flow cytometry and by setting appropriate CD4/SSC gates (Fig 1B), the proportions of CD45RA+ components within CD48+ and CD48− populations were analyzed on two-color dot plots. Results (Table 3 and Fig 3) clearly showed that CD4+CD48− lymphocytes were predominantly CD45RA+CD45R0− and that CD4+CD48+ lymphocytes showed either normal or reduced proportions of CD45RA+ components. These differences were statistically significant (paired t test:t = −18.35; df = 17; P < .0001). Similar differences were demonstrated between PNH and normal components of the CD8+ lymphocyte subset (paired t test: t = −8.11; df 17; P < .0001). The PNH component of CD8+ lymphocytes showed a predominantly naive (CD45RA+) phenotype (Table 3 and Fig 3). The CD48+ components showed normal distributions of CD4+CD45RA+ in 11 of 19 cases, increased proportions of CD4+CD45RA− cells in 6 of 19 cases, and reduced proportions in 2 of 19. For CD8+CD48+ lymphocytes, normal CD45RA expression was present in 8 of 21 cases, increased proportions of CD45RA− lymphocytes present in 8 of 21, and decreased proportions of CD45RA− lymphocytes in 5 cases. In the patient who had undergone spontaneous remission of PNH (case PNH030), the distribution of naive and memory components within both the normal and PNH components of both CD4+ and CD8+lymphocyte subsets were normal.
Comparative Expression of CD45RA by CD48+(Normal) and CD48− (GPI-Deficient) Components of CD4+ and CD8+ T-Cell Subpopulations in Patients With PNH
| Patient ID . | CD4+Lymphocytes3-150 . | CD8+ Lymphocytes3-150 . | ||||
|---|---|---|---|---|---|---|
| Size of PNH Clone % . | CD48+CD45RA+ % . | CD48−CD45RA+ % . | Size of PNH Clone % . | CD48+CD45RA+ % . | CD48−CD45RA+ % . | |
| PNH007 | 24.3 | 37 | 83 | 35.8 | 89 | 99 |
| PNH008 | 23.5 | 21 | 71 | 22.2 | 47 | 97 |
| PNH012 | 21.3 | 40 | 89 | 32.3 | 95 | 99 |
| PNH019 | 10.7 | 18 | 67 | 12.6 | 67 | 85 |
| PNH020 | 16.2 | 56 | 83 | 18.2 | 44 | 98 |
| PNH022 | 7.9 | 28 | 74 | 11.1 | 58 | 90 |
| PNH0303-151 | 14.0 | 32 | 46 | 4.3 | 63 | 59 |
| PNH033 | 21.7 | 37 | 77 | 20.7 | 42 | 93 |
| PNH034 | 1.4 | 37 | 74 | 3.2 | 64 | 89 |
| PNH042 | 0.7 | 23 | 71 | 0.7 | 36 | 85 |
| PNH043 | 5.9 | 45 | 85 | 4.4 | 63 | 89 |
| PNH047 | 0.1 | 33 | ND | 0.8 | 42 | ND |
| PNH055 | 4.3 | 61 | 78 | 8.9 | 79 | 96 |
| PNH058 | 6.1 | 51 | 90 | 7.6 | 60 | 88 |
| PNH063 | 9.6 | 7 | 45 | 10.4 | 35 | 73 |
| PNH072 | 15.9 | 40 | 87 | 26.0 | 86 | 99 |
| PNH079 | 16.7 | 42 | 72 | 20.7 | 44 | 88 |
| PNH087 | 3.7 | 43 | 95 | 2.7 | 80 | 97 |
| PNH090 | 11.8 | 45 | 93 | 16.2 | 67 | 89 |
| PNH091 | 17.5 | 16 | 71 | 14.6 | 24 | 53 |
| Median | 10.7 | 37 | 77.5 | 12.6 | 60 | 89.5 |
| Normal range3-152 | 30-52% | 54-82% | ||||
| Patient ID . | CD4+Lymphocytes3-150 . | CD8+ Lymphocytes3-150 . | ||||
|---|---|---|---|---|---|---|
| Size of PNH Clone % . | CD48+CD45RA+ % . | CD48−CD45RA+ % . | Size of PNH Clone % . | CD48+CD45RA+ % . | CD48−CD45RA+ % . | |
| PNH007 | 24.3 | 37 | 83 | 35.8 | 89 | 99 |
| PNH008 | 23.5 | 21 | 71 | 22.2 | 47 | 97 |
| PNH012 | 21.3 | 40 | 89 | 32.3 | 95 | 99 |
| PNH019 | 10.7 | 18 | 67 | 12.6 | 67 | 85 |
| PNH020 | 16.2 | 56 | 83 | 18.2 | 44 | 98 |
| PNH022 | 7.9 | 28 | 74 | 11.1 | 58 | 90 |
| PNH0303-151 | 14.0 | 32 | 46 | 4.3 | 63 | 59 |
| PNH033 | 21.7 | 37 | 77 | 20.7 | 42 | 93 |
| PNH034 | 1.4 | 37 | 74 | 3.2 | 64 | 89 |
| PNH042 | 0.7 | 23 | 71 | 0.7 | 36 | 85 |
| PNH043 | 5.9 | 45 | 85 | 4.4 | 63 | 89 |
| PNH047 | 0.1 | 33 | ND | 0.8 | 42 | ND |
| PNH055 | 4.3 | 61 | 78 | 8.9 | 79 | 96 |
| PNH058 | 6.1 | 51 | 90 | 7.6 | 60 | 88 |
| PNH063 | 9.6 | 7 | 45 | 10.4 | 35 | 73 |
| PNH072 | 15.9 | 40 | 87 | 26.0 | 86 | 99 |
| PNH079 | 16.7 | 42 | 72 | 20.7 | 44 | 88 |
| PNH087 | 3.7 | 43 | 95 | 2.7 | 80 | 97 |
| PNH090 | 11.8 | 45 | 93 | 16.2 | 67 | 89 |
| PNH091 | 17.5 | 16 | 71 | 14.6 | 24 | 53 |
| Median | 10.7 | 37 | 77.5 | 12.6 | 60 | 89.5 |
| Normal range3-152 | 30-52% | 54-82% | ||||
As determined by three-color flow cytometry using CD48/CD45RA/CD4 and CD48/CD45RA/CD8 combinations of monoclonal antibodies as described in Materials and Methods section. Values in the table are the percentage of either CD48+ or CD48− cells expressing CD45RA.
Abbreviation: ND, too few cells for reliable assessment of CD45RA staining.
T-cell subpopulations identified on the basis of strong CD4 or CD8 positivity and low SSC characteristics.
Data from this patient excluded from statistical analysis and median value.
Normal ranges derived from a study of 12 normal adult controls.
Representative dot plots comparing CD45RA and CD45R0 expression by normal (CD48+) and PNH (CD48−) lymphocytes for CD4+ (T-helper) and CD8+ (T-cytotoxic) populations. Plots A and B show that the proportion of CD45RA+ cells is significantly higher (75% and 94%, respectively) for PNH components of the CD4+ and CD8+ subpopulations when compared with the residual normal components (28% and 57%, respectively), which are predominantly CD45RA−. Plots C and D are representative examples comparing CD45R0 expression by normal (CD59+) and PNH (CD59−) lymphocytes for CD4+ (T-helper) and CD8+ (T-cytotoxic) populations. As expected, the proportion of CD45R0+ cells is significantly lower (16% and 2%, respectively) for PNH components of the CD4+ and CD8+ subpopulations when compared with the normal components (44% and 57%, respectively). Plots E and F show the proportions of CD45RA+ components within the normal and PNH fractions of the CD4+ and CD8+ lymphocyte subsets of patient PNH030, who had a spontaneous remission of PNH 20 years ago. The plots show normal distributions of naive (CD45RA+) and memory cells (CD45RA−) for all populations.
Representative dot plots comparing CD45RA and CD45R0 expression by normal (CD48+) and PNH (CD48−) lymphocytes for CD4+ (T-helper) and CD8+ (T-cytotoxic) populations. Plots A and B show that the proportion of CD45RA+ cells is significantly higher (75% and 94%, respectively) for PNH components of the CD4+ and CD8+ subpopulations when compared with the residual normal components (28% and 57%, respectively), which are predominantly CD45RA−. Plots C and D are representative examples comparing CD45R0 expression by normal (CD59+) and PNH (CD59−) lymphocytes for CD4+ (T-helper) and CD8+ (T-cytotoxic) populations. As expected, the proportion of CD45R0+ cells is significantly lower (16% and 2%, respectively) for PNH components of the CD4+ and CD8+ subpopulations when compared with the normal components (44% and 57%, respectively). Plots E and F show the proportions of CD45RA+ components within the normal and PNH fractions of the CD4+ and CD8+ lymphocyte subsets of patient PNH030, who had a spontaneous remission of PNH 20 years ago. The plots show normal distributions of naive (CD45RA+) and memory cells (CD45RA−) for all populations.
CD45R0 expression by CD59+ and CD59−subpopulations of CD4+ and CD8+ T-cell subsets.
Having shown clear differences in CD45RA expression between normal and PNH T cells, expression of CD45R0 by normal and PNH constituents of CD4+ and CD8+ lymphocyte subsets was studied in 11 patients using CD59 as the GPI-linked marker. As CD59 is ubiquitously expressed by hematopoietic cells and is therefore unsuitable for use in a whole blood lysis technique, the analysis was performed on separated mononuclear cell fractions. CD45R0 antigen is reciprocally expressed in relation to CD45RA.23 24 Analysis of two-color dot plots of CD45R0 versus CD59 for CD4+ and CD8+ lymphocyte subsets as expected showed PNH lymphocyte components to be predominantly CD45R0−(Table 4 and Fig 3). Statistically significant differences in expression of CD45R0 were demonstrated between PNH and normal components for both CD4+ and CD8+ lymphocyte subsets (paired t-test: t = 12.5; df 10; P < .0001 and t = 6.60; df 10; P< .0001, respectively).
Comparative Expression of CD45R0 by CD59+(Normal) and CD59− (GPI-Deficient) Components of CD4+ and CD8+ T-Cell Subpopulations in Patients With PNH
| Patient ID . | CD4+ Lymphocytes4-150 . | CD8+ Lymphocytes4-150 . | ||
|---|---|---|---|---|
| CD59+ CD45R0+ % . | CD59− CD45R0+ % . | CD59+ CD45R0+ % . | CD59− CD45R0+ % . | |
| PNH007 | 76 | 24 | 63 | 48 |
| PNH020 | 44 | 17 | 56 | 2 |
| PNH022 | 83 | 35 | 53 | 10 |
| PNH033 | 63 | 24 | 59 | 8 |
| PNH042 | 77 | 30 | 64 | 15 |
| PNH055 | 37 | 8 | 14 | 8 |
| PNH058 | 49 | 8 | 40 | 12 |
| PNH079 | 45 | 22 | 46 | 10 |
| PNH087 | 57 | 5 | 20 | 3 |
| PNH090 | 55 | 7 | 33 | 11 |
| PNH091 | 84 | 28 | 76 | 47 |
| Median | 57 | 22 | 53 | 10 |
| Normal range4-151 | 51-68 | NA | 18-46 | NA |
| Patient ID . | CD4+ Lymphocytes4-150 . | CD8+ Lymphocytes4-150 . | ||
|---|---|---|---|---|
| CD59+ CD45R0+ % . | CD59− CD45R0+ % . | CD59+ CD45R0+ % . | CD59− CD45R0+ % . | |
| PNH007 | 76 | 24 | 63 | 48 |
| PNH020 | 44 | 17 | 56 | 2 |
| PNH022 | 83 | 35 | 53 | 10 |
| PNH033 | 63 | 24 | 59 | 8 |
| PNH042 | 77 | 30 | 64 | 15 |
| PNH055 | 37 | 8 | 14 | 8 |
| PNH058 | 49 | 8 | 40 | 12 |
| PNH079 | 45 | 22 | 46 | 10 |
| PNH087 | 57 | 5 | 20 | 3 |
| PNH090 | 55 | 7 | 33 | 11 |
| PNH091 | 84 | 28 | 76 | 47 |
| Median | 57 | 22 | 53 | 10 |
| Normal range4-151 | 51-68 | NA | 18-46 | NA |
As determined by three-color flow cytometry using CD59/CD45R0/CD4 and CD59/CD45R0/CD8 combinations of monoclonal antibodies as described in Materials and Methods section. Values in the table are the percentage of either CD59+ or CD459− cells expressing CD45R0 determinants.
Abbreviation: NA, not applicable.
T-cell subpopulations identified on the basis of strong CD4 or CD8 positivity and low SSC characteristics. Statistical comparisons were made using a paired t test. Statistically significant differences in expression of CD45R0 were shown between CD59+ and CD59− lymphocytes.
Normal range for CD45R0 expression by T cells derived from analysis of 12 normal adult controls.
Functional studies.
As the majority of GPI-deficient T cells in PNH patients have a naive (CD45RA+R0−) phenotype, we performed in vitro functional studies on 2 patients to determine whether these cells were capable of converting to a memory cell phenotype upon activation. After 5 days in culture using an anti-CD3 solid phase to stimulate T cells, residual normal and GPI-deficient populations for both CD4+ (T-helper) and CD8+ (T-cytotoxic) subpopulations showed complete conversion to a memory cell phenotype (CD45R0+) (Fig 4). These results clearly show that absence of GPI-anchored proteins from the membrane of PNH T cells does not prevent conversion to a memory cell phenotype after activation.
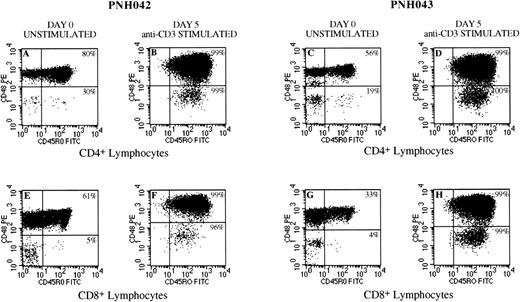
Flow cytometry dot plots from 2 patients (PNH042 and PNH 043) showing conversion of GPI-deficient T cells from a naive phenotype (CD45R0−) to a memory (CD45R0+) phenotype after stimulation in an anti-CD3 solid phase culture system. Plots A and C and E and G are unstimulated CD4+ lymphocytes and CD8+ lymphocytes, respectively. These plots clearly illustrate that the GPI-deficient (CD48−) components to be predominantly CD45R0−. After stimulation, these GPI-deficient populations convert to a memory cell phenotype with greater than 96% coexpression of CD45R0+ for CD4+ (plots B and D) and CD8+ (plots F and H) subsets in both cases studied. The residual normal components (CD48+) of CD4+ and CD8+lymphocytes although showing variable proportions of memory cells in the unstimulated controls (plots A, C, E, and G), also show maximal conversion (greater than 99%) to a memory cell phenotype in all instances (plots B, D, F, and H).
Flow cytometry dot plots from 2 patients (PNH042 and PNH 043) showing conversion of GPI-deficient T cells from a naive phenotype (CD45R0−) to a memory (CD45R0+) phenotype after stimulation in an anti-CD3 solid phase culture system. Plots A and C and E and G are unstimulated CD4+ lymphocytes and CD8+ lymphocytes, respectively. These plots clearly illustrate that the GPI-deficient (CD48−) components to be predominantly CD45R0−. After stimulation, these GPI-deficient populations convert to a memory cell phenotype with greater than 96% coexpression of CD45R0+ for CD4+ (plots B and D) and CD8+ (plots F and H) subsets in both cases studied. The residual normal components (CD48+) of CD4+ and CD8+lymphocytes although showing variable proportions of memory cells in the unstimulated controls (plots A, C, E, and G), also show maximal conversion (greater than 99%) to a memory cell phenotype in all instances (plots B, D, F, and H).
Relationship between age and absolute numbers of naive (CD45RA+) PNH T cells.
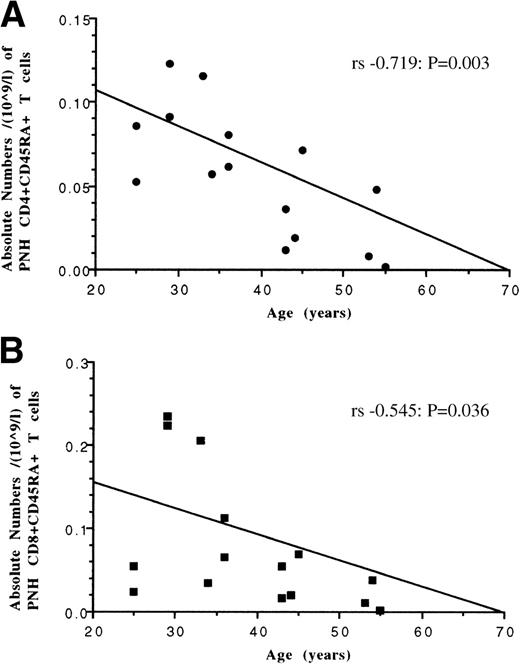
PNH T cells, which have a naive (CD45RA+CD45RO−) phenotype consistent with having undergone thymic differentiation,25 are unequivocally produced from bone marrow-derived hematopoietic progenitor cells. Therefore, in patients where the majority of hematopoiesis is derived from the PNH stem cell (ie, granulocytes predominanly GPI-deficient), the relationship between patient age and absolute numbers of these cells was examined as a potential indicator of naive T-cell production. Statistically significant inverse correlations (Fig 5) were found between patient age and absolute numbers of both naive PNH CD4 and PNH CD8 lymphocyte subpopulations (Spearman’s (rs) −0.719;P = .003) and (rs − 0.545;P = .036) respectively.
Relationship between patient age and absolute numbers of CD45RA+, GPI-deficient T-cell subsets in patients with PNH. Both CD4+ T cells (plot A) and CD8+ T cells (plot B) show an inverse relationship between increasing age and decreasing absolute numbers of naive T cells. Correlations determined by Spearman rank order (rs).
Relationship between patient age and absolute numbers of CD45RA+, GPI-deficient T-cell subsets in patients with PNH. Both CD4+ T cells (plot A) and CD8+ T cells (plot B) show an inverse relationship between increasing age and decreasing absolute numbers of naive T cells. Correlations determined by Spearman rank order (rs).
DISCUSSION
Peripheral blood T cells in a high proportion of patients with PNH comprise a variable mixture of normal cells and T cells belonging to the PNH clone.9-11 Using multicolor flow cytometry, these 2 populations can be clearly resolved by demonstrating the presence or absence of GPI-anchored proteins from the cell surface. By studying the phenotypic characteristics of these 2 populations, we have been able to resolve some of the current contentions regarding T cell lymphopoiesis, particularly the relative contributions of thymic-dependent and thymic independent pathways to the maintenance of peripheral blood T-cell numbers in adults.26
The essential role of the thymus in primary T lymphopoiesis and development of a diverse antigen receptor repertoire is an established tenet of contemporary immunology.27 Thymic-dependent generation of naive T cells occurs primarily in neonates and children, although the specific contribution of thymopoiesis to the maintenance of T-cell numbers and antigen repertoire diversity in adults is largely unknown.25,28,29 Mature, naive T cells that emerge from the thymus have a well characterized immunophenotype of CD3+CD45RA+CD45R0−CD1−CD27+with concomitant expression of either CD4 (MHC Class II) or CD8 (MHC class I) ligands.25,28,30,31 Important insights into adult T-cell lymphopoiesis were provided by the work of Mackall et al,32 who showed that the capacity to produce naive T cells after intensive chemotherapy was inversely related to patient age. In addition, they and others demonstrated that even in young adults with residual thymic function, initial recovery of T-cell numbers postchemotherapy is primarily by peripheral expansion of existing T-cell populations.32,33 This short-term T-cell regeneration is predominantly antigen driven and accompanied by a skewing in antigen receptor diversity and a subset imbalance that may persist for a considerable period of time.34 35
The exact contribution of thymopoiesis to long-term restoration of T-cell numbers remains poorly characterized and certainly in patients who have undergone chemotherapy or bone marrow transplantation, it is not possible to definitively separate T cells produced by peripheral expansion of existing mature T-cell populations from those derived de novo from HSC. Conventional studies have relied upon detecting differential expression of CD45 isoforms by flow cytomtery to identify naive (CD45RA+R0−) and memory (CD45RA−R0+) T cells.17-19,23-32 However, more recent publications have shown that these markers alone are not an entirely a reliable discriminator of naive and memory cell status, particularly for CD8+ T cells.36-38 A clearer understanding of the relative contributions of the distinct pathways of T-cell production is highly desirable for 2 main reasons. First, peripheral expansion of existing T-cell populations results in a concomitant contraction in T-cell antigenic repertoire.34 Second, a long-term failure to produce sufficient numbers of new naive T cells has obvious implications for cell-mediated immunity and mounting an effective immune response to new pathogens.
Using multicolor flow cytometry, we compared the immunophenotypic characteristics of normal and GPI-deficient populations of T cells in PNH patients with respect to activation antigen expression (HLA-DR) and the high and low molecular weight isoforms of the common leukocyte antigen, CD45RA and CD45R0. Preliminary studies showed significantly lower levels of HLA-DR expression on PNH T cells when compared with coexisting normal T cells. Furthermore, differences were found between the distributions of naive and memory cells within normal and PNH T-cell clones. PNH T cells comprised mainly naive T cells, whereas normal T cells showed either normal or increased proportions of memory cells. When this analysis was extended to CD4+ (T-helper) and CD8+ (T-cytotoxic) lymphocyte subpopulations, similar significant differences in CD45RA expression were found, with the PNH clones having a predominantly naive phenotype. Interestingly, residual normal CD4+ lymphocytes in one third of patients showed a marked increase in the proportion of cells with a memory cell phenotype, consistent with having undergone peripheral expansion. However, this contention was not supported by statistical analysis of the correlation between HLA-DR expression by T cells and CD4+ count. An inverse correlation was not found as might be expected if the increased proportions of memory cells were the result of peripheral expansion due to T-cell depletion.
As PNH T cells are unequivocally derived from a multipotent HSC with a somatic mutation of the PIG-A gene and have a naive (CD45RA+CD45R0−HLADR−) phenotype consistent with having undergone thymic differentiation, we examined the relationship between patient age and absolute numbers of these cells as a potential indicator of naive T-cell production. In those patients where hematopoiesis was predominantly maintained by the PNH HSC (ie, a predominant GPI-deficient granulocyte clone), statistical analysis of the relationship between patient age and absolute numbers of either naive PNH CD4 or PNH CD8 lymphocytes conclusively showed inverse correlations for both subsets (Spearman’s (rs) −0.709; P = .0045) and (rs− 0.572; P = .033) respectively. The data strongly support the idea that the ability to produce thymic-derived naive T cells and therefore maintain antigen receptor diversity falls dramatically with age. The fact that the PNH stem cell produces GPI-deficient neutrophils, red blood cells, and monocytes in relatively normal numbers suggests that this age-related decline in T-cell production is secondary to a decrease in thymic activity rather than a stem cell defect. This contention is further supported by data from patient 027, diagnosed as PNH 7 years previously, who at the age of 74 years had predominantly PNH hematopoiesis (ie, greater than 95% granulocyte clone) with virtually no detectable PNH T cells. Normal lymphopoietic activity in this patient is inferred by the finding that natural killer (NK) cells, known to be derived from a common lymphoid progenitor, were predominantly GPI-deficient.28 39
The interpretations of the data we present make the tacit assumption that absence of GPI antigens from PNH T cells has no detrimental effect on development or function. This supposition is supported by in vitro functional studies on 2 patients, which clearly showed that GPI-deficient CD4+ and CD8+ T cells that were predominantly CD45R0−, underwent activation and converted to a CD45R0+ phenotype. Further evidence to support normal function comes from murine studies that show (1) embryonic stem cells with a nonfunctional pig-a gene are competent for hematopoiesis and (2) a CD48 knockout mouse has only minor abnormalities in T-lymphocyte functional activity.40,41Therefore, it is highly likely that the predominantly naive phenotype of PNH T cells we describe is a reflection of recent production rather than an intrinsic inability to activate due to GPI deficiency. The predominantly naive phenotype of PNH T cells is because the process of T-cell activation and memory cell formation may take a number of years to occur, reflecting the picture seen in normal healthy infants.42 This contention is supported by studies of 1 patient (PNH030) who underwent spontaneous remission of PNH 20 years ago who still has significant numbers of PNH T cells and B cells. This patient’s GPI-deficient T-cell subsets show normal distributions of naive and memory cell components. Further studies involving serial monitoring at yearly intervals of memory T-cell formation in the individual patients reported in this study are currently in progress.
These studies provide important new insights into adult T lymphopoiesis, specifically that the ability to produce naive T cells through thymic dependent pathways declines to very low levels around the age of 40. Furthermore, the findings show that after failure of normal hematopoiesis in patients with PNH, peripheral expansion is the primary mechanism by which normal peripheral T-cell numbers are maintained. The increasing use of bone marrow transplantation and novel therapies such as monoclonal antibodies for treating a range of malignant and autoimmune diseases invariably results in a profound T-cell depletion.42 43 Therefore, further studies to promote effective immune reconstitution in adults should focus on strategies to enhance T-cell regeneration by thymic independent pathways and peripheral expansion of existing T-cell populations.
ACKNOWLEDGMENT
The authors gratefully acknowledge the support of Cymbus Biosciences for generous donation of monoclonal antibodies used in this study. We also thank Drs S. Allard, D. Bareford, M. Hamilton, S. Jowett, M. Laffan, M. Layton, P. Mahendra, D. Norfolk, D. Swirsky, A. Williams, D. Watson, and J. Yin for providing patient samples and Dr R.A. Jones (HMDS) for advice on cell culture.
Supported in part by the Leukaemia Research Fund, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephen J. Richards, PhD, Haematological Malignancy Diagnostic Service, The Algernon Firth Building, Leeds General Infirmary, Leeds, LS1 3EX, UK.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal