Abstract
Fanconi anemia (FA) is an autosomal recessive disorder characterized by birth defects, increased incidence of malignancy, and progressive bone marrow failure. Bone marrow transplantation is therapeutic and, therefore, FA is a candidate disease for hematopoietic gene therapy. The frequent finding of somatic mosaicism in blood of FA patients has raised the question of whether wild-type bone marrow may have a selective growth advantage. To test this hypothesis, a cohort radio-ablated wild-type mice were transplanted with a 1:1 mixture of FA group C knockout (FACKO) and wild-type bone marrow. Analysis of peripheral blood at 1 month posttransplantation showed only a moderate advantage for wild-type cells, but upon serial transplantation, clear selection was observed. Next, a cohort of FACKO mice received a transplant of wild-type marrow cells without prior radio-ablation. No wild-type cells were detected in peripheral blood after transplantation, but a single injection of mitomycin C (MMC) resulted in an increase to greater than 25% of wild-type DNA. Serial transplantation showed that the selection occurred at the level of hematopoietic stem cells. No systemic side effects were observed. Our results show that in vivo selection for wild-type hematopoietic stem cells occurs in FA and that it is enhanced by MMC administration.
FANCONI ANEMIA (FA) is an inherited disorder characterized clinically by pancytopenia, short stature, renal defects, defects of the thumb and radius, and hyperpigmentation.1 Pancytopenia is universally present, but the other traits can be variable in their presence and severity.2,3 On the basis of somatic cell genetic experiments it is clear that at least 8 or more complementation groups exist, termed A through H,4-7 with the genes for groups A, C, and G having been cloned.8-11 The group A and C genes do not resemble any other known genes,12 whereas group G has turned out to be XRCC9.8 The prognosis in FA is poor, with most of the morbidity and mortality due to pancytopenia. Affected individuals have a predisposition to leukemia13and other tumors, with the suggestion of an increased incidence of neoplasms in heterozygotes.14
The basic biochemical defect in FA is unknown, but FA cells are hypersensitive to DNA damage by interstrand cross-linking agents such as mitomycin C (MMC), diepoxybutane (DEB), or cis-diplatinum. This can be exploited for diagnostic testing, including prenatal diagnosis, in which these abnormalities are induced by exposure of cells to MMC or DEB.15 During cytogenetic analysis, cells display chromosomal aberrations at a very high frequency, including chromatid breaks, gaps, exchange figures, and endoreduplication. The sensitivity of FA cells to DNA cross-linking agents varies greatly between complementation groups and families, ranging from severely sensitive to almost normal. Bone marrow transplantation is currently the best therapy available for FA and has been successfully performed in FA in a number of cases.16,17 The successful use of bone marrow transplantation makes FA an obvious candidate for somatic gene therapy by transfer of the normal gene into hematopoietic stem cells.18 19
Recently, an important observation relevant to bone marrow transplantation and gene therapy in FA was reported. It was found that up to 25% of patients with FA had evidence of somatic mosaicism in peripheral blood lymphocytes.20 In these patients, populations of cells that were no longer sensitive to DNA cross-linking agents could be identified. In 1 case, the molecular mechanism by which the revertant cells arose was identified.20 An intragenic mitotic recombination resulted in a cell containing 2 mutant FA alleles on one chromosome and a wild-type on the other. Similar somatic mosaicism has previously been observed in adenosine deaminase deficiency and Bloom’s syndrome.21 22 In both cases, genetically corrected cells were found to have a selective growth advantage. The observation of frequent mosaicism in FA patients suggests that in vivo growth selection also favors wild-type cells, at least in peripheral blood. Mosaicism at the level of the hematopoietic stem cell has not yet been convincingly documented in FA, and the factor(s) that enhances selection has not been identified. The hypothesis that the growth selection in FA occurs at the stem cell level could best be tested directly in an animal model.
We have previously reported the generation of a mouse model with a targeted deletion of exon 9 of the FANCC gene.23Heterozygous FANCC knockout mice (FACKO) are normal, and mutant animals have no obvious developmental abnormalities except sterility caused by decreased numbers of germ cells.23 However, fibroblast cultures from the FACKO mice exhibited the characteristic chromosome breakage seen in human FA cells upon treatment with MMC or DEB.23 Hematologically, FACKO mice appear almost normal and have normal complete blood counts throughout life. In addition, the number of hematopoietic progenitor cells is the same in mutant and control mice as measured by in vitro culture assays23 and spleen colony-forming units.24 However, in vitro assays (burst-forming units-erythroid [BFU-E] and colony-forming unit granulocyte-macrophage [CFU-GM]) have documented marked hypersensitivity to the mitotic inhibitor interferon-γ. FACKO marrow is an order of magnitude more sensitive to interferon-γ than heterozygote controls.25
We chose 2 experimental approaches to test for both spontaneous and enhanced selection of wild-type hematopoietic stem cells (HSCs) in FACKO mice. First, we tested the repopulation capacity of FACKO and wild-type marrow in lethally irradiated recipient mice in a competition assay. Second, we transplanted wild-type marrow into nonirradiated FACKO mice and determined whether repopulation could occur in this setting. Our results show that wild-type HSCs are positively selected in FACKO mice and that this selection is enhanced by serial transplantation and the administration of sublethal doses of MMC.
MATERIALS AND METHODS
Mouse strains and animal husbandry.
All mice used in these experiments were 6 to 12 weeks old, were derived from the 129SvJ strain, and were either FACKO, as previously reported,23 or heterozygous for a deletion at the hereditary tyrosinemia type 1 (HT1) locus, as previously reported.26 The animals were housed in the Department of Animal Care (DAC) at Oregon Health Sciences University (Portland, OR). All experiments were performed in accordance to the guidelines of the Institutional Animal Care and Use Committee. Rodent chow (Purina 5010) and water were given ad libitum. All experiments were performed according to a protocol approved by the DAC.
Polymerase chain reaction (PCR) genotyping.
PCR genotyping was performed with commercially available buffers and enzymes (PE Applied Biosystems, Foster City, CA) using a Perkin-Elmer 480 thermal cycler (Perkin-Elmer, Norwalk, CT). Primer A (AAAGACAGAGGAGACGCCAC) hybridized to the sense strand of the genomic DNA 5′ to the knockout region. Primer B (AAGAGCAACACAAATGGTAAGG) hybridized to the antisense strand of the wild-type genomic DNA 3′ to primer A. Primer C (GCATGCTCCAGACTGCCTTG) hybridized to the antisense strand of the neo cassette. Primer A and B in combination give a product of 463 bp for a wild-type allele, and primers A and C give a product of 292 bp for a mutant allele. The assay is schematically depicted in Fig 1. PCR was conducted with a standard mixture including all 3 primers (100 ng DNA, 1× PCR buffer, 2 mmol/L magnesium chloride, 0.2 mmol/L dNTPs, 0.2 μmol/L each primer, 2.5 U Taq, and ddH2O to 30 μL), under standard conditions (30 cycles of 60 seconds at 95°C, 60 seconds at 58°C, and 60 seconds at 72°C and 10 minutes at 72°C). PCR products were visualized by agarose gel electrophoresis on a 1.5% gel stained with ethidium bromide. Genotyping was then scored by the presence or absence of appropriate PCR products.
Quantitative PCR assay. (A) Graphical representation of primer locations relative to the genomic structure of wild-type and transgenic knockout SV129 mice. Primers A, B, and C were used for PCR amplification, whereas primer D is for Southern blotting. The border for the knockout is the EcoRI restriction site. The lower panel shows a typical PCR reaction using primers A, B, and C in FACKO mutants (Mut) and heterozgyotes (Het). The wild-type DNA yields a 463-bp PCR product, and the mutant gives a 292-bp PCR product. (B) Ethidium bromide-stained gel of standard curve for quantifying DNA contribution. Wild-type and knockout DNA was mixed in various ratios then subjected to PCR. This gel would then be Southern-blotted and quantitated as described.
Quantitative PCR assay. (A) Graphical representation of primer locations relative to the genomic structure of wild-type and transgenic knockout SV129 mice. Primers A, B, and C were used for PCR amplification, whereas primer D is for Southern blotting. The border for the knockout is the EcoRI restriction site. The lower panel shows a typical PCR reaction using primers A, B, and C in FACKO mutants (Mut) and heterozgyotes (Het). The wild-type DNA yields a 463-bp PCR product, and the mutant gives a 292-bp PCR product. (B) Ethidium bromide-stained gel of standard curve for quantifying DNA contribution. Wild-type and knockout DNA was mixed in various ratios then subjected to PCR. This gel would then be Southern-blotted and quantitated as described.
Phenotypic analysis of bone marrow from FACKO mice.
Bone marrow was obtained from 8-week-old female mice by flushing the long bones of mice with Hank’s balanced salt solution (HBSS) supplemented with 3% fetal bovine serum and 10 mmol/L HEPES (pH 7.2). Five female mutants and 5 heterozygotes each were harvested. Using a StemSep negative cell selection system (StemCell Technologies Inc, Vancouver, British Columbia, Canada), cell preparations were enriched for hematopoietic progenitors. Marrow cells were incubated with a hematopoietic progenitor enrichment cocktail containing biotinylated lineage markers for CD5, CD45R (B220), CD11b (Mac-1), Gr-1, and TER-119, as previously described.27 Colloidal magnetic dextran iron particles were bound to labeled cells using an anti-biotin tetrameric antibody complex and a magnetic column was used to remove labeled lineage-positive cells. Unfractionated and lineage-depleted marrow was incubated with a panel of lineage marker antibodies, including B220, CD3, Mac-1, Gr-1, and TER-119 (conjugated with either allophycocyanin [APC] or phycoerythrin [PE]) in combination with either CD34-APC or c-kit-fluorescein isothiocyanate (FITC) and Sca-1-PE (Pharmingen, San Diego, CA). Cell preparations were then analyzed with 3-color flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA). Data analysis was performed using Paint-a-Gate software (Becton Dickinson).
Transplantation.
Bone marrow harvesting and transplantation was performed essentially as described.28 Mice were anesthetized with tribromoethanol where appropriate. Radioablation of recipient mice was performed by exposing the recipient animals to 1,100 rad from a 131Cs source in an evenly split dose 3 hours apart. Transplantation was then performed within 5 hours on the same day as irradiation. Bone marrow donors were killed by cervical dislocation, the fur was saturated with 70% alcohol, and the animal was moved into a sterile, laminar flow hood. Skin and muscle were removed from the hind limbs and the femurs were removed. The ends of the femurs were cut off and the femur shaft contents were expelled into a collection tube using a 27-gauge needle and RPMI 1640 supplemented with antibiotics. Unfractionated marrow cells were counted using a hemocytometer and the concentration was adjusted for injection. Cells were then injected into the retroorbital plexus of anesthetized recipient mice.
Transplant analysis.
Transplanted mice were bled periodically after transplantation and at treatment. Animals were bled from the retroorbital plexus into heparinized capillary tubes. Blood was then prepared for PCR using Chelex resin (Bio-Rad Laboratories, Hercules, CA).29 Six microliters of blood was combined with 1 mL of ddH2O and incubated at room temperature for 30 minutes. After 2 minutes of centrifugation, the supernatant was discarded and 200 μL of 5% Chelex 100 resin was added. The mixture was incubated for 30 minutes at 56°C, vortexed for 10 seconds, boiled for 8 minutes, vortexed again, and centrifuged to collect the resin at the bottom of the tube. PCR was performed on 10 μL of samples as described above. After electrophoresis, the PCR products were bound to Hybond-N+ membrane (Amersham Life Science Inc, Arlington Heights, IL) by overnight alkaline capillary transfer. Two picomoles of primer D (AGTTGGCACCTATGG) was end-labeled (2 pmol primer; 150 μCi γ-ATP; 2.5 μL 700 mmol/L Tris, pH 7.5, 100 mmol/L MgCl2, 50 mmol/L dithiothreitol [DTT], 1 mmol/L spermidine-HCl, 1 mmol/L EDTA; 10 U T4 polynucleotide kinase; water to 25 μL; incubated for 60 minutes at 37°C) and used as a probe in a 40% formamide hybridization buffer. Hybridization was allowed to run a minimum of 6 hours at 42°C; the filter was then washed once in 2× SSPE, 0.1% sodium dodecyl sulfate (SDS) at room temperature for 10 minutes. Primer D hybridizes 3′ to primer A in a region of wild-type genomic DNA. The Southern blot was visualized using a Molecular Dynamics Phosphorimager SI (Molecular Dynamics, Sunnyvale, CA) and the intensity of the bands was quantitated using the software IPLab Gel (Signal Analytics Corp, Vienna, VA). The band intensity was then compared with a standard curve to determine the percentage of contribution of each allele. Figure 1B is an example of a standard curve. Figures 2D and 4B show examples of electrophoresis and phosphorimaging data typical of this analysis.
Spleen colony analysis.
A cohort of mice were serially transplanted using original nonablated recipients as donors. Some animals were killed 12 days posttransplant; the spleen colonies were excised as closely as possible to the colony margin, and DNA was isolated. These colonies were then analyzed by quantitative PCR, as described.
Progenitor culture and analysis.
Unfractionated bone marrow cells (1 × 105) were cultured in 1 mL of MethoCult H4230 (Stem Cell Technologies), penicillin-streptomycin (Life Technologies, Rockville, MD), and 3 recombinant growth factors: human erythropoietin (2 U/mL; Amgen, Thousand Oaks, CA), murine Steel factor (10 ng/mL; R&D Systems, Minneapolis, MN), and murine interleukin-3 (10 ng/mL; R&D Systems). CFU-GM and BFU-E were cultured in 35-mm tissue culture dishes at 37°C in 5% CO2 in air. The colonies in each dish were counted. Colonies were then picked from the plate, the cells were pooled and DNA was prepared with Chelex as described, and quantitative PCR was performed as described.
RESULTS
To compare the repopulation capacity of wild-type and FACKO bone marrow cells, we performed 2 sets of experiments. The first set of experiments was to inject mixes of wild-type and FACKO bone marrow into lethally irradiated HT1 heterozygote hosts. The second set of experiments involved injection of wild-type marrow into nonablated FACKO recipients. In both cases, we also tested the effects of MMC administration and serial transplantation on the repopulation process.
Competitive repopulation of radio-ablated HT1 heterozygotes.
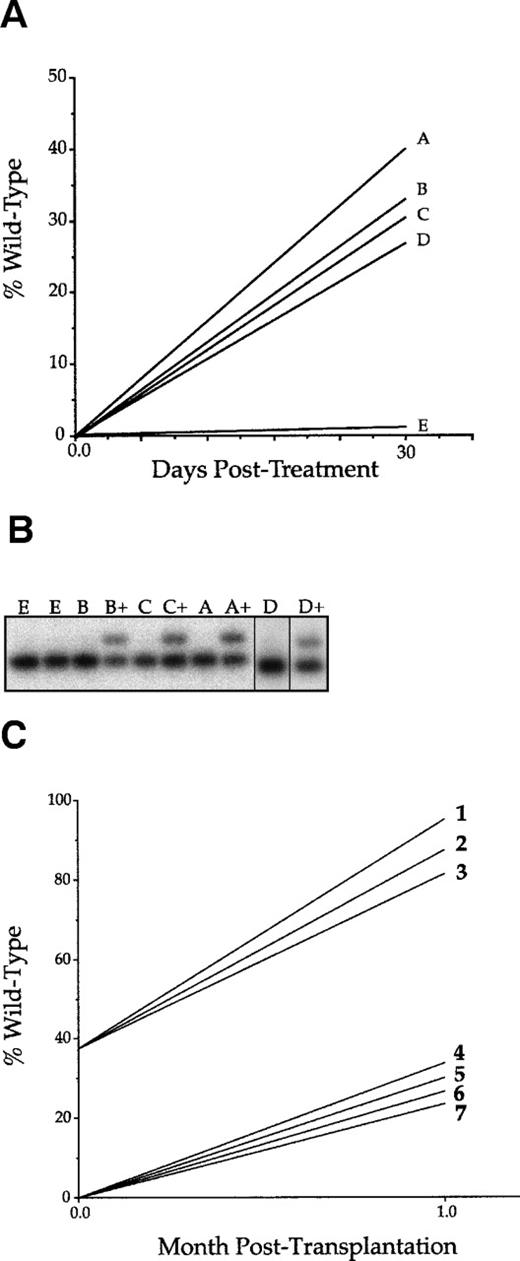
To test the hypothesis that wild-type cells have a selective advantage of Fanconi cells, cohorts of 5 mice heterozygous for an HT1 knockout were irradiated as described and transplanted with a mixture of wild-type and FACKO marrow in a 1:1 ratio for a total of 106 unfractionated cells. The use of a genetically marked (HT1 knockout) recipient allowed the unambiguous distinction of all 3 populations of hematopoietic cells in the transplanted mice by simple PCR genotyping assays. These were the original recipient (HT1 heterozygous, FANCC wild-type), wild-type donors (HT1 wild-type, FANCC wild-type), and Fanconi donors (HT1 wild-type, FANCC mutant). The mice were allowed to recover for 3 weeks when they were bled and subsequently injected intraperitonealy with 10 μg of MMC. The treated animals were bled subsequently 1 and 2 months after MMC treatment. The relative contribution of the genotypes to peripheral blood DNA was determined by PCR analysis as described above and the data are shown in Fig 1. The PCR assay for the HT1 knockout allele was negative in all cases (data not shown), indicating that only transplanted donor cells contributed significantly to peripheral blood DNA and that the irradiation dose had been sufficient for complete bone marrow ablation of the host. Figure 2A shows the MMC-treated cohort of animals over the 3-month study. One month after transplantation, this group had an average of approximately 78% of the peripheral blood cells containing wild-type DNA. After MMC treatment, 3 of the 5 animals showed a decrease, whereas 2 of 5 showed an increase in wild-type blood cells in the peripheral circulation. At 3 months posttransplantation, we saw an increase in wild-type blood cells from the prior month in all subjects. On average, the 3-month values are not significantly different than the 1-month values. Figure2B shows the control cohort to Fig 2A. These animals were transplanted in the same way and bled at the same time, but received no MMC. The graph shows an initial 1-month value of approximately 80%, a value very close to the treated cohort. The only difference between the MMC-treated and untreated cohort was that 3 of the 4 animals showed a slight decrease in wild-type contribution over the 3-month period of the experiment. These data indicate that wild-type bone marrow had only a modest advantage during repopulation (increase from 50% to 80%) and that the relative contribution of wild-type cells remained stable over 3 months.
Competitive repopulation. A cohort of mice were radio-ablated and transplanted with a 1:1 mixture of FACKO and wild-type bone marrow for a total of 1 × 106 cells. The lines connecting data points indicate the trend of change. (A) Cohort of mice treated with MMC 1 month posttransplantation and observed to 3 months posttransplantation. (B) Control mice as in (A), except with no MMC treatment. (C) Cohort of mice serially transplanted from an MMC-treated mouse and nontreated control in (B). A through E and F through J represent transplant recipients from separate donors. (D) Phosphoimager quantitative data corresponding to the datapoints after serial transplantation in (C). The high contribution of wild-type DNA is readily discernible.
Competitive repopulation. A cohort of mice were radio-ablated and transplanted with a 1:1 mixture of FACKO and wild-type bone marrow for a total of 1 × 106 cells. The lines connecting data points indicate the trend of change. (A) Cohort of mice treated with MMC 1 month posttransplantation and observed to 3 months posttransplantation. (B) Control mice as in (A), except with no MMC treatment. (C) Cohort of mice serially transplanted from an MMC-treated mouse and nontreated control in (B). A through E and F through J represent transplant recipients from separate donors. (D) Phosphoimager quantitative data corresponding to the datapoints after serial transplantation in (C). The high contribution of wild-type DNA is readily discernible.
Serial transplantation of competitively repopulated bone marrow.
Peripheral blood DNA was analyzed in the experiments described above and, therefore, it was not possible to determine whether the observed modest selective advantage occurred in peripheral blood cells only or also at the level of HSCs. For this, bone marrow was harvested from a cohort of the animals repopulated with a 1:1 mix of wild-type and FACKO marrow and 106 marrow cells were serially transplanted into lethally irradiated secondary recipients. The analysis is shown in Fig2C. Serial transplantation considerably enhanced the relative contribution of wild-type cells to peripheral blood DNA both in MMC-treated (average, 95.2%) and nontreated animals (average, 84.8%). This result shows that the selective repopulation advantage of wild-type cells did indeed occur at the stem cell level and not only in peripheral blood nucleated cells.
Phenotypic analysis of hematopoietic progenitor cells in FACKO mice.
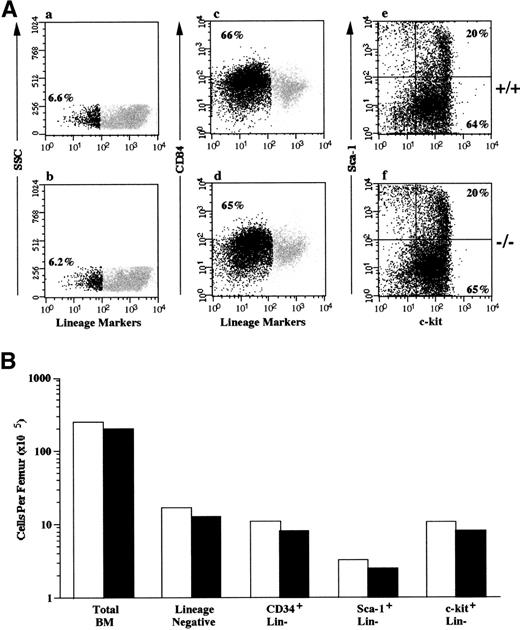
To demonstrate that the results of the competitive repopulation and serial transplantation studies were not due to a decreased number of stem cells/progenitor cells in the FACKO mice, we evaluated the frequency of phenotypically defined cells in the marrow of both FACKO and wild-type mice (Fig 3). The total bone marrow cellularity of FACKO mice and +/+ mice was similar, with one femur containing 2.5 × 107 and 2.1 × 107total nucleated cells, respectively. Populations of cells that express low or undetectable levels of lineage-specific markers are known to contain all HSC activity in the marrow. The frequency of this lineage-negative cell population was 6.6% in +/+ mice and 6.2% in FACKO mice. To determine the absolute number of phenotypically defined progenitor cells in the marrow, we enriched the lineage marker-negative population using a negative selection technique. This approach is used to exclude Sca-1–positive T cells and lineage-committed progenitor cells that continue to express low levels of c-kit as they mature. The lineage-negative fraction of the marrow from both FACKO mice and the wild-type mice contained equivalent numbers of CD34+ cells, Sca-1+ cells, and c-kit+ cells (Fig 3B). These results demonstrate that the decreased competitive repopulation activity and serial transplantation activity in the marrow of FACKO mice is not due to a decreased frequency of phenotypically defined HSCs or progenitor cells.
Phenotypic analysis of hematopoietic progenitor cells in heterozygous and FACKO mice. The bone marrow of wild type (+/+) and FACKO (−/−) mice (n = 5 animals per group) was analyzed for the presence of phenotypically defined HSC/progenitor cells. (A) Flow cytometric analysis of unfractionated marrow from wild type (a) and FACKO (b) mice. The percentage of lineage-negative cells is indicated (black dots). The frequency of lineage-negative cells after lineage depletion and incubation with antibodies to CD34 is shown in (c) and (d). The percentage of lineage-negative cells is shown (black dots). The expression of c-kit and Sca-1 on gated lineage-negative marrow cells is indicated in (e) and (f). The percentage of c-kit+, Sca-1+ cells is shown in the upper right quadrant, and the percentage of c-kit+, Sca-1− cells is shown in the lower right quadrant. (B) The total number of phenotypically defined cells per femur in wild type (□) and FACKO (▪) mice is indicated on a log scale.
Phenotypic analysis of hematopoietic progenitor cells in heterozygous and FACKO mice. The bone marrow of wild type (+/+) and FACKO (−/−) mice (n = 5 animals per group) was analyzed for the presence of phenotypically defined HSC/progenitor cells. (A) Flow cytometric analysis of unfractionated marrow from wild type (a) and FACKO (b) mice. The percentage of lineage-negative cells is indicated (black dots). The frequency of lineage-negative cells after lineage depletion and incubation with antibodies to CD34 is shown in (c) and (d). The percentage of lineage-negative cells is shown (black dots). The expression of c-kit and Sca-1 on gated lineage-negative marrow cells is indicated in (e) and (f). The percentage of c-kit+, Sca-1+ cells is shown in the upper right quadrant, and the percentage of c-kit+, Sca-1− cells is shown in the lower right quadrant. (B) The total number of phenotypically defined cells per femur in wild type (□) and FACKO (▪) mice is indicated on a log scale.
Transplantation of wild-type marrow into nonablated FACKO mice.
The selective advantage for wild-type cells observed in the competitive repopulation experiments described above raised the possibility that positive selection may be achievable even in nonirradiated FACKO recipients. Therefore, a cohort of 5 FACKO mice were used as recipients of 106 unfractionated wild-type marrow without prior ablation. After a 1-month recovery period, all 5 were bled to obtain baseline values, and then 4 mice received 10 μg MMC intraperitoneally. No adverse effects (death, weight loss, or malaise) were observed after treatment. One month after MMC, the 4 treated animals and 1 control were bled again and the blood was analyzed by quantitative PCR (Fig 4A and B). The detection limit for wild-type DNA in our quantitative assay was 1% of total DNA, and at this level of sensitivity, no wild-type DNA could be detected in any of the 5 animals before MMC injection. After MMC treatment, wild-type DNA remained undetectable in the control animal, whereas it increased to between 28% and 42% of total DNA in the treated cohort. The degree of wild-type contribution was observed to 8 months after MMC treatment and remained stable. This experiment was repeated independently with 10 FACKO mice (5 controls and 5 MMC treated) and the results were the same. About 30% of peripheral blood DNA was wild-type in MMC-treated mice (data not shown), but no wild-type DNA was found in the controls. The animals from this repeat experiment were used for serial transplantation as described below.
Repopulation in nonablated FACKO mice. (A) A cohort of FACKO mice were injected with 1 × 106 unfractionated wild-type cells. Mice A, B, C, and D were treated with a single intraperitoneal injection of 10 μg MMC. Mouse E was untreated. The lines connecting data points indicate the trend of change. (B) Phosphoimager data corresponding the data in (A) before and after (+) MMC treatment. (C) Wild-type recipients were radio-ablated and transplanted with bone marrow cells from a treated nonablated transplant recipient (1, 2, and 3) or from an untreated nonablated transplant recipient (4, 5, 6, and 7).
Repopulation in nonablated FACKO mice. (A) A cohort of FACKO mice were injected with 1 × 106 unfractionated wild-type cells. Mice A, B, C, and D were treated with a single intraperitoneal injection of 10 μg MMC. Mouse E was untreated. The lines connecting data points indicate the trend of change. (B) Phosphoimager data corresponding the data in (A) before and after (+) MMC treatment. (C) Wild-type recipients were radio-ablated and transplanted with bone marrow cells from a treated nonablated transplant recipient (1, 2, and 3) or from an untreated nonablated transplant recipient (4, 5, 6, and 7).
Serial transplantation of marrow from FACKO mice transplanted with wild-type cells.
Several assays were performed to show that the positive selection observed occurred at the level of the hematopoietic stem cell. Bone marrow from 2 randomly selected treated and nontreated transplanted FACKO mice was serially transplanted into lethally irradiated secondary congenic recipients. At the time of marrow harvest, an aliquot of bone marrow was used to establish CFU-GM and BFU-E colonies. No differences between MMC-treated mice and controls were observed in the absolute numbers of BFU-E and CFU-GM colonies (data not shown). After 2 weeks in culture, DNA was isolated from pooled colonies and analyzed by PCR genotyping. In 2 independent experiments, more than 98% of BFU-E and CFU-GM DNA was of wild-type origin in MMC-treated mice. Interestingly, wild-type DNA was also readily detectable at approximately 25% in colonies derived from nontreated mice. Thus, positive selection for wild-type cells occurred not only in peripheral blood nucleated cells, but also in early progenitors.
The secondary recipients of the serial transplants were analyzed in 2 different ways. First, day-12 spleen colonies were isolated and genotyped from 3 secondary recipients each. Second, the peripheral blood of secondary recipients was analyzed 1 and 2 months after transplantation. Seventeen of 17 (100%) of the day-12 CFU-S from MMC-treated mice were of wild-type origin. Similarly, more than 80% of peripheral blood DNA was wild-type derived in repopulated secondary recipients (Fig 4C). In contrast, only 1 of 14 (7%) CFU-S colonies from the control animals was wild-type. Taken together, these results provide strong support for selection of the level of the hematopoietic stem cell itself. Interestingly, after serial transplantation, positive selection was also observed without MMC treatment. The contribution of wild-type DNA increased from undetectable to approximately 20% (Fig4C).
DISCUSSION
The findings described here have implications regarding the pathophysiology of FA as well as its treatment by bone marrow transplantation and gene therapy. Our experiments show that the hematopoietic stem cells of FACKO mice have repopulation capacity only slightly lower than those of wild-type controls. Despite the considerable number of cell doublings required for complete marrow repopulation with only 1 × 106 donor cells, the average ratio of wild-type to mutant cells only increased from 0.5 to 0.78. Furthermore, this ratio remained stable for many months once repopulation had occurred, indicating that selection for wild-type HSCs was minimal under normal marrow turnover conditions. These results obtained in our animal model of FA are consistent with observations in human FA patients in which the time for development of clinical anemia is highly variable and can be several years, even decades.3Thus, bone marrow repopulation with healthy cells is unlikely to occur spontaneously, even in the presence of wild-type HSCs generated by mitotic recombination. Despite the only modest growth advantage during normal bone marrow renewal, situations demanding many rounds of cell doubling, such as serial transplantation, eventually lead to significant selection for wild-type cells. It is possible that hematopoietic cells from FACKO mice become progressively less able to repopulate with increasing age or increasing number of cell doublings, ie, age more rapidly. The effects of age and number of cell divisions on the ability of FACKO HSCs to compete for marrow repopulation was not addressed in the current study and will be the subject of future experimentation.
Whereas wild-type cells were only modestly selected under normal conditions, the selection was strong and rapid when direct selective pressure in the form of MMC treatment was applied. Under these conditions, even a very small number of wild-type HSCs was able to significantly repopulate the hematopoietic system of FACKO mice without any prior irradiation. Direct exposure to DNA cross-linking drugs is an unlikely occurrence and is not likely to be responsible for the gradual decrease in bone marrow function seen in human patients with FA. However, FA cells are known to be sensitive to other compounds as well as DNA cross-linkers, especially the mitotic inhibitor interferon-γ.23,25 Other cytokines (tumor necrosis factor-α and macrophage inflammatory protein-1α [MIP-1α]) have recently been added to this list.30 Thus, the physiologic selection agent damaging the hematopoietic system in human FA patients may be these cytokines that are frequently released in response to viral infections. Frequent exposure to viral infections or other selection events may allow the emergence of revertant hematopoietic stem cells or clones of committed progenitors (eg, T-cell progenitors), as is seen in human FA patients.
In most genetic disorders, hematopoietic gene therapy would require correction of a large percentage of cells to be clinically effective. For this reason, gene transfer has been combined with bone marrow ablation in successful animal experiments.28,31,32 An alternative approach would be to use in vivo selection for genetically transduced cells to avoid ablation. The strong selection for wild-type cells under MMC selection could potentially be exploited for practical applications in the treatment of FA, especially gene therapy. MMC was exquisitely and selectively toxic to hematopoietic cells, and no adverse effects to other organ systems were observed. This is likely to also be true in human FA patients, as suggested by the clinical experience with another cross-linking drug, cyclophosphamide.33 FA patients are very sensitive to this alkylating agent, but a dose that is selectively toxic to the bone marrow can be found and used in preparation for bone marrow transplantation. Thus, it is conceivable that in vivo selection could be used to enhance gene therapy FA. After ex vivo correction by gene therapy, a cohort of HSCs from an FA patient could be autologously transplanted back into the patient. After this, a selective drug, such as MMC, cytoxan, or possibly interferon-γ, would be used to expand the genetically corrected HSCs to clinically meaningful levels. Certainly the immediate enrichment to greater than 25% of peripheral blood cells as seen in our experiments is encouraging in this regard. Others have recently reported similar levels of enrichment in preclinical gene therapy experiments using dehydrofolate reductase as a selectable marker and, as selection, the drug trimetrexate together with nucleoside transport inhibitor nitrobenzylmercapto-purine riboside monophosphate.34 In FA, the biology of the disease favors selection of corrected cells. The lack of in vivo selection for corrected cells in the FANCC human gene therapy trials performed at National Institutes of Health35underscores the notion that selection occurs only very slowly or not at all without the use of specific selective pressure. Thus, it is likely that effective hematopoietic gene therapy for FA will not require complete bone marrow ablation by irradiation, but would require administration of a selective regimen. Ideally, future research in animal models will identify nongenotoxic compounds such as cytokines for this purpose.
The reported experiments are also interesting from the standpoint of transplantation biology. Previous reports have shown that significant repopulation of nonablated syngeneic recipients can be achieved with extremely high doses of donor cells.36 37 Our experiment demonstrated that engraftment occurs even with low numbers of donor cells in FACKO mice and that the engrafted cells persisted for 4 weeks or more. In all cases, MMC was administered at least 4 weeks after transplantation, and in all cases, wild-type cells were nonetheless found after selection.
MMC selection was much less effective in the competitive repopulation experiments than in the nonablated FACKO mice. We currently do not have a definitive explanation for this observation, but it can be hypothesized that selection worked better in the FACKO environment because of indirect effects of MMC on that environment. All competitive repopulation experiments were performed in wild-type recipients. This hypothesis will be tested directly in future experiments comparing FACKO and wild-type as recipients.
Supported by NHLBI Program Project Grant No. 1PO1HL48546 to M.G. and G.B. and by NRSA award 1F32HL09862 through the NHLBI to K.P.B.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Markus Grompe, MD, Department of Molecular and Medical Genetics, L103, 3181 SW Sam Jackson Rd, Portland, OR 97201.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal