In vitro maintenance and proliferation of human hematopoietic stem cells is crucial for many clinical applications. Early hematopoietic cells express low levels of FLT-3 and c-kit receptors, as well as the interleukin-6 (IL-6) receptor signal transducing element, gp130, but do not express IL-6 receptor itself. Therefore, we have attempted to maintain human cord blood or bone marrow CD34+ cells ex vivo in serum-free cultures containing stem cell factor (SCF) and FLT-3 ligand (FL) alone or together with a new recombinant molecule of soluble IL-6 receptor fused to IL-6 (IL6RIL6 chimera). The effect of IL6RIL6 chimera on the proliferation and differentiation of CD34+ cells was compared with that of each chimera component added separately. The engraftment potential of in vitro-cultured cells was determined using our recently established functional in vivo assay for primitive human severe combined immunodeficiency (SCID)-repopulating cells (SRC). We report here that IL6RIL6 chimera induced significantly higher levels of progenitors and SRC compared with SCF + FL alone or together with IL-6 and soluble IL-6 receptor. IL6RIL6 chimera prolonged in vitro maintenance of SRC for up to 14 days. Stimulation of CD34+CD38−/low enriched cells with IL6RIL6 chimera maintained the early CD34+CD38−/lowcell subpopulation, which could be detected in vitro for up to 14 days. Moreover, IL6RIL6 chimera preferentially stimulated the growth of early CD34+38−/low cells, resulting in significantly higher levels of progenitors compared with more mature CD34+38+ cells. Taken together, these findings demonstrate the importance of IL6RIL6 chimera in stimulating the proliferation of early CD34+· CD38−gp130+IL-6R−cells in vitro and extended maintenance of progenitors and SRC.
HEMATOPOIETIC stem cells continuously produce all mature blood cells by extensive proliferation and multilineage differentiation. Human hematopoietic CD34+ or CD34+CD38− cells, which are enriched for stem cells, play a major role in the long-term bone marrow (BM) reconstitution of ablated patients in autologous and allogeneic transplantations. BM transplantation and other clinical applications, such as purging of tumor cells and gene therapy, involve in vitro maintenance of CD34+ cells. Thus, many studies are focused on defining appropriate conditions for culturing CD34+cells in vitro, with particular emphasis on their engraftment and repopulation potential. Several groups have used human and murine stromal cells to support the maintenance in vitro of CD34+or CD34+CD38− cells.1-3Others replaced the stromal cells and their soluble secreted factors with recombinant human cytokines to produce a microenvironment capable of maintaining the primitive cells.4-8
The early acting cytokines stem cell factor (SCF), FLT-3 ligand (FL), and interleukin-6 (IL-6) are commonly used to maintain hematopoietic stem cells in vitro. SCF and FL were shown to improve survival and maintenance of hematopoietic progenitors.9,10 The addition of IL-3 resulted in the expansion of human long-term culture-initiating cells (LTC-IC) in vitro.5,11 IL-6 is a potent cofactor for the survival and proliferation of primitive multilineage progenitor cells in liquid cultures in vitro.12,13 Moreover, defective hematopoiesis in IL-6–deficient mice indicates that IL-6 plays such a role in vivo as well.14 IL-6 acts on cells through a receptor system comprising 2 proteins, IL-6 receptor (IL-6R, gp80) and gp130.15,16 Signal transduction is solely due to dimerization of gp13017 upon formation of a hexameric complex of 2 gp130, 2 IL-6R, and 2 IL-6 ligands.18-20Soluble forms of IL-6R (sIL-6R) are produced by cells and are found in blood and urine.21,22 These sIL-6R act as a potent agonists of IL-6 on many cell types,15,23 because they retain the ability to induce IL-6–dependent gp130 dimerization. Stimulation of human cord blood (CB) CD34+ cells by adding sIL-6R together with IL-6 to SCF24 or to FL25 led to marked in vitro stimulation of multilineage progenitors, followed by their expansion. CD34+ cells expressing gp130 but not IL-6R represent a more primitive cell population enriched for LTC-IC that can be stimulated when both sIL-6R and IL-6 are added to SCF and IL-3.26,27 By fusion of sIL-6R to IL-6, chimeric IL6RIL6 proteins were obtained that have a higher activity than the mixture of IL-6 + sIL-6R for in vitro expansion of early hematopoietic progenitors when added to SCF + IL-328 or to SCF + FL, as we recently showed.29
In previous studies, primitive human stem cells could not be maintained in vitro successfully for long periods of time due to differentiation. In addition, the repopulating potential of cultured primitive cells cannot be assayed in vitro. These difficulties led to the establishment of several animal models, thus facilitating the study of human stem cells development and biology.30-32 We have previously described a functional in vivo assay for primitive human hematopoietic cells based on their ability to repopulate the BM of sublethally irradiated SCID mice homozygous for the severe combined immunodeficiency Prkdcscid mutation and more recently nonobese diabetic (NOD)/SCID mice, after intravenous transplantation. We have defined the engrafting human cell as an SCID-repopulating cell (SRC).33,34 Purification assays demonstrated that SRC are phenotypically characterized as CD34+CD38− cells.34 Recent studies provide evidence that CD34−CD38− cells35 and CD34+CD38+6 cells also have limited engraftment potential. Kinetic studies showed that only a small fraction of the transplanted cells engraft and repopulate the murine BM by extensive proliferation and multilineage differentiation.36
Previous results with a cytokine cocktail that included IL-6, SCF, and FL quantified in 2 similar model systems showed a 2-fold increase in the levels of SRC after 4 days7 or competitive repopulating units (CRU) after 5 to 8 days6 of in vitro stimulation. However, on day 9, in vitro analysis demonstrated the loss of CD34+CD38− cells, and SRC could not be detected.7
In the present study, we investigated the potential of IL6RIL6 chimera to maintain and expand ex vivo SRC capable of repopulating the BM of NOD/SCID mice.
We describe here stroma-free culture conditions for human SRC maintenance in ex vivo cultures established for up to 14 days while maintaining and enhancing their repopulating potential. IL6RIL6 chimera increased the levels of progenitors and SRC by acting mainly on the primitive CD38−/low subpopulation of CD34+ cells. We suggest an important role for IL6RIL6 chimera in stimulating the proliferation of early human CD34+· CD38−/lowgp130+IL-6R−cells in vitro, resulting in extended maintenance of progenitors and SRC.
MATERIALS AND METHODS
Human cells preparation.
Human CB samples were obtained from full-term deliveries after informed consent was obtained. Human BM cells were obtained from harvests of normal donors for allogeneic transplantation after informed consent. Human cells were used in accordance with the procedures approved by the human experimentation and ethics committees of the Weizmann Institute (Rehovot, Israel). The blood samples were diluted 1:1 in phosphate-buffered saline (PBS) supplemented with 10% fetal calf serum (FCS), without Mg2+/Ca2+. Low-density mononuclear cells (MNC) were collected after standard separation on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) and washed in RPMI with 1% FCS. Some samples were frozen in 10% dimethyl sulfoxide (DMSO), whereas the others were used fresh. Enrichment of CD34+ cells was performed with mini MACS separation kits (Miltnyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. The purity of the enriched CD34+ cells was 60% to 80% using 1 column and greater than 97% when cells were passed over 2 columns. CD34+CD38− enrichment was performed either by a StemSep kit (StemCell Technologies Inc, Vancouver, British Columbia, Canada) according to the manufacturer’s instructions or by fluorescence-activated cell sorting (FACS; FACStar+; Becton Dickinson, San Jose, CA) after staining with monoclonal antibody (MoAb) antihuman CD34-fluorescein isothiocyanate (FITC; Becton Dickinson) and antihuman CD38-phycoerythrin (PE; Coulter, Miami, FL). The purity of the obtained subpopulations was 55% to 65% (StemSep) and greater than 99% (FACS), respectively.
Mice.
NOD/SCID mice were bred and maintained under defined flora conditions at the Weizmann Institute in sterile micro-isolator cages. All of the experiments were approved by the animal care committee of the Weizmann Institute. Sublethally irradiated (375 cGy, at 67 cGy/min), 8-week-old mice were transplanted with human cells as previously described,33 34 with minor modifications. Briefly, human cells were injected into the tail vein of irradiated mice in 0.5 mL of RPMI with 10% FCS. Nonengrafting CD34− carrier cells were irradiated (1,500 cGy) and were cotransplanted with cultured cells at a final concentration of 0.5 × 106 cells/mouse. Mice were killed 1 month after transplantation, and BM cells were flushed from the 8 bones of each mouse (femures, tibias, humeri, and pelvis).
Ex vivo cultures.
Human CD34+ enriched cells were cultured in 24-well plates (1 to 2 × 105 in 0.5 mL) containing 10% FCS and 1% bovine serum albumin (BSA; Sigma, St Louis, MO) or in serum-free media composed of IMDM, 2% BSA, 20 μg/mL human insulin (Biological Industries, Beit Haemek, Israel), 40 μg/mL human low-density lipoprotein (LDL) (Sigma), 200 μg/mL human transferrin (Sigma), 10−4 mol/L 2-mercaptoethanol (2ME), and 10 mmol/L HEPES buffer (pH 7.3). CD34+CD38−/low cells were cultured in serum-free media in 96-well plates. Ex vivo cultures contained combinations of the following cytokines: SCF at 100 ng/mL, FL at 100 ng/mL, IL-15 at 100 ng/mL (R&D Systems Inc, Minneapolis, MN), recombinant human IL-6 (rhIL-6) at 50 ng/mL, and sIL-6R at 1,280 ng/mL (InterPharm Laboratories, The Ares-Serono Group, Ness Ziona, Israel).29 The fused protein of soluble IL-6 receptor and IL-6 (IL6RIL6 chimera) was produced as described29 and used at 150 ng/mL. For natural killer (NK) cell development, BM cells from engrafted mice were cultured with serum-free media that contained 100 ng/mL of SCF and IL-15 (R&D) for 10 days. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Colony-forming unit (CFU) assay.
To detect the levels of human progenitors after cytokine stimulation in ex vivo cultures and in the marrow of transplanted mice, semisolid cultures were performed as previously described.33 34 In brief, the cells were plated in 0.9% methylcellulose (Sigma), 30% FCS, 5 × 10−5 mol/L 2ME, 50 ng/mL SCF, 5 ng/mL IL-3, 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D), and 2 U/mL erythropoietin (Orto Bio Tech, Don Mills, Ontario, Canada). Human progenitors from engrafted mice were plated in 15% FCS + 15% human plasma together with the human cytokines listed above. These conditions are selective for human colonies. Plating concentrations were as follows: for enriched CD34+ cells, 4 × 103 cells/mL; for CD34+CD38−/low cells, 800 cells/mL; and for BM cells from transplanted mice, 2 × 105cells/mL. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 and scored 14 days later for myeloid, erythroid, mixed, and blast colonies by morphologic criteria.
Flow cytometry analyses.
The purity of enriched subpopulations after magnetic beads separation was analyzed using two-color staining using MoAb antihuman CD34-FITC (Becton Dickinson) and antihuman CD38-PE (Coulter). The levels of human cells in the marrow of engrafted mice were detected by staining with MoAb antihuman CD45-FITC (Immuno Quality Products, Groningen, The Netherlands). Human pre-B cells were detected by double staining with MoAb antihuman CD45-FITC/CD19-PE, and NK cells were detected by antihuman CD45-FITC/CD56-PE (Coulter). Analysis of human gp130 was performed using anti gp130 (AM64; Pharmingen, San Diego, CA) and MoAb anti–IL-6 receptor as previously described.37 Human Fc receptors were blocked using human plasma (1:50) and murine Fc receptors by antimouse CD16/CD32 MoAb (Pharmingen). Isotype control antibodies were used to exclude false-positive cells (Coulter). BM cells from irradiated mice were used as a negative control, and human cells were used as a positive control. Dead cells were gated out by staining with propidium iodide (Sigma). Cells were washed with PBS supplemented with 1% FCS and 0.02% Azide, suspended to a volume of 1 to 5 × 105 cells/mL, stained with direct labeled MoAb, and incubated for 25 minutes on ice. After staining, cells were washed once in the same buffer and analyzed on a FACSort (Becton Dickinson). Analysis was performed using CELLquest software (Becton Dickinson).
Human DNA analysis.
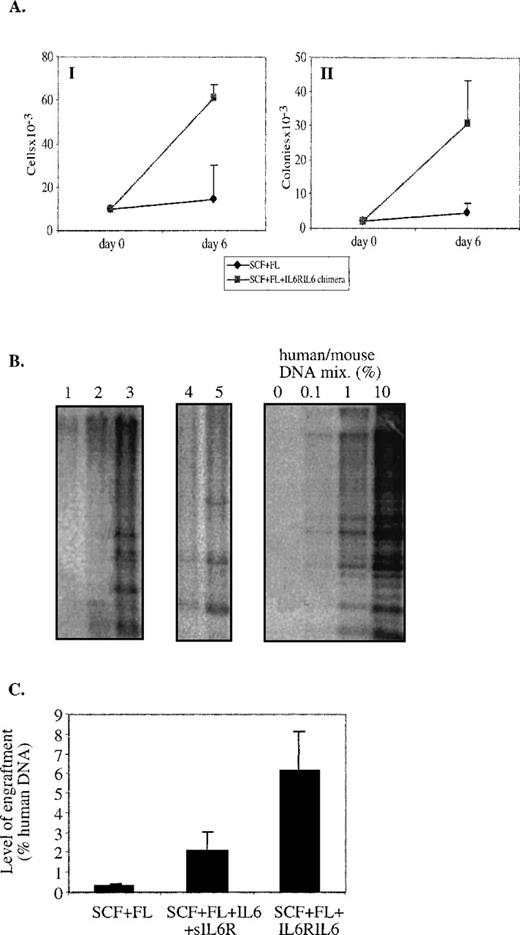
The levels of human cell engraftment were detected as previously described.33 34 Briefly, high molecular weight DNA was obtained from the BM of transplanted mice by phenol/chloroform extraction. DNA (5 μg) was digested with EcoRI, subjected to electrophoresis on 0.6% agarose gel, blotted onto a nylon membrane, and hybridized with a human chromosome 17-specific α-satellite probe (p17H8) labeled with 32P. After a random digestion withEcoRI, this probe hybridizes a characteristic multisize band pattern that is specific for human DNA. For quantification of the human DNA in the samples, the intensity was compared with that in artificial mixtures of human and mouse DNA (0%, 0.1%, 1%, and 10% human DNA) run in parallel lanes. Multiple exposures of the autoradiographs were taken to ensure sensitivity down to 0.01% human DNA.
Binding of IL6RIL6 chimeric molecule to gp130.
Monoclonal antihuman gp 130 AM64 (2 μg/mL; Pharmingen) was added in a 96-well microplate to 0.1 mL/well of PBS. After 18 hours at 4°C, the plate was washed and blocked with 1% BSA in PBS (0.1 mL/well) for 3 hours. Soluble gp130 (50 ng/mL; R&D) was added at 0.1 mL/well in PBS for 2 hours at 20°C. After washing 3 times with 0.05% Tween-20 in PBS, 0.1 mL of IL-6 (500 ng/mL) with increasing amounts of sIL-6R (from 4 to 500 ng/mL) were added for 2 hours at 20°C. In other wells, the IL6RIL6 chimeric molecule was similarly added (from 0.1 to 50 ng/mL). After washing, the bound sIL-6R was quantitated by sandwich enzyme-linked immunosorbent assay (ELISA) with polyclonal anti–sIL-6R rabbit serum and goat antirabbit Ig-horseradish peroxidase conjugate.22 Calculations of molar concentrations are based on molecular weights of 60 kD for sIL-6R and 85 kD for IL6RIL6 produced as glycoproteins in CHO cells.29
RESULTS
Proliferation and differentiation of human CD34+ cells cultured ex vivo with SCF, FL, and IL6RIL6 chimera.
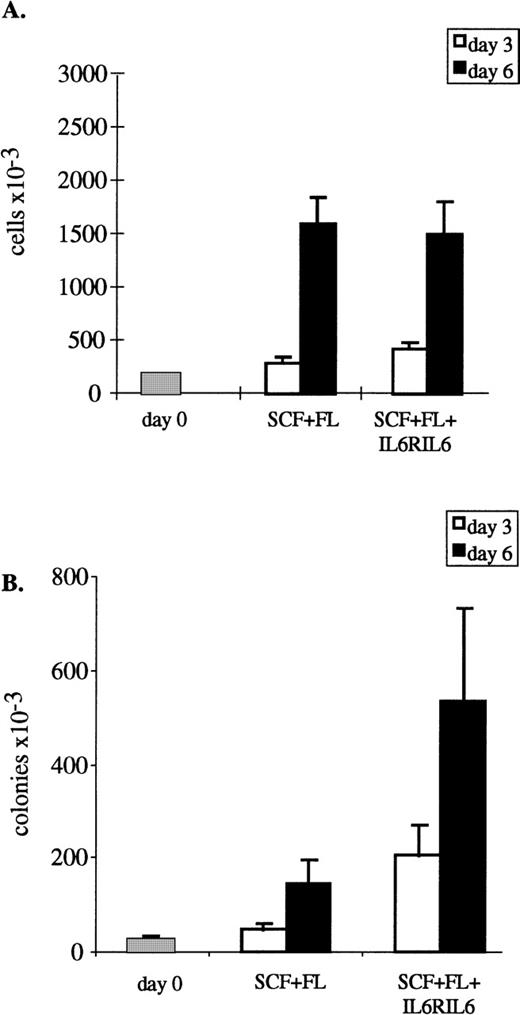
In the first experiments, human CB CD34+ cells were cultured in vitro for 3 or 6 days with SCF and FL to which the IL6RIL6 chimera was added (Fig 1A). On day 3, there was only a small increase in cell numbers compared with day 0, but on day 6 there was a 7.5- to 10-fold increase that did not vary significantly whether SCF and FL were present alone or supplemented with IL6RIL6 chimera. Similar results were obtained with media containing 10% FCS or with serum-free media. The proliferation of cultured CD34+ cells is, therefore, due mainly to SCF and FL.
Total cell numbers and progenitor levels in ex vivo-cultured CD34+ cells. CB CD34+enriched cells were cultured (2 × 105 cells/0.5 mL) in RPMI + 10% FCS + 1% BSA or in serum-free media, both supplemented with cytokines, for 3 or 6 days. Cytokines were used in the following concentrations: SCF at 100 ng/mL, FL at 100 ng/mL, and IL6RIL6 chimera at 150 ng/mL. (A) Cells were counted for viable cell numbers. (B) Ex vivo-cultured cells were seeded (4 × 103 cells/mL) into semisolid media, and colonies were scored on day 14. Progenitor levels were calculated on the basis of total cell numbers. Values shown are the mean ± SE from 10 independent experiments.
Total cell numbers and progenitor levels in ex vivo-cultured CD34+ cells. CB CD34+enriched cells were cultured (2 × 105 cells/0.5 mL) in RPMI + 10% FCS + 1% BSA or in serum-free media, both supplemented with cytokines, for 3 or 6 days. Cytokines were used in the following concentrations: SCF at 100 ng/mL, FL at 100 ng/mL, and IL6RIL6 chimera at 150 ng/mL. (A) Cells were counted for viable cell numbers. (B) Ex vivo-cultured cells were seeded (4 × 103 cells/mL) into semisolid media, and colonies were scored on day 14. Progenitor levels were calculated on the basis of total cell numbers. Values shown are the mean ± SE from 10 independent experiments.
Different results were obtained when the expansion of clonogenic progenitors was determined by plating the ex vivo-cultured CD34+ cells in semisolid media (Fig 1B). Cells that had been cultured in suspension for 3 days with SCF + FL alone had a moderate 2-fold increase in the levels of colony-forming progenitors compared with day 0; however, the increase averaged 8-fold when IL6RIL6 chimera was added. When CD34+ cells were cultured for 6 days, the increase was 5.8-fold with SCF + FL and 21-fold when IL6RIL6 was added. Taken together, IL6RIL6 chimera increased significantly the levels of progenitors compared with SCF + FL alone after 3 and 6 days of culture (P = .004 and P = .03, respectively).
Stimulation of SRC by the addition of IL6RIL6 chimera to SCF and FL.
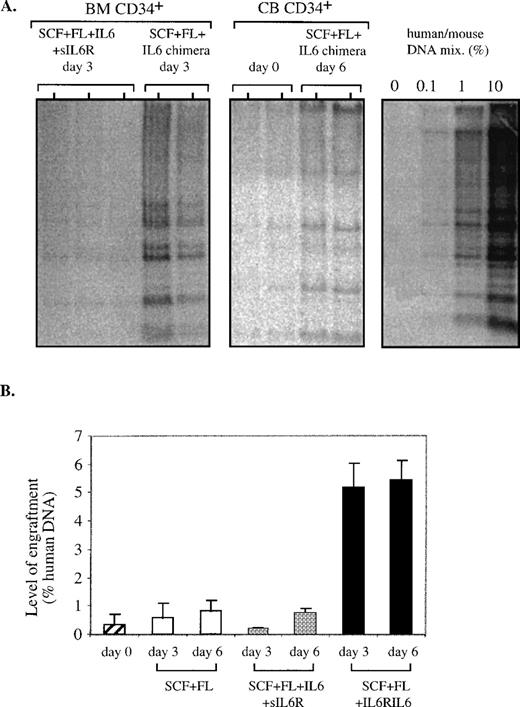
In the next set of experiments, we studied the effect of the IL6RIL6 chimera on maintenance or proliferation of the more primitive SRC in ex vivo cultures with human CB or BM cells. Enriched CD34+cells were seeded at 1 to 2 × 105 cells/well and cultured for 3 or 6 days with various cytokine combinations. At each time point, the cells of each well were collected and transplanted into one NOD/SCID mouse by intravenous injection. One month later, the levels of engraftment were determined by recovering the mouse BM cells and quantifying the percentage of human DNA by Southern blot analysis. The highest levels of engraftment were obtained when IL6RIL6 chimera was added to SCF and FL during the ex vivo cultures of CB and BM cells (Fig 2A). Figure 2B summarizes the levels of human cell engraftment in mice (7 per group) that were transplanted with ex vivo cytokine stimulated cells. As compared with the original CD34+ cells (day 0), the cells cultured ex vivo for 3 and 6 days showed increases in engraftment that were much higher when SCF and FL were complemented by the IL6RIL6 chimera. Compared with SCF + FL alone, the addition of IL6RIL6 chimera increased the engraftment levels by 9.2-fold at day 3 (P = .0002) and by 6.5-fold at day 6 (P < .0002). Furthermore, in these experiments with total CD34+ cells, IL6RIL6 chimera was superior to the mixture of IL-6 and sIL-6R, resulting in higher engraftment levels (Fig 2B).
Quantitative analysis of SRC after 3 or 6 days of ex vivo cultures. Southern blot analysis of DNA extracted from the BM of individual NOD/SCID mice transplanted with 105CD34+ uncultured cells or with their expanded progeny after ex vivo cultures. (A) Representative Southern blots. BM of mice transplanted with human BM CD34+ cells cultured for 3 days with indicated cytokine combinations. BM of mice transplanted with the original CB CD34+ cells before seeding (day 0) or with cells expanded ex vivo for 6 days with SCF + FL + IL6RIL6 chimera. DNA was extracted from the BM of transplanted mice 1 month after transplantation and was hybridized with a human-specific probe. (B) A summary of the levels of human cell engraftment by percentage of human DNA in the BM of mice transplanted with ex vivo-cultured CB and BM CD34+ cells. Cells were cultured for 3 or 6 days before transplantation with the cytokine combinations as indicated. Values shown are the mean ± SE from 7 independent experiments, with an average of 7 mice per group. The differences between SCF + FL + IL6RIL6 chimera and other combinations or noncultured day 0 cells for both time points were significant (P = .0002).
Quantitative analysis of SRC after 3 or 6 days of ex vivo cultures. Southern blot analysis of DNA extracted from the BM of individual NOD/SCID mice transplanted with 105CD34+ uncultured cells or with their expanded progeny after ex vivo cultures. (A) Representative Southern blots. BM of mice transplanted with human BM CD34+ cells cultured for 3 days with indicated cytokine combinations. BM of mice transplanted with the original CB CD34+ cells before seeding (day 0) or with cells expanded ex vivo for 6 days with SCF + FL + IL6RIL6 chimera. DNA was extracted from the BM of transplanted mice 1 month after transplantation and was hybridized with a human-specific probe. (B) A summary of the levels of human cell engraftment by percentage of human DNA in the BM of mice transplanted with ex vivo-cultured CB and BM CD34+ cells. Cells were cultured for 3 or 6 days before transplantation with the cytokine combinations as indicated. Values shown are the mean ± SE from 7 independent experiments, with an average of 7 mice per group. The differences between SCF + FL + IL6RIL6 chimera and other combinations or noncultured day 0 cells for both time points were significant (P = .0002).
Human SRC are maintained for 10 to 14 days in the presence of IL6RIL6 chimera.
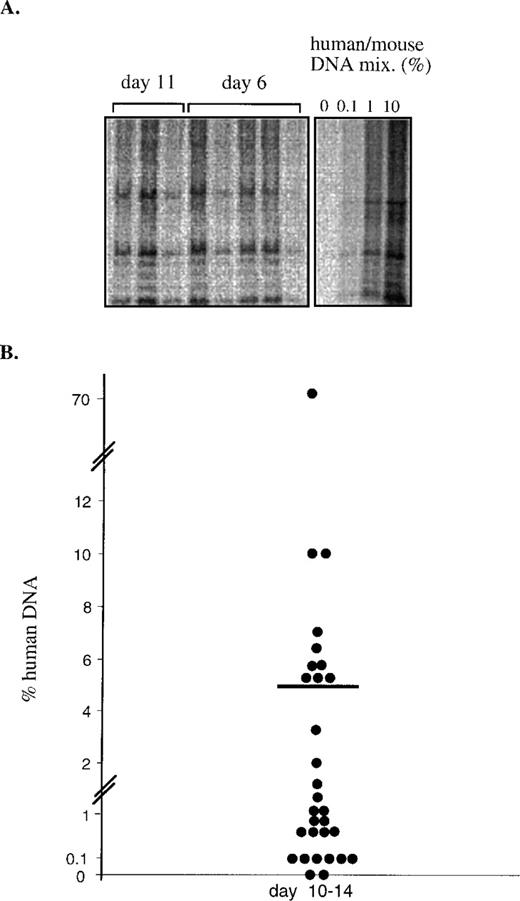
To determine if the addition of SCF, FL, and IL6RIL6 chimera could maintain SRC for longer periods of time in ex vivo cultures, NOD/SCID mice were transplanted with cells after 10 to 14 days of culture. Representative blots demonstrate that the level of engraftment in the BM of transplanted mice was as high at day 11 compared with day 6 of cultures (Fig 3A).
Quantitative analysis of SRC after 10 to 14 days of ex vivo cultures. (A) CB CD34+ cells were transplanted into NOD/SCID mice (105 cells/mouse) from replicate wells. The cells in the wells were ex vivo-cultured for 6 or 11 days with SCF + FL + IL6RIL6 chimera. At each time point, the content of 1 well containing initial cells and their expanded progeny was transplanted into a mouse. DNA was extracted from the BM of transplanted mice 1 month after transplantation and hybridized with a human-specific probe. (B) A summary of the levels of human engraftment in mice transplanted with CD34+ cells that were ex vivo-cultured for 10 to 14 days before transplantation. Data shown are from 4 independent experiments.
Quantitative analysis of SRC after 10 to 14 days of ex vivo cultures. (A) CB CD34+ cells were transplanted into NOD/SCID mice (105 cells/mouse) from replicate wells. The cells in the wells were ex vivo-cultured for 6 or 11 days with SCF + FL + IL6RIL6 chimera. At each time point, the content of 1 well containing initial cells and their expanded progeny was transplanted into a mouse. DNA was extracted from the BM of transplanted mice 1 month after transplantation and hybridized with a human-specific probe. (B) A summary of the levels of human engraftment in mice transplanted with CD34+ cells that were ex vivo-cultured for 10 to 14 days before transplantation. Data shown are from 4 independent experiments.
A total of 30 mice transplanted with CD34+ cells cultured in vitro with SCF, FL, and IL6RIL6 for 10 to 14 days were analyzed. Fourteen of the mice had levels of engraftment between 2% and 10%, whereas another 14 mice had lower levels of engraftment between 0.1% and 1% (Fig 3B). Only 2 mice showed no engraftment. For the 28 mice (93% of the animals) showing engraftment of human cells cultured in vitro for these prolonged periods of time with SCF, FL, and IL6RIL6, the mean level of human DNA was 5%. Hence, despite variations from donor to donor, a significant level of engraftment was maintained in the majority of mice, even after prolonged culture in vitro.
Multilineage differentiation of human hematopoietic cells engrafted in NOD/SCID mice with SCF, FL, and IL6RIL6 chimera.
We verified whether human lymphoid progenitor cells developed in the BM of the NOD/SCID mice engrafted with cells that were cultured with SCF, FL, and IL6RIL6 chimera. The cells recovered from the murine BM were double-stained for human-specific CD45 and for the CD19 B-cell marker. Figure 4A shows the presence of human CD45+CD19+ cells in the BM of a mouse that was transplanted with human CD34+ cells after 10 days in vitro with IL6RIL6 chimera. The BM cells recovered from the engrafted mouse were further cultured with SCF and IL-15 for an additional 10 days and stained for the NK cell marker CD56 (Fig 4B). The presence of 63% CD45+CD56+ cells demonstrates that the human lymphoid progenitors have the potential to differentiate also into NK cells. In parallel, BM cells recovered from engrafted mice gave rise to both erythroid and myeloid colonies (data not shown). Hence, CD34+ cells maintained 10 days in ex vivo cultures with SCF, FL, and IL6RIL6 chimera contain engrafting SRC/stem cells that can give rise to both myeloid and lymphoid cells in the transplanted animals.
Lymphoid differentiation of SRC ex vivo-cultured with IL6RIL6 chimera from the marrow of mice transplanted with CD34+ cells. Recovered BM cells from a highly engrafted NOD/SCID mouse transplanted with CD34+ cells that were cultured with SCF + FL + IL6RIL6 chimera for 10 days before transplantation. One month after transplantation, the murine BM was harvested. The cells were stained with lineage-specific markers and with isotype control for detection of nonspecific staining. (A) Murine BM cells were stained with antihuman CD45-FITC and antihuman CD19-PE for detection of pre-B cells. (B) BM cells from the transplanted mouse were further cultured with SCF + IL-15 for 10 days before staining with human CD45-FITC and antihuman CD56-PE for detection of NK cells.
Lymphoid differentiation of SRC ex vivo-cultured with IL6RIL6 chimera from the marrow of mice transplanted with CD34+ cells. Recovered BM cells from a highly engrafted NOD/SCID mouse transplanted with CD34+ cells that were cultured with SCF + FL + IL6RIL6 chimera for 10 days before transplantation. One month after transplantation, the murine BM was harvested. The cells were stained with lineage-specific markers and with isotype control for detection of nonspecific staining. (A) Murine BM cells were stained with antihuman CD45-FITC and antihuman CD19-PE for detection of pre-B cells. (B) BM cells from the transplanted mouse were further cultured with SCF + IL-15 for 10 days before staining with human CD45-FITC and antihuman CD56-PE for detection of NK cells.
The IL6RIL6 chimera preferentially stimulates CD34+ CD38−/low cells.
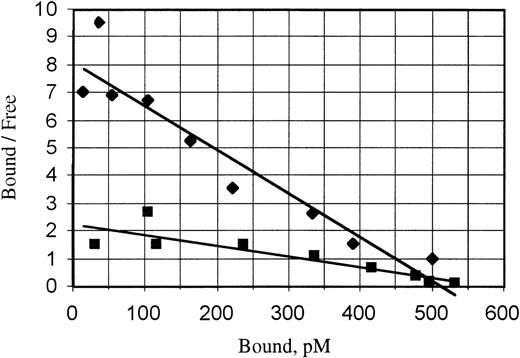
Whereas nearly all CD34+ cells from human CB were found to express gp130, there is a small fraction that do not express the IL-6R (data not shown). This finding is in line with previous reports, which found that CD34+gp130+IL-6R−cells contain higher levels of more primitive LTC-IC.26Because these cells lack membranal IL-6R, they require both IL-6 and sIL-6R to activate gp130 signaling. Previous results have demonstrated that SRC are a very small subpopulation of the CD34+cells,34 and our data show that, within the total CD34+ population, SRC can be stimulated by IL6RIL6 chimera much more efficiently than by a mixture of IL-6 + sIL-6R (Fig 2). We found that IL6RIL6 chimera binds to gp130 with much higher affinity than the mixture of IL-6 and sIL-6R added separately (Fig 5). Therefore, because of its higher affinity, IL6RIL6 chimera could also bind to the rare gp130+ SRC cell subpopulation, triggering gp130 dimerization and transduction of the IL-6–type biological signal.
Relative affinities of IL6RIL6 chimera and IL-6 + sIL-6R mixture for gp130. Scatchard plot of the dose-dependent binding of the IL6RIL6 chimera alone (⧫) or of free sIL-6R in the presence of a constant amount of IL-6 (▪). Binding measured on immobilized pure soluble gp130 as described in Materials and Methods. Calculated kd were 6 × 10−11 mol/L for IL6RIL6 chimera and 2.5 × 10−10 mol/L for the sIL-6R + IL-6 mixture.
Relative affinities of IL6RIL6 chimera and IL-6 + sIL-6R mixture for gp130. Scatchard plot of the dose-dependent binding of the IL6RIL6 chimera alone (⧫) or of free sIL-6R in the presence of a constant amount of IL-6 (▪). Binding measured on immobilized pure soluble gp130 as described in Materials and Methods. Calculated kd were 6 × 10−11 mol/L for IL6RIL6 chimera and 2.5 × 10−10 mol/L for the sIL-6R + IL-6 mixture.
To investigate the response of CD34+ subpopulations to stimulation with these cytokines, we separated the very early CD34+CD38−/low cells that contain SRC from the more differentiated CD34+CD38+population. In vitro, 6-day, serum-free cultures showed (Table 1) that the CD34+CD38−/low cells proliferated only when IL6RIL6 was added to SCF + FL (6-fold increase in cell number). In contrast, CD34+CD38+ cells stimulated with SCF + FL together with IL6RIL6 chimera produced only a small 1.5-fold increase over cells cultured with SCF + FL alone (Table 1). A similar difference was seen in the number of colony forming progenitors, which were increased 12.9-fold by IL6RIL6 chimera in the primitive CD34+CD38−/low cell population and only 1.3-fold in the more mature CD34+CD38+ cell population compared with SCF + FL alone (Table 1). In 5 different experiments, CD34+CD38−/low cells were found to respond to the addition of IL6RIL6 chimera by a large increase of both total cell numbers and progenitor levels after 6-day cultures (Fig 6A, I + II). The differences in cell growth and requirement for IL6RIL6 chimera between total CD34+ cells (Fig 1) and CD34+CD38−/low cells (Table 1 and Fig 6A) reflect the low percentage of CD38− cells in the heterogeneous population of CD34+ cells.
Cell Numbers and Progenitor Levels of CD34+CD38−/low and CD34+CD38+ Cells Cultured With IL6RIL6 Chimera for 6 Days
| Subpopulation . | Cells (×103) . | Colonies . | ||||
|---|---|---|---|---|---|---|
| Day 0 . | SCF + FL . | SCF + FL + IL6RIL6 . | Day 0 . | SCF + FL . | SCF + FL + IL6RIL6 . | |
| CD34+CD38−/low | 3.2 | 3 | 18 | 172 | 255 | 3,307 |
| CD34+CD38+ | 39 | 330 | 507 | 5,606 | 18,562 | 23,448 |
| Subpopulation . | Cells (×103) . | Colonies . | ||||
|---|---|---|---|---|---|---|
| Day 0 . | SCF + FL . | SCF + FL + IL6RIL6 . | Day 0 . | SCF + FL . | SCF + FL + IL6RIL6 . | |
| CD34+CD38−/low | 3.2 | 3 | 18 | 172 | 255 | 3,307 |
| CD34+CD38+ | 39 | 330 | 507 | 5,606 | 18,562 | 23,448 |
Cell numbers, progenitors, and engraftment levels of CD34+CD38−/low cells after ex vivo cultures. Human CD34+CD38−/low cells were cultured in serum-free media with cytokines as indicated and were assayed for cell numbers, progenitors, and levels of human cell engraftment. (A, I) Cell numbers. Mean increase over day 0: SCF + FL = 1.4, SCF + FL + IL6RIL6 = 6.1 (P = .02). (A, II) Progenitor levels. Mean increase over day 0: SCF + FL = 1.9, SCF + FL + IL6RIL6 = 12.8 (P = .03). Values shown are the mean ± SE from 5 independent experiments. (B) Representative Southern blots of the BM of mice transplanted with ex vivo-cultured CD34+CD38−/low cells that originated from 2 different donors. DNA was extracted from the BM of transplanted mice 1 month after transplantation and hybridized with a human-specific probe. The marrow of mice transplanted with 104 uncultured cells before seeding (lane 1) expanded cells cultured for 6 days with SCF + FL either alone (lane 2) or together with IL6RIL6 chimera (lane 3). The marrow of mice transplanted with 104 ex vivo-cultured for 10 days with SCF + FL either alone (lane 4) or together with IL6RIL6 chimera (lane 5). (C) A summary of the levels of human cell engraftment by percentage of human DNA in the BM of mice transplanted with ex vivo-cultured CD34+CD38−/low cells. NOD/SCID mice were transplanted with initial 104CD34+CD38−/low cells that were ex vivo-cultured for 6 to 10 days in serum-free media with SCF + FL alone compared with SCF + FL + IL-6 + sIL-6R (P = .04) or compared with SCF + FL + IL6RIL6 chimera (P = .02). Values shown are the mean ± SE from 5 independent experiments (n = 17).
Cell numbers, progenitors, and engraftment levels of CD34+CD38−/low cells after ex vivo cultures. Human CD34+CD38−/low cells were cultured in serum-free media with cytokines as indicated and were assayed for cell numbers, progenitors, and levels of human cell engraftment. (A, I) Cell numbers. Mean increase over day 0: SCF + FL = 1.4, SCF + FL + IL6RIL6 = 6.1 (P = .02). (A, II) Progenitor levels. Mean increase over day 0: SCF + FL = 1.9, SCF + FL + IL6RIL6 = 12.8 (P = .03). Values shown are the mean ± SE from 5 independent experiments. (B) Representative Southern blots of the BM of mice transplanted with ex vivo-cultured CD34+CD38−/low cells that originated from 2 different donors. DNA was extracted from the BM of transplanted mice 1 month after transplantation and hybridized with a human-specific probe. The marrow of mice transplanted with 104 uncultured cells before seeding (lane 1) expanded cells cultured for 6 days with SCF + FL either alone (lane 2) or together with IL6RIL6 chimera (lane 3). The marrow of mice transplanted with 104 ex vivo-cultured for 10 days with SCF + FL either alone (lane 4) or together with IL6RIL6 chimera (lane 5). (C) A summary of the levels of human cell engraftment by percentage of human DNA in the BM of mice transplanted with ex vivo-cultured CD34+CD38−/low cells. NOD/SCID mice were transplanted with initial 104CD34+CD38−/low cells that were ex vivo-cultured for 6 to 10 days in serum-free media with SCF + FL alone compared with SCF + FL + IL-6 + sIL-6R (P = .04) or compared with SCF + FL + IL6RIL6 chimera (P = .02). Values shown are the mean ± SE from 5 independent experiments (n = 17).
The repopulating potential of ex vivo-cultured CD34+· CD38−/low cells was assayed by transplantation into NOD/SCID mice. Representative Southern blots of individual mice show a 10-fold increase in the level of engraftment when IL6RIL6 chimera was added during the 6-day cultures (Fig 6B, lane 3) compared with SCF + FL alone (lane 2) or with noncultured day 0 cells (lane 1). The same pattern was seen after 10 days of culture (lane 5 v lane 4). Analysis of all experiments with CD34+CD38−/low cells cultured for 6 to 10 days showed that the mean level of engraftment was increased 19.8-fold by the addition of IL6RIL6 chimera to SCF + FL compared with SCF + FL alone (Fig 6C, P = .02). With the purified CD34+CD38−/low cells, a 6.4-fold increase in the levels of engraftment was also seen when the IL-6 + sIL-6R mixture (instead of IL6RIL6 chimera) was added to SCF + FL (Fig6C, P = .04). Therefore, as expected, the enrichment achieved by subfractionation of the CD34+ cells allows the SRC cells to respond better to the IL-6/sIL-6 receptor-type signal, because they now represent a higher percentage of the total gp130+population.
These results were supported by CD34/CD38 phenotype analysis of ex vivo-cultured cells. When total CD34+ cells were cultured for 11 days with SCF + FL + IL6RIL6 chimera, the CD34+CD38−/low subpopulation was twice as high than in cultures with SCF + FL alone (data not shown). Cultures of sorted CD34+CD38−/low cells were analyzed for maintenance of the primitive cells during prolonged cultures of up to 14 days. When CD34+CD38−/low cells were cultured with SCF + FL and IL6RIL6 chimera, we found 28.2%, 29.5%, and 9.1% of CD34+CD38−/low cells at days 6, 10, and 14, respectively (data not shown). Previous reports on cultures with SCF, FL, and IL-6 indicated complete loss of CD34+CD38−/low cells after 9 days.7
Increased human cell engraftment by the IL6RIL6 chimera-treated cells was also demonstrated by assaying the levels of human clonogenic progenitors recovered from the BM of transplanted NOD/SCID mice (Table 2). Human progenitor levels were increased 16-fold when IL6RIL6 chimera was added to SCF + FL in the ex vivo cultures. Furthermore, BM from mice engrafted with CD34+CD38−/low cells that had been cultured with SCF, FL, and IL6RIL6 chimera before transplantation gave rise to multilineage human progenitors that included myeloid colony-forming unit–granulocyte-macrophage (CFU-GM) as well as erythroid burst-forming unit-erythroid (BFU-E) and colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM) mixed colonies. Without IL6RIL6, mostly myeloid CFU-GM were observed (Table 2 and data not shown).
Human Progenitors in the BM of NOD/SCID Mice Transplanted With CD34+CD38−/low Cells That Were Cultured Ex Vivo for 6 Days
| Cytokine Combination . | Total Colonies* . | Primitive Colonies† . |
|---|---|---|
| SCF + FL | 5 ± 1 | 0 ± 0 |
| SCF + FL + IL6RIL6 chimera | 80 ± 10 | 11 ± 4 |
| Cytokine Combination . | Total Colonies* . | Primitive Colonies† . |
|---|---|---|
| SCF + FL | 5 ± 1 | 0 ± 0 |
| SCF + FL + IL6RIL6 chimera | 80 ± 10 | 11 ± 4 |
Data shown represent mean ± SE from 3 independent experiments (P = .01, 2 tails, paired t-test).
With SCF + FL, only macrophage colonies were found. When IL6RIL6 was added, mixed GEMM, BFU-E, and blast colonies were seen.
The maintenance activity provided by the IL6RIL6 chimera, together with the cell expansion, probably accounts for the marked increase in engraftment levels of the human hematopoietic cells in the transplanted NOD/SCID mice.
DISCUSSION
The establishment of the NOD/SCID model enables us to measure the in vivo engraftment properties of primitive human hematopoietic cells. One issue of current interest is the potential of ex vivo-cultured and expanded human hematopoietic cells to repopulate in vivo the BM by proliferation and multilineage differentiation.
Recently, several groups suggested different cytokine combinations for maintenance and expansion of primitive human hematopoietic cells in ex vivo cultures.4-8 The stem cell activities in these cytokine-stimulated cultures were evaluated in vitro by the levels of colony-forming cells (CFC) or LTC-IC6,38 as well as by expression of differentiation markers such as CD34, CD38, or HLA-DR.6,8,38 In vivo models were used in which cultured cells were transplanted into immunodeficient mice6-8 or into sheep fetuses in utero.39 In transplanted NOD/SCID mice, human SRC could be maintained in vitro for 4 days7 or human CRU for 5 to 8 days6 and still engraft the marrow of transplanted mice, but successful maintenance of SRC for longer periods was not reported, apparently due to differentiation and loss of repopulating stem cell potential.7,34 In the sheep chimera model, transplantation of cells after ex vivo culture for 14 days resulted in only short-term engraftment in the marrow of in utero fetuses, but after the fetuses were born no human engraftment was detected.39
A combination of IL-6 and sIL-6R together with either SCF24or FL25 was shown to increase the levels of CD34+ cells and progenitors in vitro, and we previously reported that this IL-6 + sIL-6R combination increases progenitors even when added to cultures containing both SCF and FL.29Therefore, despite the synergistic effect of SCF with FL,40IL-6 can further stimulate progenitors when provided with its sIL-6R agonist, which allows gp130 signaling in very early CD34+gp130+IL-6R− cell subpopulations.26 We also showed that a fused IL6RIL6 chimeric glycoprotein is very active in increasing colony-forming progenitors when added to SCF + FL,29 and another such fusion protein named Hyper–IL-6 was shown to increase progenitor levels when added to SCF + IL-3.28 The higher activity of proteins in which sIL-6R is fused to IL-6 can be explained, as we demonstrate here, by a higher affinity of IL6RIL6 chimera to gp130 compared with the IL-6 + sIL-6R mixture (Fig 5).
Our current study shows the effect of the IL6RIL6 chimera added to SCF and FL for SRC maintenance and expansion in cultures of CD34+ and CD34+CD38−/lowhuman CB and BM cells. Assayed in the NOD/SCID model, CD34+cultures containing the IL6RIL6 chimera produced higher levels of engraftment compared with day 0 noncultured cells or compared with cells stimulated with SCF + FL either alone or with a mixture of IL-6 + sIL-6R. Total levels of human cell engraftment depend on the purity of enriched transplanted cells and occasionally fluctuate upon different donors and mice, resulting in a wide range of engraftment levels in the marrow of transplanted mice. Hence, despite the relatively low engraftment levels obtained in some experiments, the described growth effect of IL6RIL6 chimera on CD34+ and CD34+CD38−/low cells was consistently observed. Furthermore, in the presence of IL6RIL6 chimera, we could prolong the period of ex vivo cultures for up to 14 days and still achieve high levels of human cell engraftment. CD34+ cells cultured with the IL6RIL6 chimera added to SCF + FL demonstrated multilineage differentiation in the murine BM, generating myeloid, erythroid, and lymphoid progenitor cells, including NK cells. Moreover, human progenitors recovered from the murine BM produced higher numbers of colonies, and particularly immature colonies such as CFU-GEMM, when the mice were transplanted with cells cultured with IL6RIL6 as compared with SCF + FL alone. Together, these features indicate the maintenance of stem cell properties in CD34+ cells ex vivo-cultured in the presence of the IL6RIL6 chimera.
A possible mechanism for IL6RIL6 chimera stimulation may be the differential expression of IL-6R and of gp130 on the cell surface of CD34+ cells. In the CD34+ population, all cells express gp130, but the levels of IL-6R vary and are undetectable in a small subpopulation of the cells. A previous study demonstrated that CD34+IL-6R− cells are more primitive than CD34+IL-6R+ cells.26 Because the IL6RIL6 chimera binds directly with high affinity to isolated gp130, it is likely that, in the CD34+ population, cells lacking IL-6R on their surface are still capable of gp130-mediated response to IL6RIL6 chimera. The more primitive cells may have an enhanced response to IL6RIL6 chimera stimulation compared with more mature cells. This was verified by examining the potential of the IL6RIL6 chimera to induce proliferation and to enhance SRC in very early CD34+CD38−/low subpopulations isolated from CB CD34+ cells.
Our study shows that IL6RIL6 chimera stimulated mainly the very early CD34+CD38−/low cells, which responded by extensive proliferation. There was even a higher increase in progenitor levels than in total cell numbers, supporting the notion that IL6RIL6 chimera is a very early acting cytokine/soluble receptor complex. Indeed, the more mature CD34+CD38+ cells were hardly affected, exhibiting only a limited increase in cell numbers and in progenitors.
Previous studies with SCF, FL, IL-3, granulocyte colony-stimulating factor (G-CSF), and IL-6 by itself reported a decrease in the engrafting potential of SRC after 8 days of in vitro culture, accompanied by loss of the early CD34+CD38− compartment as detected by flow cytometry.7 In contrast, in this report, flow cytometry analyses of sorted CD34+CD38−/low cells cultured in the presence of IL6RIL6 chimera demonstrate maintenance of these early cells after 6 and 10 days of ex vivo stimulation. Even after 14 days, a remarkable percentage of CD34+CD38−/lowcells were still observed in ex vivo cultures. This maintenance of the CD34+CD38−/low phenotype can account for the much higher levels of engraftment in the marrow of transplanted NOD/SCID mice after IL6RIL6 chimera cultures compared with cells cultured with SCF + FL alone. Engraftment by these cells, as demonstrated by DNA analysis and recovery of human progenitors from the murine BM, was higher with IL6RIL6 chimera than with the IL-6 + sIL-6R combination.
These findings demonstrate that the present CHO-produced IL6RIL6 chimera is an important hematopoietic stimulator that supports maintenance as well as proliferation of primitive CD34+CD38−/low cells in ex vivo cultures. In addition, induction of extensive proliferation of early SRC, while maintaining their repopulating potential, suggests that the IL6RIL6 chimera may be useful for clinical transplantation protocols.
ACKNOWLEDGMENT
The assistance of Zipora Marks, Rosalie Kaufman, and Nili Nissin is gratefully acknowledged. We thank D. Novick and M. Rubinstein for help in providing anti–IL-6R antibodies.
Supported in part by grants from the Israel Academy of Science (T.L.), the Balfur Peisner Bone Marrow Cancer Research Fund (O.K.), the Ares Serono group (T.L. and M.R.), and MINERVA Foundation, Munich/Germany (R.A.) and by National Institutes of Health Grant No. A130389 (L.S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tsvee Lapidot, PhD, Incumbent of the Pauline Recanati Career Development Chair of Immunology, Department of Immunology, The Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: litsvee@weizmann.weizmann.ac.il.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal