Abstract
The labile iron pool (LIP) harbors the metabolically active and regulatory forms of cellular iron. We assessed the role of intracellular ferritin in the maintenance of intracellular LIP levels. Treating K562 cells with the permeant chelator isonicotinoyl salicylaldehyde hydrazone reduced the LIP from 0.8 to 0.2 μmol/L, as monitored by the metalo-sensing probe calcein. When cells were reincubated in serum-free and chelator-free medium, the LIP partially recovered in a complex pattern. The first component of the LIP to reappear was relatively small and occurred within 1 hour, whereas the second was larger and relatively slow to occur, paralleling the decline in intracellular ferritin level (t½= 8 hours). Protease inhibitors such as leupeptin suppressed both the changes in ferritin levels and cellular LIP recovery after chelation. The changes in the LIP were also inversely reflected in the activity of iron regulatory protein (IRP). The 2 ferritin subunits, H and L, behaved qualitatively similarly in response to long-term treatments with the iron chelator deferoxamine, although L-ferritin declined more rapidly, resulting in a 4-fold higher H/L-ferritin ratio. The decline in L-ferritin, but not H-ferritin, was partially attenuated by the lysosomotrophic agent, chloroquine; on the other hand, antiproteases inhibited the degradation of both subunits to the same extent. These findings indicate that, after acute LIP depletion with fast-acting chelators, iron can be mobilized into the LIP from intracellular sources. The underlying mechanisms can be kinetically analyzed into components associated with fast release from accessible cellular sources and slow release from cytosolic ferritin via proteolysis. Because these iron forms are known to be redox-active, our studies are important for understanding the biological effects of cellular iron chelation.
MAMMALIAN CELLS maintain steady levels of metabolically active iron through the regulation of iron uptake and storage.1-9 The metabolically active forms of intracellular iron are components of a cytosolic labile iron pool (LIP), classically referred to as the chelatable iron pool.10 This pool harbors forms of iron loosely associated with macromolecular complexes and, possibly, also harbors low molecular weight forms.10-12 The levels of these LIP forms are thought to be both sensed and homeostatically controlled by iron responsive proteins (IRPs) that, by cell iron sensing, coordinately regulate the expression of the transferrin receptor (TfR) and of ferritin.1-9 The accepted view is that increased cellular iron levels appear initially in the LIP, but that excess iron is eventually safely sequestered into ferritin molecules. The ferritins are macromolecules that display a robust iron-binding capacity (up to 4,500 iron atoms per polymer molecule) with properties dictated by the protein subunit composition. Ferritin is essentially hetero-polymeric, being composed of different combinations of heavy (H = 21 kD) and light (L = 19 kD) subunits that are characteristic of tissue or cell type.13 However, in addition to its iron-scavenging capacity, ferritin seemingly serves also as a potential source of the metal for the synthesis of heme (and, possibly, iron-containing enzymes)12,14 and for catalysis of reactive oxidant species when the metal interacts with pro-oxidant species.15-17 The presumed mechanism of iron transfer from ferritin to heme, originally observed in early human erythroid precursor cells that mature in a liquid culture, was tentatively localized to an acidic cell compartment and was attributed to ferritin shell degradation by acid proteases.14 However, it has not yet been determined whether the process is associated with the normal turnover of the ferritin protein or with specific signals that respond to cellular requirements, such as a posttranslational upregulation of LIP levels via enhanced ferritin degradation. To study the potential contribution of ferritin as a donor of iron to other molecules, we have looked at the LIP as possibly the first compartment of iron released from ferritin. For that purpose, we investigated the temporal and quantitative relationships between intracellular ferritin levels and the in situ LIP levels and their impact on IRP activity as well as iron homeostasis in K562 cells.
MATERIALS AND METHODS
Cell cultures.
Human K-562 cells were cultured in α-minimal essential medium (α-MEM) containing 7% fetal calf serum (FCS), L-glutamine, and antibiotics as described previously.18Cellular LIP was measured with the fluorescent metalosensor calcein-AM as previously described.19 In brief, for calcein loading, 1 × 107/mL cells were incubated with 250 nmol/L calcein-AM for 5 minutes at 37°C in α-MEM (without bicarbonate), washed, resuspended at room temperature at a density of 1 × 106/mL in α-MEM containing 1% bovine serum albumin, and used within 1 hour. Before use, the cells were washed and finally resuspended (1 × 106/mL) in Na-HEPES (20 mmol/L) buffered saline (HBS; 145 mmol/L NaCl, pH 7.2, 37°C). Fluorescence (488 excitation, 517 nm emission) was measured in a continuous mode using a PTI fluorescence station (PTI, New Brunswick, NJ), with the cells constantly stirred and kept at 37°C. After stabilization of the baseline, anticalcein antibodies were added to quench extracellular probe fluorescence and the amount of intracellular metal bound to calcein (CA-Fe) was assessed by (1) addition in excess (100 μmol/L) of the fast permeating chelator isonicotinoyl salicylaldehyde hydrazone (referred to in figures as ISAH when used for cellular iron depletion and SIH when used for measuring the LIP; kindly donated by Dr Prem Ponka, Montreal, Quebec, Canada); (2) superimposing the calibration curve of standard free acid-calcein added to a cell suspension; and (3) calculation of LIP (given as [CA − Fe + [Fe]),12 18 using the experimental kd value of CA-Fe obtained in cells and the number of cells measured in the experimental setup.
Electromobility RNA gel retardation assays.
Cells were washed twice in ice-cold phosphate-buffered saline (PBS) and then lysed for 30 minutes on ice with a buffer containing protease and RNAse inhibitors (lysis buffer: 25 mmol/L Tris-HCl, pH 8, containing 1% Triton X-100 [Pierce, Rehovot, Israel], 40 mmol/L KCL, 10 μg/mL leupeptin, 1 μmol/L pepstatin, 10 μg/mL chymostatin, 0.023 trypsin inhibitor units [TIU]/mL aprotinin [all from Boehringer Mannheim, Mannheim, Germany], 10 μg/mL benzamidine, 3.7 μg/mL N-tosyl-L-phenylalanine chloromethyl ketone [TPCK], 3.7 μg/mL N-tosyl-L-lysine chloromethyl ketone [TLCK], 0.25 mmol/L phenylmethylsulphonyl fluoride [PMSF] [all from Sigma-Israel, Rehovot, Israel], and 10 μL/mL ACE-RNAse inhibitor). Cell nuclei and debris were precipitated by centrifugation at 10,000g for 15 minutes, and the protein content of the supernatant was analyzed by the BCA protein assay (Pierce, Rockford, IL). Freshly prepared lysates were incubated with the iron-responsive element (IRE) probe (kindly provided by Dr Tracey Rouault, National Institutes of Health, Bethesda, MD) that was transcribed and labeled with 32P as described by Haile et al.20 For each lane of the polyacrylamide gel, 10 μg protein of a freshly prepared lysate was incubated for 10 minutes at room temperature with 15 μL 32P-“IRE Probe” cocktail (containing 30,000 cpm IRE-Probe, 2 μg t-RNA, 3.3% Glycerol, and 1 μL ACE-RNAse inhibitor) in the presence or absence of 2% β-mercaptoethanol. The reaction mixture was applied onto an 8% native polyacrylamide gel and electrophoresis was allowed to proceed for 2 hours at 180 V. To standardize the 32P-IRE-IRP migration, recombinant IRP was used (obtained from Dr Tracey Rouault), and gels were fixed and subsequently dried. The intensity of the label of the 32P-IRE/IRP complex was determined by phosphorimaging.
Ferritin measurements.
Cells were harvested, washed, and lysed on ice in solubilization buffer containing 1% Triton X-100, 10 μg/mL aprotinin,10 μg/mL leupeptin, 1 μmol/L pepstatine, 10 μg/mL benzamidine, 3.7 μg/mL TPCK, 3.7 μg/mL TLCK, 0.25 mmol/L PMSF, and 0.02% sodium azide in 10 mmol/L Tris-HCl, pH 7.4. After heating for 10 minutes at 70°C followed by immediate cooling on ice, the lysates were centrifuged at 10,000g for 10 minutes and the supernatants collected and stored at −80°C until use. Ferritin standards either were isolated from human term placenta or from the spleen of a thalassemic patient obtained at surgery, as previously described.21,22Measurements of cellular ferritin were performed on cell lysates using a fluorogenic enzyme-linked immunosorbent assay (ELISA).23 The antiferritin antibodies were raised either against human spleen (L-subunits) or placental (H+L subunits) ferritin. H-ferritin levels were considered to be the difference between the results obtained with ELISAs conducted with anti–total placental ferritin and those performed with antispleen ferritin antibodies. H-ferritin levels thus obtained were identical to those found when measured by a recently developed specific ELISA for H-ferritin using H-ferritin–specific monoclonal antibodies.24
RESULTS
The LIP of K562 cells was assessed by the calcein-based method in conjunction with the fast permeating chelator isonicotinoyl salicylaldehyde hydrazone (SIH),19 a tridentate, fast-permeating, and high-affinity chelator of iron and other metals.
We have shown previously that SIH exhaustively extracts55Fe from the chelatable fraction of55Fe-labeled cells and from calcein in calcein-loaded cells.12 Calcein, which contains an EDTA-like binding moiety, binds to and is quenched by Fe(II) and (III) as well as Cu(II), Ni(II), and Co(II). However, we believe that calcein, used in conjunction with SIH, is a reliable and specific indicator of cellular LIP for 2 reasons: (1) in comparison with Fe, the total available pools of the above-noted metals are negligible in viable living cells; and (2) deferoxamine (DFO), which has a high affinity for Fe but a low affinity for the other metals mentioned above, abrogates the calcein-detectable LIP.12 The calcein-based method for LIP determination is as follows: fluorescent calcein, formed intracellularly upon cellular loading with the nonfluorescent precursor calcein-AM, binds to a fraction of the cellular iron associated with the LIP, thereby quenching its fluorescence. That fraction of iron that is exposed by the addition of the iron chelator SIH is indicated after the arrow in Fig 1 (left half). The increase in the fluorescence signal elicited by SIH reflecting cellular iron was used for calculating the values of LIP (shown as micromoles of iron per liter on the right), as described in Materials and Methods. Anticalcein fluorescence-quenching antibodies were added to the medium to bind extracellular calcein and thereby to ascertain that the fluorescence changes elicited by SIH are associated with intracellular iron.
Short-term recovery of LIP levels in iron-depleted K562 cells. K562 cells were incubated in HBS (2 × 106/mL) alone or in the presence of 100 μmol/L SIH for 10 minutes at 37°C to examine acute iron depletion. Cells were washed free of chelator and incubated for an additional recovery period either in Dulbecco’s modified Eagle’s medium (DMEM) alone or DMEM supplemented with 20 μg/mL leupeptin, 50 μg/mL transferrin, or 10% FCS. At the end of the incubation period, the cells were washed with HBS and processed for LIP measurements by loading with calcein-AM (CA), washed, and resuspended in HBS-medium. Fluorescence (left panels) was continuously monitored as described in Materials and Methods. When fluorescence readings were steady, anti-CA antibodies (ab) were added (to quench extracellular fluorescence), followed by 100 μmol/L SIH, to attain maximum recoverable fluorescence. Shown are tracings obtained after recovery periods of 0 to 6 hours (ISAH-0-6 h). The maximum value, which was equivalent to the concentration of CA-bound iron [CA-Fe], was used for calculating LIP values (in micromoles per liter). LIP values are shown in the right panels for the different treatments: (A) recovery in DMEM alone (recov), (B) recovery in DMEM supplemented with leupeptin (recov.+leup), or (C) recovery in DMEM supplemented with transferrin (Recov. + Tf) or FCS (Recov.+ FCS).
Short-term recovery of LIP levels in iron-depleted K562 cells. K562 cells were incubated in HBS (2 × 106/mL) alone or in the presence of 100 μmol/L SIH for 10 minutes at 37°C to examine acute iron depletion. Cells were washed free of chelator and incubated for an additional recovery period either in Dulbecco’s modified Eagle’s medium (DMEM) alone or DMEM supplemented with 20 μg/mL leupeptin, 50 μg/mL transferrin, or 10% FCS. At the end of the incubation period, the cells were washed with HBS and processed for LIP measurements by loading with calcein-AM (CA), washed, and resuspended in HBS-medium. Fluorescence (left panels) was continuously monitored as described in Materials and Methods. When fluorescence readings were steady, anti-CA antibodies (ab) were added (to quench extracellular fluorescence), followed by 100 μmol/L SIH, to attain maximum recoverable fluorescence. Shown are tracings obtained after recovery periods of 0 to 6 hours (ISAH-0-6 h). The maximum value, which was equivalent to the concentration of CA-bound iron [CA-Fe], was used for calculating LIP values (in micromoles per liter). LIP values are shown in the right panels for the different treatments: (A) recovery in DMEM alone (recov), (B) recovery in DMEM supplemented with leupeptin (recov.+leup), or (C) recovery in DMEM supplemented with transferrin (Recov. + Tf) or FCS (Recov.+ FCS).
Cell iron dynamics in K562 cells after fast iron depletion and subsequent recovery.
K562 cells grown in standard culture conditions at exponential phase show steady-state LIP values of 0.7 to 0.9 μmol/L as reported earlier.18 After 10 minutes of treatment with SIH, the LIP is quickly reduced to 0.2 to 0.25 μmol/L (labeled “ISAH-0h” and “recov. 0 h” in Fig 1A; a representative experiment is shown in Fig 1 and pooled data are shown in Fig 2). However, after removal of the chelator and resuspending the cells in an essentially iron-free medium, LIP gradually recovered over the following 3 to 6 hours (labeled “ISAH-3h”/“recov. 3h” and “ISAH-6h”/“recov. 6h,” respectively, in Fig 1A). After an 8-hour recovery period, LIP apparently reached a new steady-state value of 0.54 to 0.6 μmol/L, which is somewhat lower than that of the control (not shown). The recovery of LIP after the 10 minutes of iron depletion by the chelator was markedly inhibited by leupeptin (Fig 1B) and other antiproteases such as chymostatin and pepstatin (not shown). These results indicate that the process of LIP recovery was apparently dependent on intracellular proteolysis. When the recovery from the initial iron depletion was performed in the presence of an extracellular iron source, either in the form of diferric transferrin (Tf) or FCS, the LIP was restored more quickly. Thus, when Tf was added, LIP increased to levels considerably higher than those found in iron-free medium (Fig 1C). However, with 10% FCS in the cell medium, the LIP level attained was similar to that observed in stock cultures. It should be noted that we could not rule out the possibility that trace amounts of the chelator remained associated with the cell after washing. However, if this were the case, the values of LIP depicted in Fig 1, as well as the general trend in the changes of LIP with time, would be somewhat underestimated. Hence, the interpretation of these findings would be essentially unchanged.
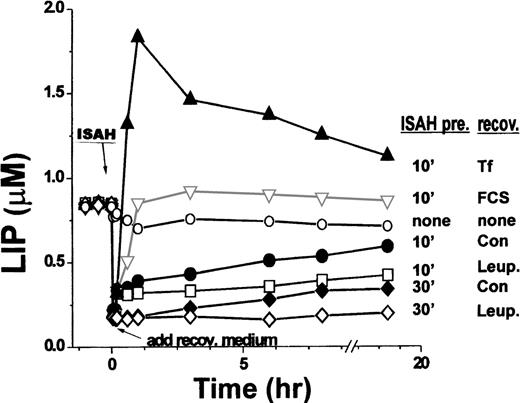
Long-term recovery of LIP levels in K562 cells after acute iron depletion. Calcein-loaded cells were resuspended in HBS and the fluorescence was monitored for 10 to 20 minutes. At 0 time, 100 μmol/L SIH was added for 10 minutes (labeled as ISAH); cells were then washed free of chelator and resuspended in recovery medium lacking SIH. The cells were incubated either alone (recov: con.) or containing the indicated additives, as in Fig 1. In some experiments, cells were incubated with SIH for 30 minutes before washing (ISAH pre: 30’ recov: Con; ISAH pre 30’ recov: Leup.). At the indicated time points, intracellular LIP concentration was measured as described.
Long-term recovery of LIP levels in K562 cells after acute iron depletion. Calcein-loaded cells were resuspended in HBS and the fluorescence was monitored for 10 to 20 minutes. At 0 time, 100 μmol/L SIH was added for 10 minutes (labeled as ISAH); cells were then washed free of chelator and resuspended in recovery medium lacking SIH. The cells were incubated either alone (recov: con.) or containing the indicated additives, as in Fig 1. In some experiments, cells were incubated with SIH for 30 minutes before washing (ISAH pre: 30’ recov: Con; ISAH pre 30’ recov: Leup.). At the indicated time points, intracellular LIP concentration was measured as described.
The time dependence of LIP recovery after initial depletion with SIH is shown in more detail in Fig 2, which combines data from Fig 1 with additional experiments. When cells were not pretreated with SIH and maintained in an essentially iron-free medium in both the pretreatment and recovery periods (indicated as none/none), the LIP levels decreased somewhat (∼30% ± 7%) and then remained stable over the following 18 hours. After a brief (10 minutes) pretreatment with SIH (indicated as “ISAH pre.10min,” Fig 2), LIP was rapidly reduced from 0.85 ± 0.07 μmol/L to 0.20 ± 0.08 μmol/L (n = 5). Upon removal of SIH, LIP recovered with time in a biphasic mode. The initial phase was completed within 2 to 10 minutes, with LIP reaching a value of 0.33 ± 0.07 μmol/L for virtually all of the cells after a brief pretreatment with the chelator. The second phase of recovery showed a peak LIP value and time response that were dependent on the presence of additives in the medium. In serum-free medium, the second phase of LIP recovery was slow as compared with the first phase (t½ of ∼8 hours), reaching about 70% of the original steady-state value within 18 hours. In the presence of leupeptin (replenished twice during the 18-hour recovery period), the second phase of recovery was markedly delayed, but the initial, relatively small recovery was unaffected. The rate of recovery and the magnitude of LIP in the second phase were markedly enhanced if either human diferric-Tf or FCS was present. Tf elicited a typical overshoot in the LIP level in the first hour and a subsequent relaxation to steady-state levels, with the latter being significantly higher than those obtained with FCS. We tentatively attributed the initial phase of LIP recovery to the replenishment of LIP by iron released from intracellular macromolecular stores that were apparently unaffected by the relatively short chelator treatment. Indeed, if the chelator treatment was prolonged from 10 to 30 minutes (indicated as ISAH-30′, Fig 2), LIP levels were reduced to less than 0.12 ± 0.08 μmol/L and remained at that level if the medium included leupeptin, which suppressed only the second phase of LIP recovery. Thus, the second phase of LIP recovery might be associated primarily with proteolytic events leading to release of iron into the LIP. An additional contribution of iron to be considered is that of a contaminant taken up from the nominally iron-free medium by a leupeptin-sensitive mechanism. However, this possibility seem unlikely, because supplementing the culture medium of a murine erythroid cell line (MEL) with 5 μmol/L iron(III)-citrate [a concentration much greater than estimated contaminating levels of iron(III)] did not have a significant effect on their LIP as measured by the calcein method (data not shown).
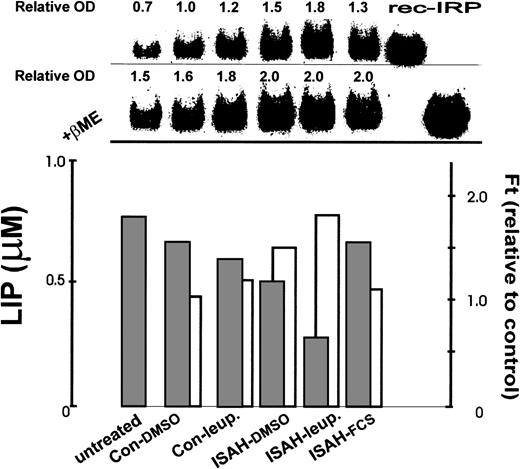
The changes in LIP levels after chelator-induced iron depletion and their susceptibility to antiproteases were compared with changes in IRP levels (Fig 3, upper panel). Indeed, after either a 10-minute chelator treatment (ISAH-DMSO) or an 8-hour treatment with leupeptin (Con-leup.), the cells showed increased IRP activities, concomitant with the decreases in LIP. Compared with the control (Con-DMSO), IRP activity increased by 50% and 20% for chelator- and leupeptin-treated cells, respectively. However, the active/total IRP ratio did not differ significantly from the control after incubation with leupeptin alone but increased by 20% after the treatment with the chelator. From Fig 3 it is apparent that part of the increased IRP activity can be attributed to an increase in total cellular IRP content. Moreover, when cells were treated with both chelator for 10 minutes and leupeptin for 8 hours (ISAH-leup.), LIP was maintained at a relatively low level and IRP activity increased to its highest level. Thus, an 80% increase in IRP activity was observed compared with the control and the active/total IRP ratio increased by 44%; concomitantly, there was a 25% increase in total cellular IRP. We interpret the results to indicate that rapid iron depletion by a fast-acting chelator results in the depletion of iron in the LIP. The accompanying change in IRP levels is the result of a LIP-sensitive process. In this process, leupeptin inhibits the degradation of intracellular ferritin and the release of iron from ferritin, as we have shown previously,14 and thereby prevents the replenishment of the cellular LIP. On the other hand, cells treated with chelator and then incubated in culture medium containing FCS rapidly regained their normal LIP levels via membrane-associated transport mechanisms and IRP activity responded accordingly. However, the total cellular IRP level remained elevated.
Correlation between total ferritin (ferritin), IRP, and LIP levels in K562 cells after acute iron depletion. K562 cells incubated for 30 minutes without additives (Con) or with 100 μmol/L SIH (ISAH) were allowed to “recover” for 8 hours at 37°C in bicarbonate-free DMEM containing the indicated additives: 0.1% dimethyl sulfoxide (DMSO) final (Con-DMSO, ISAH-DMSO), 20 μg/mL leupeptin (Con-Leup, ISAH-leup), or 10% FCS (ISAH-FCS). ‘Untreated’ cells (left bar) were grown in full medium without additives for 8 hours. After the 8-hour period, aliquots of cells were taken for determination of LIP levels by the calcein method as in Fig 1(lower panel, ▪), ferritin by ELISA (lower panel, □), and IRP by electromobility gel retardation assay (upper panel). LIP levels are given in micromoles per liter, ferritin values are expressed relative to control (which contained ∼130 ng/mg protein), and IRP is given in terms of densitometry tracings (OD) of gel shift bands relative to control. All samples in the gel were obtained from equivalent numbers of cells. Rec-IRP refers to recombinant IRP, which is used here as standard for identification purposes. The second row of the upper panel depicts gel shifts performed after treatment with β-mercaptoethanol (βME) representing total (fully activated) cellular IRP.
Correlation between total ferritin (ferritin), IRP, and LIP levels in K562 cells after acute iron depletion. K562 cells incubated for 30 minutes without additives (Con) or with 100 μmol/L SIH (ISAH) were allowed to “recover” for 8 hours at 37°C in bicarbonate-free DMEM containing the indicated additives: 0.1% dimethyl sulfoxide (DMSO) final (Con-DMSO, ISAH-DMSO), 20 μg/mL leupeptin (Con-Leup, ISAH-leup), or 10% FCS (ISAH-FCS). ‘Untreated’ cells (left bar) were grown in full medium without additives for 8 hours. After the 8-hour period, aliquots of cells were taken for determination of LIP levels by the calcein method as in Fig 1(lower panel, ▪), ferritin by ELISA (lower panel, □), and IRP by electromobility gel retardation assay (upper panel). LIP levels are given in micromoles per liter, ferritin values are expressed relative to control (which contained ∼130 ng/mg protein), and IRP is given in terms of densitometry tracings (OD) of gel shift bands relative to control. All samples in the gel were obtained from equivalent numbers of cells. Rec-IRP refers to recombinant IRP, which is used here as standard for identification purposes. The second row of the upper panel depicts gel shifts performed after treatment with β-mercaptoethanol (βME) representing total (fully activated) cellular IRP.
Cellular ferritin levels after LIP depletion.
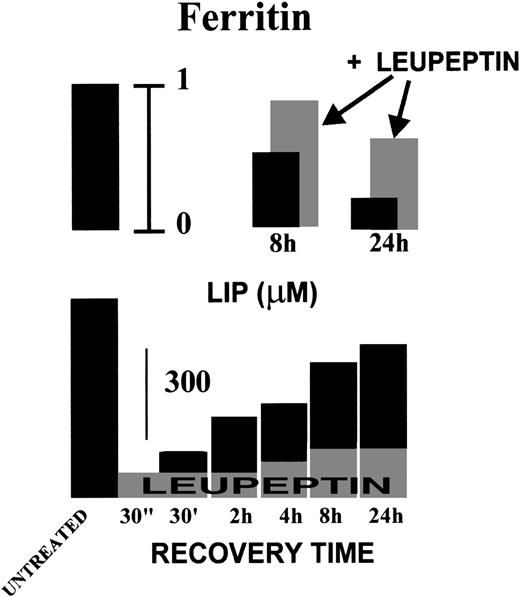
The ferritin levels of K562 cells in exponentially growing cultures were 706 ± 157 ng/mg protein for H-subunits and 100 ± 33 ng/mg protein for L-subunits (Table 1). After a 24-hour incubation with fresh medium, the respective levels of H-subunits and L-subunits increased to 1,010 ± 174 and 123.5 ± 36 ng/mg protein, respectively. In the presence of 100 μmol/L DFO, ferritin levels decreased by approximately 60% for H-subunits and 90% for L-subunits. In contrast, the antiproteases, leupeptin and chymostatin, induced increases in both H- and L-subunit levels of about 70% and 90%, respectively. The leupeptin-induced increase in total ferritin is already apparent after 8 hours and closely correlates with inhibition of LIP recovery by this agent (Fig 4).
Effect of DFO, Protease, and Lysosomal Inhibitors on Intracellular H-Ferritin and L-Ferritin Levels and H/L-Ferritin Ratios in K562 Cells
| . | H-Ferritin (ng/mg protein) . | L-Ferritin (ng/mg protein) . | H/L-Ferritin Ratio . |
|---|---|---|---|
| Before treatment | 706 ± 157 | 100.1 ± 33.1 | 7.3 ± 1.0 |
| Control | 1,010 ± 174 | 123.5 ± 36.2 | 8.4 ± 1.5 |
| DFO (100 μmol/L) | 412 ± 45 | 11.4 ± 3.8* | 38.5 ± 11.1* |
| Leupeptin (20 μg/mL) | 1,681 ± 256 | 192.5 ± 29.5 | 8.7 ± 0.2 |
| Chymostatin (100 μg/mL) | 1,887 ± 280 | 232.8 ± 23.1 | 8.2 ± 2.0 |
| Chloroquine (20 μmol/L) | 856 ± 278 | 103.6 ± 39.3 | 8.4 ± 0.7 |
| Leupeptin (20 μg/mL) + DFO (100 μmol/L) | 940 ± 455 | 110.3 ± 65.0 | 9.0 ± 1.6 |
| Chymostatin (100 μg/mL) + DFO (100 μmol/L) | 678 ± 136 | 70.4 ± 29.0 | 10.5 ± 3.3 |
| Chloroquine (20 μmol/L) + DFO (100 μmol/L) | 490 ± 62 | 23.4 ± 5.7* | 21.4 ± 2.8* |
| . | H-Ferritin (ng/mg protein) . | L-Ferritin (ng/mg protein) . | H/L-Ferritin Ratio . |
|---|---|---|---|
| Before treatment | 706 ± 157 | 100.1 ± 33.1 | 7.3 ± 1.0 |
| Control | 1,010 ± 174 | 123.5 ± 36.2 | 8.4 ± 1.5 |
| DFO (100 μmol/L) | 412 ± 45 | 11.4 ± 3.8* | 38.5 ± 11.1* |
| Leupeptin (20 μg/mL) | 1,681 ± 256 | 192.5 ± 29.5 | 8.7 ± 0.2 |
| Chymostatin (100 μg/mL) | 1,887 ± 280 | 232.8 ± 23.1 | 8.2 ± 2.0 |
| Chloroquine (20 μmol/L) | 856 ± 278 | 103.6 ± 39.3 | 8.4 ± 0.7 |
| Leupeptin (20 μg/mL) + DFO (100 μmol/L) | 940 ± 455 | 110.3 ± 65.0 | 9.0 ± 1.6 |
| Chymostatin (100 μg/mL) + DFO (100 μmol/L) | 678 ± 136 | 70.4 ± 29.0 | 10.5 ± 3.3 |
| Chloroquine (20 μmol/L) + DFO (100 μmol/L) | 490 ± 62 | 23.4 ± 5.7* | 21.4 ± 2.8* |
Cells were incubated in medium alone (control) and/or with DFO, leupeptin, chymostatin, or chloroquine for 24 hours. Leupeptin and chymostatin were added twice, at the beginning of the incubation and after 12 hours. At the end of the incubation period, the cells were washed and lysed, and total (H + L) ferritin and L-ferritin were separately determined by ELISA. H-ferritin was calculated as total minus L-ferritin. Values for cells before treatment are shown for comparison. Results are expressed as mean ± SD of 3 independent experiments run in triplicate.
P < .05: DFO at 100 μmol/L versus chloroquine at 20 μmol/L + DFO at 100 μmol/L.
Time dependence of LIP recovery and total ferritin levels in K562 cells after iron chelation. K562 cells were treated for 10 minutes with 100 μmol/L SIH and then incubated in serum-free growth medium alone (▪) or containing leupeptin 20 μg/mL (added at 0 time and at 8 hours; ▩). At the indicated times, cells were washed and analyzed for LIP (by the calcein method) and total ferritin levels (by ELISA) as described in Fig 3. Ferritin levels (upper panel) are given relative to untreated cells (extreme left bar) and LIP levels (lower panel) expressed in micromoles per liter.
Time dependence of LIP recovery and total ferritin levels in K562 cells after iron chelation. K562 cells were treated for 10 minutes with 100 μmol/L SIH and then incubated in serum-free growth medium alone (▪) or containing leupeptin 20 μg/mL (added at 0 time and at 8 hours; ▩). At the indicated times, cells were washed and analyzed for LIP (by the calcein method) and total ferritin levels (by ELISA) as described in Fig 3. Ferritin levels (upper panel) are given relative to untreated cells (extreme left bar) and LIP levels (lower panel) expressed in micromoles per liter.
The lysosomotropic agent chloroquine had only a minor effect on the ferritin subunits when added alone (not statistically significant). However, in combination with DFO, chloroquine partially reversed the inhibitory effect of the chelator on L-ferritin, but had no apparent significant effect on H-ferritin levels (Table 1). On the other hand, DFO and the antiproteases, which individually produced opposing effects on the 2 ferritin subunits, produced no net effect on subunit levels when added together. Such countereffects might indicate a possible common site of action on ferritin levels. However, the lack of a distinct effect of chloroquine alone would seemingly exclude an acidic compartment as the putative common site.
As we have shown, both ferritin subunits generally reacted towards the various agents in qualitatively and quantitatively similar fashions. However, the susceptibility of L-subunits to DFO alone or in combination with chloroquine was considerably greater (5- and 3-fold, respectively) than that of H-subunits. (Alternately, the H-subunits might be more resistant to degradation than the L-subunits.) This point is strikingly illustrated in Table 1, depicting H/L ratios that are markedly increased after DFO treatment either alone or in combination with chloroquine. It is also clear from Table 1 that chloroquine partially reversed the suppressive effect of DFO on the H/L ratio. This was due to a selective effect of chloroquine on L-ferritin, as described above (Table 1). This might suggest not only different metabolic fates, but also possibly different mechanisms of degradation of the 2 ferritin subunits after chelation.
DISCUSSION
Despite the various roles attributed to the LIP in cell iron homeostasis and in possible toxic effects, very few studies have attempted to relate these properties quantitatively with the LIP per se. Recently, it has become possible to determine the LIP in living cells with a novel fluorescence-based method.19 This method was used here for assessing some of the cellular factors involved in the maintenance of LIP within physiological limits in K562 cells. Those factors include both the translational control of ferritin and TfR expression that are IRP-dependent and ferritin degradation that is IRP-independent.
Measurements of the LIP with a method based on calcein equilibration with intracellular iron19 showed that the cellular LIP apparently consists of at least 2 kinetic components. The first, which is the major contributor to the LIP under physiological conditions (0.6 to 0.8 μmol/L), is evidently in rapid equilibrium with calcein and easily accessible to chelators. This is seen in Figs 1 and 2, which show that most of the intracellular calcein-quenchable fluorescence can be rapidly restored by a short treatment with the highly cell-membrane permeate chelator isonicotinoyl salicylaldehyde hydrazone (SIH). The second component is relatively minor (0.1 to 0.2 μmol/L); it appears after removal of SIH and increases and levels off in less than 1 hour. This minor LIP fraction is apparently in a relatively slow equilibrium with calcein and is relatively less accessible to SIH than the major one (Figs 1A and 2). This kinetically slow component can be eliminated either by pretreatments of cells with SIH or by increasing the chelator concentration (Glickstein and Cabantchik, unpublished observations). We can infer from the present studies that the slow component (which we refer to as “cryptic”) includes forms of iron that are loosely associated with cell constituents but not easily accessible to relatively large probes such as calcein or the chelator SIH. An analogous form of iron, which is inaccessible to chelators such as transferrin, DFO, or deferriprone (L1), has recently been detected in sera of iron-overloaded patients (some with even <70% transferrin iron saturation).25 If either human diferric-Tf or FCS was present in the cell medium, the rate of recovery and the magnitude of LIP in the second phase were markedly enhanced, with Tf evoking a typical overshoot. In serum-free medium, the second phase of LIP recovery was relatively slow, thus showing the high efficiency of iron delivery to the cell by Tf. Another cellular iron component, which is not in equilibrium with calcein but can contribute substantially to the LIP, is shown after iron starvation. This component replenishes approximately 50% to 70% of the original LIP, and its contribution to the LIP can be markedly reduced by protease inhibitors such as leupeptin. Thus, the major replenishment of iron in the LIP after chelation treatment might be associated with degradation of putative iron-containing proteins such as ferritin. The half-life of this component was approximately 8 hours, which is considerably shorter than that reported previously for ferritin in K562 cells.26However, the lack of an external iron source in a serum-free medium might explain the shortened half life of the ferritin. Further evidence that ferritin is a possible source for the LIP iron is inhibition of both the recovery of the LIP and the decrease in cellular ferritin levels by the protease inhibitors leupeptin and chymostatin.
It was assumed that in K562 cells the predominant mechanism for release of iron from ferritin is through the constitutive degradation of the protein in lysosomes.26 Furthermore, the presumed mechanism for iron release from ferritin and its transfer to hemoglobin originally observed in early human erythroid precursor cells that mature in a liquid culture was tentatively localized to an acidic cell compartment. The mechanism proposed was ferritin shell degradation by acid proteases, because ferritin degradation, as well as the transfer of its iron to heme, was inhibited by chloroquine and by the acid protease inhibitors chymostastin and leupeptin.14 We showed here that leupeptin inhibited long-term as well as short-term LIP recovery after LIP depletion by SIH and that it also inhibited the decrease in cellular ferritin levels (Figs 3 and 4). This means that, when there are no extracellular iron resources, ferritin is the intracellular source for iron and the iron is released by its proteolytic degradation.
Like all intracellular proteins, the level of ferritin is determined by the degree of its degradation and biosynthesis. Although ferritin degradation and the release of its iron is a proteolytic process14,17,26 27 the mechanism by which this process is regulated is still an enigma.
Cytosolic ferritin has been assumed to be degraded in lysosomes,14,17,26,27 and we have shown that in human erythroid cells developing in a liquid culture chloroquine inhibited the release of iron from ferritin and its transfer to heme.14 We have found here that this is not the case in K562 cells after chelator treatment, insofar as chloroquine had a minimal effect on preventing ferritin degradation (Table 1).
The question of whether IRPs control the cell LIP levels and, vice versa, whether LIP levels dictate IRP activities has been dealt with extensively.3,4,6-8 However, both concepts have been only inferred due to experimental difficulties in estimating the LIP.2 In the current work, IRP activity was inversely correlated with the LIP in various experimental conditions, although generally over a long time period (8 hours). This includes treatment of cells with SIH (cell iron depletion) and prevention of iron replenishment into the LIP by controlling intracellular protein degradation (Figs 3 and 4). However, in all of these studies, despite the demonstrable changes in LIP levels that are induced by chelation, there were no detectable changes in IRP activity up to 2 hours after treatment (data not shown). In fact, no substantial changes in IRP were observed earlier than 8 hours posttreatment. Therefore, we suggest that, of the 2 parameters associated with the recovery of cell iron, the LIP provides a quicker indicator than the IRP of the iron available to the cell for its metabolic needs at any given point in time.
Part of the increased IRP activity after LIP manipulation can be attributed to an increase in total cellular IRP content (Fig 3). Moreover, when cells were treated with a combination of chelator and leupeptin, LIP was at its lowest level and IRP activity increased by 80% compared with the controls incubated with DMSO alone. The active/total IRP ratio increased by 44%. Thus, 25% of the increased IRP activity could be ascribed to the augmentation in total cellular IRP content. From the data given above, we suggest that part of the IRP changes can be attributed to IRP 2, the synthesis and oxidation-dependent degradation of which is determined by the cellular iron availability and is not posttranslationally activated.28 29
The additional parameter tested, ferritin, also showed a pattern of behavior complementary to that of LIP and IRP after iron chelation treatment (Fig 4). Again, leupeptin, by inhibiting ferritin degradation, apparently reduced the recovery of LIP after cell iron depletion when no extracellular source of iron was available.
In general, inhibition of intracellular proteolysis by protease inhibitors resulted in parallel increases in both H and L subunits of ferritin (Table 1), whereas chelator treatment of cells led to decreases in the cell levels of both subunits. However, although the suppressive effect of DFO on L-ferritin subunit levels in K562 cells was relatively greater than that on H-subunits, the resulting H/L ratio in DFO-treated cells was significantly higher than in untreated cells (Table 1). These results indicate that the DFO-induced iron depletion differentially affects L-ferritin levels either by inhibiting L-ferritin synthesis (via IRP) or by stimulating L-ferritin degradation. A similar differential behavior was also observed when DFO was used in combination with chloroquine. Whereas chloroquine alone hardly affected either H- or L-subunit levels, the combination with DFO differentially reduced the L-subunit, leading to an increased H/L ratio. However, the H/L ratio after this combined treatment was still significantly lower than after DFO alone, due to selective reversal by chloroquine of the DFO-induced L-ferritin suppression. Because DFO has weak base properties, it is plausible that chloroquine counteracted DFO effects on L-subunit levels via a lysosomotropic effect, suggesting different modes of processing of each subunit.
In conclusion, this work shows that, in the absence of an extracellular iron source, K562 cells replenish their LIP by various mechanisms that include a fast release of iron from immediate intracellular resources followed by a slow release from ferritin. The release of iron from ferritin into the LIP is associated with a proteolytic event, inasmuch as protease inhibitors prevent it from occuring. LIP levels apparently dictate the IRP activity, although a considerable gap in time is found before those levels are sensed and the corrective mechanisms are eventually set in motion.
Supported in part by the Israel Research Fund, the EEC Biomed 2, National Institutes of Health Grant No. AI-20342, and the Mirsky Foundation for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Abraham M. Konijn, PhD, Department of Human Nutrition and Metabolism, The Hebrew University, Faculty of Medicine, PO Box 12272, Jerusalem 91120, Israel; e-mail: konijn@md2.huji.ac.il.

![Fig. 1. Short-term recovery of LIP levels in iron-depleted K562 cells. K562 cells were incubated in HBS (2 × 106/mL) alone or in the presence of 100 μmol/L SIH for 10 minutes at 37°C to examine acute iron depletion. Cells were washed free of chelator and incubated for an additional recovery period either in Dulbecco’s modified Eagle’s medium (DMEM) alone or DMEM supplemented with 20 μg/mL leupeptin, 50 μg/mL transferrin, or 10% FCS. At the end of the incubation period, the cells were washed with HBS and processed for LIP measurements by loading with calcein-AM (CA), washed, and resuspended in HBS-medium. Fluorescence (left panels) was continuously monitored as described in Materials and Methods. When fluorescence readings were steady, anti-CA antibodies (ab) were added (to quench extracellular fluorescence), followed by 100 μmol/L SIH, to attain maximum recoverable fluorescence. Shown are tracings obtained after recovery periods of 0 to 6 hours (ISAH-0-6 h). The maximum value, which was equivalent to the concentration of CA-bound iron [CA-Fe], was used for calculating LIP values (in micromoles per liter). LIP values are shown in the right panels for the different treatments: (A) recovery in DMEM alone (recov), (B) recovery in DMEM supplemented with leupeptin (recov.+leup), or (C) recovery in DMEM supplemented with transferrin (Recov. + Tf) or FCS (Recov.+ FCS).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2128/5/m_blod41820001x.jpeg?Expires=1769104477&Signature=ErlzgurOgiwyJN93zDpRAUUrEdAS4nYyldf-lLFPwqIt6nkpbpaabjNm8e2516~fyWeXQk30mMbikqVW9L-EYx2i~Z~nqgTUQT9Foct-tDSeu6JswY1uKFnc~STMdAMRx0Ned~kUJRszg5lL~McB4D~lbaGUU-mr8mk35JoDJmPb1HnHEoWJ93Llt2ILTdKSmRtAVTpUAlRKTafmEv~b2RoAPuXVb5pcJOgDGVTxOMMfn6jmA1lMgRPaXahWIPEgSOss9KvsAiS5WXoNxObQM-mcRYy4K3zyw6hr4auvkuDf0BPc9J0OTkhHZk2gC5eWXwYzYS2bjofHoj9Mwn2POA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal