Progression of cutaneous T-cell lymphoma (CTCL) is associated with profound defects in cell-mediated immunity and depressed production of cytokines, which support cell-mediated immunity. Because we have observed marked defects in interleukin-12 (IL-12) production in CTCL and because IL-12 is critical for antitumor cytotoxic T-cell responses, we initiated a phase I dose escalation trial with recombinant human IL-12 (rhIL-12) where patients received either 50, 100, or 300 ng/kg rhIL-12 twice weekly subcutaneously or intralesionally for up to 24 weeks. Ten patients were entered: 5 with extensive plaque, 3 with Sezary syndrome, and 2 with extensive tumors with large cell transformation. One patient with Sezary syndrome dropped out after 1 week for personal reasons. Subcutaneous dosing resulted in complete responses (CR) in 2 of 5 plaque and partial responses (PR) in 2 of 5 plaque, and 1 of 2 Sezary syndrome (overall response rate CR+PR 5 of 9, 56%). A minor response also occurred in 1 of 5 plaque patients. Intralesional dosing resulted in individual tumor regression in 2 of 2 patients. Biopsy of regressing lesions showed a significant decrease in the density of the infiltrate in all cases and complete resolution of the infiltrate among those with clinical lesion resolution. An increase in numbers of CD8-positive and/or TIA-1–positive T cells were observed on immunohistochemical analysis of skin biopsy specimens obtained from regressing skin lesions. Adverse effects of rhIL-12 on this regimen were minor and limited and included low-grade fever and headache. One patient discontinued rhIL-12 at week 6 because of depression. These results suggest that rhIL-12 may augment antitumor cytotoxic T-cell responses and may represent a potent and well-tolerated therapeutic agent for CTCL.
CUTANEOUS T-CELL lymphoma (CTCL) is a clonally derived malignant proliferation of skin-invasive CD4+ T lymphocytes.1,2 Clinical manifestations of CTCL can encompass a broad spectrum of findings ranging from limited cutaneous patches and plaques with no overt peripheral blood or lymph node involvement to extensive skin involvement with tumors or erythroderma with concomitant blood, node, or visceral disease.3 Because CTCL is a malignancy that is responsive at all stages to biological response modification,4-6 a thorough understanding of the immunopathogenesis of this disease can lead to the enhanced use of novel biologic agents, including recombinant cytokines. In this regard, it is known that during disease progression, a variety of immunologic abnormalities become evident including prominent defects in cell-mediated immunity.7,8These abnormalities are typically associated with the depressed ability of peripheral blood cells to produce the T-helper type I cytokines interferon-γ (IFN-γ) and interleukin-2 (IL-2).7,9 The pathogenesis of these cytokine defects may be related to the excess production of T-helper type 2 cytokines (IL-4, -5, and -10) by the malignant clonal T-cell population, which can antagonize the function and production of IFN-γ and IL-2.10 11
IL-12 is a recently identified cytokine, which is a powerful inducer of IFN-γ production12 and which exerts potent Th1-inducing effects.12,13 Furthermore, IL-12 augments natural killer cell cytotoxicity14 and cytotoxic T-cell proliferation and function,15 activities that may be beneficial in regard to the abnormal Th2 clonal proliferation observed in advanced CTCL. Recent studies16 have demonstrated a deficiency in production of IL-12 by peripheral blood cells from patients with advanced CTCL. Notably, culture of these patients’ cells with recombinant IL-12 (rIL-12) in vitro leads to a restoration of IFN-γ production and a marked enhancement of cell-mediated cytotoxicity. Because lesional infiltrating cytolytic T cells have been shown in early skin lesions of CTCL17 and are believed to play a role in retarding the progression of disease, the ability to enhance their generation via the administration of exogenous IL-12 could be important for the therapy of CTCL. Moreover, the restoration of IFN-γ production, as well as the enhancement of cell-mediated cytotoxicity, serve as primary rationale for the use of IL-12 as therapy for this T-cell malignancy.
For these reasons, a phase I trial using human recombinant IL-12 (rhIL-12) to treat patients diagnosed with CTCL was initiated. We report here that rhIL-12 administered subcutaneously twice weekly was well tolerated and induced significant clinical responses in 5 of 9 patients. Furthermore, serial immunohistochemical studies of involved skin lesions before and during therapy indicated that lesion regression was associated with tumor infiltrating cytotoxic T-cell responses.
MATERIALS AND METHODS
Patients.
Patients 18 years of age or older with the clinical and histological diagnosis3 of CTCL with plaques, tumors, or erythroderma with an expected survival of at least 6 months were eligible for entry into the study. Individuals with seropositivity against human immunodeficiency virus (HIV), human T-cell lymphotrophic virus (HTLV)-I, or hepatitis C were excluded, as were individuals with a past history of gastrointestinal hemorrhage or serious autoimmune, cardiac, or renal disease. CTCL patients with documented visceral involvment, other than bone marrow involvment, were also excluded. No more than 3 systemic therapies were permitted during the previous 12 months.
Study design.
This was an open-label, nonrandomized, single center, phase I trial to test the safety and efficacy of rhIL-12. Dose levels of 50 ng/kg, 100 ng/kg, or 300 ng/kg were administered 2 times a week by subcutaneous injection. In some cases, rhIL-12 was administered directly into discrete plaques or tumors. Treatment was performed for up to 24 weeks. An initial group of 2 patients was entered at 50 ng/kg. Safety at this dose was evaluated at 4 weeks before entering patients at the 100 ng/kg dosing level. Similarly, an initial group of 4 patients was entered at the 100 ng/kg dose and safety was evaluated at 4 weeks before entering patients at the 300 ng/kg dose range. Toxicity grading of adverse experiences was in accordance with the modified National Cancer Institute (NCI) Common Toxicity Criteria Scale. If after 4 weeks of therapy, adverse effects were mild and the level of disease was stable or progressive, escalation of the dose to the next higher level was possible at the discretion of the investigator. Before initiating therapy with rhIL-12, all other topical and systemic therapies, with the exclusion of 1% hydrocortisone cream or ointment, which could be applied to less than 5% of the skin surface area daily, were to be discontinued for at least a 3-week period. Therapies other than 1% hydrocortisone preparations were not permitted once rhIL-12 was started.
Patient evaluation.
Before initiation of rhIL-12, all patients had a complete history and physical examination. In addition, a chest x-ray, electrocardiogram, complete skin photographs, skin biopsy of 1 or more lesions for hematoxylin and eosin (H&E) analysis and for immunohistochemistry, complete blood count, serum chemistries, urinalysis, serology for HIV, HTLV-I, hepatitis C, and fluorescence-activated cell sorting (FACS) analysis of peripheral blood were performed. Routine physical exams, blood counts, and chemistries were repeated weekly during the first month of treatment, then monthly thereafter. Repeat skin biopsies were obtained at the time of initial lesion regression and on complete clinical regression. A Sezary cell preparation was performed on 1-μm sections of pelleted, fixed buffycoat specimens at baseline and at the final study visit for patients with no blood involvement at baseline or every 2 months for patients with blood involvement at baseline. Blood was also obtained for T-cell receptor gene rearrangement testing at baseline, at the time of a complete response of skin disease, and at completion of the study.18
Patients were evaluated for treatment efficacy by skin lesion measurements after every 4 weeks during the period of drug administration and at 1 month after completion of treatment and photography at the conclusion of therapy. A complete clinical response (CR) was defined as complete disappearance of all measurable and evaluable lesions for at least 1 month. A CR was documented by rebiopsy of a previously involved skin site and by reevaluation of previously involved peripheral blood by gene rearrangement studies and Sezary count. A partial clinical response (PR) was defined as at least 50% disappearance of all CTCL skin lesions or decrease in area of skin involvement for at least 1 month. A minor response was defined as 25% to 49% disappearance of all CTCL skin lesions for at least 1 month, while stable disease was defined as less than 25% disappearance of all measurable and evaluable lesions or stabilization of all existent lesions for at least 1 month. Progressive disease was defined as worsening on 2 consecutive visits 4 weeks apart with at least a 50% increase in measured cutaneous disease burden from baseline.
Immunohistochemistry.
Representative portions of lesional skin biopsy specimens were snap-frozen, stored at −70°C, cryostat-sectioned, and acetone-fixed as described previously.19 Sections were immunostained with a 3-stage monoclonal antibody/biotin/avidin method with 3,3-diaminobenzidine chromagen and counterstained with methylene blue as reported previously.19 The antibody panel included T-cell subset markers (CD3, CD4, CD7, CD8), IL-2 receptor (CD25), cytolytic granule marker (TIA-1), class II major histocompatibility antigen (HLA-DR), and proliferating cell marker (Mib-1). Controls included deletion of various immunostaining stages, replacement of first stage monoclonal antibodies with irrelevant antibodies of similar isotype, and staining of normal skin and reactive lymphoid tissues. Stained sections were evaluated using a standard light microscope.
RESULTS
Patient characteristics.
Ten patients with the clinical and histological diagnosis of CTCL began therapy with rhIL-12. The characteristics of the patients are shown in Table 1. Three of 5 with plaque type disease had more than 30% of their skin surface area involved with typical plaques, while 1 of these patients (patient 7) had a detectable T-cell receptor gene rearrangement in the peripheral blood at baseline. Patients 1 and 5 with evidence of numerous skin tumors each had evidence of large cell transformation on histological analysis of biopsy specimens from skin tumors.20 Patients 3, 4, and 10 had Sezary syndrome with 70%, 80%, and 85% of circulating mononuclear cells as Sezary cells. All patients with tumors and Sezary syndrome had received a mean of 3 previous therapies and thus could be considered as heavily pretreated before starting rhIL-12.
Patient Characteristics and Treatment Outcome
| Patient No. . | Age/Sex . | Skin Stage* . | Previous Therapy . | IL-12 Dose (ng/kg)† . | Weeks Treated . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | 69/M | T3 (large cell transformation) | topical mechlorethamine, retinoids, CHOP, IFN, photopheresis | 50-100 | 6 | Local response |
| 2 | 44/F | T2 | PUVA, topical mechlorethamine | 50-100 | 13 | Minor response |
| 3 | 69/M | T4 | IFN, retinoids, photopheresis | 100 | 1 | Withdrew |
| 4 | 76/F | T4 | IFN, photopheresis | 100-300 | 24 | Partial response |
| 5 | 70/F | T3 (large cell transformation) | Radiation, methotrexate | 100-300 | 8 | Local response |
| 6 | 71/M | T2 | PUVA, topical mechlorethamine | 100-300 | 24 | CR |
| 7 | 50/F | T2 | Topical steroids | 100 | 24 | CR |
| 8 | 80/M | T1 | PUVA, topical BCNU, mechlorethamine | 100 | 24 | PR |
| 9 | 56/M | T1 | Topical mechlorethamine, PUVA | 300 | 24 | PR |
| 10 | 64/M | T4 | Photopheresis, IFN, retinoids, GM-CSF | 300 | 10 | No response |
| Patient No. . | Age/Sex . | Skin Stage* . | Previous Therapy . | IL-12 Dose (ng/kg)† . | Weeks Treated . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | 69/M | T3 (large cell transformation) | topical mechlorethamine, retinoids, CHOP, IFN, photopheresis | 50-100 | 6 | Local response |
| 2 | 44/F | T2 | PUVA, topical mechlorethamine | 50-100 | 13 | Minor response |
| 3 | 69/M | T4 | IFN, retinoids, photopheresis | 100 | 1 | Withdrew |
| 4 | 76/F | T4 | IFN, photopheresis | 100-300 | 24 | Partial response |
| 5 | 70/F | T3 (large cell transformation) | Radiation, methotrexate | 100-300 | 8 | Local response |
| 6 | 71/M | T2 | PUVA, topical mechlorethamine | 100-300 | 24 | CR |
| 7 | 50/F | T2 | Topical steroids | 100 | 24 | CR |
| 8 | 80/M | T1 | PUVA, topical BCNU, mechlorethamine | 100 | 24 | PR |
| 9 | 56/M | T1 | Topical mechlorethamine, PUVA | 300 | 24 | PR |
| 10 | 64/M | T4 | Photopheresis, IFN, retinoids, GM-CSF | 300 | 10 | No response |
Abbreviations: PUVA, psoralen, ultraviolet A; BCNU, carmustine.
T1 represents patch/plaque disease involving 10% or less of the skin surface, T2 is patch/plaque disease involving greater than 10% of the skin surface, T3 represents multiple tumors and T4 represents erythroderma.
Range of treatment doses indicates patient was dose escalated at week 4 of therapy.
Clinical response to rhIL-12.
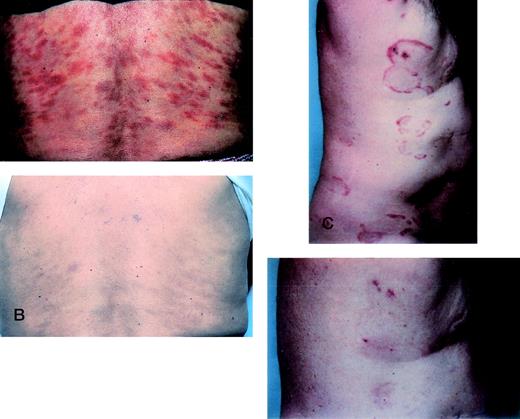
All patients with plaque disease had measurable clinical improvement while receiving rhIL-12 (Table 1). Two patients experienced a CR at weeks 7 and 8, respectively (Fig 1A through D). A PR was documented in each by 5 weeks of therapy. Both received 100 ng/kg rhIL-12. Each CR was documented by histological evidence of clearing of previously involved areas of the skin. Furthermore, a previously detectable T-cell receptor gene rearrangement in the blood of patient 7 became undetectable at the time of the documented CR and remained undetectable at the conclusion of treatment. This patient had more than 80% of her skin surface involved with patches and plaques before initiating rhIL-12. She maintained the CR for 8 weeks while on therapy (Fig 1A and B). Recurrence of skin lesions was characterized by the development of 2 faint patches each 2 cm in diameter located on the lateral ankles, which persisted throughout the conclusion of the 24 weeks of therapy. However, at follow-up 1 month after concluding rhIL-12, all skin abnormalities had again resolved. Recurrence of skin lesions in patient 6 was also associated with minimal disease with several small patches observed, which were limited to the thighs. One month after concluding rhIL-12, more extensive patches had recurred on the body, but the extent of skin disease was still significantly less than at the treatment initiation.
CRs of 2 patients during treatment with rhIL-12. (A) Patient 7 before starting rhIL-12. (B) Patient 7 at the conclusion of treatment with rhIL-12 showing complete clearing of skin lesions on the trunk. (C) Patient 6 before initiating rhIL-12. (D) The same patient at week 10 of therapy with complete clearing of skin lesions.
CRs of 2 patients during treatment with rhIL-12. (A) Patient 7 before starting rhIL-12. (B) Patient 7 at the conclusion of treatment with rhIL-12 showing complete clearing of skin lesions on the trunk. (C) Patient 6 before initiating rhIL-12. (D) The same patient at week 10 of therapy with complete clearing of skin lesions.
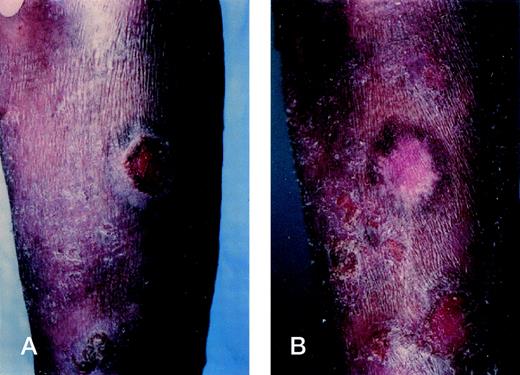
Intralesional injections of rhIL-12 result in tumor regression. (A) Tumor of the right forearm at baseline in patient 5. (B) Complete flattening of the tumor at week 3 after 6 injections of rhIL-12 at a dose of 100 ng/kg into the tumor. Histologic examination of the flattened tumor showed complete resolution of the malignant infiltrate.
Intralesional injections of rhIL-12 result in tumor regression. (A) Tumor of the right forearm at baseline in patient 5. (B) Complete flattening of the tumor at week 3 after 6 injections of rhIL-12 at a dose of 100 ng/kg into the tumor. Histologic examination of the flattened tumor showed complete resolution of the malignant infiltrate.
In addition to the 2 plaque patients with CRs, 2 plaque patients experienced a PR, which was established at 12 weeks and 7 weeks of therapy, respectively. The former received 300 ng/kg, while the other received 100 ng/kg during 24 weeks of therapy. The PR were maintained for the remainder of the 24 weeks of therapy. One plaque patient had a minor response noted at week 12 while receiving 50 ng/kg, but she elected to discontinue therapy at week 13 due to increasing pruritus. The pruritus persisted after discontinuing rhIL-12 and was thus attributed to her disease.
Among the 3 patients with Sezary syndrome, defined as such by the presence of erythroderma and circulating malignant cells detected by flow cytometry or morphological analysis of buffy coats, 1 elected to discontinue therapy for personal reasons after only 2 injections of rhIL-12, 1 discontinued therapy at week 10 with stable skin and blood disease at a dose of 100 ng/kg, and 1 had a documented PR at week 13 with clearing of erythema from large areas of the trunk. This patient started at 100 ng/kg and dose escalated at week 4 to 300 ng/kg. Histological analysis of improving areas of the skin further confirmed the response by showing a significant reduction in the cutaneous lymphoid infiltrate compared with baseline. In addition, an overall decrease in scaling was also observed. These improvements in skin disease were maintained throughout the remainder of the 24 weeks of therapy. The total white blood count decreased from 18,000/μL at baseline to 9,000/μL at study completion, and the relative percentage of circulating Sezary cells decreased from 80% to 50% during the treatment period. Moreover, the absolute lymphocyte count dropped by one half, suggesting a significant decrease in the total number of circulating Sezary cells.
Each of the 2 tumor-stage patients had rapidly progressive disease with numerous skin tumors at the time of initiation of rhIL-12. Direct intralesional therapy into tumors was undertaken with each patient. Patient 1 began at a dose of 50 ng/kg and was dose escalated to 100 ng/kg at week 4, while patient 5 began rhIL-12 at 100 ng/kg and escalated to 300 ng/kg at week 4. Although as shown in Fig 2, each experienced flattening and complete resolution of several injected tumors, new lesions continued to develop beyond the injection sites and, thus, each had progressive disease. Patient 1 discontinued therapy at week 6, while patient 5 discontinued treatment at week 8.
Immunohistochemistry.
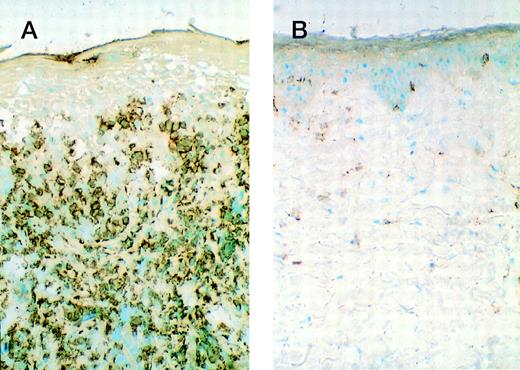
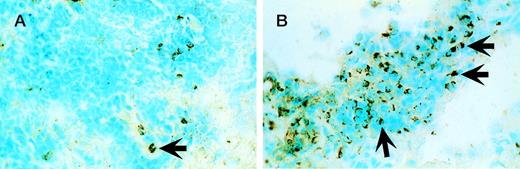
The 5 patients with significant clinical responses had immunohistological analysis of biopsy material from involved skin lesions obtained at baseline and during lesion regression. All pretreatment samples demonstrated epidermal hyperplasia and increased keratinocyte HLA-DR expression. The epidermal and dermal lymphocytic infiltrate contained a predominantly CD3+CD4+T-cell infiltrate deficient in CD7 expression with a minority of CD8+ cells admixed. Repeat biopsies were obtained within 1 to 2 weeks of initially noting lesion regression while on rhIL-12 treatment. In 5 of 5 patients analyzed, a number of alterations were noted during lesion regression that were considered significant differences from baseline. All cases showed a 2- to 3-fold reduction in total CD3+ T cells (Fig 3A and B) associated with a 2- to 3-fold increase in the proportion of CD8+ cells (cytotoxic T cells). A 2- to 3-fold increase in the porportion of TIA-1+ cells (cytotoxic cell marker) in 2 of 3 evaluable cases was detected (Fig 4A and B). In addition, there was a trend toward normalization of the epidermis with reduction in epidermal hyperplasia, scale, and/or ulceration in each case, associated with a reduction in epidermal HLA-DR expression in 2 of 3 evaluable cases. The other 2 cases were missing epidermis in some specimens due to lesional ulceration or loss of epidermis during subdivision of the biopsy specimen for various assays.
CD3+ cells within involved skin lesions decrease during lesion regression while receiving rhIL-12. (A) Biopsy specimen of involved skin from patient 6 at baseline. (B) Biopsy specimen of same lesion as in (A) when lesion was nearly completely resolved during rIL-12 treatment.
CD3+ cells within involved skin lesions decrease during lesion regression while receiving rhIL-12. (A) Biopsy specimen of involved skin from patient 6 at baseline. (B) Biopsy specimen of same lesion as in (A) when lesion was nearly completely resolved during rIL-12 treatment.
TIA-1+ cells increase significantly within regressing skin lesions during rhIL-12 treatment. (A) Involved skin at baseline from patient 8 with small numbers of TIA-1+cells as indicated by the arrow. (B) A 2- to 3-fold increase in the proportion of TIA-1+ cells as indicated by the arrows within the same lesion during regression associated with rhIL-12 treatment.
TIA-1+ cells increase significantly within regressing skin lesions during rhIL-12 treatment. (A) Involved skin at baseline from patient 8 with small numbers of TIA-1+cells as indicated by the arrow. (B) A 2- to 3-fold increase in the proportion of TIA-1+ cells as indicated by the arrows within the same lesion during regression associated with rhIL-12 treatment.
Adverse effects of rhIL-12.
The adverse effects associated with rhIL-12 treatment during this trial are shown in Table 2. Most adverse effects were mild and were short-lived with a duration of 24 to 36 hours after the initial injection. Constitutional symptoms consisting of fatigue, headache, or myalgias typically did not recur after subsequent doses of rhIL-12. Exceptions included patient 10 who entered the trial at a dose of 300 ng/kg, but who experienced severe fatigue lasting 1 week after the initial injection, which necessitated a dose decrease to 100 ng/kg. Similarly, patient 7 experienced severe fatigue when she was dose escalated from 100 ng/kg to 300 ng/kg at week 4. Because of this, the dose was deescalated to the original level after a single injection at 300 ng/kg. Subsequently, it was determined that significant alcohol ingestion had occurred on the day of the dose escalation.
Adverse Effects of IL-12
| Event . | Number . |
|---|---|
| Myalgias | 5 |
| Chills | 4 |
| Fatigue* | 2 |
| Loose stools | 2 |
| Elevated liver enzymes† | 2 |
| Loss of appetite | 2 |
| Headache | 2 |
| Depression‡ | 1 |
| Leukopenia | 1 |
| Event . | Number . |
|---|---|
| Myalgias | 5 |
| Chills | 4 |
| Fatigue* | 2 |
| Loose stools | 2 |
| Elevated liver enzymes† | 2 |
| Loss of appetite | 2 |
| Headache | 2 |
| Depression‡ | 1 |
| Leukopenia | 1 |
Fatigue occurred in one patient entered at 300 ng/kg and in one patient dose-escalated to 300 ng/kg necessitating dose decrease in both cases.
Elevated liver enzymes occurred in the context of alcohol ingestion at a dose of 300 ng/kg and rapidly returned to baseline values with the holding of a single dose of rhIL-12.
Depression occurred at a dose of 100 ng/kg necessitating discontinuation of rhIL-12. This patient had experienced severe depression during the previous administration of recombinant IFN-α.
Patients 9 and 10 each had elevations in serum hepatic enzyme levels measured during week 2 of therapy on doses of 300 ng/kg. In each case, alcohol ingestion occurred within 24 hours of the rhIL-12 dosing. Both patients had 1 dose of rhIL-12 omitted and, within 1 week, serum levels of hepatic enzymes returned to normal. No additional abnormalites in these laboratory parameters were noted during the duration of rhIL-12 therapy for these 2 patients.
Patient 1 experienced depression at week 5 of therapy after dose escalation from 50 ng/kg to 100 ng/kg at week 4. Because of the severity of the depression, he elected to discontinue treatment with rhIL-12 after 6 weeks of therapy. The depression resolved within 1 week of discontinuation of the rhIL-12. It is noteworthy that this patient also experienced severe depression during treatment with recombinant IFN-α, which also resolved on discontinuation of this cytokine, thus suggesting that this apparent idiosyncratic adverse effect was not unique to rhIL-12.
DISCUSSION
Our results with a small number of patients with mycosis fungoides and Sezary syndrome suggest that rhIL-12, in the dosing schedule used, is both efficacious and without serious adverse effects. Five of five patch/plaque type patients had a clinical response, with 2 of the 5 experiencing a complete clinical response associated with histological clearing. One of 2 Sezary syndrome patients who remained on protocol for more than 2 months also experienced a significant reduction in their disease burden. While the 2 tumor-stage patients with large cell transformation did not experience a significant overall response, intralesional injections of rhIL-12 resulted in regression of the injected tumors, indicating that rhIL-12 can exert biological activity even in aggressive forms of CTCL.
The observation that the plaque type patients more frequently responded to rhIL-12 than patients with Sezary syndrome or multiple tumors may relate to the overall degree of immunological integrity in each subset of patients. Those with plaque type disease tend to have a more intact cell-mediated immune response than do patients with multiple tumors or with Sezary syndrome.21,22 Those with more advanced disease will have a larger tumor burden consisting of CD4+malignant T cells that produce the immunosuppressive cytokines IL-4 and IL-10, which exhibit the capacity to blunt an antitumor cytolytic T-cell response.23 24 In addition, the tumor stage patients had been heavily pretreated with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy and high dose methotrexate, which may depress cell-mediated immunity and which might be expected to counteract the immune enhancing effects of rhIL-12. Although the numbers of patients participating in our study were small and definitive conclusions regarding response rates of different patient subtypes cannot be made, it is possible that a more substantial immunological substrate existed among patch/plaque stage patients, which presumably facilitated the response to rhIL-12 administration.
The findings of significantly increased numbers of CD8+ or TIA-1+ cytotoxic cells within skin lesions undergoing regression strongly suggest that clinical responses were a consequence of the immunomodulatory effects of rhIL-12 to induce antitumor cytolytic T cells. The reservoir for these tumor infiltrating T cells is undoubtedly derived from circulating normal CD8+ T cells with skin-homing potential. These observations are consistent with our recent studies that indicate that rhIL-12 is not likely to be acting directly on the Sezary T cells.25 We have shown that peripheral blood Sezary T cells express the β1, but not the β2, component of the IL-12 receptor.25 The β2 component appears to be necessary for both high-affinity cell surface binding of IL-12, as well as for the intracellular transmission of the IL-12 signal.26,27 When high concentrations of rhIL-12 are added to cultures of Sezary patient-derived purified malignant CD4+ T cells, it fails to result in activation of IL-12 responsive Jak and Stat pathways essential for mediating IL-12 effects on lymphocytes.25 In contrast to the rIL-12 refractory malignant T-cell population, Sezary patient CD8+ T cells, as well as peripheral blood lymphocytes of patch/plaque patients, do appear to possess the β2 component of the IL-12 receptor and do exhibit activation of IL-12 responsive signal transduction pathways when cultured with rhIL-12.25 These studies suggest that rhIL-12 is most likely to exert its effects in CTCL by mediating activation of normal immune cells. Thus, the in vivo responses and our in vitro data support the hypothesis that those patients with the most intact cell-mediated immune response are most likely to display a benefit from rIL-12.
Although previous studies of rhIL-12 administered intravenously have been associated with a greater incidence of adverse experiences,28 the current study has demonstrated that rhIL-12 is well tolerated when administered subcutaneously twice weekly in the doses used. The high response rate of patients with plaque stage CTCL and the lack of serious toxicity of rhIL-12 in this patient population has prompted the development of future phase II/III clinical trials. These studies should delineate those categories of patients most likely to benefit from the effects of rhIL-12. Another future goal of these trials should be to determine whether the beneficial antitumor effects of rhIL-12 can be synergistically enhanced by the concomitant use of other cytokines, which augment cell-mediated immunity.
ACKNOWLEDGMENT
The authors thank Dr Giorgio Trinchieri for the inspiration for this study.
Supported in part by grants from the Leukemia Society of America and by Genetics Institute, Inc, and by a grant from the National Institutes of Health to the General Clinical Research Center (5-MO1RR0040).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alain H. Rook, MD, Department of Dermatology, University of Pennsylvania, 3600 Spruce St, Philadelphia, PA 19104; e-mail: arook@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal