Abstract
In an attempt to gain insight into the molecular mechanisms involved in interleukin-9 (IL-9) activities, representational difference analysis (RDA) was used to identify messages that are induced by IL-9 in a murine T-helper–cell clone. One of the isolated genes encodes for the newly described M-Ras or R-Ras3, which is part of the Ras gene superfamily. M-Ras expression was found to be induced by IL-9 but not IL-2 or IL-4 in various murine T-helper–cell clones, and this induction seems to be dependent on the JAK/STAT pathway. Contrasting with the potent upregulation of M-Ras expression, M-Ras was not activated by IL-9 at the level of guanosine triphosphate/guanosine diphosphate (GTP/GDP) binding. However, IL-3 increased GTP binding to M-Ras, suggesting that M-Ras induction might represent a new mechanism of cooperativity between cytokines such as IL-3 and IL-9. Constitutively activated M-Ras mutants induced activation of Elk transcription factor by triggering the MAP kinase pathway and allowed for IL-3–independent proliferation of BaF3 cells. Taken together, these results show that cytokines such as IL-9 can regulate the expression of a member of the RAS family possibly involved in growth-factor signal transduction.
INTERLEUKIN-9 (IL-9) is a TH2 cytokine that was discovered based on its growth-promoting effect on T-helper cells.1 Subsequently, several other activities were attributed to this protein, including IgE production by B cells, differentiation of hematopoietic and neuronal progenitor cells, and proliferation as well as differentiation of mast cells.2,3In addition, some observations suggest the involvement of IL-9 in both human and murine tumorigenesis. IL-9 overexpression indeed results in a high susceptibility to the development of T-cell lymphomas in vivo,4 and constitutive IL-9 expression has been shown in many human Hodgkin lymphomas.5 More recently, the involvement of IL-9 has been suggested in asthma,6-9 in line with the effect of this cytokine on IgE production10,11 and mast cell differentiation.12
To further characterize the biological activity of IL-9, we established a panel of T-helper–cell clones that can proliferate in response to either IL-2, IL-4, or IL-9, and thus provide us with a simple model to compare functional characteristics of these cytokines.13 In this report, using 1 of these T-cell clones, we employed a subtractive hybridization approach to identify genes whose expression is induced by IL-9 and may be involved in the IL-9 pathway to regulate proliferation and/or apoptosis in T-helper cells. Using this strategy, we found that the expression of the guanosine triphosphate (GTP)-binding protein M-Ras can be selectively induced by IL-9 but not by IL-2 or IL-4. Biochemical analysis of constitutively expressed M-Ras in lymphoid cells failed to detect its activation by IL-9 in contrast to IL-3, suggesting that this molecule is not involved in IL-9 signal transduction in effector cells. However, these data suggest a mechanism by which M-Ras activity can be regulated at the mRNA level in addition to the posttranslational level of GTP binding and GTPase activity.
MATERIALS AND METHODS
Cell cultures and cytokines.
T-helper–cell clones TS2 and TS313 were grown in Dulbecco’s modified Eagle’s medium (DMEM) medium supplemented with 10% fetal calf serum (FCS), 50 μmol/L 2-mercaptoethanol, 0.55 mmol/L L-arginine, 0.24 mmol/L L-asparagine, and 1.25 mmol/L L-glutamine. These factor-dependent cell lines were able to grow in the presence of either IL-2, IL-4, or IL-9 without antigen and feeder cells.1 TS2-h9R cells were obtained by electroporation of TS2 cells with the pEF-BOS.puro vector containing the wild-type human IL-9 receptor cDNA and selection in puromycin (4 μg/mL; Sigma, St Louis, MO) and human IL-9 (500 U/mL). TS2-h9Rphe116 cells were derived using the same procedure with a mutated human IL-9 receptor cDNA, where Tyr116 of the cytoplasmic tail is changed to a Phe residue. As described previously, this mutation abolished STAT (signal transducer and activator of transcription) activation in response to human IL-9.14 BaF3 cells15 were cultured in DMEM supplemented with 10% FCS and mouse IL-3 (1 ng/mL). HEK293 cells, derived from human embryonic kidney, were cultured in DMEM medium with 10% FCS. The following cell lines were kindly provided as listed: T-helper clone ST2K916 by Dr E. Schmitt (Gütenberg University, Mainz), IL-9– or IL-3–dependent mast cell line L138.8A12by Dr L. Hültner (GSF, München, Germany), IL-3–dependent mast cell line MC9 by Dr C. Petit-Frère (Institut Henri Beaufour, Les Ullys, France), macrophage cell line PU5.8 by Dr L. Franssen (Innogenetics, Gent, Belgium), and thymic lymphoma EL4 by Dr H.R. MacDonald (Ludwig Institute for Cancer Research, Lausanne, Switzerland).
Spleen cells were isolated from naive 12-week-old C57BL/6 mice by aseptic removal of spleen and resuspension in DMEM supplemented with 10% heat-inactivated FCS. Cells (106/mL) were incubated with or without 5 μg/mL of Conconavalin A (Sigma). After 24 or 48 hours, cells were harvested for RNA isolation using the Trizol reagent (GIBCO-BRL, Ghent, Belgium) according to the manufacturer’s recommendations.
Mouse and human recombinant IL-9 (4 × 107 U/mg and 2 × 107 U/mg, respectively) were produced in our laboratory by expression in the baculovirus system and purified as described.17 Human recombinant IL-2 (3.5 × 106 U/mg) was given by Dr W. Fiers (State University of Ghent, Ghent, Belgium), and mouse recombinant IL-3 (2 × 107 U/mg) was a gift of Dr J.Y. Bonnefoy (Glaxo, Geneva, Switzerland).
Subtractive hybridization.
Total RNA was prepared from TS2 cells stimulated with IL-2 (200 U/mL) or IL-9 (200 U/mL) for 48 hours, using guanidium isothiocyanate lysis and CsCl gradient centrifugation.18 Polyadenylated RNA was purified from total RNA with oligo(dT) cellulose columns. Double-stranded cDNA was generated from 5 μg polyA+ RNA using an oligo(dT) primer and the SuperScript Choice System for cDNA synthesis according to the manufacturer’s recommendations (GIBCO-BRL). Representational difference analysis was performed as described by Hubank and Schatz19: cDNAs were digested with DpnII (New England Biolabs, Beverly, MA), ligated to R-Bgl-12/24 adapters, and polymerase chain reaction (PCR) amplified to generate representations. The R-Bgl oligonucleotides were removed byDpnII digestion, and J-Bgl-12/24 adapters were ligated to the cDNA from TS2/IL-9 cells. For the first cycle of substractive hybridization, 0.4 μg of J-Bgl-ligated TS2/IL-9 cDNA was mixed with 40 μg TS2/IL-2 cDNA (1/100 ratio). After hybridization for 20 hours at 67°C, the ends of the resulting hybrids were filled in, and a PCR amplification was performed with the J-Bgl-24 oligonucleotide, which was further removed by DpnII digestion. The second and third cycles of subtraction were performed similarly with a ratio of 1/800 and 1/400,000, respectively.
After these 3 rounds of subtraction, final difference products were digested with DpnII and cloned into the BamHI site of pTZ19R. Double-stranded plasmid DNA was prepared and sequenced with a Thermo-sequenase Sequencing kit (Amersham, Arlington Heights, IL). Sequence comparisons with the GenBank and European Molecular Biology Laboratory (EMBL) databases were performed with the BLAST search program. Oligo(dT)-primed cDNA libraries generated from the mouse T-cell clone TS2 stimulated with IL-9, and from human testis were screened with the mouse M-Ras DpnII fragment and with a human reverse transcription (RT)-PCR product, respectively.
Preparation of mRNA, Northern, and RT-PCR analysis.
Total cellular RNA was fractionated by electrophoresis in a 1.3% agarose gel containing 2.2 mol/L formaldehyde and was transferred onto a Hybond-C Extra nitrocellulose membrane (Amersham). cDNA inserts were labeled using the Multiprime DNA labeling kit from Amersham. Hybridizations and washes were performed as described.13The M-Ras probe was a 1.2-kb cDNA containing the complete coding sequence for this protein. After autoradiography, all blots were reprobed with a chicken β-actin probe to control for even loading of RNA. To analyze the tissue distribution of M-Ras expression, a premade nitrocellulose multiple tissue filter covering 8 tissues was used (Clontech, Palo Alto, CA).
RT was performed on 5 μg total RNA with an oligo(dT) primer. cDNA corresponding to 20 ng of total RNA was amplified for 20 to 35 cycles by PCR with specific primers as indicated in Table 1. The post-PCR products were analyzed in ethidium bromide–stained 1% agarose gel. Specific amplification was confirmed after blotting (Zeta-Probe membrane; Biorad, Hercules, CA) and hybridization of the post-PCR results with internal radioactive probes. For β-actin the internal probe is 5′-GTCCACGACATCATGCTACTG-3′, and for the mouse M-Ras, 5′-GGATGTTCTGGACACAGCCGG-3′. Radioactive signals were quantified by Phosphorimager (Molecular Dynamics, Sunnyvale, CA) and the M-Ras/actin ratios were calculated. Human M-Ras was amplified from a human brain cDNA with the oligonucleotides designed from the mouse sequence and shown in Table 1.
Specific Primers Used for PCR Amplification of Members of the Ras Family
| Gene . | Sense Primer . | Antisense Primer . | Annealing Tm . | Product Size (bp) . |
|---|---|---|---|---|
| M-Ras | 5′-GGCCTGACTACCAGAAACATG-3′ | 5′-TGCCCAGGGCTTCAGGCTG-3′ | 58 | 645 |
| H-Ras | 5′-TCATTGATGGGGAGACATGTC-3′ | 5′-GTCCTGGGCCTGCCGAGACTC-3′ | 62 | 262 |
| N-Ras | 5′-TGATTGATGGTGAGACCTGC-3′ | 5′-CAGTTCGTGGGCTTGCTTTG-3′ | 60 | 265 |
| R-Ras | 5′-ACTGTGGACGGCATCCCTGC-3′ | 5′-GAAAGAGGAGGCTTCAGACCG-3′ | 60 | 269 |
| β-Actin | 5′-ATGGATGACGATATCGCTGC-3′ | 5′-GCTGGAAGGTGGACAGTGAG-3′ | 60 | 471 |
| Gene . | Sense Primer . | Antisense Primer . | Annealing Tm . | Product Size (bp) . |
|---|---|---|---|---|
| M-Ras | 5′-GGCCTGACTACCAGAAACATG-3′ | 5′-TGCCCAGGGCTTCAGGCTG-3′ | 58 | 645 |
| H-Ras | 5′-TCATTGATGGGGAGACATGTC-3′ | 5′-GTCCTGGGCCTGCCGAGACTC-3′ | 62 | 262 |
| N-Ras | 5′-TGATTGATGGTGAGACCTGC-3′ | 5′-CAGTTCGTGGGCTTGCTTTG-3′ | 60 | 265 |
| R-Ras | 5′-ACTGTGGACGGCATCCCTGC-3′ | 5′-GAAAGAGGAGGCTTCAGACCG-3′ | 60 | 269 |
| β-Actin | 5′-ATGGATGACGATATCGCTGC-3′ | 5′-GCTGGAAGGTGGACAGTGAG-3′ | 60 | 471 |
M-Ras–specific antibody production.
A synthetic peptide beginning with an NH2-terminal cysteine residue followed by the M-Ras sequence 187-204 (CKKKTKWRGDRATGTHKLQ), a region that showed no homology with other members of the Ras family, was conjugated to maleimide-activated KLH (Imject-Maleimide activated Carrier proteins; Pierce, Rockford, IL). Two rabbits were each immunized with 150 μg of peptide-KLH conjugate in 4 vol of Freund complete adjuvant by multiple-site intradermal injection. At 3-week intervals, the rabbits were boosted with 100 μg of peptide-conjugate in 4 vol of Freund incomplete adjuvant by multisite subcutaneous injection. Fifteen days after the second boost, the animals were test bled, and after the third boost the recovery period between injections was increased to 4 weeks, with test bleedings 10 to 14 days post-boost. The production of peptide-specific antibodies (JAL-4) was assessed by enzyme-linked immunosorbent assay (ELISA).
Electrophoretic mobility shift assay.
Nuclear extracts were prepared as described,20 with minor modifications. Briefly, cells (1.5 × 107) stimulated with murine (m) IL-2 (200 U/mL), mIL-9 (200 U/mL), or hIL-9 (500 U/mL) for 10 minutes, were washed with phosphate-buffered saline (PBS), and resuspended in 1 mL ice-cold hypotonic buffer ‘A’ for 15 minutes (10 mmol/L HEPES buffer, pH 7.5, containing 10 mmol/L KCl, 1 mmol/L MgCl2, 5% glycerol, 0.5 mmol/L EDTA, 0.1 mmol/L EGTA, 0.5 mmol/L dithiothreitol, 1 mmol/L Pefabloc [Boehringer Mannheim, Mannheim, Germany], 10 mg/mL aprotinin, 1 mmol/L Na3VO4, 5 mmol/L NaF). The cells were lysed by adding 65 mL NP-40 10% and vortexing. Nuclei were pelleted (30 seconds at 14,000 rpm) and extracted for 30 minutes in 100 mL hypertonic buffer ‘B’ (buffer ‘A’ supplemented with HEPES [20 mmol/L], glycerol [20%], and NaCl [420 mmol/L]). Nuclear debris were removed through a 2-minute centrifugation. Analysis of DNA binding activity was performed using 32P-labeled GRR oligonucleotide probes (from FcγRI gene promoter): upper strand: 5′-ATGTATTTCCCAGAAA-3′; bottom strand: 5′-CCTTTTCTGGGAAATAC-3′.
Isolation of BaF3 transfectants.
cDNA encoding wild-type (M-Ras), activated mutants of mouse M-Ras (M-Ras/22V, M-Ras/22K, or M-Ras/71K) were cloned into the pEF-BOS plasmid,21 in which a puromycin resistance gene had been inserted for selection of transfected cells.14 Mutagenesis was performed in pBluescript using appropriate oligonucleotides followed by subcloning into pEF-BOS (Stratagene, La Jolla, CA; Chameleon Double-Stranded, site-directed Mutagenesis Kit) and the coding sequence of each mutant was checked with a Thermo-sequenase Sequencing kit (Amersham). Plasmid DNA was extracted with a Nucleobond AX kit (Macherey-Nagel, Duren, Germany).
BaF3 were transfected by electroporation (1,500 μF, 74 W, and 300 V) with 50 μg of sterile DNA. Pools of transfected cells were selected with puromycin (3 μg/ml; Sigma). M-Ras and M-Ras/22V–transfected BaF3 cells were further transfected with pRG8 plasmid containing the human IL-9 receptor cDNA and selected with G418 (1 mg/mL; GIBCO-BRL). Selected cells were maintained in growth medium supplemented with 20 ng/mL human IL-9 or 1 ng/mL of murine IL-3.
GTP-, GDP-bound RAS assay.
Cells were washed 3 times in PBS, resuspended in LB (DMEM without sodium phosphate or sodium pyruvate [GIBCO-BRL] containing 20 mmol/L HEPES, 0.1% bovine serum albumin [BSA], and 0.3 mCi/mL of phosphorus-32 [Amersham]), and incubated for 4 hours at 37°C. Two to 3 × 107 cells were used for each experimental point. Cells were stimulated for 5 minutes with the appropriate cytokine and immediately washed with ice-cold PBS. Cells were lysed in 1 mL of buffer 1 (50 mmol/L HEPES pH 7.5, 100 mmol/L NaCl, 01% Triton X-114 [Sigma], 5 mmol/L MgCl2, 0.1% BSA, 1X “Complete” protease inhibitor mix [Boehringer Mannheim]) and lysates were cleared by centrifugation. Supernatant was transferred in a fresh tube containing 100 μL of 5 mol/L sodium chloride, incubated for 2 minutes at 37°C, and centrifuged. The aqueous layer was discarded and the detergent layer (50 μL) was resuspended in 1 mL of buffer 2 (50 mmol/L Tris pH 7.5, 5 mmol/L MgCl2, 1% Triton X-100, 0.5% deoxycholate, 0.05% sodium dodecyl sulfate [SDS], 0.5 mol/L NaCl, 1 mmol/L EGTA pH 8.0, 1 mmol/L dithiothreitol [DTT], 0.1% BSA, 1X “Complete” protease inhibitor mix) and 25 μL of protein A+G agarose-conjugated beads were added. Lysates were precleared for 5 minutes. The anti–M-RAS antiserum (JAL-4) was added (1/100) and lysates were tumbled for 45 minutes at 4°C. Subsequently, 20 μL of protein A+G agarose-conjugated beads were added and lysates were tumbled at 4°C. Beads were washed 5 times in washing buffer (50 mmol/L HEPES pH 7.5, 0.5 mol/L NaCl, 0.1% Triton X-100, 0.005% SDS, 5 mmol/L MgCl2), resuspended in 12 μL of elution buffer (2 mmol/L EDTA, 2 mmol/L DTT, 0.2% SDS, 5 mmol/L GTP [Sigma], 5 mmol/L guanosine diphosphate [GDP; Sigma]) and incubated at 68°C for 20 minutes. Samples were then centrifuged and 8 μL was loaded in a PEI-cellulose TLC plate (Merck, Darmstadt, Germany) that was subsequently developed in 0.75 mol/L potassium phosphate pH 3.4 for 1.5 hours.
Western blot.
Cells (2 × 105) were lysed in modified RIPA buffer (Tris HCl 50 mmol/L, pH 8, NaCl 150 mmol/L, Triton X-100 1%, sodium deoxycholate 0.25%, EGTA 1 mmol/L, NaF 1 mmol/L, leupeptin 10 mg/mL, Pefabloc 2 mmol/L) and cell debris was removed by centrifugation. Lysates were fractionated on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) polyacrylamide gels (14%) and electrophoretically transferred onto PVDF membranes (Amersham). Membranes were blocked in 5% nonfat milk, washed, and probed with rabbit polyclonal M-Ras antiserum (JAL-4, 1/500) and with horseradish peroxidase–linked anti-rabbit antibody (1/5,000; Amersham). The ECL detection kit (Amersham) was used for detection.
Signal transduction pathway assay.
Wild-type or mutated M-Ras constructs were cotransfected using a lipid transfection method into HEK293 cells together with the pathway-specific trans-activator vector and the reporter vector (Pathdetect trans-reporting system; Stratagene). The HEK293 cells were seeded at a density of 5 × 105/well in 12-well tissue-culture plates, and incubated overnight at 37°C. Each assay was performed with 3 plasmids: (1) 25 ng of the fusion activator plasmid (pFA vector) encoding a pathway-specific trans-activator protein consisting of the DNA binding domain of the yeast GAL4 and the activation domains of Elk; (2) 500 ng of the pFR-Luc reporter plasmid in which expression of the luciferase gene is controlled by a synthetic promoter that contains the yeast GAL4 binding site; (3) 500 ng of either empty pEF-BOS or pEF-BOS plasmid containing wild-type or mutated M-Ras. pFC-MEK1 plasmid (25 ng) encoding constitutively activated kinase was used as positive control plasmid for the MAPK-pathways. pFC-dbd (encoding the GAL4 DNA-binding domain) was used as a negative control for the pFA vector to ensure that the observed effects were not due to the GAL4 DNA binding domain.
Target cells were washed once with serum-free medium (OPTI-MEM; GIBCO), and transfected with the DNA constructs in 50 μL serum-free medium that had been mixed with 20 μg of lipofectamine reagent (GIBCO-BRL) in 50 μL of OPTI-MEM medium. Twenty-four hours after transfection, cells were washed twice with PBS before lysis and analysis of luciferase activity using the “Luciferase Reporter Gene Assay” kit according to the manufacturer’s instructions (Boehringer Mannheim).
RESULTS
Isolation of an IL-9–induced gene homologous to Ras proto-oncogenes.
Although IL-9 and IL-2 have T-cell growth activity for mouse T-helper clones, some experiments suggest that distinct activation pathways are involved.22 Based on the hypothesis that different biological activities of these cytokines reflect their ability to transcriptionally activate distinct target genes in T cells, we performed a representational difference analysis of gene expression on TS2 cells that were stimulated with either IL-2 or IL-9. Oligo(dT)-primed cDNAs prepared from cells stimulated with both cytokines were digested with DpnII and used to generate the respective amplicons. After 3 rounds of subtractive hybridization, the third difference product (DP3) was cloned and 57 clones were sequenced. Five independent IL-9–induced genes were identified, one of them showing a significant sequence identity with members of the Ras family. To obtain the full-length sequence of this mRNA, we screened an oligo-dT primed cDNA library generated from IL-9–stimulated TS2 cells with a 200-bp DpnII restriction fragment recovered from the subtractive hybridization. The largest clone isolated contained 1,128 nucleotides including a 624-bp open reading frame (ORF) that encodes for a 208 amino acid residue protein with a molecular weight (Mr) of 23,886 (GenBank accession no. AF043581).
A human homologue was identified from human brain cDNA by RT-PCR amplification using oligonucleotides based on the mouse sequence. Several cDNA clones were subsequently isolated from a human testis cDNA library. The largest of these cDNA clones contained a 1,081-bp insert with an ORF that encodes a protein of 208 amino acids with a calculated molecular mass of 23,831 daltons (GenBank accession no.AF043938). Sequence alignment of the mouse and human genes showed an identity of 89% at the nucleotide level and 97% at the protein level. During the characterization of these genes, the cloning of the mouse and human cDNAs was independently reported by 2 groups, based on homologies with other Ras genes.23 24 When compared with our sequence, the published mouse M-Ras cDNA sequence differs by only 3 nucleotide substitutions in the 3′ untranslated region. The human M-Ras cDNA shows 3 differences in the coding sequences and 2 of them result in a change of the amino acid sequence (glutamic acid-166 is replaced by a lysine and alanine-199 by a glycine in our sequence). These discrepancies may result from the low fidelity of Taq polymerase since the published M-Ras/R-Ras3 sequences were obtained by PCR amplification, whereas our sequences were found in several cDNA clones from conventional cDNA libraries. Alternatively, this could correspond to true allelic variations of the same gene.
A comparison of the amino acid sequence of murine M-Ras with those of murine H-Ras and R-Ras showed an amino acid identity of 49% and 46% with p21 H-Ras and p23 R-Ras, respectively. Conserved residues include an amino-terminal domain with a putative GTP-binding site (amino acids 20-27) corresponding to amino acids 10 to 17 in the H-Ras sequence. The carboxy-terminus was characterized by the presence of the consensus prenylation signal CAAX (where C is a cysteine, A is a hydrophobic residue, and X can be any residue) known to play an important role in the plasma membrane localization of Ras.25 In addition, a cluster of 8 basic residues (lysine) located upstream of this motif may mediate membrane attachment by electrostatic interaction with acidic phospholipids on the inner leaflet membrane, as shown previously for K-Ras.26
IL-9 inducibility of mouse M-Ras expression.
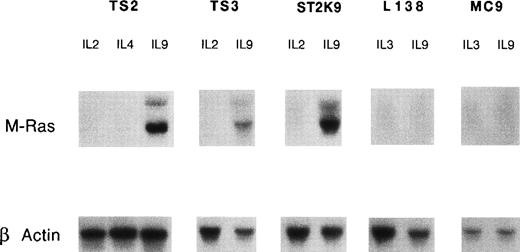
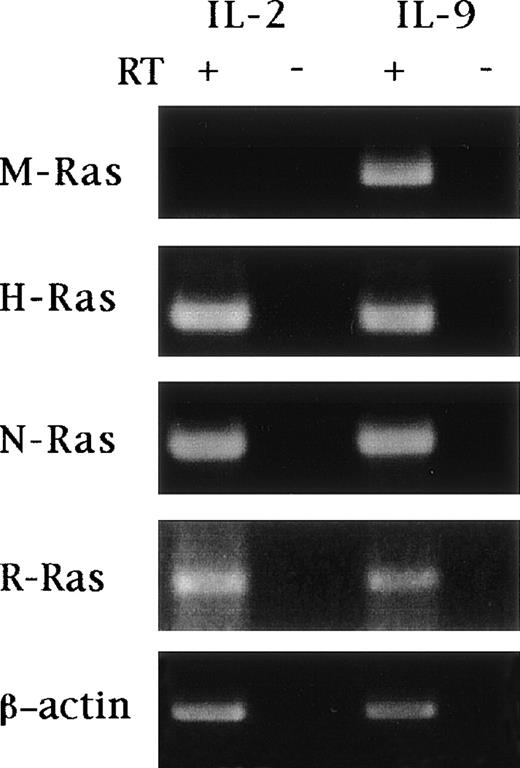
To confirm the inducibility of M-Ras by IL-9, we analyzed a series of IL-9–responsive cell lines including T-helper clones (TS2, TS3, and ST2K9) and mast cell lines (MC9 and L138). The murine M-Ras transcripts (2 bands of 1.3 and 4.4 kb) were observed in the T-cell clone TS2 cultured in the presence of IL-9 whereas neither IL-2– nor IL-4–stimulated cells had detectable amounts of M-Ras transcript (Fig 1). Induction of M-Ras expression by IL-9, but not by IL-2, was confirmed in 2 additional T-helper–cell clones (TS3 and ST2K9). By contrast, we failed to detect M-Ras expression in the mast cell lines MC9 and L138 stimulated with IL-9, indicating that IL-9 induced the expression of this gene in only a subset of IL-9–responsive cells. The inducibility by IL-9 is a specific characteristic of M-Ras since other members of the Ras family, such as H-Ras, N-Ras, and R-Ras, were expressed at similar levels in TS2 cells stimulated by IL-9 or IL-2 (Fig2).
IL-9 induces expression of M-Ras. IL-9–responsive cell lines were cultured for 10 days in medium containing saturating concentrations of the indicated cytokines: 100 U/mL IL-2, 200 U/mL IL-4, or 200 U/mL IL-9 for T-helper–cell clones (TS2, ST2K9, and TS3), and 200 U/mL of IL-3 or 200 U/mL of IL-9 for mast cell lines (L138 and MC9). After electrophoresis of 10 μg of total RNA and transfer on nitrocellulose, filters were hybridized with a 32P-labeled mouse M-Ras cDNA probe. Hybridization with a β-actin probe confirmed that comparable amounts of RNA had been loaded in each lane.
IL-9 induces expression of M-Ras. IL-9–responsive cell lines were cultured for 10 days in medium containing saturating concentrations of the indicated cytokines: 100 U/mL IL-2, 200 U/mL IL-4, or 200 U/mL IL-9 for T-helper–cell clones (TS2, ST2K9, and TS3), and 200 U/mL of IL-3 or 200 U/mL of IL-9 for mast cell lines (L138 and MC9). After electrophoresis of 10 μg of total RNA and transfer on nitrocellulose, filters were hybridized with a 32P-labeled mouse M-Ras cDNA probe. Hybridization with a β-actin probe confirmed that comparable amounts of RNA had been loaded in each lane.
IL-9 does not regulate expression of other members of the Ras family. TS2 cells were cultured for 3 days in the presence of 100 U/mL IL-2 or 200 U/mL IL-9. Total RNA was extracted and RT-PCR amplification was performed as described in Materials and Methods, using specific oligonucleotides listed in Table 1. Amplifications were performed at 94°C for 30 seconds, 58°C to 62°C for 1 minute, 72°C for 1.5 minutes, with a total number of 18 cycles for β-actin or 35 cycles for M-, H-, N-, and R-Ras. Samples where the reverse transcriptase (RT) was omitted were used as negative controls for each condition. The post-PCR products were analyzed in ethidium bromide–stained 1.5% agarose gel. The specificity of the PCR amplification was checked by sequencing the PCR products.
IL-9 does not regulate expression of other members of the Ras family. TS2 cells were cultured for 3 days in the presence of 100 U/mL IL-2 or 200 U/mL IL-9. Total RNA was extracted and RT-PCR amplification was performed as described in Materials and Methods, using specific oligonucleotides listed in Table 1. Amplifications were performed at 94°C for 30 seconds, 58°C to 62°C for 1 minute, 72°C for 1.5 minutes, with a total number of 18 cycles for β-actin or 35 cycles for M-, H-, N-, and R-Ras. Samples where the reverse transcriptase (RT) was omitted were used as negative controls for each condition. The post-PCR products were analyzed in ethidium bromide–stained 1.5% agarose gel. The specificity of the PCR amplification was checked by sequencing the PCR products.
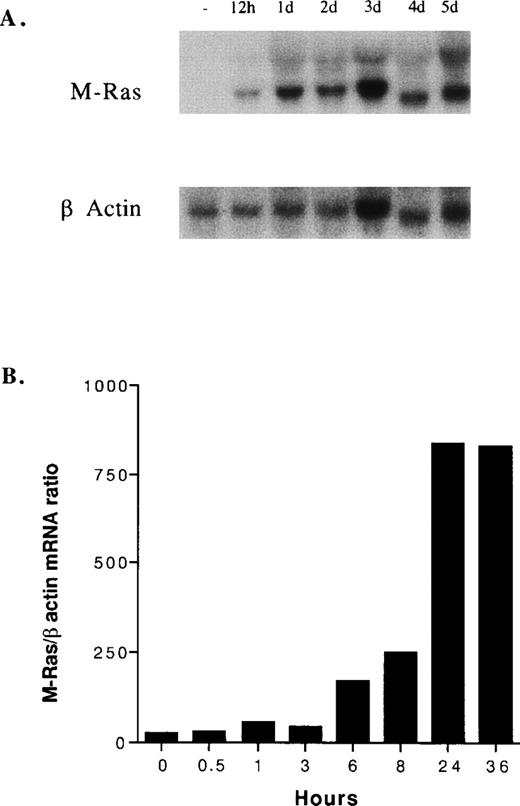
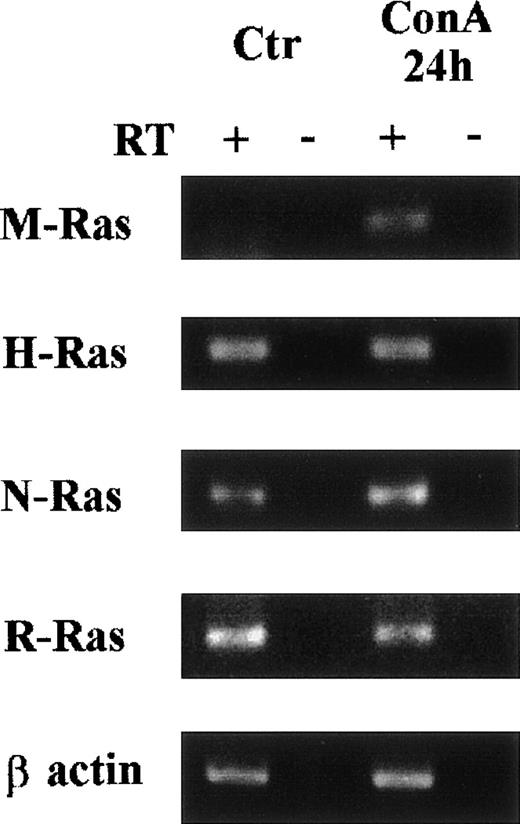
The kinetics of IL-9–induced M-Ras induction in TS2 cells found its expression increased at 6 hours and reached a maximal level at 24 hours (Fig 3). We next investigated the expression of M-Ras in different tissues and found that the highest expression of M-Ras level could be detected in brain, although a significant M-Ras expression was also found in kidney and skeletal muscle. In the other tissues tested, M-Ras messenger was barely detectable (Fig 4A). Tissue expression was also analyzed on IL-9 transgenic mice (Tg5) that overexpress IL-9 in all tissues (data not shown) and in its congenic background strain (FVB). No significant difference was observed between normal and IL-9 transgenic mice (Fig 4B). Noticeably, IL-9 overexpression in these transgenic mice does not induce normal T-cell hyperplasia, probably because resting T cells do not respond to IL-9. By contrast, IL-9 induces intestinal mastocytosis with increased expression of other IL-9–regulated genes such as granzymes and proteases.27Thus, the lack of M-Ras upregulation in the gut of IL-9–transgenic mice supports the observation made on cell lines that IL-9 induction of M-Ras was restricted to some cell types and/or conditions, such as activated T cells. In this respect, M-Ras induction is not limited to IL-9–stimulated T cells, as this gene is also upregulated upon ConA stimulation of freshly isolated spleen cells (Fig 5).
Kinetics of induction of M-Ras in TS2 cell. TS2 cells growing in the presence of IL-2 (100 U/mL) were washed and incubated with 200 U/mL of IL-9. Two independent experiments are shown. (A) Northern blots were prepared with 10 μg of total RNA isolated from cells stimulated for the indicated times, in hours (h) or in days (d). Filters were probed with a 32P-labeled mouse M-Ras cDNA. (B) M-Ras expression was monitored by RT-PCR and normalized with β-actin expression as described in Materials and Methods. M-Ras/β-actin ratios are arbitrary units.
Kinetics of induction of M-Ras in TS2 cell. TS2 cells growing in the presence of IL-2 (100 U/mL) were washed and incubated with 200 U/mL of IL-9. Two independent experiments are shown. (A) Northern blots were prepared with 10 μg of total RNA isolated from cells stimulated for the indicated times, in hours (h) or in days (d). Filters were probed with a 32P-labeled mouse M-Ras cDNA. (B) M-Ras expression was monitored by RT-PCR and normalized with β-actin expression as described in Materials and Methods. M-Ras/β-actin ratios are arbitrary units.
Tissue distribution of mRNA for mouse M-Ras. (A) Northern blot of a multiple tissue [2 μg of mouse poly(A)+ RNA per lane] (Clontech) was hybridized with a 32P-labeled mouse M-Ras cDNA probe. The size of the mRNAs detected are indicated to the left of the panel. (B) Northern blots containing 10 μg per lane of total RNA from adult tissues of FVB mice or IL-9–transgenic mice (Tg5) were hybridized with a 32P-labeled mouse M-Ras cDNA probe. Ethidium bromide staining of the gel is shown as control for loading.
Tissue distribution of mRNA for mouse M-Ras. (A) Northern blot of a multiple tissue [2 μg of mouse poly(A)+ RNA per lane] (Clontech) was hybridized with a 32P-labeled mouse M-Ras cDNA probe. The size of the mRNAs detected are indicated to the left of the panel. (B) Northern blots containing 10 μg per lane of total RNA from adult tissues of FVB mice or IL-9–transgenic mice (Tg5) were hybridized with a 32P-labeled mouse M-Ras cDNA probe. Ethidium bromide staining of the gel is shown as control for loading.
Induction of M-Ras expression by ConA stimulation of freshly isolated spleen cells. Spleen cells were cultured for 24 hours with or without ConA (5 μg/mL). Total RNA was extracted and RT-PCR amplification was performed as described for Fig 2. Samples where the reverse transcriptase (RT) was omitted were used as negative controls for each condition. The post-PCR products were analyzed in ethidium bromide–stained 1.5% agarose gel.
Induction of M-Ras expression by ConA stimulation of freshly isolated spleen cells. Spleen cells were cultured for 24 hours with or without ConA (5 μg/mL). Total RNA was extracted and RT-PCR amplification was performed as described for Fig 2. Samples where the reverse transcriptase (RT) was omitted were used as negative controls for each condition. The post-PCR products were analyzed in ethidium bromide–stained 1.5% agarose gel.
STAT proteins mediate IL-9 induction of M-Ras.
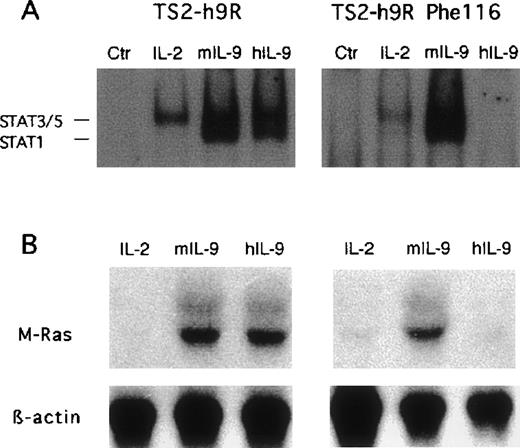
To determine the mechanisms by which IL-9 upregulate M-Ras, we transfected TS2 cells with the wild-type human IL-9 receptor (h9R) or its mutated form, that lacks the ability to activate STAT transcription factor due to substitution of a single tyrosine residue with phenylalanine (h9Rphe116). This residue is known to be phosphorylated by JAK tyrosine kinases and to serve as a docking site for STAT transcription factors.14 Both tranfectants, but not the parental cells, were able to proliferate in response to human IL-9 (data not shown). As shown in Fig 6A, hIL-9 activated STAT transcription factors (STAT-1, -3, -5) only in cells expressing the wild-type human IL-9R, and not in cells expressing the phenylalanine mutant. The expression of M-Ras was induced by hIL-9 only in cells expressing the wild-type receptor (Fig 6B). These data suggest that STAT activation is required for M-Ras upregulation.
IL-9–induced M-Ras expression in TS2 cells expressing wild-type or mutant hIL-9R. (A) Electromobility shift assay using a STAT-binding oligonucleotide. TS2 transfectants were stimulated for 10 minutes with IL-2, mIL-9, or hIL-9 (which does not bind the endogenous murine receptor) and nuclear extracts were prepared, and the electromobility shift assay was performed with the GRR oligonucleotide as described in Materials and Methods. (B) Northern blot analysis of M-Ras expression. The same cells were cultured for 48 hours in the presence of 200 U/mL of either IL-2 or murine IL-9, or 500 U/mL of human IL-9. Northern blots were prepared with 10 μg of total RNA and hybridized as described in Fig 1.
IL-9–induced M-Ras expression in TS2 cells expressing wild-type or mutant hIL-9R. (A) Electromobility shift assay using a STAT-binding oligonucleotide. TS2 transfectants were stimulated for 10 minutes with IL-2, mIL-9, or hIL-9 (which does not bind the endogenous murine receptor) and nuclear extracts were prepared, and the electromobility shift assay was performed with the GRR oligonucleotide as described in Materials and Methods. (B) Northern blot analysis of M-Ras expression. The same cells were cultured for 48 hours in the presence of 200 U/mL of either IL-2 or murine IL-9, or 500 U/mL of human IL-9. Northern blots were prepared with 10 μg of total RNA and hybridized as described in Fig 1.
IL-3 but not IL-9 induces activation of M-Ras.
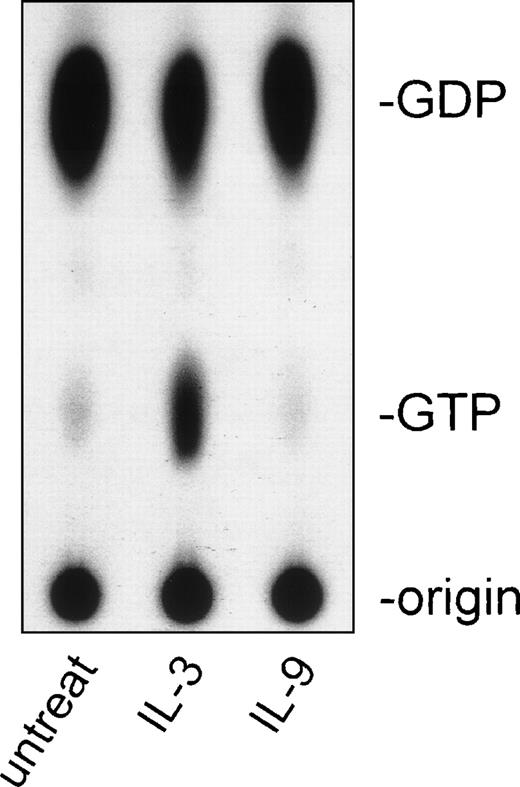
Binding of cytokines such as IL-2 or IL-3 to their receptors has been shown to cause activation of Ras-p21. To study whether IL-9 could also trigger activation of M-Ras, BaF-3 cells constitutively expressing wild-type M-Ras were further transfected with the human IL-9 receptor. Cells were starved in serum-free medium before stimulation with mIL-3 or human (h) IL-9, M-Ras was immunoprecipitated, and the GTP/GDP binding was analyzed by thin-layer chromatography. Figure 7 shows that IL-9 is unable to activate M-Ras while IL-3 stimulation increased the amount of GTP-bound M-Ras, thus showing that IL-9 does not use this Ras pathway to mediate signaling in downstream effector cells. These data are concordant with the finding that IL-9 does not elicit activation of Ras-p21 in lymphoid cells (Grasso L, Demoulin JB, Atkins JM, Louahed J, Renauld JC, Levitt RC, Nicolaides NC: submitted for publication) and suggests a potential synergy between IL-9 and IL-3, where IL-9 induces the expression of M-Ras, which in turn can be activated by another growth factor.
Activation of M-Ras by IL-3 but not by IL-9. BaF3 cells constitutively expressing the hIL-9 receptor and wild-type M-Ras were cytokine-starved 8 hours in serum-free medium before stimulation with 500 U/mL of either hIL-9 or 3 ng/mL of mIL-3. Thin-layer chromatography of in vivo 32P-labeled GDP and GTP was perfomed after immunoprecipitation of M-Ras. The result is representative of 3 independent experiments. untreat, untreated cells.
Activation of M-Ras by IL-3 but not by IL-9. BaF3 cells constitutively expressing the hIL-9 receptor and wild-type M-Ras were cytokine-starved 8 hours in serum-free medium before stimulation with 500 U/mL of either hIL-9 or 3 ng/mL of mIL-3. Thin-layer chromatography of in vivo 32P-labeled GDP and GTP was perfomed after immunoprecipitation of M-Ras. The result is representative of 3 independent experiments. untreat, untreated cells.
M-Ras activates the MAPKinase pathway.
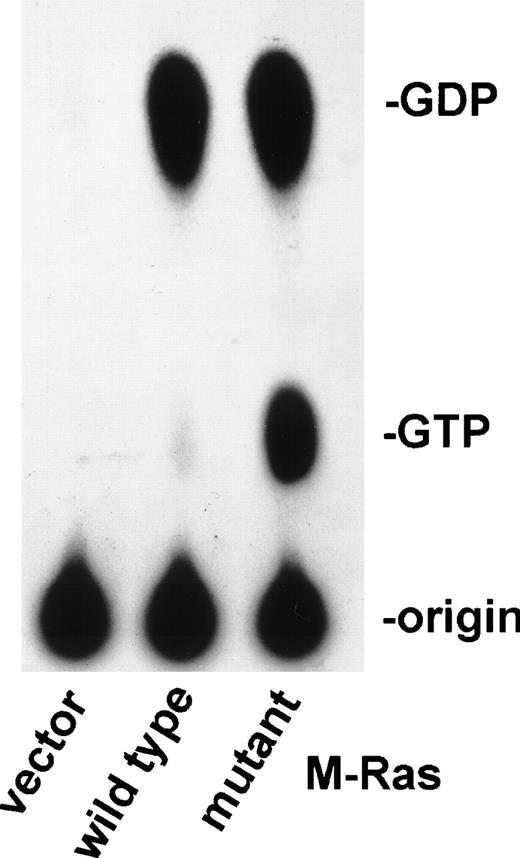
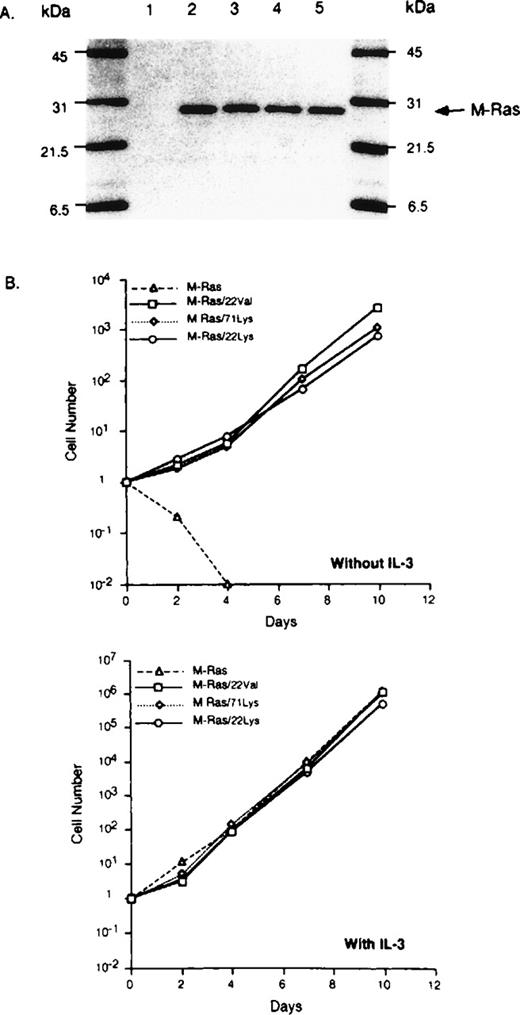
To further elucidate the biological role of M-Ras in lymphoid cells, we generated murine cDNAs carrying the oncogenic mutations on codons 22 or 71. The M-Ras Gly 22 was replaced by a valine (M-Ras/22V) or a lysine (M-Ras/22K), and M-Ras Glu 71 was replaced by a lysine (M-Ras/71K). The corresponding mutants of p21 H-Ras have been shown to persist in the GTP-bound form, which is the active form of Ras.28 Figure 8 shows a thin-layer chromatography of in vivo 32P-labeled GDP and GTP eluted from M-Ras immunoprecipitated from BaF3 transfected cells. As expected, the dominant active form of M-Ras bound more GTP than the wild-type M-Ras.
Constitutive active M-Ras mutants bind GTP. Thin-layer chromatography of in vivo 32P-labeled GDP and GTP eluted from M-Ras immunoprecipitated from BAF-3 cells transfected with the indicated experimental plasmids encoding the wild-type or activated mutant M-Ras/22 Val. Same results were obtained with M-Ras/71Lys and M-Ras/22Lys.
Constitutive active M-Ras mutants bind GTP. Thin-layer chromatography of in vivo 32P-labeled GDP and GTP eluted from M-Ras immunoprecipitated from BAF-3 cells transfected with the indicated experimental plasmids encoding the wild-type or activated mutant M-Ras/22 Val. Same results were obtained with M-Ras/71Lys and M-Ras/22Lys.
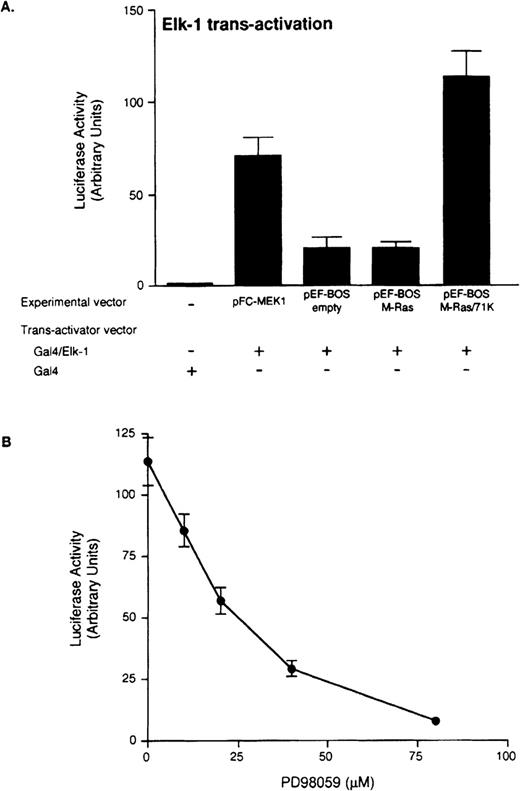
To gain a further understanding on the signal transduction pathway of M-Ras, we tested the ability of our mutants to activate the MAPK in an in vivo signal transduction pathway reporting system. This system allows for the indirect measurement of the phosphorylation of a chimeric transcriptional trans-activator consisting of the GAL4 DNA binding domain fused to the activation domain of Elk (target of the MAPK pathway; pFA-Elk). HEK293 cells were cotransfected with the following 3 constructs: (1) a luciferase reporter gene (pFR-Luc) controlled by a promoter containing 4 copies of a GAL4 binding motif, (2) a plasmid inducing expression of the fusion protein mentioned above, (3) a plasmid encoding the wild-type or mutated M-Ras. Lysates of cells coexpressing GAL4-Elk fusion protein and the constitutively activated MEK1 (used as positive control) contained high luciferase activity (Fig 9A). Similarly, transfection with mutant M-Ras/71K resulted in a 5.5-fold increase of Elk-mediated transcription, compared with the empty vector or wild-type M-Ras. The M-Ras–mediated transcriptional activation of Elk was blocked by the MEK1 inhibitor PD9805929 (Fig 9B), while activation of the JNK pathway by constitutively activated MEKK was unaffected even at the highest concentration tested (80 μmol/L) (data not shown).
Activated M-Ras protein induces Elk-1–mediated transcriptional activation. (A) HEK293 cells (5 × 105) were transiently cotransfected by lipofection with 500 ng of pFR-Luc reporter plasmid, 500 ng of the indicated experimental plasmids encoding the wild-type or activated mutants M-Ras, and 25 ng of the pathway-specific trans-activator plasmids (Gal4/Elk-1). pFC-MEK1 plasmid encodes constitutively activated MEK1 and was used as a positive control for Elk-1–trans-activation, and pFC-dbd as a negative control (Gal4). Luciferase activity was determined 24 hours after transfection. Data represent the means ± SD from an experiment performed in triplicate. The experiment was repeated 3 times with similar results. (B) HEK293 cells (5 × 105) were cotransfected with the reporter plasmid, pFR-Luc, the trans-activator plasmid pFA-Elk-1 and an expression vector encoding activated M-Ras/71K. After 5 hours of transfection, increasing amounts of PD98059 were added to the cell cultures and luciferase activity was measured after 24 hours. Similar results were obtained with other activated M-Ras mutants (data not shown). As a toxicity control, HEK293 cells were transfected with the reporter plasmid, pFR-Luc, the trans-activator plasmid pFA-Jun, and an expression vector encoding activated MEKK with or without PD98059, and no inhibition was observed (data not shown).
Activated M-Ras protein induces Elk-1–mediated transcriptional activation. (A) HEK293 cells (5 × 105) were transiently cotransfected by lipofection with 500 ng of pFR-Luc reporter plasmid, 500 ng of the indicated experimental plasmids encoding the wild-type or activated mutants M-Ras, and 25 ng of the pathway-specific trans-activator plasmids (Gal4/Elk-1). pFC-MEK1 plasmid encodes constitutively activated MEK1 and was used as a positive control for Elk-1–trans-activation, and pFC-dbd as a negative control (Gal4). Luciferase activity was determined 24 hours after transfection. Data represent the means ± SD from an experiment performed in triplicate. The experiment was repeated 3 times with similar results. (B) HEK293 cells (5 × 105) were cotransfected with the reporter plasmid, pFR-Luc, the trans-activator plasmid pFA-Elk-1 and an expression vector encoding activated M-Ras/71K. After 5 hours of transfection, increasing amounts of PD98059 were added to the cell cultures and luciferase activity was measured after 24 hours. Similar results were obtained with other activated M-Ras mutants (data not shown). As a toxicity control, HEK293 cells were transfected with the reporter plasmid, pFR-Luc, the trans-activator plasmid pFA-Jun, and an expression vector encoding activated MEKK with or without PD98059, and no inhibition was observed (data not shown).
Activated M-Ras induces IL-3–independent proliferation of the mouse pro-B lymphocyte cell line BaF3.
The M-Ras mutants were tested for their ability to support growth factor–independent cell proliferation by transfecting BaF3 cells with wild-type or dominant active forms of M-Ras. After selection in puromycin, Western blot analysis of transfected BaF3 cells confirmed comparable but elevated levels of M-Ras, while no expression was detected in the parental, untransfected BaF3 cells (Fig 10A). Parental BaF3 cells undergo apoptosis in absence of IL-3 (data not shown). Overexpression of wild-type M-Ras did not affect survival or proliferation of the transfectant cells (Fig 10B). By contrast, M-Ras/22V, M-Ras/22K, or M-Ras/71K-transfected BaF3 cells continued to proliferate in the absence of IL-3. This proliferation does not appear to result from an autocrine loop due to the activation of the IL-3 gene by M-Ras, because the supernatant of M-Ras–transfected cells did not induce proliferation of the parental BaF3 cells (data not shown). In addition, IL-3 further increased the proliferation rate of BaF3 cells expressing activated M-Ras, probably reflecting the fact that IL-3 induces an additional growth-promoting pathway.30
Activated M-Ras induces cytokine-independent proliferation. (A) BaF-3 transfected cells with empty vector (lane 1), wild-type M-Ras (lane 2), constitutively activated M-Ras/22Val (lane 3), M-Ras/22Lys (lane 4), or M-Ras/71Lys (lane 5) were lysed and analyzed by SDS-PAGE (14% acrylamide, 2 × 105 cells per lane). M-Ras proteins were detected by immunoblotting. (B) Transfected BaF3 cells (105 per well) were cultured with or without IL-3 (200 U/mL). Cells were counted and sequentially diluted as appropriate. The results represent the mean of 2 separate cultures that varied by less than 10%.
Activated M-Ras induces cytokine-independent proliferation. (A) BaF-3 transfected cells with empty vector (lane 1), wild-type M-Ras (lane 2), constitutively activated M-Ras/22Val (lane 3), M-Ras/22Lys (lane 4), or M-Ras/71Lys (lane 5) were lysed and analyzed by SDS-PAGE (14% acrylamide, 2 × 105 cells per lane). M-Ras proteins were detected by immunoblotting. (B) Transfected BaF3 cells (105 per well) were cultured with or without IL-3 (200 U/mL). Cells were counted and sequentially diluted as appropriate. The results represent the mean of 2 separate cultures that varied by less than 10%.
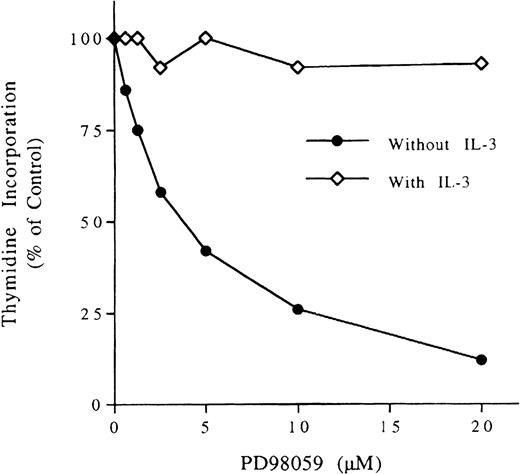
To assess whether the MAPK pathway, which is induced by constitutive M-RAS mutants in HEK293 cells (Fig 9), was responsible for this M-Ras–induced proliferation, we treated BaF3 cells expressing M-Ras/71K with the MEK inhibitor PD98059. As shown in Fig 11, the proliferation induced by activated M-Ras was reduced to 12% by 20 μmol/L of PD98059 compared with the untreated control. These data suggest that MAPK activation is required for M-Ras–induced proliferation of BaF3 cells. By contrast, in the presence of IL-3, the growth of these BaF3 cells was maintained at about 90% of control, thus showing that the effect of PD98059 on M-Ras–dependent proliferation was not due to nonspecific cytotoxicity (Fig 11) and confirming that, in BaF3 cells, the MAPK pathway is dispensable for IL-3–driven proliferation.30 The observation that active M-Ras promoted survival and proliferation of IL-3–starved pro-B lymphocytes is suggestive of the oncogenic activity of these M-Ras mutations.
Inhibition of M-Ras–induced proliferation of BaF3 by MAP kinase inhibitor. BaF3 cells expressing constitutively activated M-Ras/71K (3,000/well) were incubated with increasing concentrations of PD98059 in the presence or absence of 200 U/mL of IL-3. Thymidine incorporation was measured after 3 days. The results are presented as the percentage of the proliferation without PD98059 (means of triplicate wells). The same results were obtained with other activated M-Ras mutants (data not shown).
Inhibition of M-Ras–induced proliferation of BaF3 by MAP kinase inhibitor. BaF3 cells expressing constitutively activated M-Ras/71K (3,000/well) were incubated with increasing concentrations of PD98059 in the presence or absence of 200 U/mL of IL-3. Thymidine incorporation was measured after 3 days. The results are presented as the percentage of the proliferation without PD98059 (means of triplicate wells). The same results were obtained with other activated M-Ras mutants (data not shown).
DISCUSSION
In an attempt to identify genes selectively induced by IL-9 in mouse T-helper–cell clones, we found that IL-9 upregulates the expression of M-Ras, the newest member of the Ras superfamily. This gene was cloned independently by 2 other groups on the basis of sequence homologies within this family of proteins and designated M-Ras or R-Ras3.23,24 Interestingly, this induction by IL-9 seems to depend on the activation of STAT transcription factors because a mutated IL-9 receptor, which lacks STAT activity but is able to induce proliferation and activation of various kinases (JAK-1, JAK-3, PI-3 kinase, etc),14 failed to mediate IL-9–induced M-Ras gene expression. This observation, together with the rapid induction in response to IL-9, suggests that M-Ras is regulated at the transcriptional level. Because IL-2 activates STAT-5, and IL-9 activates STAT-1, -3, and -5, it is most likely that M-Ras gene induction results from the activation of STAT-1 and/or STAT-3, as recently observed for other IL-9–induced genes such as Granzyme A and Ly6A2.31
Contrasting with the potent induction of M-Ras expression by IL-9, this cytokine failed to induce M-Ras activation, as measured by its GTP/GDP binding state. This suggests that M-Ras is not a direct component of IL-9–dependent signal transduction pathways. However, we show here that IL-3, a cytokine known to synergize with IL-9,12 is a potent activator of M-Ras. Taken together, these observations point to a role for IL-9 as competence factor, upregulating the ability of effector cells to respond to Ras-activating cytokines. Interestingly, other T-cell activators, such as Concanavalin A, similarly upregulate M-Ras expression in mouse spleen cells, indicating that M-Ras upregulation is not limited to the IL-9 response but takes part in various T-cell activation processes.
M-Ras is closely related to R-Ras, TC21, H-Ras, K-Ras, and N-Ras, and can therefore be considered as a member of the “true” Ras subfamily. Members of the Ras subfamily are thought to control cell growth, apoptosis, and transformation. Here, we studied the effect of M-Ras activation on proliferation of BaF3 cells transfected with constitutively activated mutants of M-Ras. Activated M-Ras was found to mediate cytokine-independent survival and proliferation of the cells, a result similar to the one reported for activated R-Ras that prevented cell death of BaF3 cells caused by IL-3 deprivation.32However, the activity of R-Ras in these cells was dependent on the presence of serum or IGF-1, while, in our hands, M-Ras induced cell survival even in the absence of serum (data not shown). The ability of activated M-Ras to support the proliferation of BaF3 cells is likely to be mediated by the MAPK pathway. A transient expression system using HEK293 cells showed that M-Ras activates the MAPK pathway via the activation of c-Raf (data not shown), which eventually results in Elk-dependent transcriptional activation. Moreover, the M-Ras–induced proliferation of BaF3 was blocked by the MEK inhibitor PD98059, indicating that this function of M-Ras requires MAPK activity. This is in agreement with the previous finding that activation of the Raf/MAPK pathway in IL-3–dependent cells by expression of an oncogenic Raf or a Ras mutant prevented apoptosis following IL-3 deprivation.33 The fact that aberrant M-Ras activation can cause malignant transformation suggests that deregulation of M-Ras function may also contribute to spontaneous malignancies, particularly in IL-9 transgenic mice. In these mice, constitutive IL-9 overexpression results in a high susceptibility to the development of T-cell lymphomas upon exposure to low doses of a chemical mutagen, which was reported to induce Ras mutations.4 Interestingly, the fact that M-Ras expression was also found in mitotically quiescent tissues such as brain and kidney suggests that M-Ras plays a role in other physiological processes as well.
To our knowledge, it is the first time that a cytokine has been shown to induce the expression of a member of the Ras superfamily. In contrast to H-, N-, or R-Ras, which are constitutively expressed in TS2 cells (data not shown), M-Ras is only expressed upon IL-9 stimulation. Although IL-9 by itself cannot activate M-Ras, our observations illustrate a new cross-talk mechanism between JAK/STAT and MAPK-dependent pathways, which might explain, for instance, some of the activities of IL-9 in synergy with other cytokines such as IL-3 or stem cell factor.
ACKNOWLEDGMENT
The authors thank Drs S. Nagata, M. Gueguen, and A. Burgess for their generous donations of reagents; Dr V. Stroobant for peptide synthesis; and Dr M. Lackmann for helpful discussions and critical reading of the manuscript.
Supported in part by the Belgian Federal Service for Scientific, technical and cultural affairs and the Opération Télévie. J.L. is a scientific associate (Télévie) and J.-C.R. a research associate with the Fonds National de la Recherche Scientifique, Belgium.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jean-Christophe Renauld, MD, Ludwig Institute for Cancer Research, Avenue Hippocrate, 74, UCL 7459, B-1200 Brussels, Belgium; e-mail: renauld@licr.ucl.ac.be.

![Fig. 4. Tissue distribution of mRNA for mouse M-Ras. (A) Northern blot of a multiple tissue [2 μg of mouse poly(A)+ RNA per lane] (Clontech) was hybridized with a 32P-labeled mouse M-Ras cDNA probe. The size of the mRNAs detected are indicated to the left of the panel. (B) Northern blots containing 10 μg per lane of total RNA from adult tissues of FVB mice or IL-9–transgenic mice (Tg5) were hybridized with a 32P-labeled mouse M-Ras cDNA probe. Ethidium bromide staining of the gel is shown as control for loading.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1701/5/m_blod41729004w.jpeg?Expires=1765924471&Signature=LXji4eIvS9wsFnlLixroOdNmCM7hkkyjpotjdJ0ha3kW59PSCQMpv4y8qqC3QTzKx~K29dRngecva5~~a4709wJ4t~zt36cCg1vJsmNHs7c~Vw-D7~QybcRskuf0E~yD2lPh8VQoLesD9NJIzPhDXee1iZEmfobvu~Smpw2IWw1LTCjq-jTMxC3rpoc6qUQbx~txqAj55xAA9NKfpDYl04Ir-Tv9xMvwiV5ICN8R0B-JtYuZlxerYfjUEo-wGJC5UwFdC6lFXZYdRH6LUITOIkupUFZgo9AckUrB8o-3D91FylKcR8FXisuhMw7kUeq7~5S3TCUg4P8NrXxqGs5eog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal