T-lymphocyte migration into tissues requires focal degradation of the basement membrane. In this study, we show that transient adherence to fibronectin induces the production of activated forms of matrix metalloproteinase-2 (MMP-2) and MMP-9, as well as downregulation of tissue inhibitor of metalloproteinase-2 (TIMP-2) by T-cell lines. MMP-2 activation was likely achieved by inducing a coordinated expression of membrane-type matrix metalloproteinase-1 (MMP-14), a major activator of MMP-2. Blocking monoclonal antibodies against 4, 5, and v integrins strongly reduced MMP-2 and MMP-9 production induced by fibronectin. Disrupting actin cytoskeleton organization by cytochalasin D strongly enhanced fibronectin-induced MMP-2 and MMP-9 expression. Inhibiting Src tyrosine kinases with herbimycin A reduced MMP-2 and MMP-9 production with no effect on cell attachment. By contrast, G-protein inhibition by pertussis toxin, or transfection with a dominant negative mutant of Ha-Ras strongly increased fibronectin-induced MMP-2 and MMP-9. Inhibition of PI3 kinase, MAPkinase (MEK1), or p38 MAPkinase by wortmannin, PD 98059, or SB 202190, respectively, strongly promoted fibronectin-induced MMP2 and MMP-9. Cells at high density lost their ability to synthesize MMP-2 and MMP-9 in response to fibronectin and MMP expression was restored by transfection with a dominant-negative mutant of Ha-Ras or by treatment with wortmannin, PD 98059, or SB 202190. Our findings suggest that adhesion to fibronectin transduces both stimulatory (through Src-type tyrosin kinases) and inhibitory signals (through Ras/MAPKinase signaling pathways) for MMP-2 and MMP-9 expression by T lymphocytes and that their relative predominance is regulated by additional stimuli related to cell adhesion, motility, and growth.
THE DEVELOPMENT OF inflammatory infiltrates in target tissues is a common pathologic substrate in many chronic inflammatory and autoimmune diseases.1 Tissue infiltration by T lymphocytes requires dynamic and finely regulated interactions between lymphocytes, endothelial cells, and the underlying basement membrane mediated via a complex array of surface receptors.2-4 Lymphocyte receptors participating in the interactions with endothelial cells belong to two major classes of proteins: selectins that mediate the very initial interactions with carbohydrate ligands on the endothelial cell surface, and integrins that are responsible for the tight adhesion and further transmigration through the endothelial cell junctions.
Once the transmigration process is accomplished, lymphocytes begin to interact with the underlying basement membrane and interstitial matrix.5-7 The basement membrane is a specialized extracellular matrix structure synthesized by epithelial and endothelial cells composed essentially of collagen type IV, laminin, and perlecan. Additional important components are nidogen/entactin, collagen type V, and fibronectin among other glycoproteins and proteoglycans.8 Its structural organization in multilayers conforms a resistant barrier.
Vectorial motility across the basement membrane and interstitial matrix requires coordinated series of adhesion-release steps and focal matrix degradation.7,9,10 Lymphocyte integrins have a major role in mediating interactions with extracellular matrix proteins during the transmigration process.5,7,9-11 However, the mechanisms involved in basement membrane degradation by transmigrating lymphocytes are much less understood. Recent contributions suggest an important role for matrix metalloproteinases in basement membrane disruption by T lymphocytes.12
The matrix metalloproteinase (MMP) family includes a growing family of endopeptidases that have been classified according to their substrate specificity into gelatinases, stromelysins, and collagenases.10,13,14 Recently, a new subclass of transmembrane MMP (MT-MMP) expressed on the surface of invasive tumor cells or in the surrounding stromal cells has been identified.14 MMP are synthesized in an inactive form or zymogen with an aminoterminal propeptide that must be cleaved to yield the active enzyme.10,13 15
The 92 kD gelatinase (gelatinase B, MMP-9) and the 72 kD gelatinase (gelatinase A, MMP-2), efficiently degrade native collagen types IV and V, fibronectin, entactin, and elastin. Therefore, these proteases are believed to be of crucial importance in processes requiring basement membrane disruption such as tumor invasion and metastasis10,13-16 and, presumably, tissue infiltration by leukocytes, the pathologic substrate of chronic inflammatory diseases. Gelatinases are secreted in association with specific inhibitors of MMP or TIMP (MMP-2/TIMP-2 and MMP-9/TIMP-1) that bind to the carboxy-terminal domain of progelatinases.10,13,15 17
The precise mechanisms of gelatinase activation are beginning to be understood. Other members of the MMP family have been proposed as putative physiologic activators of progelatinases.18Recently, membrane-bound MMP MT1-MMP (MMP-14) has been shown to be a potent activator of progelatinase A by cleaving the propeptide, a process that also promotes gelatinase-A autoproteolytic activation.14,19 Gelatinase A, in turn, is able to process interstitial collagenase (MMP-1)20 and collagenase-3 (MMP-13).21
It has been recently shown that T lymphocytes constitutively produce small amounts of gelatinase B (MMP-9), which is highly upregulated upon phorbol ester22 or interleukin-2 (IL-2) stimulation.22 T- and B-lymphoblastoid cell lines also exhibit baseline production of MMP-9.24 In T-lymphoblastoid cell lines chemokines, such as MIP-1α and RANTES, and cytokines, such as IL-2 and IL-4, upregulate MMP-9 expression.25 Functional studies have shown that increased MMP-9 production leads to an enhanced invasiveness through reconstituted basement membrane Matrigel.23,25 Gelatinase-A (MMP-2) production by T lymphocytes has only been detected under much more restricted conditions such as IL-2 stimulation for long periods of time23 or upon VCAM-1–dependent adhesion to cultured endothelial cells.26 However, the mechanisms underlying MMP expression and activation by T lymphocytes have not been investigated.
According to the hypothesis that attachment to the extracellular matrix proteins and their subsequent degradation may be related events, the purpose of our study was to determine whether basement membrane components are able to modulate MMP synthesis by human lymphocytes. Herein, we show that, among several basement membrane constituents, only intact fibronectin possesses the information necessary not only for inducing MMP-9 and MMP-2 production by T-cell lines but also for promoting MMP-2 activation, probably by inducing coordinated expression of MT1-MMP.
MATERIALS AND METHODS
Monclonal antibodies (MoAb), chemicals, extracellular matrix proteins, and peptides.
Blocking MoAb anti-β1 chain (CD29) K20 and anti-α5 chain (CD49e) SAM1 were purchased from Immunotech (Marseille, France). Blocking MoAb anti-α4 (CD49d) HP 2/1 and anti-αv (CD51) 17E6 were kindly provided by Dr F. Sánchez-Madrid (Hospital La Princesa, Madrid, Spain) and Dr F. Mitjans (Merck Biomedical Research Laboratory, Barcelona, Spain), respectively. MoAb 93.1B3 (anti-CD20) was kindly provided by Dr R. Vilella (Hospital Clı́nic, Barcelona, Spain). Anti–MMP-2 (Ab-3), polyclonal Ab anti–MMP-14 (Ab-2), anti–TIMP-1 (Ab-2), and anti–TIMP-2 (Ab-1) were purchased from Oncogene Research Products (Cambridge, MA).
Human plasma fibronectin was obtained from Sigma Chemical Co (St Louis, MO). Mouse laminin-1 was a generous gift from Dr H.K. Kleinman (National Institute of Dental Research, Bethesda, MD). Type-I and type-IV collagen, as well as polylysine were purchased from Collaborative Research (Bedford, MA). Fibronectin-derived synthetic peptides GRGDSPC and EILDVPST and control peptide GRGES were obtained from Peninsula Laboratories (Belmont CA).
Cytochalasin D, EGTA, herbymicin A, pertussis toxin, phorbol 12-myristate 13-acetate (PMA), and p-aminophenylmercuric acetate (APMA) were purchased from Sigma Chemical Co. Wortmannin, 2′amino-3′methoxy-flavone (PD 98059), and 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole (SB202190) were obtained from Calbiochem-Novabiochem (La Jolla, CA).
Cell culture.
Human T-cell lines (CCRF-CEM, Jurkat, Molt-4) were obtained from the European Collection of Cell Cultures (Salisbury, UK) and grown in RPMI 1640 (GIBCO Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine, and 50 μg/mL gentamycin at 37°C, 5% CO2, up to a maximal cell density of 8 × 105 cells/mL at which point the cells were passed 1:10. Unless otherwise indicated, cells were used in experiments at a concentration of 2 to 3 × 105 cells/mL. Human umbilical vein endothelial cells (HUVEC) were obtained from freshly delivered cords and grown as reported.28 Cells at passage 4 to 6 were used for experiments.
Adhesion assays.
Extracellular matrix proteins were diluted in phosphate-buffered saline (PBS) at 100 μg/mL. 96-well tissue culture plates were coated with either laminin-1, type-I collagen, type-IV collagen, fibronectin, or polylysine at 5 μg/well. Laminin-1 was incubated for 1 hour at 37°C whereas fibronectin, type-I collagen, type-IV collagen, and polylysine were incubated overnight at 4°C. Then, the remaining fluid was aspirated. Cells were suspended in serum-free RPMI medium, placed on coated plates at 1.5 × 105 cells/well and incubated at 37°C for 90 minutes. In some experiments, cells were preincubated in suspension with the mentioned chemicals for 30 minutes at 37°C before the attachment step was performed. Nonadherent cells were removed by aspiration and wells were washed once with warm, serum-free medium. Adherent cells were fixed and stained with 0.2% crystal violet in 20% methanol in PBS for 20 minutes and then washed repeatedly with distilled H2O. After solubilization with 1% SDS, the optical density was measured with a spectrophotometer at 600 nm wavelength.
Gelatin zymography.
Lymphoblastoid cell lines were washed and resuspended in serum-free RPMI medium and cultured in suspension or in dishes coated with different extracellular matrix proteins or fibronectin-derived synthetic peptides. Alternatively, in some experiments fibronectin or synthetic peptides were added in solution. Twenty-four hours later, the conditioned medium was collected, centrifuged, and stored at −20°C. In some experiments, cells were preincubated with purified MoAb at 10 μg/mL for 30 minutes at 4°C. In additional experiments, cells were incubated at 37°C with chemicals. Cytochalasin D was applied at 20 μmol/L; EGTA at 1 mmol/L; herbimycin A at 2 μmol/L; pertussis toxin at 1μg/mL; PMA at 10 ng/mL; wortmannin at 10 nmol/L; PD98059 at 5 μmol/L; and SB 202190 at 0.5 μmol/L. These products were added 30 minutes before exposure to fibronectin. Experiments with chemicals were run for only 6 hours to ensure cell viability that was always confirmed by trypan blue exclusion.
The conditioned medium obtained from 107 cells was concentrated 200-fold using Urifil-10 concentrator devices (Millipore, Molsheim, France) at 4°C, and subjected to SDS-PAGE through 10% polyacrylamide gels copolymerized with 0.2 g/mL gelatin (Bio-Rad Laboratories, Hercules, CA). Gels were washed with 2.5% Triton-X-100, rinsed with 10 mmol/L Tris and incubated overnight at 37°C in 50 mmol/L Tris, 5 mmol/L CaCl2, and 1 μmol/L ZnCl2. Gels were fixed and stained with 0.2% Coomassie blue R250. After destaining, gelatinolytic signals were quantified by densitometry (Gel Analysis Program SW5000 software, Ultraviolet Products, Cambridge, UK).
Unconcentrated conditioned medium from HUVEC was used as a control in some zymograms either freshly obtained or treated with 1 mmol/L p-APMA for 3 hours at 37°C.
Western blot analysis.
Conditioned medium from 108 CEM cells per condition was passed through a 10 mL gelatin-Sepharose column (Sigma). The column was washed with 10 volumes of 50 mmol/L Tris, pH 7.6, 0.5 mol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij35 (Sigma) and the gelatin-bound proteins were eluted with 5 volumes of 50 mmol/L Tris, pH 7.6, 0.5 mol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij35, and 10% dimethyl sulfoxide (DMSO). The eluted solution was dialyzed against 50 mmol/L Tris, pH 7.6, 0.2 mol/L NaCl, 5 mmol/L CaCl2 , 0.02% Brij35, and concentrated.
Concentrated samples were electrophoresed under nonreducing conditions through a 10% polyacrylamide gel and transferred to a methanol-treated Hybond-PVDF membrane. After an overnight blocking step with 5% nonfat powdered milk in tris-buffered saline (TBS) with 0.05% Tween 20 (TBS-T) at 4°C, the membrane was incubated with 1 μg/mL of Ab-3 (anti-MMP-2) MoAb in 0.05% nonfat powdered milk in TBS-T for 1 hour at room temperature. After two washes with TBS-T, the membrane was incubated with an horseradish peroxidase (HRP)-conjugated goat antimouse polyclonal antibody at 0.2 μg/mL for 1 hour at room temperature, washed as above, incubated 1 minute with ECL Western blotting detection reagants (Amersham International plc, Little Chalfont, UK) and exposed to x-ray film.
Flow cytometry.
CEM cells (105 per condition) were incubated with either anti–MMP-2, anti–TIMP-1, anti–TIMP-2, or anti–MMP-14 at 5 μg/mL in PBS containing 2% FCS and 0.01% NaN3 for 20 minutes at 4°C. Cells were washed twice in 2% FCS, 0.01% NaN3 in PBS, and incubated with a biotinylated rabbit antimouse antibody (Dakopatts, Denmark) at 1:100 dilution for 20 minutes at 4oC. After two washes, cells were incubated as above with Streptavidin Tricolor fluorescent conjugate (Caltag Laboratories, Burlingame, CA) diluted 1:100 and washed. Fluorescence was quantified with a FACStar Plus fluorescence activated cell sorter. The point 0 of the scale was adjusted to the background fluorescence displayed by biotinylated bovine serum albumin (BSA).
Reverse transcriptase-polymerase chain reaction (RT-PCR).
Total cellular RNA was extracted from 2 × 107 CEM cells by lysis with 4 mol/L guanidin-isothiocyanate, ultracentrifugation through a CsCl gradient, and phenol-chloroform extraction.28 First-strand complementary DNA (cDNA) was synthesized from 5 μg of RNA with T-primed first-strand kit (Pharmacia Biotech Inc, Upsala, Sweden) according to the manufacturer’s instructions. A 1:100 final dilution of the reaction was used as a template for PCR.
Oligonucleotide primers for PCR amplification were designed according to published sequences for MMP-229 and MMP-1414spanning one intron-exon junction. MMP-2 upstream primer was 5′ GGC ACC CAT TTA CAC CTA CAC CAA 3′ (position 1216-1239) and MMP-2 downstream primer was 5′ GCT TCC AAA CTT CAC GCT CTT CAG 3′ (position 1909-1886). The predicted size of the amplification product was 694 bp. MMP-14 upstream primer was 5′ CTC CTG CTC CCC CTG CTC ACG 3′ (position 142-162). MMP-14 downstream primer was 5′ CTC ACC CCC ATA AAG TTG CTG 3′ (position 969-949). The expected size of the amplicon was 828 bp. TIMP-1 and TIMP-2 were amplified using previously published primers.23 24 Amplification of β2-microglobulin cDNA was used as control of template loading among conditions.
Amplification of MMP-2, TIMP-1, TIMP-2, and β2-microglobulin cDNA was performed with 0.5 μmol/L of each primer, 0.25 mmol/L dNTPs, 1 U of Expand High-Fidelity PCR System (Roche Diagnostics GmbH, Germany) enzyme mix and a 1:10 dilution of the reaction buffer supplied by the manufacturer. Amplification of MMP-14 cDNA was performed with 0.25 mmol/L dNTPs, 0.5 μmol/L of each primer, 1 U of Expand enzyme mix, 15 mmol/L NH4SO4, 1.5 mmol/L MgCl2, and 60 mmol/L Tris, pH 9.5. After a hotstart step, in which the polymerase was added, each cycle consisted of 30 seconds of denaturation at 95oC, 1 minute of annealing at 55°C (TIMP-1, β2-microglobulin), 58°C (TIMP-2), or 60°C (MMP-2, MMP-14), and 1 minute of extension at 72°C. Reactions were run for 25 cycles (β2-microglobulin), 35 cycles (TIMP-1, TIMP-2), or 40 cycles (MMP-2 and MMP-14), followed by a final elongation step of 10 minutes.
The specificity of the PCR reactions was confirmed with either nested PCR or endonuclease restriction analysis.
Transfection of CEM T cells.
The plasmid pMMTVrasH (Asn-17) was kindly provided by Dr E. Santos (National Cancer Institute, Bethesda, MD). pMMTVrasH (Asn-17) is an expression plasmid in which the dominant negative mutant of Ha-Ras Asn-17 is under control of a mouse mammary tumor virus LTR and is inducible by dexamethasone. The plasmid contains also a Neor gene constitutively expressed from the simian virus 40 early promoter.30 CEM cells were transfected using DMRIE-C reagent (GIBCO Life Technologies) according to the instructions of the manufacturer. Transfected cells were exposed to fibronectin for 24 hours in serum-free conditions and the supernatant fluid was subjected to gelatin zymography as described above. When the expression of Ha-Ras Asn 17 was desired, transfected cells were exposed to dexamethasone (Calbiochem-Novabiochem) at 0.5 μmol/L for 24 hours before exposure to fibronectin, being sustained during the assay as well.
RESULTS
Fibronectin induces gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in lymphocyte cell line supernatants.
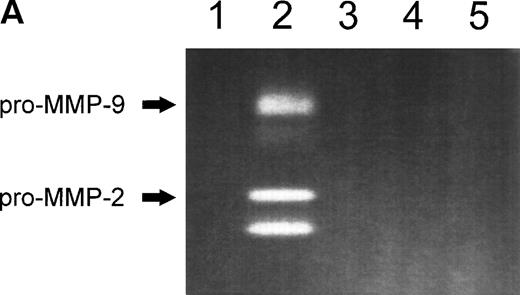
In serum-free conditions, CEM, and Jurkat T-cell lines transiently adhered to various extracellular matrix proteins (laminin-1, fibronectin, type-IV collagen) and to adhesion-supporting polymers such as polylysine. The strongest adhesion was observed on fibronectin in which CEM and Jurkat T cells fully spread. Maximal adhesion was observed at 60 to 90 minutes and decreased thereafter (data not shown). Zymography analysis of the conditioned media obtained from cells plated on fibronectin showed the presence of gelatinase activity that was absent from the media conditioned by cells plated on other extracellular matrix proteins (Fig 1A). Two major bands at 92 kD and 72 kD were observed consistent with the zymographic pattern of gelatinase B (MMP-9) and gelatinase A (MMP-2) proenzymes, respectively.
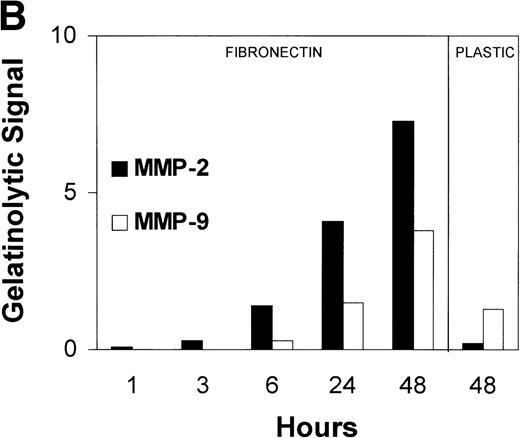
(A) MMP-2 and MMP-9 induction by fibronectin. CEM T cells were cultured on several extracellular matrix proteins for 24 hours. The figure shows the zymographic pattern of concentrated conditioned medium of 107 cells plated on polylysine (lane 1), fibronectin (lane 2), laminin-1 (lane 3), type-I collagen (lane 4), and type-IV collagen (lane 5). (B) Accumulation of MMP-2 and MMP-9 in the conditioned medium over time. 107 cells were incubated on plastic or on fibronectin for different periods of time. The conditioned medium was concentrated and subjected to gelatin zymography. The figure shows the gelatinolytic signal quantified as described in the Materials and Methods section. (C) CEM cells produce activated forms of MMP-2 in response to fibronectin. Unconcentrated conditioned medium obtained from HUVEC confluent monolayers cultured on plastic was subjected to gelatin zymography in the absence (−) or in the presence (+) of p-aminophenylmercuric acetate (APMA). Concentrated conditioned medium from 107 CEM cells cultured on fibronectin (Fn) for 24 hours disclosed an identical zymographic pattern.
(A) MMP-2 and MMP-9 induction by fibronectin. CEM T cells were cultured on several extracellular matrix proteins for 24 hours. The figure shows the zymographic pattern of concentrated conditioned medium of 107 cells plated on polylysine (lane 1), fibronectin (lane 2), laminin-1 (lane 3), type-I collagen (lane 4), and type-IV collagen (lane 5). (B) Accumulation of MMP-2 and MMP-9 in the conditioned medium over time. 107 cells were incubated on plastic or on fibronectin for different periods of time. The conditioned medium was concentrated and subjected to gelatin zymography. The figure shows the gelatinolytic signal quantified as described in the Materials and Methods section. (C) CEM cells produce activated forms of MMP-2 in response to fibronectin. Unconcentrated conditioned medium obtained from HUVEC confluent monolayers cultured on plastic was subjected to gelatin zymography in the absence (−) or in the presence (+) of p-aminophenylmercuric acetate (APMA). Concentrated conditioned medium from 107 CEM cells cultured on fibronectin (Fn) for 24 hours disclosed an identical zymographic pattern.
Gelatinase B was sometimes detected in conditioned medium from CEM cells grown on uncoated plastic. In contrast, traces of gelatinase A could only be observed when cells were maintained for long incubation periods (Fig 1B). Gelatinase A and B production increased dramatically after a few hours of exposure to fibronectin and continued accumulating in the conditioned medium over a 48-hour period (Fig 1B). Even though the specific activity of gelatinase B is 25 times higher than the gelatinase A one,22 the intensity of the gelatinase A gelatinolytic signal elicited by fibronectin was severalfold over that for gelatinase B (Fig 1A and B). In addition, the increase in gelatinolytic signal induced by 48-hour exposure to fibronectin was 30-fold for gelatinase A and only threefold for gelatinase B (Fig 1B). The absolute amount of gelatinase A and B released to the supernate, even after exposure to fibronectin, was very small. Their zymographic detection required 200 times concentration of the conditioned medium and both MMP-2 and MMP-9 were repeatedly undetectable by enzyme-linked immunoassay of nonconcentrated medium using high sensitivity (pg/mL) commercial kits (Biotrak; Amersham).
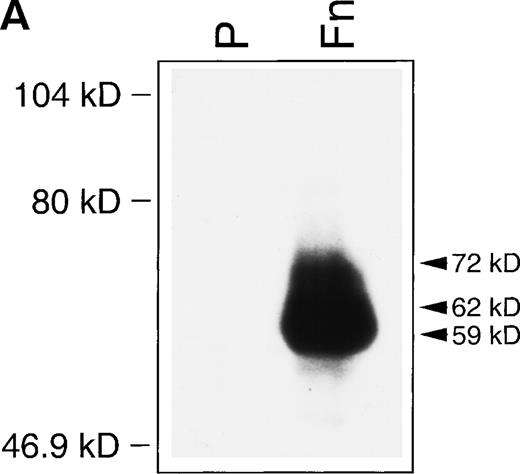
Although gelatinase B (MMP-9) synthesis by T lymphocytes has been repeatedly recognized,21-25 gelatinase A (MMP-2) production has only been identified under very restricted conditions.22,26 To further characterize the lower molecular weight species observed in gelatin zymograms of T-cell–conditioned media, a comparative zymogram was performed with conditioned media from HUVEC treated with APMA. The production of the 72 kD gelatinase-A proenzyme by HUVEC has been well characterized31 and APMA induces the activation of MMPs by promoting the autolytic cleavage of N-terminal peptide sequences. As shown in Fig 1C, the low molecular weight gelatinolytic bands obtained from CEM supernates were identical to the 62 and 59 kD products obtained by APMA treatment of HUVEC-conditioned medium. Additional confirmation that the 72 kD gelatinolytic band and the lower molecular weight species corresponded to MMP-2 was obtained by Western blot analysis. Given the lower sensitivity of Western Blot compared with gelatin zymography, a previous gelatin-affinity purification step of conditioned medium was required for MMP-2 detection (Fig2A). Western blotting of gelatin-affinity–purified HUVEC-conditioned medium, used as control, displayed only the 72 kD proenzyme (data not shown).
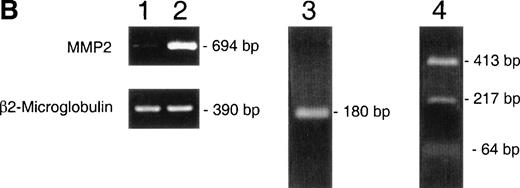
(A) Western blot identification of MMP-2 in CEM-conditioned medium. Gelatin affinity-purified–conditioned medium from 108 CEM cells cultured for 24 hours on plastic (P) or in the presence of soluble fibronectin at 10 μg/mL (Fn) was subjected to Western blot analysis with the MoAb Ab-3 against MMP-2. (B) RT-PCR demonstration of MMP-2 transcripts in CEM cells. PCR-amplified cDNA obtained from resting CEM cells (lane 1) and CEM cells exposed to fibronectin at 10 μg/mL for 4 hours (lane 2). Simultaneous amplification of the housekeeping gene β2-microglobulin is shown to ensure an equivalent amount of template in both conditions. Lane 3 shows the nested PCR product obtained from the 694 bp fragment using previously published internal primers.22 Lane 4 shows the Sac I restriction fragments of the 694 bp fragment.
(A) Western blot identification of MMP-2 in CEM-conditioned medium. Gelatin affinity-purified–conditioned medium from 108 CEM cells cultured for 24 hours on plastic (P) or in the presence of soluble fibronectin at 10 μg/mL (Fn) was subjected to Western blot analysis with the MoAb Ab-3 against MMP-2. (B) RT-PCR demonstration of MMP-2 transcripts in CEM cells. PCR-amplified cDNA obtained from resting CEM cells (lane 1) and CEM cells exposed to fibronectin at 10 μg/mL for 4 hours (lane 2). Simultaneous amplification of the housekeeping gene β2-microglobulin is shown to ensure an equivalent amount of template in both conditions. Lane 3 shows the nested PCR product obtained from the 694 bp fragment using previously published internal primers.22 Lane 4 shows the Sac I restriction fragments of the 694 bp fragment.
To determine whether fibronectin induced MMP-2 transcription or just its release into the supernatant fluid, the presence of MMP-2 messenger RNA (mRNA) was investigated by RT-PCR. As shown in Fig 2B, MMP-2 mRNA was barely detectable in cells in suspension, whereas a clear band of the expected size was detected in cells exposed to fibronectin. Nested PCR and restriction fragment analysis confirmed the specificity of the PCR amplification of MMP-2.
Fibronectin induction of MMP-2 is mediated by interaction with α4, α5, and αv-bearing lymphocyte integrins.
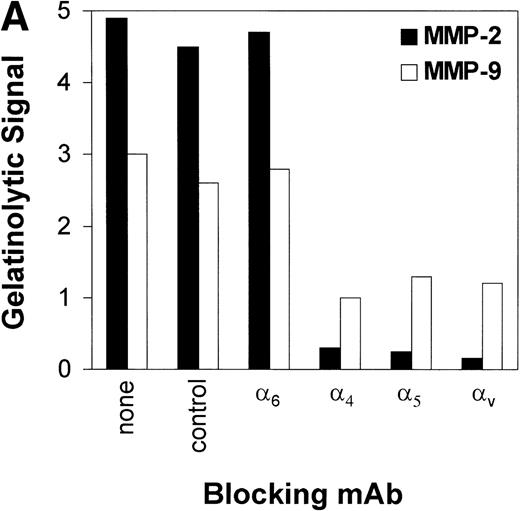
To investigate which fibronectin receptors participate in fibronectin-mediated induction of MMP synthesis, CEM T cells were preincubated with blocking MoAb against several integrins. As shown in Fig 3A, blocking α4, α5, and αv but not α6 chains dramatically reduced MMP-2 production. A less-apparent reduction in MMP-9 was also observed. Anti-α6 integrin chain (a laminin receptor) and anti-CD20 (a B-cell lineage marker) were used as negative controls.
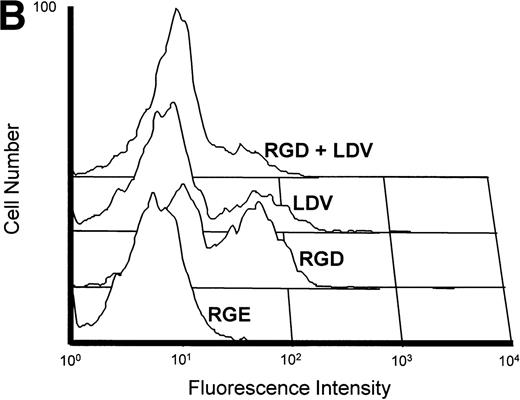
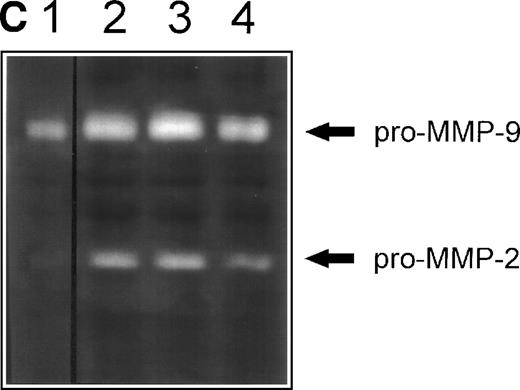
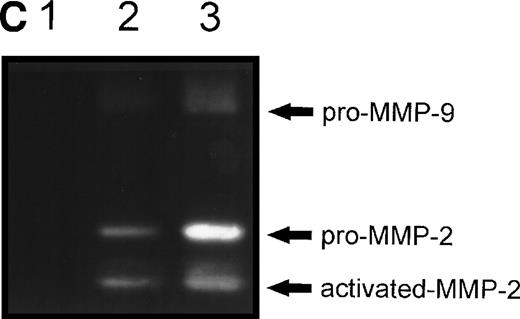
(A) Inhibition of fibronectin-induced MMP-2 and MMP-9 by blocking MoAb against integrin chains. 107 CEM cells per condition were preincubated with medium alone or with MoAb against 4, 5, and V integrin chains (fibronectin receptors); 6 integrin chain (a laminin receptor); or the anti-CD20 93.1B3 (a B-cell lineage marker) before exposure to fibronectin. Conditioned medium was concentrated and subjected to gelatin zymography. Graph shows variation in gelatinolytic signals compared with the signal obtained with untreated cells exposed to fibronectin. (B) Membrane-associated MMP-2 expression induced by fibronectin-derived synthetic peptides. CEM cells were exposed to GRGDSPC, EILDVSPT, a combination of both, or to the control peptide GRGES at 100 μg/mL for 16 hours. Histograms show distribution of fluorescence intensity in 5 × 103 cells per condition incubated with the MoAb Ab-3 recognizing MMP-2. (C) Pro-MMP-2 and pro-MMP-9 production stimulated by fibronectin-derived synthetic peptides. 2 × 107 CEM cells were cultured on plastic (lane 1) or on plastic coated with GRGDSPC (lane 2), EILDVSPT(lane 3), or both (lane 4) at 100 μg/mL. The conditioned medium obtained after 48 hours was concentrated and subjected to gelatin zymography.
(A) Inhibition of fibronectin-induced MMP-2 and MMP-9 by blocking MoAb against integrin chains. 107 CEM cells per condition were preincubated with medium alone or with MoAb against 4, 5, and V integrin chains (fibronectin receptors); 6 integrin chain (a laminin receptor); or the anti-CD20 93.1B3 (a B-cell lineage marker) before exposure to fibronectin. Conditioned medium was concentrated and subjected to gelatin zymography. Graph shows variation in gelatinolytic signals compared with the signal obtained with untreated cells exposed to fibronectin. (B) Membrane-associated MMP-2 expression induced by fibronectin-derived synthetic peptides. CEM cells were exposed to GRGDSPC, EILDVSPT, a combination of both, or to the control peptide GRGES at 100 μg/mL for 16 hours. Histograms show distribution of fluorescence intensity in 5 × 103 cells per condition incubated with the MoAb Ab-3 recognizing MMP-2. (C) Pro-MMP-2 and pro-MMP-9 production stimulated by fibronectin-derived synthetic peptides. 2 × 107 CEM cells were cultured on plastic (lane 1) or on plastic coated with GRGDSPC (lane 2), EILDVSPT(lane 3), or both (lane 4) at 100 μg/mL. The conditioned medium obtained after 48 hours was concentrated and subjected to gelatin zymography.
To further evaluate the role of lymphocyte integrins in MMP production, T cells were incubated with synthetic peptides containing minimal fibronectin sequences recognized by αv and α5 (RGD) and α4 (LDV) integrins. As shown in Fig 3B, GRGDSPC and EILDVPST fibronectin peptides but not the control peptide GRGES increased the expression of membrane-associated MMP-2, as assessed by flow cytometry. However, when added alone, synthetic peptides were not able to elicit the strong MMP-2 and MMP-9 secretion achieved by fibronectin. Only at saturating concentrations32 and with long incubation periods MMP-9 and MMP-2 proenzymes could be detected as 92 kD and 72 kD gelatinolytic bands, respectively, and no activated forms were observed (Fig 3C). These findings indicate that ligand occupancy is not sufficient to elicit full production, secretion, and activation of MMPs and that ligand clustering or concomitant interaction with other fibronectin sequences are required.32
Fibronectin does not influence TIMP-1 production but downregulates TIMP-2 expression by T-cell lines.
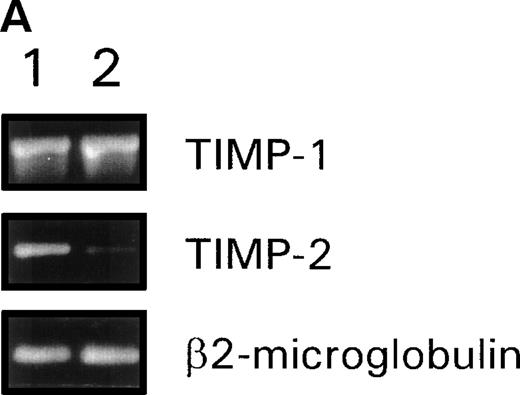
Given the importance of TIMPs on the biological activity of MMPs, we studied whether TIMP-1 and TIMP-2 were influenced by fibronectin. In resting CEM cells, we found constitutive mRNA expression of both TIMP-1 and TIMP-2 (Fig 4A). When CEM cells were exposed to fibronectin a decrease in the mRNA level of TIMP-2 could be observed, whereas the amount of TIMP-1 mRNA remained unaltered (Fig4A). We further investigated the effect of fibronectin-derived peptides on membrane-associated TIMP-1 and TIMP-2 by flow cytometry analysis (Fig 4B). A baseline membrane expression of both TIMPs was found in CEM incubated with the control peptide GRGES, being the level of TIMP-1 relatively higher than that of TIMP-2. Neither GRGDSPC nor EILDVPST were able to modify the expression of any TIMP. However, their combination induced a clear downregulatory effect on the surface expression of TIMP-2, whereas no effect on TIMP-1 membrane expression was observed.
(A) RT-PCR analysis of TIMP-1 and TIMP-2 mRNA levels in CEM cells stimulated with fibronectin. cDNA obtained from resting CEM cells (lane 1) or stimulated with soluble fibronectin at 10 μg/mL for 4 hours (lane 2). β2-microglobulin amplification is used to control template quantity in each condition. (B) Membrane expression of TIMP-1 and TIMP-2 in CEM cells treated with fibronectin-derived peptides. Flow cytometry analysis of CEM cells cultured with a combination of GRGDSPC and EILDVSPT in solution at 100 μg/mL for 16 hours. The peptide GRGES was used as control. Histograms show distribution of fluorescence intensity in 5 × 103 cells per condition.
(A) RT-PCR analysis of TIMP-1 and TIMP-2 mRNA levels in CEM cells stimulated with fibronectin. cDNA obtained from resting CEM cells (lane 1) or stimulated with soluble fibronectin at 10 μg/mL for 4 hours (lane 2). β2-microglobulin amplification is used to control template quantity in each condition. (B) Membrane expression of TIMP-1 and TIMP-2 in CEM cells treated with fibronectin-derived peptides. Flow cytometry analysis of CEM cells cultured with a combination of GRGDSPC and EILDVSPT in solution at 100 μg/mL for 16 hours. The peptide GRGES was used as control. Histograms show distribution of fluorescence intensity in 5 × 103 cells per condition.
Fibronectin induces the expression of MT1-MMP (MMP-14) by T-cell lines.
Because CEM cells exposed to fibronectin secreted activated forms of MMP-2, we investigated whether CEM cells expressed MMP-14, a membrane-bound MMP which is believed to be one of the major activators of MMP-214,18 and which has not been previously shown to be expressed in lymphoid cells. MMP-14 was not expressed constitutively by CEM cells (Fig 5A and 5B). When cells were exposed to fibronectin, MMP-14 mRNA could be detected by RT-PCR (Fig5A). MMP-14 surface expression was absent from resting CEM cells but could be detected by flow-cytometry when cells were treated with fibronectin-derived synthetic peptides (Fig 5B). The observation that, even at saturating concentrations, synthetic peptides were not able to elicit the secretion of activated forms of MMP-2, indicates that besides MMP-14 expression, other factors may be required for full MMP-2 activation, as shown in other cell types.33
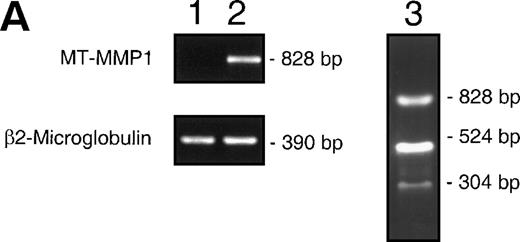
(A) Induction of MMP-14 expression in CEM cells by fibronectin. MMP-14 (MT1-MMP) transcripts obtained from baseline CEM cells (lane 1) and CEM cells exposed to fibronectin (10 μg/mL) for 4 hours (lane 2). Concomitant amplification of β2-microglobulin shows equivalent amounts of template in both samples. Lane 3 shows the Nco I restriction fragments of the 828 bp PCR product. (B) Surface expression of MMP-14 induced by fibronectin-derived peptides in CEM cells. CEM cells were incubated with either GRGDSPC, EILDVSPT, a combination of both, or the control peptide GRGES at 100 μg/mL for 16 hours and subjected to flow cytometry. Histograms show fluorescence intensity distribution among 5 × 103 cells immunostained with the polyclonal antibody Ab-2 recognizing MMP-14.
(A) Induction of MMP-14 expression in CEM cells by fibronectin. MMP-14 (MT1-MMP) transcripts obtained from baseline CEM cells (lane 1) and CEM cells exposed to fibronectin (10 μg/mL) for 4 hours (lane 2). Concomitant amplification of β2-microglobulin shows equivalent amounts of template in both samples. Lane 3 shows the Nco I restriction fragments of the 828 bp PCR product. (B) Surface expression of MMP-14 induced by fibronectin-derived peptides in CEM cells. CEM cells were incubated with either GRGDSPC, EILDVSPT, a combination of both, or the control peptide GRGES at 100 μg/mL for 16 hours and subjected to flow cytometry. Histograms show fluorescence intensity distribution among 5 × 103 cells immunostained with the polyclonal antibody Ab-2 recognizing MMP-14.
Fibronectin-activated signaling pathways provide both stimulatory and inhibitory signals for MMP-2 and MMP-9 expression.
As stated above, in serum-free conditions, CEM cell adhesion and spreading on fibronectin was transient. Disrupting Rho-dependent actin cytoskeleton organization triggered by attachment to fibronectin by cytochalasin D34 increased both MMP-2 and MMP-9 production (Fig 6A). Moreover, when cells were exposed to soluble fibronectin in suspension, a much higher production of both MMP-2 and MMP-9 was detected (Fig 6B). However, although accelerated detachment induced by EGTA strongly promoted MMP-2 production, MMP-9 production was inhibited, indicating that fibronectin-induced signaling pathways leading to MMP-2 and MMP-9 expression are partially independent (Fig 6A). According to this concept, protein kinase C (PKC) activation by PMA was not able to increase fibronectin-induced MMP-2 production whereas fibronectin-induced MMP-9 expression was strongly increased by PMA (Fig 6A). PMA alone was also able to elicit MMP-9 production by CEM cells without exposure to fibronectin, as it has been previously described in other lymphoid cell lines.21However, treatment with calphostin C did not result in an inhibition of fibronectin-induced MMP-9 (Fig 6A), indicating that induction of MMP-9 by fibronectin does not occur through PKC-dependent signaling pathways.
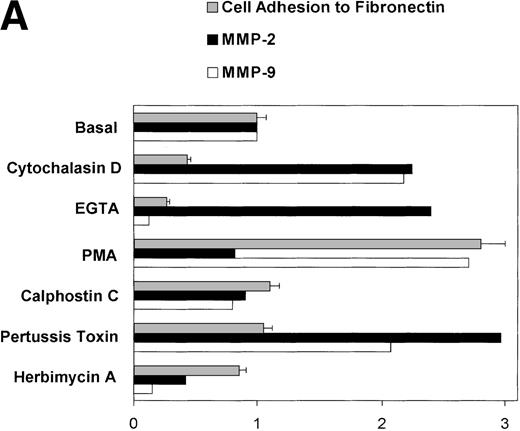
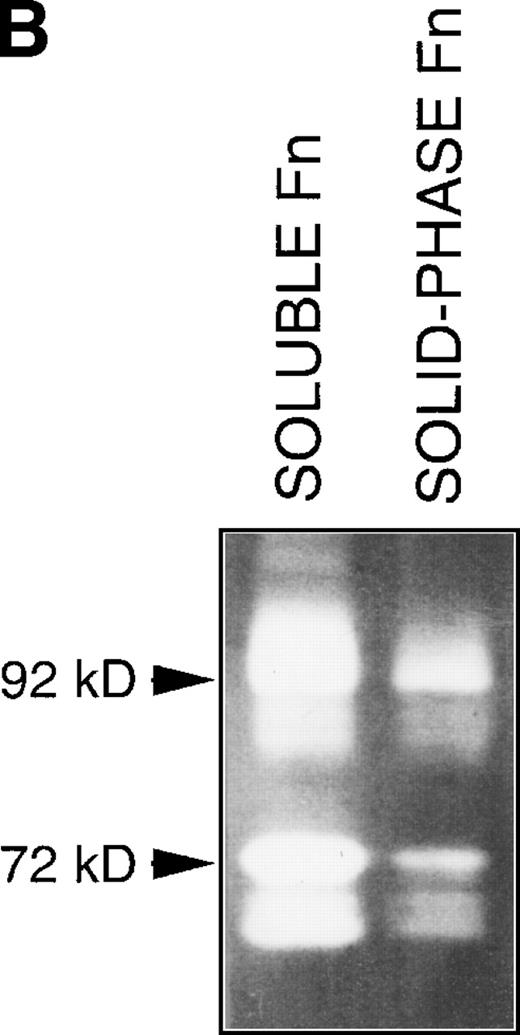
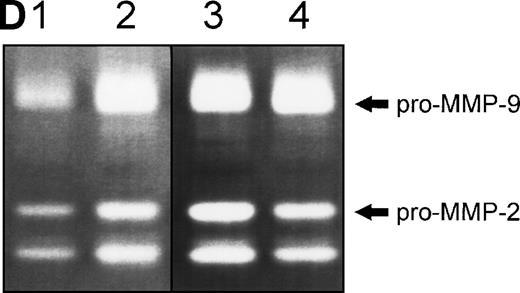
(A) Changes in fibronectin-induced MMP-2 and MMP-9 production achieved by manipulating integrin-mediated signal transduction pathways. 107 CEM cells were exposed to the above-described chemicals 30 minutes before exposure to fibronectin. Conditioned medium was obtained after 6 hours, concentrated, and subjected to gelatin zymography. Graph shows the variation in the intensity of the fibronectin-induced gelatinolytic signal induced by treatment with the different products and their simultaneous effect on cell adhesion, both expressed as a percentage of those obtained by fibronectin alone (labeled basal). (B) Intensity of MMP-2 and MMP-9 production induced by exposure to soluble or solid-phase fibronectin. 107 CEM cells were exposed to soluble (10 μg/mL) or solid-phase (plastic surfaces coated with 100 μg/mL) fibronectin for 24 hours. Conditioned media were concentrated and subjected to gelatin zymography. (C) Fibronectin-induced MMP-2 and MMP-9 production by cells transfected with a dominant negative mutant of Ha-Ras. 107CEM cells transfected with Ha-Ras-Asn 17, cloned in the dexamethasone-inducible vector pMMTV, were exposed to soluble fibronectin for 24 hours. Conditioned medium from transfected cells was concentrated and subjected to gelatin zymography. Lane 1 shows absence of gelatinolytic activity in conditioned medium from transfected cells stimulated with 0.5 μmol/L dexamethasone without exposure to fibronectin. Lanes 2 and 3 display gelatinolytic signals provided by conditioned medium from untreated transfected cells (lane 2) and from transfected cells treated with 0.5 μmol/L dexamethasone (lane 3), both exposed to fibronectin. (D) Effect of PI-3K, MEK-1/2, and p38 MAPK inhibition on fibronectin-induced MMP-2 and MMP-9 production. 2 × 107 CEM cells were preincubated with several very specific inhibitors of PI-3K (wortmannin), ERK-1/2 kinase (PD 98059), or p38 MAPK (SB 202190) for 30 minutes before exposure to soluble fibronectin at 10 μg/mL. Conditioned medium was obtained 6-hours later, concentrated, and subjected to gelatin zymography. The picture shows the gelatinolytic signals provided by conditioned medium from untreated cells exposed to fibronectin (lane 1), or pretreated with PD 98059 (lane 2), wortmannin (lane 3), or SB 202190 (lane 4).
(A) Changes in fibronectin-induced MMP-2 and MMP-9 production achieved by manipulating integrin-mediated signal transduction pathways. 107 CEM cells were exposed to the above-described chemicals 30 minutes before exposure to fibronectin. Conditioned medium was obtained after 6 hours, concentrated, and subjected to gelatin zymography. Graph shows the variation in the intensity of the fibronectin-induced gelatinolytic signal induced by treatment with the different products and their simultaneous effect on cell adhesion, both expressed as a percentage of those obtained by fibronectin alone (labeled basal). (B) Intensity of MMP-2 and MMP-9 production induced by exposure to soluble or solid-phase fibronectin. 107 CEM cells were exposed to soluble (10 μg/mL) or solid-phase (plastic surfaces coated with 100 μg/mL) fibronectin for 24 hours. Conditioned media were concentrated and subjected to gelatin zymography. (C) Fibronectin-induced MMP-2 and MMP-9 production by cells transfected with a dominant negative mutant of Ha-Ras. 107CEM cells transfected with Ha-Ras-Asn 17, cloned in the dexamethasone-inducible vector pMMTV, were exposed to soluble fibronectin for 24 hours. Conditioned medium from transfected cells was concentrated and subjected to gelatin zymography. Lane 1 shows absence of gelatinolytic activity in conditioned medium from transfected cells stimulated with 0.5 μmol/L dexamethasone without exposure to fibronectin. Lanes 2 and 3 display gelatinolytic signals provided by conditioned medium from untreated transfected cells (lane 2) and from transfected cells treated with 0.5 μmol/L dexamethasone (lane 3), both exposed to fibronectin. (D) Effect of PI-3K, MEK-1/2, and p38 MAPK inhibition on fibronectin-induced MMP-2 and MMP-9 production. 2 × 107 CEM cells were preincubated with several very specific inhibitors of PI-3K (wortmannin), ERK-1/2 kinase (PD 98059), or p38 MAPK (SB 202190) for 30 minutes before exposure to soluble fibronectin at 10 μg/mL. Conditioned medium was obtained 6-hours later, concentrated, and subjected to gelatin zymography. The picture shows the gelatinolytic signals provided by conditioned medium from untreated cells exposed to fibronectin (lane 1), or pretreated with PD 98059 (lane 2), wortmannin (lane 3), or SB 202190 (lane 4).
Adhesion of different cell types to fibronectin activates the Ras/Raf-1/Mitogen-activated protein kinase (MAPK), Phosphatidil inositol-3 kinase (PI-3K), and p38 MAPK signaling pathways through integrin-mediated signal transduction.35-37 CEM transfection with the dominant inhibitory mutant Ha-Ras Asn-17 increased fibronectin-induced MMP-2 and MMP-9 production (Fig 6C). Because transfected cells were exposed to dexamethasone to induce Ha-Ras Asn-17 expression, the effect of dexamethasone on fibronectin induction of the MMPs was tested. Dexamethasone alone had no effect on fibronectin-induced MMP-2 or MMP-9 expression (data not shown). Ha-Ras Asn-17 expression did not stimulate MMP-2 or MMP-9 production without exposure to fibronectin (Fig 6C). Inhibition of PI-3K by wortmannin, MAPK kinase 1 and 2 (MEK-1/2) by PD98059, and p38 MAPK by SB 202190 all strongly increased fibronectin-induced MMP-2 and MMP-9 production (Fig6D), indicating that signals transduced through PI-3K and MAPKs inhibit MMP-2 and MMP-9 production by T lymphocytes. None of these inhibitors by themselves were able to induce MMP-2 or MMP-9 without exposure to fibronectin (data not shown). By contrast, herbimycin A, an inhibitor of Src-type tyrosine kinases, strongly inhibited both MMP-2 and MMP-9 production (Fig 6A). None of the kinase inhibitors substantially modified cell attachment, indicating that they influence integrin-signaling pathways involved in MMPs production that are independent or are located downstream of the signaling pathways involved in cell attachment and spreading.
The existence of PI-3K– and MAPK-transduced inhibitory pathways suppressing MMP-2 and MMP-9 production was further supported by the observation that cells cultured at high density (1 × 106/mL or more) lost their ability to produce MMP-2 and MMP-9 when exposed to fibronectin (Fig 7A). Inhibition of MMP-2 and MMP-9 production by overgrown cells was not produced by the accumulation of a soluble inhibitory factor because conditioned medium obtained from cells cultured at high density did not downregulate MMP-2 or MMP-9 production by cells plated on fibronectin at low density (data not shown). Moreover, the lack of response to fibronectin was not due to a downregulation of α4, α5, or αv integrin expression because their surface expression in cells at high density was identical to that observed in cells plated at low density, as assessed by flow cytometry (Fig 7B). Similarly, it was not caused by a decrease in integrin avidity because adhesion and spreading of cells on fibronectin was even higher in cells cultured at high density compared with that of cells plated at low density (Fig 7A). Taken together, these data indicate a dominance of pathways inhibiting MMP-2 and MMP-9 expression in overgrown cells.
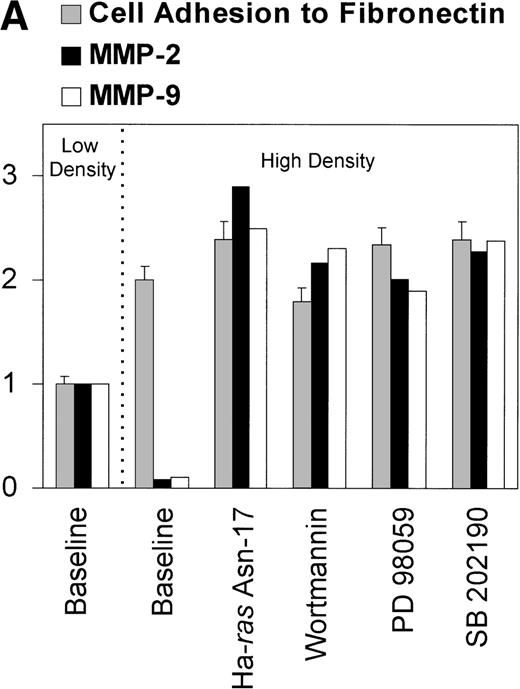
(A) Abrogation of MMP-2 and MMP-9 production in response to fibronectin by high-cell density and its reversion by inhibiting PI-3K and different elements of the Ras/MAPK–signaling pathway. 2 × 107 CEM cells were cultured at a 2 × 105cells/mL or at 1 × 106 cells/mL and exposed to fibronectin. Conditioned medium was obtained after 6 hours, concentrated, and subjected to gelatin zymography. Graph shows the intensity of the gelatinolytic band in untreated cells (baseline), cells expressing Ha-Ras Asn-17, and cells treated with wortmannin, PD 98059, or SB 202190. Concomitant effect on cell binding to fibronectin is also shown. Both adhesion and intensity of the gelatinolytic bands are expressed as fold increase or decrease over that observed with the untreated cells exposed to fibronectin at low density. (B) Effect of cell density on integrin cell-surface expression. Flow cytometry analysis of membrane expression of fibronectin receptors in CEM cells cultured at low density (filled histograms) versus cells cultured at high density (clear histograms). Histograms show fluorescence-intensity distribution among 5 × 103 cells immunostained with MoAb raised against several integrin chains and the common β chain.
(A) Abrogation of MMP-2 and MMP-9 production in response to fibronectin by high-cell density and its reversion by inhibiting PI-3K and different elements of the Ras/MAPK–signaling pathway. 2 × 107 CEM cells were cultured at a 2 × 105cells/mL or at 1 × 106 cells/mL and exposed to fibronectin. Conditioned medium was obtained after 6 hours, concentrated, and subjected to gelatin zymography. Graph shows the intensity of the gelatinolytic band in untreated cells (baseline), cells expressing Ha-Ras Asn-17, and cells treated with wortmannin, PD 98059, or SB 202190. Concomitant effect on cell binding to fibronectin is also shown. Both adhesion and intensity of the gelatinolytic bands are expressed as fold increase or decrease over that observed with the untreated cells exposed to fibronectin at low density. (B) Effect of cell density on integrin cell-surface expression. Flow cytometry analysis of membrane expression of fibronectin receptors in CEM cells cultured at low density (filled histograms) versus cells cultured at high density (clear histograms). Histograms show fluorescence-intensity distribution among 5 × 103 cells immunostained with MoAb raised against several integrin chains and the common β chain.
CEM transfection with a dominant inhibitory mutant of Ha-Ras, inhibition of PI-3K by wortmannin, of MAPKK by PD98059, and of p38 MAPK by SB 202190, all restored the ability of overgrown cells to produce MMP-2 and MMP-9 in response to fibronectin (Fig 7A). Together, these findings support the concept that integrin-mediated signaling pathways transduce both activating and repressing signals for MMP-2 and MMP-9 production and their relative predominance is controlled by additional factors, probably related to cell migration and cell growth.
DISCUSSION
Tissue infiltration by lymphocytes requires transmigration through endothelial cell junctions and, subsequently, through the basement membrane and interstitial matrix.1,7 This later step likely requires adhesion to extracellular matrix proteins and their focalized degradation.7,9 10 In the present study, we show that interaction with fibronectin induces the synthesis, activation, and secretion of MMP-2 and MMP-9 by lymphoid cell lines from T lineage. In addition, fibronectin downregulates TIMP-2 expression, a process that may enhance the proteolytic activity of MMP-2.
Previous studies have shown the ability of extracellular matrix proteins to induce their own degradation by promoting the production and release of several matrix metalloproteinases by several adherent cell types.38-43 In our study, intact fibronectin induced MMP-2 and MMP-9 expression whereas fibronectin-derived peptides containing an RGD or an LDV sequence elicited only a slight response, even at saturating concentrations, indicating that receptor occupancy alone is not able to optimally induce MMP-2 and MMP-9 and/or that other sequences in fibronectin are also required for optimal MMP-2 and MMP-9 production by T cells.
Fibronectin induced the secretion of activated forms of MMP-2 by lymphoid cell lines that often predominated over the 72 kD proenzyme form. Previous reports showing MMP-2 production by T lymphocytes triggered by soluble factors such as IL-2 have only provided zymographic detection of the 72 kD proenzyme with no evidence of activated forms.23 By contrast, the 62 kD activated form has been detected in T lymphocytes adherent to VCAM-1 expressing endothelial cells,26 a process probably mediated also by α4 integrins. Recently, the membrane-associated metalloproteinase MT1-MMP (MMP-14) has been shown to be one of the major activators of MMP-2 in invasive tumors14,19 and their coordinated expression has been shown in different settings such as development,44 angiogenesis,42 and tumor progression.14,45 Several soluble factors such as concanavalin A and phorbol esters can upregulate MMP-14 production.33 46 Herein, we show for the first time that T-cell lines, indeed, produce MMP-14 and that, in addition to soluble factors, interactions with matrix molecules such as fibronectin are able to induce MMP-14 expression.
In our study, fibronectin-induced MMP-2 and MMP-9 production by T-cell lines was mediated by signaling triggered by engagement of α4, α5, and αv-bearing integrins that have all been shown to serve as fibronectin receptors.47 Integrins mediating MMP induction by matrix proteins, as well as the type of MMP produced vary among different cell types.41,43,48 In synovial fibroblasts, for instance, α5β1 engagement induces MMP-1 and MMP-3 expression whereas α4β1 seems to have an inhibitory role on α5β1-mediated MMP expression.38,43 A predominant function of α4β1 in this cell type might then account for the inability of intact fibronectin to induce MMP expression by synovial fibroblasts.43 α4β1has virtually no role in fibronectin-induced MMP-9 expression by macrophages.49 By contrast, according to our results, α4β1 seems to induce MMP-2 and MMP-9 expression by T lymphocytes because blocking MoAb against α4 highly reduced the MMP production induced by intact fibronectin. Recent work has shown the production of MMP-2 by lymphocytes adherent to endothelial cells expressing VCAM-1 and to recombinant VCAM-1 itself,26 further supporting the concept that engagement of α4β1 triggers MMP-2 production by T lymphocytes.
Integrins mediate a variety of important cell functions such as adhesion, migration, anchorage-dependent growth, and gene expression.47 The complex signaling pathways triggered by integrin interactions with their ligands are beginning to be understood. Integrins activate small-G proteins of the Rho subfamily that regulate focal adhesion formation and actin cytoskeleton organization.50 Rho, in turn, activates, PI-5K and the MAPK JNK, whereas other members of the Rho subfamily, Rac-1 and cdc42, activate PI-3K.51 Inhibition of small-G protein activation by cytochalasin D34 led to an increase in fibronectin-induced MMP-2 and MMP-9 indicating that small-G proteins of the Rho subfamily transduce inhibitory signals for gelatinase induction. Integrin-mediated cell adhesion leads to PKC activation.52 However, PKC is not probably involved in MMP-2 expression because phorbol esters did not modify MMP-2 production by T lymphocytes in accordance with the absence of phorbol-responsive elements in the MMP-2 gene.53 PKC activation by phorbol esters is known to induce MMP-9 expression in a variety of cell types, including T lymphocytes and phorbol-responsive elements, including AP-1 binding sites, have been well characterized in the MMP-9 promoter.54 Nevertheless, PKC activation is not probably involved in fibronectin-induced MMP-9 expression by T-cell lines, because PKC inhibition by calphostin C did not result in a reduction of fibronectin-induced MMP-9 production. According to this observation it has been shown that T-lymphocyte attachment to fibronectin activates AP-1 in a PKC-independent manner.55
Integrin activation results in phosphorylation of the module protein Shc creating binding sites for the adaptor protein Grb-2, which recruits the guanidine nucleotide exchanging factor Sos activating the Ras/Raf-1/MAPK (Erk-1 and -2)–signaling cascade.35Integrin engagement may also activate Erks through PI-3K activation downstream of Ras.37 Furthermore, activation of p38 MAPK can also be triggered by integrin-mediated signaling through independent pathways.35 In our system, inhibition of Ha-Ras by transfection with a dominant negative mutant, and inhibition of PI-3K and MAPKs increased fibronectin-induced MMP-2 and MMP-9 expression suggesting that inhibitory signals for the expression of these MMPs are transduced through Ras/Raf-1/MAPK pathways. Upstream inhibition of the MAPK cascade by the G-protein inhibitor pertussis toxin also resulted in an increased MMP-2 and MMP-9 production.
Finally, integrin-mediated signaling leads to tyrosine phosphorylation of FAK which, in turn, creates sites for interaction with proteins displaying SH2 domains such as Src-type tyrosine kinases.56Inhibition of Src-type tyrosine kinases with herbimycin A significantly reduced MMP-2 and MMP-9 expression without a significant change in cell adhesion suggesting an important role for Src-type tyrosine kinases in integrin-mediated signaling leading to MMP-2 and MMP-9 expression by T lymphocytes.
Gelatinase expression is regulated by cytokines, growth factors, and matrix components through complex signaling pathways that are beginning to be understood. Mechanisms involving fibronectin-induced gelatinase expression by T-lymphoid cell lines largely differ from those mediating gelatinase production by transformed cells derived from solid tumors. Some transformed cell lines constitutively express MMP-9, MMP-2, or both54,57,58 and activated Ras seems to play a role in spontaneous gelatinase expression by some of them.56,59 It has been shown that v-Src can activate the expression of MMP-9 through mechanisms independent from that triggered by some cytokines and growth factors.60 According to our data, fibronectin induction of gelatinases in T-cell lines was mediated through integrin-mediated signaling pathways probably involving Src-type tyrosine kinases and not by other pathways employed by G-protein–coupled growth-factor receptors, which, in fact, had an inhibitory function. Contrarily to other cell responses,36 T-lymphocyte integrins do not cooperate with growth factors in gelatinase induction.
When undisrupted, cell interaction with fibronectin promotes cell attachment and spreading. As mentioned, integrin-mediated adhesion to fibronectin leads to the activation of the MAPK cascade in different cell types.35-37 MAPK activation, in turn, has been shown to be crucial for cell migration through complex and largely unknown mechanisms, among which direct phosphorylation of myosin light chains has been recently identified.61 On the other hand, excessive adherence to substrates reduces cell motility, and circumstances that halt integrins in an adherent status lead to a decreased cell migration.59 Recently, it has been shown that MAPKs mediate an interesting self-inhibitory loop. Excessive MAPK activation achieved by transfecting cells with constitutively active H-Ras or Raf-1 decreases integrin avidity.59 Decreasing integrin avidity by activated MAPKs may allow the coordinated series of adhesion-deadhesion events required for cell motility. In our system, lymphocyte adhesion to fibronectin was transient suggesting the existence of a similar kind of negative feedback triggered by interaction with fibronectin itself. Similarly, in our setting, adhesion to fibronectin was a necessary stimulus for MMP-2 and MMP-9 production (presumably mediated by Src-type tyrosine kinases) but signals transduced through other pathways (PI-3K and MAPKs activation) provided inhibitory signals for MMP-2 and MMP-9 expression. As mentioned, several stimuli promoting cell release increased fibronectin-induced MMP-2 and MMP-9 production by T lymphocytes whereas a high-density status that was associated with a higher cell adherence, suppressed MMP-2 and MMP-9 induction, suggesting a connection between cell migration, a phenomenon requiring coordinated series of adhesion and deadhesion events, and gelatinase production. Our findings suggest that migrating T lymphocytes in contact with fibronectin release tightly regulated pulses of very small amounts of activated gelatinases which results in focalized matrix degradation, allowing their progression through the basement membrane and interstitial matrix.
ACKNOWLEDGMENT
The authors thank Drs F. Sánchez-Madrid, F. Mitjans, and R. Vilella for providing MoAbs HP2/1, 17E6, and 93.1B3, respectively. Dr E. Santos provided the Ha-Ras-Asn-17 dominant negative mutant. We also thank Drs M.L. Corcoran, H.K. Kleinman, C. López-Otin, E. Santos, and L.M. Whal for useful discussions and constructive comments.
Supported by grants from Fondo de Investigación Sanitaria (FIS 00/0689, FIS 98/0301, FIS 96/2145) and Televisió de Catalunya (95/3009).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Maria C. Cid, MD, Department of Internal Medicine, Hospital Clı́nic, Villarroel 170, 08036-Barcelona, Spain.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal