Abstract
Bone marrow transplantation (BMT) is relatively effective for the treatment of lysosomal storage diseases. To better understand the contribution of specific hematopoietic lineages to the efficacy of BMT, we transplanted β-glucuronidase–positive mononuclear phagocytes derived from either the peritoneum or from bone marrow in vitro into syngeneic recipients with mucopolysaccharidosis type VII (MPS VII). Cell surface marking studies indicate that the bone marrow-derived cells are less mature than the peritoneal macrophages. However, both cell types retain the ability to home to tissues rich in cells of the reticuloendothelial system after intravenous injection into MPS VII mice. The half-life of both types of donor macrophages is approximately 7 days, and some cells persist for at least 30 days. In several tissues, therapeutic levels of β-glucuronidase are present, and histopathologic analysis demonstrates that lysosomal storage is dramatically reduced in the liver and spleen. Macrophages intravenously injected into newborn MPS VII mice localize to the same tissues as adult mice but are also observed in the meninges and parenchyma of the brain. These data suggest that macrophages play a significant role in the therapeutic efficacy of BMT for lysosomal storage diseases and may have implications for treatments such as gene therapy.
LYSOSOMAL STORAGE diseases are a group of congenital disorders characterized by the intracellular accumulation of undegraded catabolites and are usually caused by a single gene defect. Mucopolysaccharidosis type VII (MPS VII; Sly Syndrome) is caused by the absence of β-glucuronidase activity and results in the accumulation of undegraded glycosaminoglycans.1-3 MPS VII is a progressive disease and the clinical features manifest themselves within the first few months to years of life in humans and include facial dysmorphism, skeletal deformities, mental retardation, hepatosplenomegaly, corneal clouding, hearing defects, and a shortened life span.
In addition to occurring in humans, murine,3feline,4 and canine5 models of MPS VII exist. The mouse model has been extensively characterized and is known to be caused by a single basepair deletion in exon 10 of the β-glucuronidase gene.6 The MPS VII mouse model shares most of the clinical and pathologic features of the human disease and therefore makes an excellent model for the study of lysosomal storage diseases and for the development of novel therapies.3 7
Bone marrow transplantation (BMT) has been shown to be relatively effective in treating murine MPS VII.8-10 Adult MPS VII mice receiving BMT demonstrate a marked reduction in lysosomal storage in many tissues, including the liver, spleen, cornea, and meninges, and have a dramatically extended life span.8 Because of the progressive nature of the disease, more effective therapy is achieved by initiating treatment in the neonatal period. Bone marrow transplantation in neonatal mice reduces storage in bones, joints, and periarticular tissue and also reduces the hearing loss associated with MPS VII.9 10
Although BMT is effective for the treatment of MPS VII, it is unclear which hematopoietic cell type or cell types are responsible for the therapeutic response. Our current hypothesis is that donor-derived mononuclear phagocytes account for a large proportion of the therapeutic enzyme produced in the host. In this study, we examine the distribution, apparent half-life, enzyme levels, and effect on the disease of fully differentiated peritoneal or bone marrow-derived macrophages after intravenous injection into adult and newborn MPS VII mice.
We demonstrate that, although the 2 cell types have different cell surface markers, both cell types retain the ability to home to the same tissues and have a distribution similar to that of endogenous macrophages. Both cell types have similar half-lives in vivo and can deliver sufficient quantities of enzyme to reduce lysosomal distension in the liver and spleen. Experiments performed in neonatal MPS VII mice also demonstrate that the donor macrophages are capable of delivering enzyme to the brain.
These experiments suggest that both fully differentiated and less mature macrophages behave similarly in vivo. In addition, macrophages may be responsible for a significant portion of the therapeutic efficacy of BMT.
MATERIALS AND METHODS
Animals.
Homozygous mutant (mps/mps) mice were obtained from a B6.C-H-2bm1/ByBir-gusmps/+ colony maintained by M.S.S. at Washington University (St Louis, MO). Homozygous normal donor mice were obtained either from a separately bred group of syngeneic normal (+/+) mice or from heterozygote matings after distinguishing homozygous normal from heterozygotes (+/mps) by polymerase chain reaction (PCR).11Homozygous mutants were identified at birth by the absence of β-glucuronidase activity using a fluorometric assay on a small sample of tissue obtained by toe clipping.9
Isolation, differentiation, and injection of mononuclear phagocytes.
Homozygous normal mice were injected intraperitoneally with 1 mL of thioglycollate (DIFCO Laboratories, Detroit, MI). Peritoneal cells were harvested 5 days after thioglycollate injection by killing the donor and performing peritoneal lavage twice, using a total of 10 mL of Ca2+- and Mg2+-free phosphate-buffered saline (PBS). The cells were counted and 5 × 105 cells were frozen for later biochemical analysis. Cytospins of 1 × 105 cells were made and stained with Wright Giemsa stain (Sigma, St Louis, MO). The cytospins were examined immediately to determine if the lavage fluid contained a preponderance of macrophages that appeared to be in an activated state. The presence of large cytoplasmic vacuoles in 80% to 90% of the mononuclear cells was the criteria for injection of thyoglycollate-stimulated macrophages into MPS VII recipients.
Unfractionated bone marrow from +/+ donors was cultured in Dulbecco’s modified Eagles medium supplemented with 10% fetal calf serum (GIBCO-BRL, Grand Island, NY), 2 mmol/L L-glutamine, 100 U penicillin, 100 μg/mL streptomycin, 250 ng/mL fungizone, 1 μg/mL murine interleukin-3 (IL-3), and 10 ng/mL murine macrophage colony-stimulating factor (M-CSF; R & D Systems, Minneapolis, MN). Bone marrow cells were cultured in 100-mm petri dishes at a density of 2.5 × 106 cells/mL. Fresh media was added every 48 hours. After 4 to 6 days in culture, the nonadherent cells were removed and discarded. The adherent cells were washed 3 times in ice-cold PBS and then incubated in 5 mL of ice-cold PBS with 0.02% EDTA (Sigma) for 20 minutes on a rocking platform at 4°C. Cells were resuspended in PBS, and a sample was saved for cytospins and biochemical analysis.
Adult mps/mps recipients ranging in age from 50 to 123 days were injected intravenously through the tail vein with 400 μL of macrophages (1 × 107 cells) from a donor of the same sex. Twelve adult mutants each were injected with either peritoneal or progenitor-derived macrophages and 3 of the mice from each group were killed at each of the following time points: 1, 7, 15, and 30 days after injection. An additional 5 adult mutants were injected with the same number of peritoneal macrophages and killed at 15 minutes, 1 hour, 3 hours, 6 hours, and 12 hours after injection.
Neonatal mps/mps mice were injected intravenously with 100 μL (2.5 × 106 cells) of peritoneal macrophages from a same-sex donor through the superficial temporal vein.12 Six neonates were injected on day 1 or 2 of life. Three were killed 24 hours after injection and 3 were killed 3 weeks after injection.
Biochemical analysis.
The liver, spleen, lung, thymus, and 1 kidney were harvested from the injected animals as well as from 3 uninjected positive control (+/+) and 3 uninjected negative control (mps/mps) animals, and the total weight of each organ was determined. Fractions for biochemical analysis were taken from each tissue and also weighed. Samples of heart, brain, mesenteric lymph node, skeletal muscle, and bone marrow from 1 femur were also collected. Samples of liver, spleen, kidney, lung, heart, brain, and thymus were taken from the neonates. The tissues were homogenized with a motorized pestle in 10 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 0.2% Triton X-100, and 1 mmol/L dithiothreitol and centrifuged in a microfuge at 14,000 rpm for 1 minute to remove debris. β-Glucuronidase, α-galactosidase, and β-hexoseaminidase activities were measured fluorometrically using the substrates 4-MU-β-D-glucuronide, 4-MU-α-D-galactoside, and 4-MU-N-acetyl-β-D-glucosaminide (Sigma), respectively.13A portion of each homogenate was incubated at 37°C for 1 hour with 100 μL substrate and the reactions were stopped with 1 mL 0.1 mol/L sodium carbonate. The protein concentrations of the homogenates were determined using the Coomasie dye-binding assay14 (Bio-Rad, Hercules, CA). The specific activities (1 unit [U] = 1 nmole substrate cleaved/hr/mg protein) of the lysosomal enzymes in each tissue at each time point were calculated. The total β-glucuronidase activity in the weighed tissues was also calculated. The total β-glucuronidase activity in a sample of the injected macrophages (1 × 105 cells) was measured to determine the total activity introduced into each animal. Statistical significance was calculated using the Student’s t-test.
Histochemistry, immunofluorescence, and immunohistochemistry.
A portion of the liver, spleen, kidney, lung, heart, brain, thymus, mesenteric lymph node, skeletal muscle, and ribs was obtained from each experimental adult and frozen in embedding medium for histochemical and immunohistochemical analysis. Samples of the small intestine, colon, and foot pad were also taken from a single adult mutant 24 hours after injection. The same tissues, with the exception of the mesenteric lymph nodes, were collected from MPS VII mice injected as neonates. Ten-micron–thick sections of each tissue were obtained. Slides of peripheral white blood cells were also prepared for histochemical staining from the 5 adult animals killed from 15 minutes to 12 hours after injection. Cytospins of donor macrophages were prepared for histochemical and immunohistochemical analysis. The slides were stored at −70°C until analyzed.
The staining procedure for enzymatically active β-glucuronidase was performed as previously described using naphthol-AS-BI-β-D-glucuronide (Sigma) as a substrate.11The surface markers Mac-1 (CD11b), Mac-3, F4/80, and macrophage metalloelastase (MME) on the donor cells were identified by immunohistochemical and immunofluorescent techniques (Mac-1 and Mac-3 primary antibodies [Pharmingen, San Diego, CA], F4/80 antibody [Biosource International, Camarillo, CA], and MME antibody [kind gift of S. Shapiro15]). Sections were fixed for 20 minutes at 4°C with a solution of 70% acetone, 0.3% chlorohydrate, and 6% neutral buffered formalin. Endogenous biotin was blocked with commercially available reagents (Vector Laboratories, Burlingame, CA). The sections were incubated for 30 minutes at room temperature in antibody blocking buffer (PBS, 1% bovine serum albumin, 0.2% powdered milk, and 0.03% Triton X-100). The sections were then incubated overnight at 4°C with primary antibody followed by 30 minutes of incubation in the appropriate secondary antibody. A biotinylated rat antimouse secondary antibody was used for detection of Mac-1, Mac-3, and F4/80, and a biotinylated goat antirabbit secondary antibody was used to detect MME (Vector Laboratories). Streptavidin-conjugated alkaline phosphatase was used to detect Mac-1, and fluorescein conjugated to streptavidin was used to detect F4/80 and Mac-3. Streptavidin-conjugated horseradish peroxidase was used to detect MME. Control slides were incubated with the appropriate preimmune sera and then stained as described above.
To perform dual immunohistochemistry for Mac-1 and histochemistry for β-glucuronidase activity on spleen sections, the antibody staining was performed first. The pH of the sections was then lowered to approximately 4.5 for histochemical staining.
Histopathology.
Portions of liver, spleen, kidney, lung, heart, thymus, and eye were taken from each of the 24 adult mice in the 30-day study as well as from the neonatal mice and preserved in 4% paraformaldehyde and 2% glutaraldehyde in PBS. These tissue blocks were then embedded in Spurr’s resin, and 0.5-μm sections were stained with toluidine blue.7
RESULTS
Donor mononuclear phagocytes.
Cytospins of thioglycollate-stimulated peritoneal cells stained with Wright’s stain demonstrate the characteristic morphology of activated cells. Approximately 90% of the cells are mononuclear with extensive cytoplasmic vacuolation (Fig1A). The population of adherent cells derived from bone marrow in vitro is composed of approximately 90% mononuclear cells with more homogeneous cytoplasm (Fig 1B). All of the peritoneal and progenitor-derived macrophages stain intensely for enzymatically active β-glucuronidase (data not shown). Immunohistochemical and immunofluorescent staining of the donor cells demonstrates that greater than 90% of the peritoneal cells express relatively high levels of Mac-1, Mac-3, and F4/80 and that approximately 5% to 10% express high levels of MME (Table 1). Bone marrow-derived macrophages also express relatively high levels of Mac-1 and Mac-3 but express neither F4/80 nor MME. Dual in situ histochemical and immunohistochemical staining demonstrates the colocalization of β-glucuronidase activity and Mac-1 in individual cells in sections of spleen obtained 24 hours and 15 days after injection (Fig 1C and 1D).
The morphology of thioglycollate-stimulated peritoneal macrophages (A) and in vitro-differentiated bone marrow-derived macrophages (B) injected into MPS VII recipients are shown after Wright Giemsa staining. Dual histochemical staining for β-glucuronidase activity (diffuse red stain) and immunohistochemical staining for Mac-1 (black cell surface stain) of spleen sections from MPS VII mice 24 hours (C) and 15 days (D) after injection of peritoneal macrophages demonstrates the colocalization of the 2 markers (white arrows). Mac-1–positive spleen cells that are β-glucuronidase negative (solid arrows) are also seen. ([A] and [B], original magnification × 1,145; [C] and [D], original magnification × 467).
The morphology of thioglycollate-stimulated peritoneal macrophages (A) and in vitro-differentiated bone marrow-derived macrophages (B) injected into MPS VII recipients are shown after Wright Giemsa staining. Dual histochemical staining for β-glucuronidase activity (diffuse red stain) and immunohistochemical staining for Mac-1 (black cell surface stain) of spleen sections from MPS VII mice 24 hours (C) and 15 days (D) after injection of peritoneal macrophages demonstrates the colocalization of the 2 markers (white arrows). Mac-1–positive spleen cells that are β-glucuronidase negative (solid arrows) are also seen. ([A] and [B], original magnification × 1,145; [C] and [D], original magnification × 467).
Cell Surface Markers on Donor Macrophages
| Marker . | Peritoneal* . | Progenitor† . | ||
|---|---|---|---|---|
| % Positive . | Intensity‡ . | % Positive . | Intensity‡ . | |
| Mac-1 | ≥90 | +-+++ | ≥90 | +-+++ |
| Mac-3 | ≥90 | +++ | ≥90 | +-++ |
| F4/80 | ≥90 | +-+++ | 0 | 0 |
| MME | 5-10 | +++ | 0 | 0 |
| Marker . | Peritoneal* . | Progenitor† . | ||
|---|---|---|---|---|
| % Positive . | Intensity‡ . | % Positive . | Intensity‡ . | |
| Mac-1 | ≥90 | +-+++ | ≥90 | +-+++ |
| Mac-3 | ≥90 | +++ | ≥90 | +-++ |
| F4/80 | ≥90 | +-+++ | 0 | 0 |
| MME | 5-10 | +++ | 0 | 0 |
Peritoneal macrophages refer to cells obtained from the peritoneum of mice 5 days after thioglycollate stimulation.
Progenitor macrophages refer to mononuclear cells derived from bone marrow exposed to IL-3 and M-CSF in vitro.
0, no staining; +, minimal; ++, moderate; +++, intense staining above background.
The total β-glucuronidase activity in 1 × 107peritoneal and progenitor macrophages was 5,775 and 11,900 U, respectively. This level represents approximately 10% to 20% of the total activity measured in liver, spleen, kidneys, lungs, thymus, and marrow from a normal mouse.
Tissue distribution and kinetics.
The distribution of β-glucuronidase positive donor cells in various tissues is similar after injection with either peritoneal or progenitor-derived macrophages. The livers of injected adult mice demonstrate a uniform distribution of cells 24 hours after injection (Fig 2A). In contrast, the majority of β-glucuronidase positive cells are localized in the red pulp of the spleen and appear to surround the germinal centers (Fig 2B). There are also numerous β-glucuronidase–positive cells observed in the lung and bone marrow (Fig 2C and D). In the kidney, the introduced cells appear to localize mainly to the glomeruli (Fig 2E). There are also rare positive cells present in the thymus, in the lamina propria of the intestine, and in the myocardium (data not shown). There are no β-glucuronidase–positive cells observed in the peripheral circulation of any of the 5 adult MPS VII mice killed from 15 minutes to 12 hours after injection (data not shown).
Histochemical staining for β-glucuronidase activity (red) was performed on tissues from adult MPS VII mice 24 hours after injection with progenitor-derived macrophages derived from normal donors. β-Glucuronidase–positive cells assume a relatively uniform distribution in the liver (A), lung (B), and bone marrow (C). Enzyme-positive cells in the spleen are present predominantly in the red pulp, with rare positive cells observed in the germinal centers (D). In the kidney, the donor cells appear to be present primarily in the glomeruli (E). The newborn brain contains multiple β-glucuronidase–positive cells that are observed in both the meninges and the parenchyma (F). ([A], [C], [D], and [F], original magnification × 188; [B] and [E], original magnification × 300).
Histochemical staining for β-glucuronidase activity (red) was performed on tissues from adult MPS VII mice 24 hours after injection with progenitor-derived macrophages derived from normal donors. β-Glucuronidase–positive cells assume a relatively uniform distribution in the liver (A), lung (B), and bone marrow (C). Enzyme-positive cells in the spleen are present predominantly in the red pulp, with rare positive cells observed in the germinal centers (D). In the kidney, the donor cells appear to be present primarily in the glomeruli (E). The newborn brain contains multiple β-glucuronidase–positive cells that are observed in both the meninges and the parenchyma (F). ([A], [C], [D], and [F], original magnification × 188; [B] and [E], original magnification × 300).
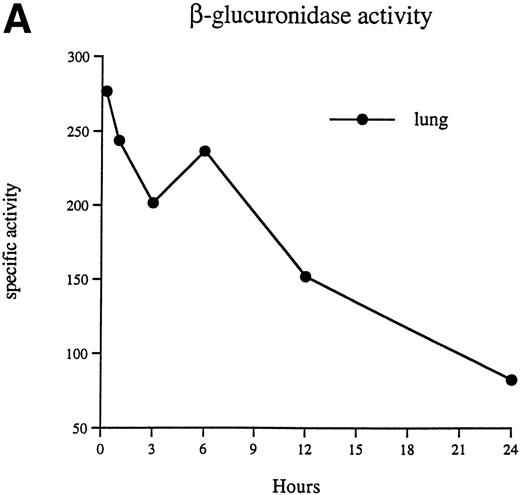
The specific activity observed in the lung of an MPS VII mouse 15 minutes after injection is 277 U (Fig 3A), which is equal to or greater than the levels seen in the lungs of normal mice. The activity associated with the lung decreases rapidly to approximately 83 U by 24 hours after injection. In contrast, the activity associated with the liver, spleen, and bone marrow increases during the first 24 hours after injection (Fig 3B).
β-Glucuronidase–specific activities (nanomoles of substrate cleaved per hour per milligram of protein) are shown in the lung (A), liver, spleen, and bone marrow (B) at 15 minutes, 1 hour, 3 hours, 6 hours, 12 hours, and 24 hours after injection of 1 × 107 donor peritoneal macrophages.
β-Glucuronidase–specific activities (nanomoles of substrate cleaved per hour per milligram of protein) are shown in the lung (A), liver, spleen, and bone marrow (B) at 15 minutes, 1 hour, 3 hours, 6 hours, 12 hours, and 24 hours after injection of 1 × 107 donor peritoneal macrophages.
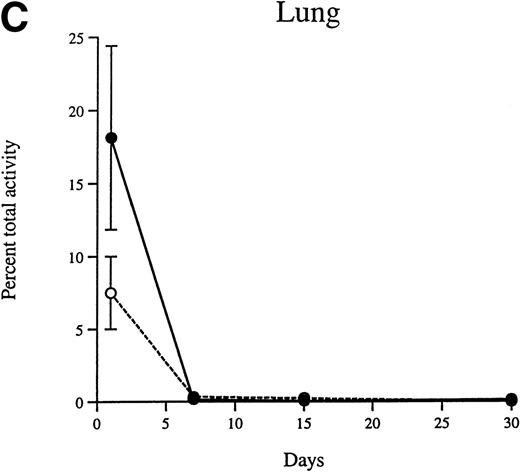
The percentage of the injected activity that is recovered in the liver, spleen, lung, kidney, thymus, and bone marrow 24 hours after injection of peritoneal and progenitor macrophages was 68.1% and 67.3%, respectively. The total amount of β-glucuronidase activity present in the adult liver, spleen, and lung of recipients decreases as time progresses (Fig 4A through C). The rate of decrease is similar for peritoneal and progenitor macrophages. The half-lives of both types of cells over the entire 30-day period is approximately 7 days. However, the decrease in enzyme activity does not obey strict first order kinetics, and the approximate half-lives over the first 15 days after injection is approximately 4 days, and from 15 to 30 days is approximately 20 days.
The decrease in β-glucuronidase activity in the liver (A), spleen (B), and lung (C) of MPS VII mice between 24 hours and 30 days after injection is shown for the peritoneal macrophages (•) and the progenitor-derived macrophages (○). The error bars represent ±1 standard deviation from the mean.
The decrease in β-glucuronidase activity in the liver (A), spleen (B), and lung (C) of MPS VII mice between 24 hours and 30 days after injection is shown for the peritoneal macrophages (•) and the progenitor-derived macrophages (○). The error bars represent ±1 standard deviation from the mean.
Although the liver accounted for the majority of the total activity (67% of the recovered activity is detected in the liver at all time points beyond 24 hours), the specific activities of tissues rich in cells of the reticuloendothelial system are similar. At day 1, the specific activities in the liver and spleen are 15.2 and 17.9 U, respectively. By days 7 and 30, the specific activities in the liver and spleen decrease to 8.1 and 9.7 and to 1.9 and 1.6 U, respectively.
The majority of activity is detected in the liver, spleen, and lung after injection of either cell type. However, measurable amounts of β-glucuronidase activity are detected in other tissues for a substantial period of time. At 24 hours after injection, the brain, kidney, heart, skeletal muscle, thymus, mesenteric lymph nodes, and bone marrow have measurable enzyme levels. By 1 week after injection, the lymph nodes and bone marrow still have detectable enzyme levels, but the remaining tissues have levels that were indistinguishable from background. However, β-glucuronidase–positive cells are detected histochemically in many of these tissues at 1 week and beyond.
Affect of donor macrophages on MPS VII.
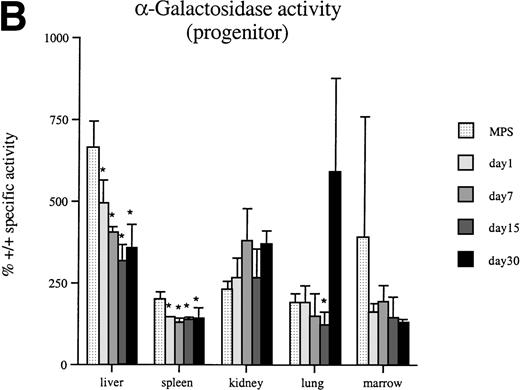
In some lysosomal storage diseases such as MPS VII, the levels of unaffected lysosomal enzymes increase, in some cases as much as several hundred percent above normal.8 Results from α-galactosidase assays show that the secondary elevation of this enzyme is reduced in liver, spleen, and lung by 30 days after injection (Fig 5A and B). Furthermore, both peritoneal and progenitor-derived macrophages result in similar reductions in enzyme levels. Corresponding reductions in the secondary elevation of the lysosomal enzyme β-hexoseaminidase are also observed in these tissues 30 days after macrophage injections (data not shown).
Thirty days after a single injection of 1 × 107 β-glucuronidase–positive macrophages into adult MPS VII mice, -galactosidase levels are significantly decreased in several tissues. A significant decrease is produced by both peritoneal-(A) and progenitor-derived (B) cells. An asterisk (*) indicates a significant (P < .05) decrease in specific activity compared with untreated MPS VII (MPS) mice.
Thirty days after a single injection of 1 × 107 β-glucuronidase–positive macrophages into adult MPS VII mice, -galactosidase levels are significantly decreased in several tissues. A significant decrease is produced by both peritoneal-(A) and progenitor-derived (B) cells. An asterisk (*) indicates a significant (P < .05) decrease in specific activity compared with untreated MPS VII (MPS) mice.
Microscopic examination of sections of liver and spleen confirm the biochemical data, suggesting that the injected macrophages result in partial correction of the disease (Fig 6). In the livers of treated animals, lysosomal storage is reduced in Kupffer cells at 7 days and is virtually eliminated by day 15. However, by day 30, lysosomal distension is apparent, but at a lower level than that observed in untreated MPS VII mice. In the spleens of injected animals, lysosomal storage is reduced at a slightly slower rate than in the liver but is virtually eliminated by day 30. There was no significant reduction in storage observed in the meninges, cornea, or retinal pigment epithelial cells of treated adult MPS VII mice.
Histopathologic analysis demonstrates no reduction in lysosomal storage in the liver (A) or spleen (F) 24 hours after injection of peritoneal macrophages. A dramatic decrease in lysosomal storage is observed in the liver 7 (B), 15 (C), and 30 (D) days after injection. A similar reduction in storage is observed in the spleen 7 (G), 15 (H), and 30 (I) days after injection. Progenitor-derived macrophages provide an equivalent level of correction. The histopathological appearance of the liver (E) and spleen (J) from an untreated age-matched MPS VII mouse is shown. (Original magnification × 242.)
Histopathologic analysis demonstrates no reduction in lysosomal storage in the liver (A) or spleen (F) 24 hours after injection of peritoneal macrophages. A dramatic decrease in lysosomal storage is observed in the liver 7 (B), 15 (C), and 30 (D) days after injection. A similar reduction in storage is observed in the spleen 7 (G), 15 (H), and 30 (I) days after injection. Progenitor-derived macrophages provide an equivalent level of correction. The histopathological appearance of the liver (E) and spleen (J) from an untreated age-matched MPS VII mouse is shown. (Original magnification × 242.)
Macrophage injection in newborn MPS VII mice.
Histochemical staining of tissues from newborn mice killed 24 hours after injection demonstrate β-glucuronidase–positive cells in all tissues examined. The distribution of the cells in the various tissues is similar to that observed in the adult animals (data not shown). One difference between newborn and adult injections is the presence of β-glucuronidase–positive cells in the brains of the injected newborn animals. At 24 hours after injection, there are only rare β-glucuronidase–positive cells observed in the adult brain, and those appeared to be localized primarily to the meninges or what appear to be vascular structures. In contrast, the newborn brain has multiple positive cells per field that are located in both the meninges and parenchyma (Fig 2F). At 3 weeks after injection, the brains from mice injected as newborns still have approximately 0.8% normal levels of activity. The brains of MPS VII mice injected as adults have undetectable levels of enzyme beyond 24 hours. The histopathologic correction after macrophage injection in newborns is similar to that observed in adult animals. There is no apparent reduction in lysosomal storage in the meninges or neurons of the brain.
DISCUSSION
β-Glucuronidase–positive macrophages localize in many tissues after intravenous injection. We believe that these cells are of donor origin. However, another possible source of the positive cells is from the release of enzyme from dead donor cells and the subsequent receptor-mediated uptake by host cells.16-18 Results from enzyme replacement studies suggest that this is not the case. If enzyme is released into the circulation and endocytosed by recipient cells, the distribution of β-glucuronidase activity should be more diffuse and the half-life should be similar to that observed after enzyme replacement (2 to 4 days rather than 7 days after macrophage injection).19,20 It is also possible that endogenous macrophages engulf the donor cells. Although the distribution of activity should be similar to what we observe in this study, we believe that this explanation is unlikely for 3 reasons. First, the donor cells are from syngeneic, sex-matched animals, so there are no histocompatibility mismatches. Second, it seems likely that the half-life of the enzyme from an engulfed cell should mimic that of directly administered enzyme. Finally, a recent report suggests that mature bone marrow-derived monocytic cells can engraft and repopulate recipient animals in a pattern similar to endogenous macrophages.21
Macrophages harvested from the peritoneum or derived from bone marrow in vitro appear to be at different stages of maturation. Both populations express relatively high levels of β-glucuronidase and are comprised primarily of mononuclear cells that express the macrophage markers Mac-1 and Mac-3.22,23 However, the thioglycollate-stimulated peritoneal cells have the morphology of activated macrophages and also express the surface antigens F4/80 and MME that are present on more mature or activated mononuclear phagocytes.15 24 Interestingly, both cell populations retain the ability to home to the same tissues when transplanted into a syngeneic recipient. The distribution of both types of donor cells is similar to that of endogenous bone marrow-derived macrophages. The donor cells are localized in a pattern similar to Kupffer cells of the liver, mesangial cells of the kidney, and phagocytic cells in the red pulp of the spleen. Donor macrophages also localize in lower numbers to regions in which antigen-presenting cells are normally found, such as the lung, thymus, white pulp of the spleen, and the lamina propria of the intestine.
In the case of the thioglycollate-stimulated peritoneal cells, it is unclear where the cells are recruited from, but it is interesting that they can home to many different sites in the recipient animal. This suggests that, once the cells are activated and migrate to a site of inflammation or irritation, they still retain the signals necessary to migrate to other sites in the body. It is also interesting that mononuclear phagocytes differentiated in vitro acquire the correct signals that mediate migration in vivo to sites where endogenous macrophages are normally localized.
The half-life of metabolically labeled endogenous macrophages in normal mice ranges from 20 to 30 days.25 26 The apparent half-life of the transplanted macrophages in these experiments is considerably shorter. There may be several reasons for this apparent difference. First, the half-lives determined in this set of experiments are indirect measurements based on β-glucuronidase activity. The state of differentiation, activation, or the localization of the macrophages in the recipient animal may affect the expression of β-glucuronidase, thereby affecting the apparent half-life of the donor cells. Second, it is possible that a fraction of the donor cells undergo apoptosis and are eliminated after becoming more mature or activated cells. However, it seems unlikely that this is the major cause of the shortened half-life, because the less mature progenitor-derived cells have essentially the same half-life as the thioglycollate-stimulated cells. Third, these experiments involve the injection of normal macrophages into a disease model. It is possible that many of the donor cells die prematurely after being faced with the abnormally large accumulation of glycosaminoglycans in the recipient animal. Finally, a macrophage that is manipulated ex vivo and then reintroduced into another animal may have an intrinsically shorter life span.
Donor macrophages from β-glucuronidase–positive animals have a significant impact on the disease in MPS VII mice. A reduction in the secondary elevations of other lysosomal enzymes is a relatively sensitive indicator of a positive response to therapy.27Although the reductions in α-galactosidase activity are not statistically significant in every tissue 30 days after injection, the levels are consistently decreased in the liver and spleen. As little as 1% of normal levels of β-glucuronidase may be sufficient to reduce lysosomal storage in some tissues in the MPS VII mouse.27-29 Although enzyme levels sufficient to reduce lysosomal storage are present in several tissues 24 hours after injection, no histopathologic improvement is observed at that time. It appears that several weeks may be required for the relatively low levels of activity to reduce accumulated lysosomal storage. This can be seen in the liver and spleen, which have 2% to 5% normal enzyme levels, where a significant reduction in lysosomal storage is not observed until 7 to 15 days after injection. In addition, there is little or no reduction of lysosomal storage in hepatocytes at any time after injection. This suggests that either the amount of enzyme transferred from the donor macrophages is insufficient to reduce storage or the half-life of the donor macrophages in the liver did not allow for a significant reduction in hepatocytes.
Lysosomal storage diseases are progressive in nature and often there is little evidence of disease at birth.1 Studies in MPS VII mice treated with bone marrow transplantation have shown that the disease can be more effectively treated when the transplants are performed at birth.9,10 However, in this study, there is little difference between injecting β-glucuronidase–positive macrophages in adult or newborn MPS VII mice. One of the few differences was observed in the brain. There are more donor cells present, and higher specific activity in the brains of newborn compared with adult MPS VII mice. In addition, the cells are present in the meninges as well as in the parenchyma of newborn brains. In contrast, the positive cells associated with the adult brains appear to be localized primarily to the meninges or vascular structures. The enzyme activity also persisted for at least 3 weeks in the newborn brain, whereas no enzyme activity is detected in the adult brain beyond 24 hours. One explanation for this difference could be that the newborn MPS VII mice were injected with approximately 5 times more cells per gram of body weight. Alternatively, it is possible that more macrophages are able to enter the brain of a newborn mouse before the blood-brain barrier is fully formed,30 and once there, they can survive for some time, perhaps becoming resident microglia. Similarly, the lack of β-glucuronidase–positive cells in the adult brain may be due to the maturity of the blood-brain barrier. Several studies show that repopulation of bone marrow-derived macrophages in the brains of adult mice after BMT was dramatically delayed relative to the repopulation of fixed tissue macrophages in other tissues such as liver and spleen.31 32 The delay in macrophage repopulation of the brain after BMT may account for the lack of positive cells in the adult mice in this study. The life span of the donor macrophages simply may not be long enough for them to gain access to the brain. Although enzyme activity persists in the brains of newborn MPS VII mice, it is insufficient to reduce lysosomal storage in the meninges or neurons.
BMT has been shown to be relatively effective in humans and animal models of lysosomal storage diseases.8-10,33-37 The hematopoietic lineage or lineages responsible for the correction in these diseases are uncertain. These data show that mature peritoneal macrophages and less mature bone marrow-derived monocytic phagocytes can localize to many tissues and persist for at least 30 days after transplantation. Levels of β-glucuronidase sufficient to reduce lysosomal storage in the liver and spleen of MPS VII mice are delivered after a single intravenous injection of macrophages from normal donor mice. These data suggest that the macrophage lineage is a major source of corrective enzyme after BMT for lysosomal storage diseases. Because macrophages are capable of delivering significant amounts of lysosomal enzymes to many tissues, macrophage-directed therapy38 or the use of macrophage-specific promoters in a gene transfer setting39 may enhance the efficacy of hematopoietic-directed therapies for these diseases.
ACKNOWLEDGMENT
The authors thank Steve Shapiro (Washington University School of Medicine, St Louis, MO) for helpful discussions and for performing the macrophage metalloelastase immunohistochemistry assays.
Supported in part by National Institutes of Health Grants No. DK50158 and HD33671 to M.S.S.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mark S. Sands, PhD, Washington University School of Medicine, Department of Internal Medicine, Box 8007, 660 S Euclid Ave, St Louis, MO 63110.

![Fig. 1. The morphology of thioglycollate-stimulated peritoneal macrophages (A) and in vitro-differentiated bone marrow-derived macrophages (B) injected into MPS VII recipients are shown after Wright Giemsa staining. Dual histochemical staining for β-glucuronidase activity (diffuse red stain) and immunohistochemical staining for Mac-1 (black cell surface stain) of spleen sections from MPS VII mice 24 hours (C) and 15 days (D) after injection of peritoneal macrophages demonstrates the colocalization of the 2 markers (white arrows). Mac-1–positive spleen cells that are β-glucuronidase negative (solid arrows) are also seen. ([A] and [B], original magnification × 1,145; [C] and [D], original magnification × 467).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2142/5/m_blod418free01z.jpeg?Expires=1767709422&Signature=01txTyPE8m9OEIMPt4RBcnwLmOAkpbuP72FwG1oxDaVKaVyJAsnJ1UYRGAeJm5eedxY4QoVH0dHyXawEtsYVwc~uNjiGRAvUgVNj725Chn1WtrM160Z-4uE~5G9YJxQXPmybvd2OvwDCbBq86DcZnPGZkdSkRHB4egMzyi~mtp5Q~kWKm6ey4SjuHv6UVrH9D7d7BOtRZof6x-7bAs2pZceZ4A~8cfGIQLpFvRG6W0Zjjdb9LiNisz~iub1Iz9uymurr2waC-IDcCx4-LWDTskz3FtW7irT8fbZcnz2C5Ir8kjSZXSo6oQps1F5i2IvUFQrDVAOWndAYA6HemT1gDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Histochemical staining for β-glucuronidase activity (red) was performed on tissues from adult MPS VII mice 24 hours after injection with progenitor-derived macrophages derived from normal donors. β-Glucuronidase–positive cells assume a relatively uniform distribution in the liver (A), lung (B), and bone marrow (C). Enzyme-positive cells in the spleen are present predominantly in the red pulp, with rare positive cells observed in the germinal centers (D). In the kidney, the donor cells appear to be present primarily in the glomeruli (E). The newborn brain contains multiple β-glucuronidase–positive cells that are observed in both the meninges and the parenchyma (F). ([A], [C], [D], and [F], original magnification × 188; [B] and [E], original magnification × 300).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2142/5/m_blod418free02z.jpeg?Expires=1767709422&Signature=d0yN-EXVA4SIYTzOIQ6vdf-RSdpDyVmyacdRHwchjvT2cjaBW8~WmOFmZzqqik6aNOl0XmHS7CTdip~b~8x2hc~lhOHBuumnkOz1jifX93l3GZuOG6DjodVd531BzQJVcMRvHbAOJXYSjRzyMK-AWJelQHDI6v12GlBKgbxDCR3ubqwZiGNSEa1SozH32p5ACVrIcjpgvim1n-pOMWwk~hgkb5Hn-qhdva5vVZ~AaPFdgxkLnbhrEzpH6O~B4MW7aU~zR2FoVFM0~fItz~6oLBoUeEnM8qJDLL11xGzuiCMr81tJlTTXCZO6zm5AB-9G~WX1EjRUyn3uyXj0HtHAHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal