A novel inositolphosphate-binding protein has been identified and shown to be an immunophilin. This protein, which was isolated from human erythrocyte membranes and from K562 (human erythroleukemia) cell membranes, has robust peptidylprolyl cis-trans isomerase activity that is strongly inhibited by nanomolar concentrations of FK506 or rapamycin, indicating a member of the FKBP (FK506-binding protein) class. However, unlike the cytosolic FKBP12, the isomerase activity of this membrane-associated immunophilin is strongly inhibited by nanomolar concentrations of inositol 1,4,5-trisphosphate (IP3), inositol 1,3,4,5-tetrakisphosphate (IP4), and phosphatidylinositol 4- and 4,5-phosphates, which are suggested to be physiological ligands. The demonstration of a single 12-kD protein that binds both IP4 or IP3and anti-FKBP12 provides strong support for the inositolphosphate-binding immunophilin having an apparent mass of 12 kD, and it is suggested that the protein might be called IPBP12 for 12-kD inositol phosphate binding protein. When an internal tryptic peptide derived from IPBP12 was sequenced, a sequence also present in human cytokeratin 10 was identified, suggesting a cytoskeletal localization for the immunophilin. While purifying IPBP12, it was found that it is immunoprecipitated with specific proteins that include a protein kinase and a phosphoprotein phosphatase. The latter is indicated to be phosphoprotein phosphatase 2A (PP-2A). It is suggested that immunophilins promote the assembly of multiprotein complexes that often include a protein kinase or a phosphoprotein phosphatase or both.
IMMUNOPHILINS ARE high-affinity receptors for the immunosuppressant drugs rapamycin,1cyclosporin,2 and FK506.3,4 These drugs are used clinically to prevent or delay the rejection of allografts after tissue or organ transplantation. Immunophilins that bind rapamycin or FK506 are classified as FKBPs (FK506-binding proteins). Those that bind the cyclosporins belong to the cyclophilin class. Initially, immunophilins were recognized as peptidylprolyl cis-transisomerases, ie, enzymes that catalyze cis-transisomerization about a restricted peptidylprolyl (Xaa-Pro) bond. However, studies designed to identify the mechanism(s) of their actions in immunosuppression have established that immunophilins are key participants in signal transduction pathways that govern cell cycle progression. When an immunophilin binds rapamycin, FK506, or cyclosporin its peptidylprolyl cis-trans isomerase activity is inhibited and the protein undergoes a gain of function that promotes tight binding between the immunophilin/drug complex and one or more specific signal transduction elements. As a result of this association, the element fails to transmit the appropriate signal. The FKBP12/FK506 or cyclophilin/cyclosporin A binary complex has been shown to bind the multisubunit phosphoprotein phosphatase calcineurin (phosphatase 2B or PP-2B), which results in its phosphatase activity being noncompetitively inhibited.5 In the T lymphocyte, this prevents assembly of the NF-AT and/or OAP-OCT transcription factor complexes and failure to upregulate the interleukin-2 (IL-2) promoter.6,7 The resulting failure to enter G1 of the cell cycle is the basis for immunosuppression induced by FK506 or cyclosporin A. However, rather than targeting calcineurin, the FKBP12/rapamycin complex binds a 289-kD protein kinase/scaffolding protein known as FRAP (FKBP-rapamycin associated protein) in humans,8 or RAFT1 (rapamycin and FKBP12 target 1) in the rat,9 or mTOR (mammalian target of rapamycin)10because of its homology with the yeast TOR proteins. FRAP/RAFT1/mTOR bound by FKBP12/rapamycin fails to undergo autophosphorylation and activation, with consequences that relate to the initiation of translation. Two of the proximal targets of FKBP12/rapamycin-inactivated FRAP/RAFT1/mTOR are the S6 kinase, p70S6K, and the eukaryotic (translation) initiation factor 4E-binding protein 1, 4E-BP1. Each fails to become phosphorylated in the manner required for progression through G1. Failure of p70S6K to achieve the activated phosphorylation status results in its failure to phosphorylate the 40S ribosomal protein S6. Among the consequences is a failure to promote the translation of mRNAs whose 5′-UTRs contain polypyrimidine tracts.11-14Failure of 4E-BP1 to achieve the appropriate phosphorylation status, however, results in its failure to dissociate from translation initiation factor eIF-4E. Dissociation of 4E-BP1 and liberation of eIF-4E are required for assembly of the m7G cap-binding complex that facilitates general cap-dependent translation and, in particular, the translation of a subset of mRNAs whose 5′-UTRs are rich in secondary structure.15-18 In both of these instances, FKBP12/rapamycin induced inactivation of FRAP/RAFT1/mTOR results in the dephosphorylation of critical serine/threonine residues of p70S6K 19-22 or 4E-BP1.23 Having observed that FRAP can phosphorylate phosphoprotein phosphatase 2A (PP-2A) in vitro, Peterson et al24 proposed a model in which autophosphorylation and activation of FRAP/RAFT1/mTOR is required to restrain the phosphatase activity of PP-2A. Hence, when FKBP12/rapamycin prevents this activation, PP-2A is no longer restrained and FRAP-dependent, rapamycin-sensitive dephosphorylation of p70S6K or 4E-BP1 occurs. However, whereas the appropriate phosphorylation status of p70S6K and 4E-BP1 appears essential for progression through G1, passage through the G1/S checkpoint, and immunosuppression appears to be critically dependent on another phosphorylation-dependent event, the downregulation of p27Kip1.25 In late G1 concentrations of the broad-specificity, stoichiometric inhibitor p27Kip1 are determined primarily by its phosphorylation status since phosphorylation on threonine results in ubiquitination-dependent protelysis.26 Inappropriately high concentrations of p27Kip1 in late G1 allow the inhibitor to target cyclin E/CDK2 and prevent the G1 to S transition.27,28 Because RhoA has been reported to influence the phosphorylation status of p27Kip129 and because in yeast (Saccharomyces cerevisiae) TOR2 promotes the activation of Rho G-proteins,30 a possible link between FRAP/RAFT1/mTOR and the degradation of p27Kip1 is suggested.
Whereas the role of immunophilins as components of immunophilin/immunosuppressant binary complexes has been the focal point of a wide variety of investigations, immunophilins are also known to associate with signal transduction elements in the absence of an immunosuppressant drug. FKBP12 and cyclophilin A each bind calcineurin in the absence of an immunosuppressant drug.31 FKBP12 also binds the (type I) transforming growth factor (TGF) β receptor in the absence of a drug32 and, in this instance, the association is disrupted by FK506.33 FKBP12 also binds the inositol 1,4,5-trisphosphate (IP3) receptor/Ca2+ channel in the absence of a drug, and this association can also be disrupted by FK506 or rapamycin.34 Furthermore, it has been shown that FKBP2535 and FKBP5236 each bind casein kinase II, that an unidentified FKBP binds v-Raf,37 and that FKBP65 binds c-Raf.38 In addition, using a 2-hybrid screen, the yeast fpr1 gene product, FKBP12, was shown to associate with the enzyme aspartokinase.39 Collectively, these observations indicate that FKBPs bind specific signal-transduction, or regulatory, elements in the absence of an immunosuppressant drug and, in the examples cited, this element is either a phosphoprotein phosphatase or a protein kinase. Hence, it was thought to be of some significance when it was found that the membrane-associated IPBP12, identified in our laboratory,40 appears to associate with both a protein kinase and a phosphoprotein phosphatase. Complexes comprising an FKBP, a protein kinase, and a phosphoprotein phosphatase are not without precedent, however, since an assembly comprising FKBP12, the TGFβ receptor (type I), and calcineurin has been described,41 as well as one comprising FKBP12, the IP3 receptor, protein kinase C, and calcineurin.42
MATERIALS AND METHODS
Reagents.
The [γ-32P]ATP was obtained from DuPont NEN (Boston, MA). The substrate for peptidylprolyl cis-trans isomerase determinations, Suc-Ala-Leu-Pro-Phe-p-nitroanilide, was from BACHEM Feinchemikalien AG (Bubendorf, Switzerland). The monoclonal antibody against the catalytic subunit of PP-2A (anti-PP-2Ac, clone 46), biotin-conjugated RC20 (a recombinant, monoclonal antiphosphotyrosine, lacking much of the Ig constant region), and agarose-linked, monoclonal antiphosphotyrosine, clone PY20, were obtained from Transduction Laboratories (Lexington, KY). Immobilized streptavidin was from Pierce (Rockford, IL). Goat polyclonal anti-FKBP12 (C-19), anti-FKBP12 (N-19), and Protein G PLUS-Agarose were from Santa Cruz Biotechnology (Santa Cruz, CA). ECL detection system used with immunoblots was from Amersham (Arlington Heights, IL). Monoclonal antiphosphotyrosine, clone PT-66 (with or without conjugation to agarose), and reagents for immunoprecipitation, Bicinchoninic acid protein determinations, plasma membrane isolation, protein kinase determinations, and markers for calibrating the Sephacryl S-300 column were obtained from Sigma Chemicals (St Louis, MO). Also from this source were the IP3, inositol 1,3,4,5-tetrakisphosphate (IP4), inositol 1,3,4-trisphosphate, phosphatidylinositol, phosphatidylinositol 4-phosphate, and phosphatidylinositol 4,5-bisphosphate. Molecular mass markers for polyacrylamide gels were from Novex (San Diego, CA).
Isolation of a membrane-associated inositolphosphate-binding immunophilin from human erythrocytes or K562 cells.
Blood was obtained from normal human donors according to protocols approved by the UMDNJ Internal Review Board (Newark, NJ). Conducting all operations at 4°C, erythrocytes were isolated, washed, and subjected to hypotonic lysis in 16.7 mmol/L Tris-HCl buffer (pH 7.5) containing 1.0 mmol/L EDTA. Membranes were isolated and subsequently solubilized using 1% Nonidet P-40 for 30 minutes at 4°C. With 40 mL of whole blood as starting material, approximately 20 mg of solubilized protein in a 16 mL volume was generally obtained. Aliquots of solubilized protein (6.0 to 7.5 mg in 6 mL) were applied to a 1.5 × 90 cm column of Sephacryl S-300. Elution with 50 mmol/L HEPES buffer (pH 7.5) containing 1.0 mmol/L magnesium acetate, 4.0 mmol/L 2-mercaptoethanol, and 0.1% Nonidet P-40 yielded 110 fractions, 1.8 mL each. Aliquots (100 μL each) were examined for peptidylprolylcis-trans isomerase activity, as described.40
K562 cells, growing logarithmically in RPMI 1640 media supplemented with 10% fetal calf serum (FCS), were isolated by centrifugation. Cells were washed and then ruptured by incubating them for 40 minutes with 16.7 mmol/L Tris-HCl (pH 7.5) containing 10.0 mmol/L sodium chloride and 1.0 mmol/L EDTA, followed by 10 downward strokes in a glass homogenizer. The resulting suspension was centrifuged (20 minutes at 3,000g) to remove cell and nuclear debris. The supernatant was then centrifuged at 100,000g for 30 minutes, which produced a particulate fraction containing assorted membranous components. This particulate was washed and then partially solubilized in the presence of 50.0 mmol/L HEPES buffer (pH 7.5) containing 2.0 mmol/L magnesium acetate, 5.0 mmol/L 2-mercaptoethanol, and 1% Nonidet P-40. After 45 minutes at 4°C, the suspension was centrifuged (100,000g for 45 minutes) and the supernatant containing solubilized membrane proteins was isolated. Starting with 4.0 × 108 K562 cells, approximately 2.0 mL of washed packed cells could be isolated and these yielded approximately 2.8 mg of solubilized membrane protein. Aliquots containing 20 to 25 μg of solubilized protein were examined for peptidylprolyl cis-transisomerase activity as described.40
Immunoprecipitation of an inositolphosphate-binding immunophilin from solubilized erythrocyte or K562 cell membrane preparations.
Fractions no. 40 through 51 from the Sephacryl S-300 column were combined and concentrated (6- to 8-fold). When immunoprecipitation was with agarose-linked PT-66 antiphosphotyrosine, an aliquot (1250 μL) of the concentrated preparation was incubated (30°C for 15 minutes) in a medium containing 11.0 mmol/L magnesium acetate, 1.0 mmol/L EDTA, 5.0 mmol/L 2-mercaptoethanol, 0.1% Nonidet P-40, and 2 μmol/L (unlabeled) ATP. The phosphorylation by endogenous protein kinases was stopped by the addition of a 1.0 mmol/L excess of EDTA and the resulting reaction mixture was then incubated (gentle rocking for 14 hours at 4°C) with 500 mL of a slurry of agarose-linked PT-66. After their isolation by centrifugation, the immunoprecipitated, immobilized proteins were washed extensively (50 mmol/L HEPES buffer [pH 7.5] containing 50 mmol/L NaCl, 0.1% Nonidet P-40, and 20% glycerol) and suspended in approximately 1,100 μL of an appropriate buffer. When the immobilized proteins were to be examined for peptidylprolyl cis-trans isomerase activity, a 20 μL aliquot of the suspension was added to the assay buffer (40 mmol/L HEPES, pH 7.9, at 20°C containing 0.015% Triton X-100 and 5.0 mmol/L 2-mercaptoethanol). When the immobilized proteins were to be examined for endogenous protein kinase activity, a 90 μL aliquot was added to protein kinase buffer (see below). Negative controls were prepared using antibody that had not been exposed to the membrane preparation or antibody that had been exposed to a preparation of bovine serum albumin (BSA).
When solubilized K562 cell membranes were used for immunoprecipitation with antiphosphotyrosine, a sample (400 μg of K562 membrane protein) was allowed to undergo phosphorylation by endogenous protein kinases and an optimized amount of antiphosphotyrosine was added. The reaction mixture was then incubated at 4°C for 2 to 6 hours, depending on the protein concentration. When agarose-linked PT-66 was used, the immobilized proteins were isolated by centrifugation and washed extensively using 50 mmol/L HEPES buffer (pH 7.5) containing 50 mmol/L NaCl, 0.1% Nonidet P-40, and 20% glycerol. When biotin-conjugated RC20 was used, agarose-linked streptavidin (20 μL for each 2 μg/8 μL antibody) was added to allow immobilization. Proteins were then washed extensively in 10 mmol/L Tris-HCl buffer (pH 7.4) containing 50 mmol/L NaCl, 1% Triton X-100, 1.0 mmol/L EDTA, 1.0 mmol/L EGTA, 0.2 mmol/L sodium vanadate, 0.2 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 0.5% Nonidet P-40 (according to protocols provided by Transduction Laboratories). When (goat) polyclonal anti-FKBP12 (C-19) was used, phosphorylation by endogenous protein kinases was omitted and samples were incubated at 4°C for 4 to 6 hours with an optimized amount of the antibody. Immunoreactive proteins were immobilized by adding Protein G-Plus-Agarose (20 μL for each 1 μg/5 μL antibody). Aliquots of 20 μL of this suspension were examined for peptidylprolyl cis-trans isomerase activity.40 Aliquots of 100 to 200 μL were used when examining for protein kinase activity.
Negative controls for all procedures were prepared from appropriate reagents that had been incubated with BSA or histones, rather than membrane preparations.
Determination of protein kinase activity.
Proteins were examined for protein kinase activity in protein kinase buffer (50 mmol/L HEPES buffer [pH.7.5] containing 3.0 to 20.0 mmol/L magnesium acetate [or chloride] and/or manganese acetate [or chloride], 1.0 mmol/L EDTA, 5.0 mmol/L 2-mercaptoethanol, and 0.1% Nonidet P-40) augmented with 1.0 to 2.0 μmol/L [γ-32P]ATP (2 × 106 to 4 × 107 dpm). The higher concentrations of divalent cations and the higher specific activities of ATP were used when immobilized proteins were being examined. Total reaction volumes were generally 100 to 200 μL. Incubation was at 30°C for 15 to 60 minutes. Reactions were stopped by the addition of sodium dodecyl sulfate (SDS; final concentration, 1%) in 50 mmol/L Tris-HCl buffer (pH 6.8) containing 5.0 mmol/L 2-mercaptoethanol unless the proteins were to remain nondenatured. Under these conditions, the reaction was stopped by the addition of a 1 to 2 molar excess of EDTA.
RESULTS
Identification of a 12-kD inositolphosphate-binding protein.
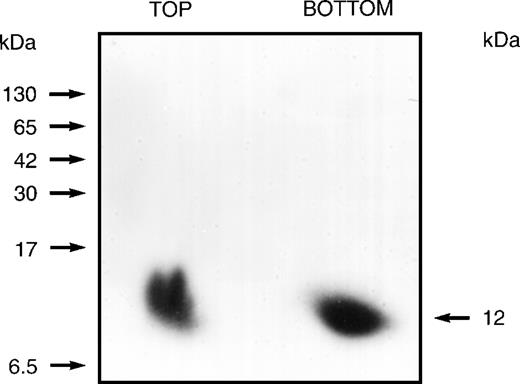
Previous studies had suggested that the human erythrocyte membrane contains proteins whose phosphorylation status is modestly influenced by the second messenger IP3. After conventional binding studies43 had indicated that solubilized erythrocyte membrane preparations contain IP3- or IP4-binding proteins, we undertook their identification using an 125I-labeled arylazido inositolphosphate analogue44 as a photoaffinity-labeling probe. This probe (a generous gift from Dr Clinton E. Ballou, University of California, Berkeley, CA) was an analogue of not only IP3and IP4, but also of phosphatidylinositols with 4- and 4,5-phosphoryl groups. After labeling it with 125I, it was used in cross-linking studies, which demonstrated that only fractions no. 40 through 51, of the total 110, contained proteins that became heavily labeled. Binding specificity was demonstrated by showing that125I-labeling was diminished 85% after exposure to a 7.5-fold excess of unlabeled IP3 or IP4 before incubation with the labeled probe. When the 125I-labeled products were examined, first on 3% to 17% gradient, nondenaturing (Nonidet P-40) polyacrylamide gels and then on 10% to 20% gradient SDS polyacrylamide gels, a single heavily labeled 12-kD protein was identified, as shown in Fig 1. These studies identified an inositolphosphate-binding protein that was present as a component of a slowly migrating complex or aggregate and also as a rapidly migrating 12-kD monomer (or dimer).
Autoradiogram showing the 12-kD protein that is cross-linked by an 125I-labeled arylazido inositolphosphate analogue. The 110 fractions from the Sephacryl S-300 gel filtration column were divided into 11 groups of 10 each and combined and concentrated (6-fold). These preparations were then loaded onto 3% to 17% gradient, nondenaturing (Nonidet P-40) polyacrylamide gel. After development, gel segments containing slowly migrating aggregates or complexes (estimated at 400 to 600 kD) were cut from the gel and eluted. After dialyzing each eluate against 50 mmol/L HEPES buffer (pH 7.5) containing 0.1% Nonidet P-40 to remove sulfhydryl reagents, 125 μL aliquots were preincubated for 60 minutes with the125I-labeled probe (2.0 × 107 dpm) and then irradiated (254 nm) for 2 minutes at a distance of 3.5 cm using a Mineralight UVSL source (Ultra-violet Products, San Gabriel, CA). Unreacted azido reagent was destroyed with 5% 2-mercaptoethanol in 50 mmol/L HEPES containing 0.1% Nonidet P-40. Reaction mixtures were then applied to 3% to 17% gradient, nondenaturing (Nonidet P-40) polyacrylamide gels that were used to prepare autoradiograms. Gel segments containing the slowly migrating or the rapidly migrating heavily labeled components demonstrated for fractions no. 41 through 50 were cut from the nondenaturing gel and inserted at the top of a 10% to 20% gradient SDS polyacrylamide gel. Autoradiograms prepared from the SDS gels identified a labeled 12-kD protein derived from the rapidly migrating component (BOTTOM) and a labeled 12-kD protein derived from the slowly migrating component (TOP). Positions of molecular mass markers are indicated on the left.
Autoradiogram showing the 12-kD protein that is cross-linked by an 125I-labeled arylazido inositolphosphate analogue. The 110 fractions from the Sephacryl S-300 gel filtration column were divided into 11 groups of 10 each and combined and concentrated (6-fold). These preparations were then loaded onto 3% to 17% gradient, nondenaturing (Nonidet P-40) polyacrylamide gel. After development, gel segments containing slowly migrating aggregates or complexes (estimated at 400 to 600 kD) were cut from the gel and eluted. After dialyzing each eluate against 50 mmol/L HEPES buffer (pH 7.5) containing 0.1% Nonidet P-40 to remove sulfhydryl reagents, 125 μL aliquots were preincubated for 60 minutes with the125I-labeled probe (2.0 × 107 dpm) and then irradiated (254 nm) for 2 minutes at a distance of 3.5 cm using a Mineralight UVSL source (Ultra-violet Products, San Gabriel, CA). Unreacted azido reagent was destroyed with 5% 2-mercaptoethanol in 50 mmol/L HEPES containing 0.1% Nonidet P-40. Reaction mixtures were then applied to 3% to 17% gradient, nondenaturing (Nonidet P-40) polyacrylamide gels that were used to prepare autoradiograms. Gel segments containing the slowly migrating or the rapidly migrating heavily labeled components demonstrated for fractions no. 41 through 50 were cut from the nondenaturing gel and inserted at the top of a 10% to 20% gradient SDS polyacrylamide gel. Autoradiograms prepared from the SDS gels identified a labeled 12-kD protein derived from the rapidly migrating component (BOTTOM) and a labeled 12-kD protein derived from the slowly migrating component (TOP). Positions of molecular mass markers are indicated on the left.
Identification of an inositolphosphate-binding immunophilin.
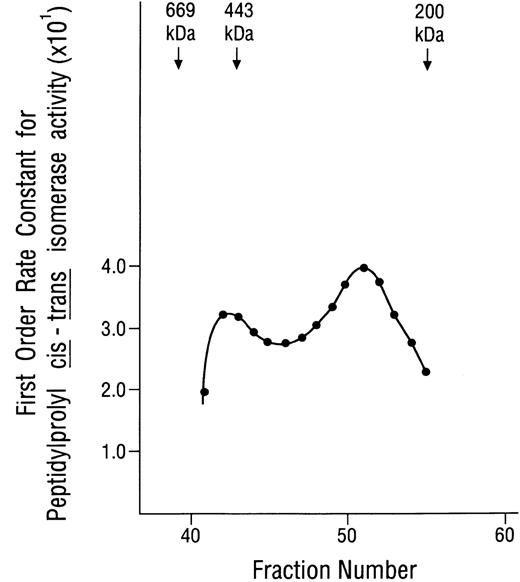
The plasma membrane localization and 12-kD apparent mass of the inositolphosphate-binding protein suggested that it was novel and, hence, its function(s) might be distinctly different from those of previously identified IP3- or IP4-binding proteins. Because we had found that all preparations with inositolphosphate-binding activity also had protein kinase activity, it was speculated that the 12-kD protein might be an immunophilin, because immunophilins were known to associate specifically with several well known protein kinases.32,33,35,37,45 When preparations containing both inositolphosphate-binding and protein kinase activities were examined on immunoblots prepared with a polyclonal antibody against FKBP12 (a generous gift from Dr Andrew R. Marks, College of Physicians and Surgeons of Columbia University, New York, NY) a positive signal at 12 kD was obtained (data not shown). With this suggestion of the presence of an immunophilin, these preparations were examined for the peptidylprolyl cis-trans isomerase activity that characterizes all immunophilins, and robust activity that was strongly inhibited by nanomolar concentrations of rapamycin or FK506 was demonstrated. These data identified an immunophilin belonging to the FKBP class, as suggested by the anti-FKBP12 immunoblots. However, most significantly, it was found that the isomerase activity of these preparations was strongly inhibited by nanomolar concentrations of IP3 and IP4,40 which distinguished this membrane-associated FKBP from previously identified immunophilins. The 110 Sephacryl S-300 column fractions were then examined systematically for peptidylprolyl cis-trans isomerase activity, and it was found that this activity could only be demonstrated for fractions no. 40 through 51. Data are shown in Fig 2. The bimodal nature of this activity curve suggested that the immunophilin(s) was associated with aggregates or complexes, one with a mass of approximately 490 kD (peak fraction no. 42) and the other with a mass of approximately 280 kD (peak fraction no. 51). Although the curve shown was generated using kobs values for the uninhibited reaction, the isomerase activity of each of these fractions was strongly inhibited by rapamycin, FK506, IP3, and IP4 (data not shown). These studies identified an inositolphosphate-binding immunophilin.
Peptidylprolyl cis-trans isomerase activity displayed by fractions from the Sephacryl S-300 gel-filtration column. Aliquots of 100 μL each (from 1.8-mL fractions) were examined, without alteration, for peptidylprolyl cis-trans isomerase activity using Suc-Ala-Leu-Pro-Phe-p-nitroanilide as the substrate, as described previously.40 Standards used for calibrating the gel-filtration column were thyroglobulin, 669 kD; apoferritin, 443 kD; β-amylase, 200 kD; and yeast alcohol dehydrogenase, 150 kD. The peak at fraction no. 42 corresponds to a mass of approximately 490 kD. The peak at fraction no. 51 corresponds to a mass of approximately 280 kD.
Peptidylprolyl cis-trans isomerase activity displayed by fractions from the Sephacryl S-300 gel-filtration column. Aliquots of 100 μL each (from 1.8-mL fractions) were examined, without alteration, for peptidylprolyl cis-trans isomerase activity using Suc-Ala-Leu-Pro-Phe-p-nitroanilide as the substrate, as described previously.40 Standards used for calibrating the gel-filtration column were thyroglobulin, 669 kD; apoferritin, 443 kD; β-amylase, 200 kD; and yeast alcohol dehydrogenase, 150 kD. The peak at fraction no. 42 corresponds to a mass of approximately 490 kD. The peak at fraction no. 51 corresponds to a mass of approximately 280 kD.
The inositolphosphate-binding immunophilin can be immunoprecipitated with antiphosphotyrosine.
When Sephacryl S-300 column fractions no. 40 through 51 were incubated with protein kinase buffer augmented with 2 μmol/L [γ-32P]ATP (4 × 106 dpm), an endogenous protein kinase activity catalyzed the labeling of 8 to 10 proteins. Prominent among these phosphoproteins was a 12-kD component that was cut from the gel and analyzed for phosphoamino acids.46 These determinations identified phosphotyrosine and phosphoserine as the principal phosphoamino acids in a hydrolysate prepared from the 12-kD protein, as shown in Fig 3. Because ligand binding by a protein-kinase substrate frequently alters its phosphorylation, IP3 was included in some of the labeling reactions. Finding that phosphotyrosine was diminished when phosphorylation was conduced in the presence of IP3 (see Fig 3) indicated that the 12-kD protein undergoing phosphorylation on tyrosine was, in fact, an IP3-binding protein. Also, the identification of phosphotyrosine and phosphoserine as the major phosphoamino acids suggested a similarity to Fpr3, an FKBP from Saccharomyces cerevisiae, which undergoes phosphorylation on tyrosine and serine that is catalyzed by casein kinase II (CK II).47 We, therefore, undertook the isolation of the inositolphosphate-binding immunophilin using antiphosphotyrosine. When agarose-linked monoclonal antiphosphotyrosine (clone PT-66) was used, the immunoprecipitate obtained displayed robust isomerase activity that was qualitatively and quantitatively comparable to that seen when examining column fractions no. 40 through 51. When an equation for characterizing inhibition by a tight-binding inhibitor48 was used to calculate inhibition constants for the isomerase activity displayed by these immobilized immunoprecipitates, the following values were obtained: rapamycin, Ki,app = 2.3 ± 0.2 nmol/L; FK506, Ki,app = 3.2 ± 0.03 nmol/L; IP4, Ki,app = 1.5 ± 0.2 nmol/L; and IP3, Ki,app = 4.1 ± 0.15 nmol/L. Neither IP3 nor IP4 had a similar effect on the isomerase activity of human recombinant FKBP12 (a generous gift from Dr Stuart L. Schreiber, Harvard University, Cambridge, MA). Furthermore, inositol 1,3,4-trisphosphate, which has vicinal phosphoryl groups like IP3 and IP4, but is not a second messenger, had a negligible effect on the isomerase activity of these preparations. Finding that the values obtained for Ki,app were of the same order of magnitude, or less, as concentrations of IP3 or IP4 that produce second-messenger responses (eg, 32 nmol/L is the threshold for IP3 action upon the IP3 receptor49and 30.8 nmol/L is the Kd for IP4 binding by GAP1IP4BP50) suggested that IP3and IP4 may be physiological ligands for the membrane-associated, inositolphosphate-binding immunophilin.
Autoradiogram showing results of phosphoamino acid analyses on the 12-kD erythrocyte membrane protein. Sephacryl S-300 column fractions no. 40 through 51 were concentrated 6-fold and allowed to undergo phosphorylation in the presence of protein kinase buffer containing 1 μmol/L [γ-32P]ATP (1 × 107dpm), 4 mmol/L magnesium acetate, and 1 mmol/L manganese acetate, without (−) or with (+) 1 μmol/L IP3. Reaction mixtures were subsequently applied to 3% to 17% gradient SDS polyacrylamide gels and a 12-kD phosphoprotein was identified on autoradiograms. Gel segments containing this component were cut out and the phosphoproteins were hydrolyzed in 6 N HCl at 110°C for 65 minutes. Liberated phosphoamino acids were separated by thin-layer chromatography using the system absolute ethanol:25% ammonia (3.5:1.6).46 Authentic standards (phosphoserine, phosphothreonine, and phosphotyrosine) were cospotted in each sample lane and subsequently visualized with ninhydrin.
Autoradiogram showing results of phosphoamino acid analyses on the 12-kD erythrocyte membrane protein. Sephacryl S-300 column fractions no. 40 through 51 were concentrated 6-fold and allowed to undergo phosphorylation in the presence of protein kinase buffer containing 1 μmol/L [γ-32P]ATP (1 × 107dpm), 4 mmol/L magnesium acetate, and 1 mmol/L manganese acetate, without (−) or with (+) 1 μmol/L IP3. Reaction mixtures were subsequently applied to 3% to 17% gradient SDS polyacrylamide gels and a 12-kD phosphoprotein was identified on autoradiograms. Gel segments containing this component were cut out and the phosphoproteins were hydrolyzed in 6 N HCl at 110°C for 65 minutes. Liberated phosphoamino acids were separated by thin-layer chromatography using the system absolute ethanol:25% ammonia (3.5:1.6).46 Authentic standards (phosphoserine, phosphothreonine, and phosphotyrosine) were cospotted in each sample lane and subsequently visualized with ninhydrin.
When immobilized immunoprecipitates were eluted and examined on SDS polyacrylamide gels that were then stained with silver, a signal at 12 kD was obtained, as shown in lane 2 of Fig4. Also shown in this figure are the signal at 12 kD that was obtained when fractions no. 40 through 51 (combined and concentrated) were examined on SDS polyacrylamide gels stained with silver (lane 1), the signal at 12 kD that was obtained when immunoprecipitated, immobilized proteins were allowed to undergo phosphorylation by the endogenous protein kinase(s) (lane 4) and the signal at 12 kD that was obtained when immunoprecipitated proteins were blotted and overlaid with rabbit polyclonal anti-FKBP12 (lane 5). (Lanes 3 and 6 are positive controls prepared from hrFKBP12.) These data identify a 12-kD protein that can be stained with silver, a 12-kD protein that undergoes phosphorylation while immobilized on agarose-linked antiphosphotyrosine, and a 12-kD protein that is recognized on anti-FKBP12 immunoblots.
Identification of a 12-kD protein on gels, on autoradiograms, and on blots. Proteins present in Sephacryl S-300 column fractions no. 40 through 51 (concentrated 6-fold) were separated on 3% to 17% gradient nondenaturing (Nonidet P-40) polyacrylamide gels and slowly migrating components (400 to 600 kD) were eluted and then applied to 3% to 17% gradient SDS polyacrylamide gels that were subsequently silver stained. The signal at 12 kD is shown (lane 1). The slowly migrating components (400 to 600 kD) were eluted and used for immunoprecipitation with agarose-linked antiphosphotyrosine (PT-66) and the resulting immunoprecipitate was eluted onto 3% to 17% gradient SDS polyacrylamide gels that were subsequently silver stained. Signals in the lower region of the gel are shown (lane 2). hrFKBP12 was applied to a 3% to 17% gradient SDS polyacrylamide gel that was subsequently silver stained (lane 3). Immunoprecipitated immobilized proteins were allowed to undergo phosphorylation by endogenous protein kinase(s) in the presence of 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. After the separation of labeled proteins on 3% to 17% gradient SDS polyacrylamide gels, autoradiograms were prepared. The lower region of the gel is shown (lane 4). Immobilized immunoprecipitated proteins were eluted from the solid support onto 3% to 17% SDS polyacrylamide gels. After development, proteins were transferred to a membrane and these blots were then overlaid with rabbit polyclonal anti-FKBP12 (1 to 10,000). Goat antirabbit antibody conjugated to horseradish peroxidase (1 to 70,000) was added and detection was by enhanced chemiluminescence (ECL). The lower region of the blot is shown (lane 5). hrFKBP12 was applied to a 3% to 17% gradient SDS polyacrylamide gel and transferred to a membrane, and the blot was overlaid with anti-FKBP12 and detected as above (lane 6). The 6.5-kD molecular mass markers are shown.
Identification of a 12-kD protein on gels, on autoradiograms, and on blots. Proteins present in Sephacryl S-300 column fractions no. 40 through 51 (concentrated 6-fold) were separated on 3% to 17% gradient nondenaturing (Nonidet P-40) polyacrylamide gels and slowly migrating components (400 to 600 kD) were eluted and then applied to 3% to 17% gradient SDS polyacrylamide gels that were subsequently silver stained. The signal at 12 kD is shown (lane 1). The slowly migrating components (400 to 600 kD) were eluted and used for immunoprecipitation with agarose-linked antiphosphotyrosine (PT-66) and the resulting immunoprecipitate was eluted onto 3% to 17% gradient SDS polyacrylamide gels that were subsequently silver stained. Signals in the lower region of the gel are shown (lane 2). hrFKBP12 was applied to a 3% to 17% gradient SDS polyacrylamide gel that was subsequently silver stained (lane 3). Immunoprecipitated immobilized proteins were allowed to undergo phosphorylation by endogenous protein kinase(s) in the presence of 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. After the separation of labeled proteins on 3% to 17% gradient SDS polyacrylamide gels, autoradiograms were prepared. The lower region of the gel is shown (lane 4). Immobilized immunoprecipitated proteins were eluted from the solid support onto 3% to 17% SDS polyacrylamide gels. After development, proteins were transferred to a membrane and these blots were then overlaid with rabbit polyclonal anti-FKBP12 (1 to 10,000). Goat antirabbit antibody conjugated to horseradish peroxidase (1 to 70,000) was added and detection was by enhanced chemiluminescence (ECL). The lower region of the blot is shown (lane 5). hrFKBP12 was applied to a 3% to 17% gradient SDS polyacrylamide gel and transferred to a membrane, and the blot was overlaid with anti-FKBP12 and detected as above (lane 6). The 6.5-kD molecular mass markers are shown.
The inositolphosphate-binding immunophilin is immunoprecipitated in association with a specific set of proteins.
As reported in 1991, when a glutathione S-transferase-FKBP12 fusion protein was generated and incubated with calf brain extracts and the resulting reaction mixture was then passed over a Glutathione-Sepharose column in the presence of FK506, proteins with relative masses of 15, 17, 57, and 61 kD were identified.51 These proteins, which had become associated with the FKBP12/FK506 binary complex, were subsequently identified as the calcineurin B subunit, the calmodulin subunit, a proteolytic product of the calcineurin A subunit, and the calcineurin A subunit, respectively. This early observation suggested that an FKBP can be a component of a functional multiprotein complex. Finding that inositolphosphate-sensitive isomerase activity is associated with species with apparent masses of 280 and 490 kD (Fig 2) suggested that the inositolphosphate-binding immunophilin can also be a component of a multiprotein complex. As a means of identifying proteins that associate with the inositolphosphate-binding immunophilin, immobilized immunoprecipitates displaying both inositolphosphate-sensitive isomerase activity and protein kinase activity were incubated with protein kinase buffer augmented with 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. Phosphoproteins with apparent masses of 12, 30, 36, 42, 60, 72, and 165 kD were subsequently identified on autoradiograms (Fig 5). Because CK II, which binds and phosphorylates FKBP25,35FKBP52,36 and (yeast) Fpr3,47 is known to be a component of the human erythrocyte membrane,52 the presence of a 42-kD phosphoprotein suggested that this might be the catalytically competent53 42-kD α subunit of CK II.
Autoradiogram showing phosphoproteins generated by protein kinase(s) present in immobilized erythrocyte immunoprecipitates. Sephacryl S-300 column fractions no. 40 through 51, combined and concentrated 6-fold, were incubated with agarose-linked antiphosphotyrosine (PT66) in the presence of low ionic strength buffers. Immobilized immunoprecipitates were incubated with protein kinase buffer augmented with 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. The 12-, 30-, 36-, 42-, 60-, 72-, and 165-kD phosphoproteins are indicated on the right. Molecular mass markers (6.5 to 204 kD) are on the left.
Autoradiogram showing phosphoproteins generated by protein kinase(s) present in immobilized erythrocyte immunoprecipitates. Sephacryl S-300 column fractions no. 40 through 51, combined and concentrated 6-fold, were incubated with agarose-linked antiphosphotyrosine (PT66) in the presence of low ionic strength buffers. Immobilized immunoprecipitates were incubated with protein kinase buffer augmented with 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. The 12-, 30-, 36-, 42-, 60-, 72-, and 165-kD phosphoproteins are indicated on the right. Molecular mass markers (6.5 to 204 kD) are on the left.
Phosphorylation of a 12-kD protein is altered by both inositolphosphate second messengers and anti-FKBP12.
A 12-kD inositolphosphate-binding protein had been identified using a photoaffinity labeling probe (Fig 1), a 12-kD protein had been recognized on immunoblots prepared with anti-FKBP12 (lane 5 of Fig 4), and a robust inositolphosphate-sensitive peptidylprolylcis-trans isomerase activity had been demonstrated for immunoprecipitates containing a 12-kD protein. Collectively, these data suggested, but did not prove, that the inositolphosphate-binding immunophilin has a mass of 12 kD. However, since the inositolphosphate-binding immunophilin undergoes phosphorylation, it was suggested that this phosphorylation might be perturbed if the reaction were conducted in the presence of one of its ligands, ie, IP3, IP4, or anti-FKBP12. Hence, aliquots of the immobilized immunoprecipitate were incubated in protein kinase buffer augmented with 2 μmol/L [γ-32P]ATP and 20 mmol/L magnesium acetate or 10 mmol/L manganese acetate, with or without an inositolphosphate and/or anti-FKBP12. Data obtained showed that both 1 nmol/L IP4 (see Table 1) and 2 μmol/L IP3(data not shown) induce modest increases (25% and 35%, respectively) in the labeling of a 12-kD protein, although inositol 1,3,4-trisphosphate, which is not a second messenger, failed to have a similar effect (not shown). Although the magnitude of these increases is modest, it is of the order that would be expected when the protein-kinase substrate, rather than the enzyme, is the ligand’s target. When effects of adding anti-FKBP12 were determined, it was found that the antibody induced a 44% diminution in labeling, as shown in Table 1. However, phosphorylation of proteins other than the 12-kD protein was unaffected by these ligands. (Data for the 36- and 42-kD proteins, as examples of this, are given in Table 1.) Most significantly, when both an inositolphosphate and anti-FKBP12 were added, labeling of the 12-kD protein was greater than when anti-FKBP12 was added alone but less than when IP4 (or IP3, not shown) was added alone, implying that the inositolphosphate and the antibody bound the same protein. Collectively, these data argue persuasively for a 12-kD inositolphosphate-binding immunophilin, because they identify a 12-kD protein that binds both inositolphosphate second messengers and anti-FKBP12.
Effects of IP4 and/or Anti-FKBP12 on the Labeling of a 12-kD Erythrocyte Membrane Protein
| Anti-FKBP12 1.0 nmol/L IP4 . | Sum Above Background . | ||||||
|---|---|---|---|---|---|---|---|
| Mg2+ . | Mn2+ . | ||||||
| − − . | − + . | + − . | + + . | − − . | − + . | + − . | |
| 12 kD | 18,254 | 22,879 | 10,381 | 16,892 | 14,812 | 18,563 | 8,178 |
| 36 kD | 8,878 | 8,512 | 8,438 | 9,356 | 6,358 | 6,427 | 6,240 |
| 42 kD | 16,789 | 16,367 | 16,666 | 16,299 | 9,287 | 9,685 | 9,365 |
| Anti-FKBP12 1.0 nmol/L IP4 . | Sum Above Background . | ||||||
|---|---|---|---|---|---|---|---|
| Mg2+ . | Mn2+ . | ||||||
| − − . | − + . | + − . | + + . | − − . | − + . | + − . | |
| 12 kD | 18,254 | 22,879 | 10,381 | 16,892 | 14,812 | 18,563 | 8,178 |
| 36 kD | 8,878 | 8,512 | 8,438 | 9,356 | 6,358 | 6,427 | 6,240 |
| 42 kD | 16,789 | 16,367 | 16,666 | 16,299 | 9,287 | 9,685 | 9,365 |
Human erythrocyte membranes were solubilized using 1% Nonidet P-40 and subjected to gel-filtration on Sephacryl S-300, and fractions no. 40 through 51 were then used for immunoprecipitation with antiphosphotyrosine (PT66). Aliquots (90 μL) of the immunoprecipitated, immobilized proteins were incubated in protein kinase buffer containing 1.0 μmol/L [γ-32P]ATP (3.7 × 107 dpm) and 20.0 mmol/L magnesium acetate or 10.0 mmol/L manganese acetate, in the absence or presence of 1 nmol/L IP4 and/or anti-FKBP12 (1 to 1,500), as indicated. After stopping the reactions, proteins were applied to 3% to 17% gradient SDS polyacrylamide gels. Labeling was quantitated by the densitometric scanning (Molecular Dynamics Model 300B computing densitometer) of autoradiograms. Data are reported in arbitrary units (sum above background). Labeling of the 30-, 36-, 42-, 60-, 72-, and 165-kD proteins was virtually unaffected by these ligands, and data for the 36- and 42-kD proteins are shown. Aliquots of the suspension of agarose-linked antiphosphotyrosine (PT66) that had not been exposed to the protein preparation were carried through these procedures to assess background labeling and/or nonspecific binding. These studies were repeated 4 times with different membrane preparations, and the data obtained were quantitatively the same.
Isolation of the inositolphosphate-binding immunophilin from K562 cell membranes.
The inositolphosphate-binding immunophilin was also isolated from K562 (human erythroleukemia) cell membranes. This cell was chosen because it uses a variety of well-characterized signal transduction pathways while undergoing proliferation, differentiation, and apoptosis. The peptidylprolyl cis-trans isomerase activity displayed by the K562 cell membrane preparations was qualitatively and quantitatively similar to that displayed by the erythrocyte membrane preparations. Rapamycin, FK506, IP3, and IP4 were, as had been seen for erythrocyte preparations, strongly inhibitory at nanomolar concentrations. Another similarity between the K562 cell membrane preparations and the erythrocyte membrane preparations was that the activity displayed by the solubilized membrane preparations was less sensitive to the drugs and inositolphosphates when compared with immunoprecipitated preparations. Because earlier observations (see Fig 2) had indicated that in solubilized membrane preparations the inositolphosphate-binding immunophilin is associated with complexes with apparent masses of approximately 280 or 490 kD, it was suggested that under these conditions the immunophilin’s inhibitor-binding sites might be obscured. To examine this issue, we determined the effects of the lipid/protein kinase inhibitor wortmannin upon the isomerase activity displayed by solubilized membrane preparations. If a wortmannin-sensitive molecule were tightly associated with the immunophilin, wortmannin binding might induce conformational changes not only in its target, but also in the immunophilin. When isomerase determinations were conducted in the presence of 100, 300, and 600 nmol/L wortmannin, inhibition was seen, as shown in Table 2. Because these concentrations are specifically effective for FRAP/RAFT1/mTOR54 or for phosphatidylinositol 4-kinase (type 3),55-57 it was suggested that a component such as one of these might be associated tightly with the inositolphosphate-binding immunophilin in solubilized membrane preparations.
Peptidylprolyl cis-trans Isomerase Activity of the Inositolphosphate-Binding Immunophilin From K562 Cells
| . | kobs (s−1) . |
|---|---|
| Membrane preparation | |
| No additions | 0.3924 ± 1.6 × 10−3 |
| 2.5 nmol/L IP4 | 0.2382 ± 8 × 10−4 |
| 10 nmol/L rapamycin | 0.2282 ± 1.0 × 10−3 |
| 100 nmol/L wortmannin | 0.3285 ± 9 × 10−4 |
| 300 nmol/L wortmannin | 0.2709 ± 6 × 10−4 |
| 600 nmol/L wortmannin | 0.2391 ± 4 × 10−4 |
| Immobilized immunoprecipitate | |
| No additions | 0.3211 ± 1 × 10−5 |
| 20.0 nmol/L IP3 | 0.0064 ± 1 × 10−5 |
| 1.0 nmol/L IP4 | 0.0064 ± 1 × 10−5 |
| 20.0 nmol/L PI 4,5-bisP | 0.0060 ± 1 × 10−5 |
| 20.0 nmol/L PI 4-P | 0.0060 ± 2 × 10−5 |
| 200.0 nmol/L PI 4,5-bisP | 0.0059 ± 1 × 10−5 |
| 200.0 nmol/L PI 4-P | 0.0059 ± 1 × 10−5 |
| hrFKBP12 | 0.0453 ± 1 × 10−4 |
| 20.0 nmol/L IP3 | 0.0425 ± 1 × 10−4 |
| hrFKBP12 | 0.0405 ± 4 × 10−4 |
| 200.0 nmol/L PI 4-P | 0.0364 ± 1 × 10−4 |
| 2.0 mmol/L PI 4-P | 0.0359 ± 1 × 10−4 |
| Uncatalyzed reaction | 0.0052 ± 2 × 10−5 |
| . | kobs (s−1) . |
|---|---|
| Membrane preparation | |
| No additions | 0.3924 ± 1.6 × 10−3 |
| 2.5 nmol/L IP4 | 0.2382 ± 8 × 10−4 |
| 10 nmol/L rapamycin | 0.2282 ± 1.0 × 10−3 |
| 100 nmol/L wortmannin | 0.3285 ± 9 × 10−4 |
| 300 nmol/L wortmannin | 0.2709 ± 6 × 10−4 |
| 600 nmol/L wortmannin | 0.2391 ± 4 × 10−4 |
| Immobilized immunoprecipitate | |
| No additions | 0.3211 ± 1 × 10−5 |
| 20.0 nmol/L IP3 | 0.0064 ± 1 × 10−5 |
| 1.0 nmol/L IP4 | 0.0064 ± 1 × 10−5 |
| 20.0 nmol/L PI 4,5-bisP | 0.0060 ± 1 × 10−5 |
| 20.0 nmol/L PI 4-P | 0.0060 ± 2 × 10−5 |
| 200.0 nmol/L PI 4,5-bisP | 0.0059 ± 1 × 10−5 |
| 200.0 nmol/L PI 4-P | 0.0059 ± 1 × 10−5 |
| hrFKBP12 | 0.0453 ± 1 × 10−4 |
| 20.0 nmol/L IP3 | 0.0425 ± 1 × 10−4 |
| hrFKBP12 | 0.0405 ± 4 × 10−4 |
| 200.0 nmol/L PI 4-P | 0.0364 ± 1 × 10−4 |
| 2.0 mmol/L PI 4-P | 0.0359 ± 1 × 10−4 |
| Uncatalyzed reaction | 0.0052 ± 2 × 10−5 |
Where indicated (membrane preparation), aliquots (100 μL) of the solubilized K562 cell membrane preparation were used directly, without immunoprecipitation, and the effects of IP4, rapamycin, and wortmannin were determined. To prepare immunoprecipitates, solubilized K562 cell membranes were incubated with biotin-conjugated, recombinant antiphosphotyrosine (RC20) in the presence of buffers containing 50 mmol/L NaCl. Immunoreactive proteins were isolated using agarose-linked streptavidin. The phosphatidylinositol 4-phosphate (PI 4-P) and phosphatidylinositol 4,5-bisphosphate (PI 4,5-bisP) were sonicated under an atmosphere of nitrogen immediately before being added to reaction media. Aliquots (20 μL) of immunoprecipitated, immobilized proteins were examined for peptidylprolyl cis-transisomerase activity using the substrate Suc-Ala-Leu-Pro-Phe-p-nitroanilide as described.40 Values for kobs were calculated using the equation for a first order reaction with offset (EnzFitter; BIOSOFT, Cambridge, UK). Reactions were observed for 7 to 9 half-lives. A sampling interval of 0.2 seconds was used for uninhibited reactions and 1.5 seconds for strongly inhibited reactions. The extent of the uncatalyzed reaction was assessed by conducting the determination using biotin-conjugated RC20 that had not been exposed to the K562 membrane proteins.
Isomerase activity of the inositolphosphate-binding immunophilin is inhibited by phosphatidylinositol phosphates.
The isomerase activity displayed by the inositolphosphate-binding immunophilin is inhibited to a similar degree by IP3 and IP4. This lack of specificity for one or the other of these second messengers suggested that this protein is neither an IP3- nor an IP4-binding protein per se. Rather, these data are more consistent with the presence of a pleckstrin homology (PH) domain. Because PH domains bind not only inositolphosphates but also phosphatidylinositols,58 we determined the effects of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate upon the isomerase activity of the immunophilin. As seen in Table 2, nanomolar concentrations of the phosphatidylinositol phosphates were inhibitory, producing effects similar to those seen in the presence of IP3 or IP4. This suggested that the membrane-associated inositolphosphate-binding immunophilin has a PH domain.
Immunoprecipitates from K562 cell membranes comprise a set of proteins similar to that identified for erythrocyte immunoprecipitates.
When immunoprecipitated, immobilized preparations of the inositolphosphate-binding immunophilin from K562 cell membranes were allowed to undergo phosphorylation by endogenous protein kinases and then examined on autoradiograms, proteins with relative apparent masses of 12, 36, 42, 60, 72, and 165 kD were demonstrated, as shown in Fig 6. This set of phosphoproteins is similar to that seen when erythrocyte membrane preparations were examined, with the single exception that a 30-kD protein present in erythrocyte preparations is absent here. Hence, proteins with apparent masses of 12, 36, 42, 60, 72, and 165 kD are common to immunoprecipitates from both sources. After observing that the composition of our immunoprecipitates remained constant over an extended period of time (>5 years and >120 different membrane preparations), it seemed likely that these proteins constitute specific erythrocyte or K562 cell multiprotein complexes.
Autoradiogram showing phosphoproteins generated in K562 cell membrane immunoprecipitates and effects of okadaic acid. K562 membrane proteins were immunoprecipitated using biotin-conjugated antiphosphotyrosine (RC20) in the presence of low ionic strength buffers. Immunoreactive proteins were immobilized using agarose-linked streptavidin. Immobilized immunoprecipitates were incubated with protein kinase buffer augmented with 1.0 μmol/L [γ-32P]ATP (4.4 × 106 dpm) and 10 mmol/L magnesium acetate and varied concentrations of okadaic acid (OA). After labeling, reaction mixtures were applied to 12.5% SDS polyacrylamide gels and autoradiograms were subsequently prepared. Lane 1, OA absent; lane 2, 0.02 nmol/L OA; lane 3, 0.2 nmol/L OA; lane 4, 20 nmol/L OA. Phosphoproteins identified are indicated on the right. Molecular mass markers (17 to 204 kD) are indicated on the left.
Autoradiogram showing phosphoproteins generated in K562 cell membrane immunoprecipitates and effects of okadaic acid. K562 membrane proteins were immunoprecipitated using biotin-conjugated antiphosphotyrosine (RC20) in the presence of low ionic strength buffers. Immunoreactive proteins were immobilized using agarose-linked streptavidin. Immobilized immunoprecipitates were incubated with protein kinase buffer augmented with 1.0 μmol/L [γ-32P]ATP (4.4 × 106 dpm) and 10 mmol/L magnesium acetate and varied concentrations of okadaic acid (OA). After labeling, reaction mixtures were applied to 12.5% SDS polyacrylamide gels and autoradiograms were subsequently prepared. Lane 1, OA absent; lane 2, 0.02 nmol/L OA; lane 3, 0.2 nmol/L OA; lane 4, 20 nmol/L OA. Phosphoproteins identified are indicated on the right. Molecular mass markers (17 to 204 kD) are indicated on the left.
The inositolphosphate-binding immunophilin associates with phosphatase 2A.
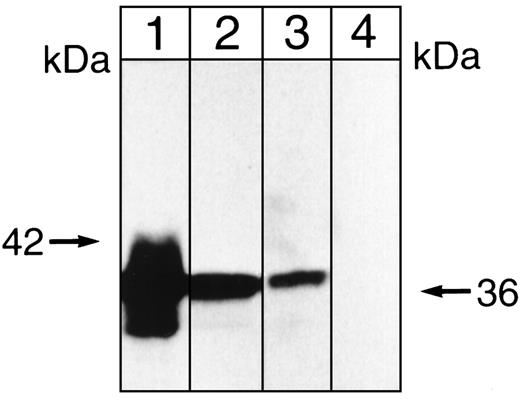
Whereas the cytosolic FKBP12 associates with the multisubunit phosphatase calcineurin, the presence of proteins with relative masses of 36, 60, and 72 kD in these immunoprecipitates suggested another multisubunit phosphatase, ie, phosphatase 2A (PP-2A). PP-2A, which can be either cytosolic or membrane-associated,59 comprises a 36-kD catalytic subunit (C) that undergoes phosphorylation on tyrosine and threonine, a 60-to 65-kD (A) subunit that functions as a scaffolding protein,60 and a B (or B′) subunit that is recruited by the scaffolding protein. A 72-kD B′ subunit is reportedly characteristic of erythroid cells.61 Although a 42-kD α4 subunit of PP-2A has also been identified,62-64its presence in a terminally differentiated cell has not been reported. To obtain initial evidence of the presence of PP-2A in our immunoprecipitates, we determined the effects of the inhibitor okadaic acid, because it inhibits PP-2A with an I50 of 0.2 nmol/L, while inhibiting PP-1 with an I50 of 20 nmol/L and calcineurin (PP-2B) with an I50 of approximately 5 μmol/L.61 When immobilized immunoprecipitates were allowed to undergo phosphorylation by endogenous protein kinase(s) in the presence of 0.02, 0.2, or 20 nmol/L okadaic acid, labeling appeared enhanced by the 0.2 nmol/L concentration of the inhibitor (compare lanes 2 and 3 in Fig 6). This was consistent with the presence of PP-2A, rather than PP-1 or calcineurin. When the proteins present in these immunoprecipitates were then separated on SDS polyacrylamide gels, blotted onto membranes, and overlaid with a monoclonal antibody against the catalytic subunit of PP-2A, a strong positive signal at 36 kD was obtained, as shown in lane 3 of Fig7. Interestingly, when immunoprecipitation was conducted in the presence of rapamycin, this signal could no longer be demonstrated (see lane 4 of Fig 7). Collectively, these data indicated that PP-2A accompanies the inositolphosphate-binding immunophilin in immobilized immunoprecipitates obtained in the absence of rapamycin.
Immunoblot showing that anti-PP-2A recognizes a 36-kD protein. Aliquots of a K562 cell lysate (lane 1) or a solubilized K562 cell membrane preparation (lane 2) were applied to 7.5% SDS polyacrylamide gels along with samples of low-ionic strength, antiphosphotyrosine (RC20) immunoprecipitates (lane 3) and samples of the immunoprecipitate obtained in the presence of 200 nmol/L rapamycin (lane 4). After development of these gels, proteins were blotted onto polyvinylidene difluoride (PVDF) membranes and blots were overlaid with (1 to 5,000) monoclonal anti-PP-2Ac (clone 46, IgG1). Second antibody was (1 to 5,000) goat antimouse IgG conjugated with horseradish peroxidase and detection was by enhanced chemiluminescence (ECL). The 42-kD molecular mass marker is shown on the left.
Immunoblot showing that anti-PP-2A recognizes a 36-kD protein. Aliquots of a K562 cell lysate (lane 1) or a solubilized K562 cell membrane preparation (lane 2) were applied to 7.5% SDS polyacrylamide gels along with samples of low-ionic strength, antiphosphotyrosine (RC20) immunoprecipitates (lane 3) and samples of the immunoprecipitate obtained in the presence of 200 nmol/L rapamycin (lane 4). After development of these gels, proteins were blotted onto polyvinylidene difluoride (PVDF) membranes and blots were overlaid with (1 to 5,000) monoclonal anti-PP-2Ac (clone 46, IgG1). Second antibody was (1 to 5,000) goat antimouse IgG conjugated with horseradish peroxidase and detection was by enhanced chemiluminescence (ECL). The 42-kD molecular mass marker is shown on the left.
Separation of isomerase activity and protein kinase activity of immobilized immunoprecipitates.
The peptidylprolyl cis-trans isomerase activity and the protein kinase activity that are displayed by immobilized immunoprecipitates were separated by conducting immunoprecipitation in the presence of buffers with increased ionic strength. When buffers were augmented with 150 to 300 mmol/L NaCl, rather than the 50 mmol/L NaCl that had been used, the immunoprecipitates displayed the characteristically robust, inositolphosphate-sensitive peptidylprolyl cis-trans isomerase activity (data presented in Table 3); however, protein kinase activity could no longer be demonstrated. Interestingly, when low ionic strength buffers were augmented with increasing concentrations of rapamycin (10 to 200 nmol/L), in addition to isomerase activity being inhibited as expected (see Table 3), protein kinase activity was again diminished. Silver-stained SDS gels indicated that the 12-kD protein was present however (data not shown). This observation in conjunction with data presented in Fig 7 suggest that, although the inositolphosphate-binding immunophilin can be immunoprecipitated with both a protein kinase and PP-2A, these components can be dissociated from the immunoprecipitate by high ionic strength or by rapamycin.
Effects of Ionic Strength and Rapamycin on the Peptidylprolyl cis-trans Isomerase Activity of Immunoprecipitates Obtained From K562 Cell Membranes
| . | kobs (s−1) . |
|---|---|
| IP in presence of 150 mmol/L NaCl | |
| No additions | 0.3942 ± 5 × 10−4 |
| 20 nmol/L IP3 during determination, only | 0.0094 ± 5 × 10−5 |
| IP with 10.0 nmol/L rapamycin | 0.2973 ± 6 × 10−4 |
| IP with 10.0 nmol/L rapamycin, 20.0 nmol/L IP3 during determination | 0.0314 ± 8 × 10−4 |
| IP in presence of 50 mmol/L NaCl | |
| No additions | 0.3234 ± 4 × 10−4 |
| IP with 10.0 nmol/L rapamycin | 0.2022 ± 3 × 10−4 |
| IP with 200.0 nmol/L rapamycin | 0.0051 ± 5 × 10−5 |
| Uncatalyzed reaction | 0.0046 ± 2 × 10−5 |
| . | kobs (s−1) . |
|---|---|
| IP in presence of 150 mmol/L NaCl | |
| No additions | 0.3942 ± 5 × 10−4 |
| 20 nmol/L IP3 during determination, only | 0.0094 ± 5 × 10−5 |
| IP with 10.0 nmol/L rapamycin | 0.2973 ± 6 × 10−4 |
| IP with 10.0 nmol/L rapamycin, 20.0 nmol/L IP3 during determination | 0.0314 ± 8 × 10−4 |
| IP in presence of 50 mmol/L NaCl | |
| No additions | 0.3234 ± 4 × 10−4 |
| IP with 10.0 nmol/L rapamycin | 0.2022 ± 3 × 10−4 |
| IP with 200.0 nmol/L rapamycin | 0.0051 ± 5 × 10−5 |
| Uncatalyzed reaction | 0.0046 ± 2 × 10−5 |
Solubilized K562 membranes were incubated with biotin-conjugated, recombinant antiphosphotyrosine (RC20) under high (150 mmol/L NaCl) or low (50 mmol/L NaCl) ionic strength conditions, as indicated. Rapamycin and IP3 were present during the immunoprecipitation or added at the time of the isomerase determination, as indicated. Immune complexes were isolated using agarose-linked streptavidin. Aliquots of immobilized proteins (20 μL) were examined for peptidylprolylcis-trans isomerase activity using the substrate Suc-Ala-Leu-Pro-Phe-p-nitroanilide, as described.40 Values for kobs were calculated using the equation for a first order reaction with offset (EnzFitter; BIOSOFT). Reactions were observed for 7 to 9 half-lives. A sampling interval of 0.2 seconds was used with uninhibited reactions; it was 1.5 seconds when immunoprecipitation had been conducted in the presence of 200 nmol/L rapamycin or when the reaction was inhibited by 20 nmol/L IP3. The extent of the uncatalyzed reaction was assessed by conducting the determination using biotin-conjugated RC20 that had not been exposed to the K562 cell membrane preparation.
Abbreviation: IP, immunoprecipitation.
Immunoprecipitation of the inositolphosphate-binding immunophilin with anti-FKBP12.
The inositolphosphate-binding immunophilin was immunoprecipitated from K562 cell membrane preparations using a goat polyclonal antibody against the 19 C-terminal amino acids (90-108) of human FKBP12. This C-terminal region includes conserved sequences that are characteristic of the FKBPs. However, antibodies against the 19 N-terminal amino acids of FKBP12 failed to recognize the inositolphosphate-binding immunophilin, suggesting that, as seen with other members of the FKBP class, the inositolphosphate-binding immunophilin has conserved C-terminal sequences but distinctive N-terminal sequences. Immobilized immunoprecipitates obtained using (C-19) anti-FKBP12 had qualitatively the same composition as immunoprecipitates obtained using antiphosphotyrosine (data not shown). They also displayed comparable peptidylprolyl cis-trans isomerase activity as immunoprecipitates obtained with antiphosphotyrosine. Values obtained for kobs when examining anti-FKBP12 immunoprecipitates were 0.3575 ± 5 × 10−4 s−1 and 0.0058± 2 × 10−5 s−1 for the uninhibited and the inhibited (20 nmol/L IP3) reactions, respectively. These values can be compared with the values of 0.3942 ±5 × 10−4 s−1and 0.0094 ± 5 × 10−5 s−1obtained for the uninhibited and inhibited reactions when immunoprecipitation was with antiphosphotyrosine (the latter were reported in Table 3).
The proteins in immunoprecipitates obtained using anti-FKBP12 in the presence of high-ionic strength buffers were separated on SDS polyacrylamide gels and samples of the 12-kD protein were isolated and used for amino acid sequencing. When tryptic peptides were generated, one yielded a 20 amino acid sequence with 100% identity to a sequence near the N-terminus of human cytokeratin 10. Finding a sequence that is shared with this cytoskeletal protein, which had been cloned from a human peripheral blood cDNA library,65 suggested a cytoskeletal localization for the inositolphosphate-binding immunophilin. Interestingly, the sequence identified also has approximately 53% identity and 94% homology with a sequence near the middle of leukophysin,66 an RNA helicase A-related protein present in membranes of cytotoxic T cells. Alignments are shown below.
Collectively, the data presented here identify a novel member of the FKBP class of immunophilins that is membrane-associated and capable of binding nanomolar concentrations of IP3, IP4, and phosphatidylinositol phosphates. It is suggested that this protein might be called IPBP12, for 12-kD inositol phosphate binding protein.
DISCUSSION
The data presented here identify a novel membrane-associated member of the FKBP class of immunophilins. Collectively, these studies have (1) identified a 12-kD inositolphosphate-binding protein (Fig 1), (2) identified a 12-kD protein that is recognized on immunoblots prepared with anti-FKBP12 (lane 5, Fig 4), (3) identified a 12-kD protein that binds both IP4 and anti-FKBP12 (Table 1), and (4) demonstrated a robust peptidylprolyl cis-trans isomerase activity that is strongly inhibited by nanomolar concentrations of rapamycin, FK506, IP3, IP4, and phosphatidylinositol phosphates (Tables 2 and 3). This immunophilin, which I have suggested might be called IPBP12, is distinguished from FKBP12 by displaying peptidylprolyl cis-trans isomerase activity that is inhibited by nanomolar concentrations of IP3, IP4, and phosphatidylinositol phosphates and by undergoing phosphorylation by an associated protein kinase. IP3, IP4, and phosphatidylinositol 4- and 4,5-phosphates are suggested to be physiological ligands for IPBP12. It might be noted that the second messenger cyclic ADP-ribose is reported to be a physiological ligand for FKBP12.6.67 The observation that IPBP12 binds the inositolphosphate second messengers and phosphatidylinositol phosphates similarly, and in the low nanomolar range, suggests that the membrane-associated immunophilin has a pleckstrin homology (PH) domain, however. Interestingly, when the structure of the PH domain of (mouse brain) spectrin was examined, it was found to comprise a ligand-binding pocket moderately related to the drug-binding pocket of FKBP12.68 In FKBP12, the residues Y26, F46, V55, I56, W59, I76, and F99 form a hydrophobic pocket into which the pipecolinyl ring of rapamycin or FK506 fits snugly.69 The indole ring of W59 forms the bottom of this pocket. Critical residues of the ligand-binding pocket of the spectrin PH domain were identified as R7, V26, F37, V56, K59, and Y86. When these were aligned with residues of the FKBP12 drug-binding pocket, K59 of the PH domain corresponded to W59 in the immunophilin drug-binding pocket. The presence of a positively charged lysine residue in the spectrin PH domain, in place of tryptophan in FKBP12, suggests why the former binds negatively charged inositolphosphates and phosphatidylinositol phosphates, whereas the latter does not. It is tempting to speculate that IPBP12 will be found to differ from FKBP12 by having a critical lysine (or arginine) residue in its ligand-binding pocket.
IPBP12 can be immunoprecipitated from human erythrocyte or K562 cell membrane preparations accompanied by proteins with relative masses of 36, 42, 60, 72, and 165 kD. It is suggested that this immunoprecipitate, which displays both protein kinase activity and phosphatase 2A activity, constitutes a specific multiprotein complex. Although our data have provided no insight into the identity of the 165-kD protein, the 36-, 60-, and 72-kD proteins are suggested to be the C, A, and B′ subunits, respectively, of PP-2A. Because it has been reported that, in quiescent K562 cells, PP-2A is bound to and phosphorylated by the 42-kD α subunit of CKII,70 it is suggested that the 42-kD protein in these immunoprecipitates might be this α subunit. In support of this, CK II has been shown to be associated with the human erythrocyte membrane53 and CK II has also been shown to bind and phosphorylate FKBP25,35FKBP52,36 and Fpr3.47
The observation that immunoprecipitated IPBP12 is accompanied by PP-2A and that this association appears rapamycin sensitive is of particular interest because Peterson et al24 have suggested that the FRAP-dependent, rapamycin-sensitive event occurring during late G1 of the cell cycle entails FRAP binding to and restraining the activity of PP-2A. An association between FRAP and PP-2A is quite feasible, because both FRAP/RAFT1/mTOR and the A subunit of PP-2A have protein-interaction motifs sometimes referred to as HEAT repeats.71 Because the A subunit of PP-2A binds its catalytic subunit by means of interactions involving these motifs,72 FRAP/RAFT1/mTOR might bind the catalytic subunit of PP-2A by the same means. Furthermore, regarding their premise that association between FRAP and PP-2A inhibits the phosphatase activity of the latter, it is recalled that the 240-kD cain (for calcineurin inhibitor) associates with the phosphatase calcineurin, thereby inhibiting its activity.73 Finally, our indications that PP-2A can associate with an FKBP (IPBP12) suggests that assembly of a cytosolic FKBP12/FRAP/PP-2A complex or a membrane-associated IPBP12/CK II/PP-2A complex could be a critical event in cell signaling and that rapamycin, by binding the FKBP, can prevent this. Further studies on IPBP12 are expected to provide an opportunity to examine our broad hypothesis that immunophilins act by facilitating the assembly of multiprotein complexes that participate in signal transduction.
ACKNOWLEDGMENT
I wish to express my gratitude to Dr Stuart L. Schreiber for his suggestions at the very beginning of these studies and to Dr Robert F. Standaert, who gave us the benefit of his expertise and insight concerning the determination of peptidylprolyl cis-transisomerase activity. Furthermore, I gratefully acknowledge the particular skills of Verrell M. Randolph, Joseph Fernicola, George Szu-Wei Lin, Vaishali Patel, Weikang Tao, Bin Tian, Deepmala Yadav, Dr Louis A. Scala, and Rashida Mc Cain at various stages of this work. I thank Dr Mukund J. Modak, Dr Michael B. Mathews, and Dr Surren N. Sehgal for critically reading this manuscript. I am, in addition, indebted to Dr Ihor Berkersky (Fujisawa USA, Deerfield, IL) for FK506 and to Dr Suren N. Sehgal (Wyeth-Ayerst Research, Princeton, NJ) for the rapamycin used in these studies.
Supported in part by a Career Advancement Award (DCB-910391) from the National Science Foundation, by Wyeth-Ayerst Research, and by Biomedical Research Support from UMDNJ-New Jersey Medical School.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Earlene Brown Cunningham, PhD, Department of Biochemistry and Molecular Biology, UMDNJ-New Jersey Medical School, 185 S Orange Ave, Newark, NJ 07103-2714; e-mail: cunnineb@umdnj.edu.

![Fig. 3. Autoradiogram showing results of phosphoamino acid analyses on the 12-kD erythrocyte membrane protein. Sephacryl S-300 column fractions no. 40 through 51 were concentrated 6-fold and allowed to undergo phosphorylation in the presence of protein kinase buffer containing 1 μmol/L [γ-32P]ATP (1 × 107dpm), 4 mmol/L magnesium acetate, and 1 mmol/L manganese acetate, without (−) or with (+) 1 μmol/L IP3. Reaction mixtures were subsequently applied to 3% to 17% gradient SDS polyacrylamide gels and a 12-kD phosphoprotein was identified on autoradiograms. Gel segments containing this component were cut out and the phosphoproteins were hydrolyzed in 6 N HCl at 110°C for 65 minutes. Liberated phosphoamino acids were separated by thin-layer chromatography using the system absolute ethanol:25% ammonia (3.5:1.6).46 Authentic standards (phosphoserine, phosphothreonine, and phosphotyrosine) were cospotted in each sample lane and subsequently visualized with ninhydrin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2778.420k10_2778_2789/7/m_blod42010003w.jpeg?Expires=1769156137&Signature=Wt5hglpgvbPaVqG6luw5BVXa7wm9dLDVvTMKCVxgla55q5EBjtDXTBgx8calbum-VlIEk0mLPX22k5PUCrZ7K8wMgTKkd0qAEYFF5DgZERIT6ORgWJ04UOlqn4hS84Uvcp-D~1PfMiNNCk0hq68CXltoQgD46wGPtXUF0jcBjcNafFcfwTbE4qSc1PE1iyCeivf00FLk3txTcTgzDJChFX9i0QznJlSuVJ~jdva7JkXWVtSdbDRfshDghgSc0ES5prZ9QeyZMU7jD49Q1wooF5VJIeV9-xLGMRvxFm8g0it87qpE48vGzqVY3CpPspb9q5lmTIxhHJjdm0SrMu1yTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Identification of a 12-kD protein on gels, on autoradiograms, and on blots. Proteins present in Sephacryl S-300 column fractions no. 40 through 51 (concentrated 6-fold) were separated on 3% to 17% gradient nondenaturing (Nonidet P-40) polyacrylamide gels and slowly migrating components (400 to 600 kD) were eluted and then applied to 3% to 17% gradient SDS polyacrylamide gels that were subsequently silver stained. The signal at 12 kD is shown (lane 1). The slowly migrating components (400 to 600 kD) were eluted and used for immunoprecipitation with agarose-linked antiphosphotyrosine (PT-66) and the resulting immunoprecipitate was eluted onto 3% to 17% gradient SDS polyacrylamide gels that were subsequently silver stained. Signals in the lower region of the gel are shown (lane 2). hrFKBP12 was applied to a 3% to 17% gradient SDS polyacrylamide gel that was subsequently silver stained (lane 3). Immunoprecipitated immobilized proteins were allowed to undergo phosphorylation by endogenous protein kinase(s) in the presence of 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. After the separation of labeled proteins on 3% to 17% gradient SDS polyacrylamide gels, autoradiograms were prepared. The lower region of the gel is shown (lane 4). Immobilized immunoprecipitated proteins were eluted from the solid support onto 3% to 17% SDS polyacrylamide gels. After development, proteins were transferred to a membrane and these blots were then overlaid with rabbit polyclonal anti-FKBP12 (1 to 10,000). Goat antirabbit antibody conjugated to horseradish peroxidase (1 to 70,000) was added and detection was by enhanced chemiluminescence (ECL). The lower region of the blot is shown (lane 5). hrFKBP12 was applied to a 3% to 17% gradient SDS polyacrylamide gel and transferred to a membrane, and the blot was overlaid with anti-FKBP12 and detected as above (lane 6). The 6.5-kD molecular mass markers are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2778.420k10_2778_2789/7/m_blod42010004w.jpeg?Expires=1769156137&Signature=KagfD2NJaSyXtlA2HiEw2h6FkhGW8bV74MW-mRw0Sd1OsNCqZuU8dNodBaTnO-eWsdIfJPboT74jLCtYxUMGahUOmLHAjc12ZT-tXELSN-q2mwfENZHOTX7mjyddIP9mIvzgu6x6dUpC-e9QQ3Y9UKpH39c-TOdgYTfKMppsiYRPLgZsoL1YOXnHVWXkeptj8gz-o2baG4qWGDKmrITDyI-xJ12kx~7QHlEKY~hKf4buKRM1JCUVdHUTVm8ZZZi3Cf27DZ2DCyZBmuYm~n6BnlfjA-yOxJqF8IUD7QITb4QixfuFKsIa1UzDj8qYE16vkzvbaFi5FyfWf-tN6mD5Zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Autoradiogram showing phosphoproteins generated by protein kinase(s) present in immobilized erythrocyte immunoprecipitates. Sephacryl S-300 column fractions no. 40 through 51, combined and concentrated 6-fold, were incubated with agarose-linked antiphosphotyrosine (PT66) in the presence of low ionic strength buffers. Immobilized immunoprecipitates were incubated with protein kinase buffer augmented with 1 μmol/L [γ-32P]ATP (4 × 107 dpm) and 20 mmol/L magnesium acetate. The 12-, 30-, 36-, 42-, 60-, 72-, and 165-kD phosphoproteins are indicated on the right. Molecular mass markers (6.5 to 204 kD) are on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2778.420k10_2778_2789/7/m_blod42010005w.jpeg?Expires=1769156137&Signature=BlaYHqQAHCSYHaajRjNWFp-NTEQfssVVrXRzAZ7~vySTKg7ej~eSQStgAriri-OIJcwO8t1Ph9VreQDlpl6KctTUK0vR73vDf23av4xWJ7oAqsZ8BQpFpfGrX09cyOS3clbPtuYjzORCQL~XUZCNO5MO2kjMZvZ-E4LMh0fnXTg-AldqwgpSDiir9gMSu4PKrlRdnvgiYLxSgB5QWp0G4PvNSbIoAXWfa-VYP3s51FGT68RakohfLieaaoFJU6HmMIeNfuNrEqtYCZJOF6c8SeXr-9ecTESSebGf92SgxfMiuYsC8hNDAXOo9ELW6qBPgG03aqS8N9dFhn84arZJ6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Autoradiogram showing phosphoproteins generated in K562 cell membrane immunoprecipitates and effects of okadaic acid. K562 membrane proteins were immunoprecipitated using biotin-conjugated antiphosphotyrosine (RC20) in the presence of low ionic strength buffers. Immunoreactive proteins were immobilized using agarose-linked streptavidin. Immobilized immunoprecipitates were incubated with protein kinase buffer augmented with 1.0 μmol/L [γ-32P]ATP (4.4 × 106 dpm) and 10 mmol/L magnesium acetate and varied concentrations of okadaic acid (OA). After labeling, reaction mixtures were applied to 12.5% SDS polyacrylamide gels and autoradiograms were subsequently prepared. Lane 1, OA absent; lane 2, 0.02 nmol/L OA; lane 3, 0.2 nmol/L OA; lane 4, 20 nmol/L OA. Phosphoproteins identified are indicated on the right. Molecular mass markers (17 to 204 kD) are indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2778.420k10_2778_2789/7/m_blod42010006w.jpeg?Expires=1769156137&Signature=eNtXwIcxwG-jmCbW1CiLGcmpX2n2mQ1JLo-FKGlU6uAcpt3hwvkUJ9izBrASSr5DJ1XFKQkiWKPxKbitk4-BEc69nYrVuzvrFDXchNIIWfRwhY6s3tF6yY-GJq6z-tfHg5m5VxE0A5GTPlvCm4Li5CEJbBuf1EfbkzoybJ4UAwCbIvn9zvDiwjSaorEU9v-NuiD8YMvpvM1POdP-3wnxTzxdg-xQnC5nwPubzVmVnkbgbCEJllNz3Cb1hRlBGBKBOxRXxQMpcWPcs8xJxDCS3oXsQAhi0YfaxiEv45YmrmRByWTgDjC7JUfvwgJEJaR8GDy84w7oIqOJfJrMPvwMNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal