Activated protein C resistance (APCR) in the absence of alterations in the factor V gene has been observed during pregnancy, in patients on oral contraceptives, in the presence of antiphospholipid antibodies, and in patients with ischemic stroke. We report a 49-year-old woman with recurrent major venous and arterial thromboses who displayed pronounced APCR, yet no changes in the activated protein C (APC) cleavage sites of factor V. The APCR values determined by four different assays were similar to those obtained in plasma from a homozygote for factor V Q506. Addition of IgG isolated from the patient’s serum to normal plasma lowered the APCR ratio from 2.4 to 1.6. Incubation of patient’s IgG with normal APC resulted in a profound change in the mobility of APC in crossed immunoelectrophoresis. APC was also shown to bind to patient’s IgG immobilized on a protein A agarose column. Factor Va inactivation by APC was inhibited by patient’s IgG, but not by control IgG in the presence or absence of either phospholipids or protein S. These results provide evidence for the existence of an acquired antibody against APC in the patient’s plasma, which gave rise to the APCR phenotype and was probably responsible for the major thrombotic events. We suggest that acquired APCR due to anti-APC antibodies be considered a potential cause for severe venous and arterial thromboses.
A POOR ANTICOAGULANT response to activated protein C (APC) resulting in familial thrombophilia was first described by Dahlback et al.1 The predominant cause for this hereditary APC resistance (APCR) is a G1691A mutation in the factor V gene that abolishes the APC cleavage site at Arg506 due to Arg → Gln substitution.2-4 The heterozygous state for Arg506Gln confers a 2.7- to 7-fold increase in the relative risk toward venous thromboembolism5,6 and in homozygotes, the relative risk is substantially higher with odds ratios of 30 to 140.6,7 Other changes in the factor V gene that lead to APCR are a rare mutation, G1091C, in the second APC cleavage site at Arg306 (Arg306Thr substitution),8 and an array of polymorphisms designated HR2 haplotype.9
Acquired APCR has been observed during pregnancy,10 in patients taking oral contraceptives,11 patients with a lupus anticoagulant,12,13 and patients with stroke.14,15 The mechanisms by which APCR is generated in these conditions have not been elucidated. The presence of autoantibodies against protein C has been proposed as one possible mechanism for acquired APCR,1 and Mitchell et al16 demonstrated antibodies against the functional activities of protein C in a patient with a double monoclonal gammopathy. This patient had chronic disseminated intravascular coagulation for several years and finally died of extensive venous and arterial thrombosis, as well as spontaneous skin necrosis.
In the present study, we describe another patient with extensive venous and arterial thrombotic events and skin necrosis that were associated with profound APCR. We identified and characterized in this patient an IgG that specifically bound to APC, but not to protein C, inhibited the inactivation of factor Va by APC, and caused the resistance to APC.
MATERIALS AND METHODS
Plasma Samples
Blood samples were anticoagulated with 0.129 mol/L buffered sodium citrate, and platelet-free plasma was obtained by centrifugation, first at 3,000g and then at 10,000g. Plasma samples were stored at −35°C for assays that were not performed immediately. Blood samples were obtained from the patient in 1992, 1993, 1995, and 1998 while she was on enoxaparine treatment.
Coagulation assays.
Prothrombin time (PT) was measured using a recombinant tissue thromboplastin, Innovin, and activated partial thromboplastin time (APTT) was measured using Actin FS, both purchased from Dade (Miami, FL). Thrombin time was measured by standard techniques using bovine thrombin (Dade, Miami, FL). Atroxin time was measured after addition of 0.1 mL atroxin (Sigma, St Louis, MO) to 0.2 mL plasma. Protein C activity was assayed after activation of plasma protein C by a specific snake venom and hydrolysis of a specific chromogenic substrate by the formed APC (Baxter Dade, Bonnstrasse, Switzerland). Antithrombin (AT) activity was measured by a chromogenic assay (Chromogenix, Molndal, Sweden) and free protein S antigen was measured by enzyme-linked immunosorbent assay (ELISA) (Gradipore Elisa PS kit, North Ryde, Australia). Circulating anticoagulant was assayed by three methods: Staclot-LA kit (Diagnostica Stago, Asnieres, France), Kaolin APTT ratio,17 and dilute Russell’s viper venom (RVV) ratio (Gradipore). Anticardiolipin antibody (Autozyme ACL kit, Cambridge Life Sciences, Cambridge, UK), antiphosphatidylserine antibody and antiphosphatidylethanolamine antibody were assayed as described previously.18 19
APC resistance assays.
APCR was determined using the following four different assays: (1) an APTT-based assay in which APC is added at the last stage of the assay together with calcium ions (Coatest, Chromogenix, Molndal, Sweden); (2) an APTT-based assay in which protein C is activated by the addition of a snake venom (Protac, ProC Global; Behring Diagnostics, Marburg, Germany); (3) an RVV-based assay in which plasma is preincubated with APC (PC Impedence; Gradipore); (4) an RVV-based assay in which protein C is activated by snake venom (Factor V [Leiden] test; Gradipore). Some of the APCR tests were performed after dilution of patient’s or normal plasma with either factor V– or factor VIII–deficient plasmas. The results were expressed as a ratio of clotting time of the sample plasma in the presence of APC over the clotting time in the absence of APC. The normalized APCR ratio was the patient’s APCR ratio divided by control plasma APCR ratio. Normal plasma (Unicalibrator, Diagnostica Stago) served as reference plasma.
Purification of IgG
IgG fractions from plasma of healthy controls not bearing Q506-factor V, from plasma of an asymptomatic subject with homozygous Q506-factor V, and from plasma of the patient (obtained in 1995 and 1998) were purified by affinity chromatography on a protein A agarose column according to the manufacturer’s technical bulletin (Bio-Rad, Hercules, CA). The IgG fractions were dialyzed against 50 mmol/L Tris-HCl and 0.15 mmol/L NaCl, pH 7.5 (TBS) and concentrated by Centricon 30 ultrafiltration (Amicon, Beverly, MA) to approximately 14 mg/mL. IgG samples appeared homogenous on both mercaptoethanol-reduced and nonreduced 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The effect of IgG on APC activity determined by a chromogenic assay.
Equal volumes of 5 nmol/L APC (Haematologic Technologies Inc, Essex Junction, VT) and control or patient’s IgG (14 mg/mL) in TBS containing 1 mg/mL of bovine serum albumin (TBS-BSA) were incubated for 30 minutes at 37°C. A total of 30 μL of the reaction mixture was then added to 80 μL of a chromogenic substrate (Protein C kit; Diagnostica Stago) and ΔΑ/min was measured at 405 nm.
The effect of IgG on the inactivation of factor Va by APC.
For measurement of the effect of the patient’s IgG on the rate of inactivation of factor Va by APC, human factor Va (Haematologic Technologies Inc) and control or patient’s IgG, in TBS-BSA containing 5 mmol/L CaCl2, were incubated with APC in the presence or absence of phospholipids (rabbit brain cephalin; Sigma) and protein S (Enzyme Research, South Bend, IN). At selected time intervals, aliquots of the mixture were diluted in TBS-BSA and assayed for residual factor Va activity in a one stage PT-based assay using factor V–deficient plasma (Diagnostica Stago). Standard curves for residual factor V activity were prepared from clotting times of dilutions of control plasma in factor V–deficient plasma in a one stage PT-based assay. A detailed description of the reaction mixtures is given in the legend to Fig 3.
Binding of factor Va and APC to immobilized IgG.
The ability of the patient’s IgG to bind purified factor Va or APC was examined by passing these components through a protein A agarose column from which the IgG of the patient or a control had not been eluted. In these experiments, 1 μg of factor Va (20 to 25 U) in 250 μL TBS-BSA stabilized with 2.5 mmol/L CaCl2 or 200 ng APC in 200 μL TBS-BSA, were applied to either control or patient’s protein A-IgG columns (total volume, 1 mL) and collected into 250-μL fractions at 4°C. The amount of factor Va or APC retained on the columns, expressed as percent, was calculated from measurements of factor Va and APC concentrations, respectively, in samples applied and those that passed through the columns.
The concentration of APC was measured by incubating test samples with 3 nmol/L factor Va, 40 nmol/L protein S, 5% vol/vol phospholipids, and 5 mmol/L CaCl2 in TBS-BSA at 37°C for 1 minute and assaying the residual factor Va activity. APC activity was calculated from a standard curve using purified APC.
After the binding experiments were completed, the amounts of patient’s and control IgG on their respective protein A columns were calculated following elutions with 0.1 mol/L glycine, pH 2.8, and measurement of the optical density at 280 nm. The quantities of control and patient’s IgG were 8.2 mg and 11.6 mg, respectively.
Crossed immunoelectrophoresis.
Crossed immunoelectrophoreses of purified APC and plasma protein C was performed in 1% agarose (Seakem ME; FMC Bioproducts, Rockland, ME) in Tris-Tricine buffer (81 mmol/L Tris-HCl and 24 mmol/L tricine, pH 8.6).20 Rabbit antihuman protein C antiserum (Diagnostica Stago) was included in the agarose at a final concentration of 0.2% for electrophoresis in the second dimension. The antigen-antibody complexes (arcs) were visualized by autoradiography after immersing the agarose plates in TBS-BSA containing 100,000 cpm 125I Protein A/mL (NEN Life Science Products, Boston, MA) followed by excessive washing with distilled water.
Western blotting.
Purified IgG, factor V, factor Va, protein S, and APC were analyzed on 10% nonreduced SDS-PAGE gels according to the method of Laemmli.21 Proteins were transblotted onto Immobilon-P transfer membrane (Millipore, Bedford, MA) according to the method of Towbin et al.22 To examine whether the patient’s IgG could bind denatured nonreduced proteins, patient’s plasma that had been heat-inactivated at 56°C for 30 minutes was diluted 1 to 100 in TBS and applied to the membrane. IgG binding was detected using biotinylated goat antihuman IgG, ABC-peroxidase kit, and the substrate DAB (Vector, Burlingame, CA) according to the manufacturer’s instructions.
Search for Prothrombotic Polymorphisms
Genomic DNA was isolated from whole blood by a standard method.23 The Arg506Gln and Arg306Thr were analyzed by polymerase chain reaction (PCR) amplifications and enzyme digestions as previously described.8,24 The nucleotide C677T substitution in the methylenetetrahydrofolate reductase (MTHFR) gene and the G20210A substitution in the factor II gene were examined as previously described.25 26
Sequence Analysis of Factor V cDNA
Total RNA was obtained from peripheral blood mononuclear cells by TRI reagent kit (Molecular Research Center, Cincinnati, OH). mRNA was purified by QuickPrep Micro mRNA purification kit (Pharmacia, Uppsala, Sweden). First-strand cDNA was generated from mRNA using random hexamers (Boehringer Mannheim, Mannheim, Germany) and Avian Myeloblastosis virus reverse transcriptase (Promega, Madison, WI) and was sequenced by Sequenase II (US Biochemical, Cleveland, OH).
Sequence alterations from the published factor V cDNA27were confirmed by PCR amplification and restriction enzyme digestion. The sequence variations, A327G and G495A, are polymorphisms that were described elsewhere.28 The PCR-amplified product of exon 4 containing G495A was digested with the restriction enzyme AvaI to identify a new polymorphism G642T. An additional unpublished polymorphism in exon 13, C4300T, was identified by PCR amplification and DdeI digestion using forward 5′ATGCCCCTCTTTGCAGATCTCAG and reverse 5′AGTCACCTGGCTGAGGTCTGGG primers.
Case Report
The patient was 32 years old when she first presented in 1981 with idiopathic deep vein thrombosis (DVT) of the right femoral vein. Her past history was unremarkable. She has never smoked or taken oral contraceptives. Hypertension, diabetes mellitus, and dyslipidemia were excluded. At age 39, the patient experienced another episode of DVT at the same site and as a result, long-term warfarin treatment was instituted. Four years later (September, 1992), extensive skin necrosis of the left thigh and foot occurred and the patient was referred to our center. Warfarin treatment was discontinued and daily subcutaneous injections of enoxaparine 40 mg were administered. Repeated skin grafting failed to arrest progressive necrosis, and consequently below-knee amputation was performed. Despite this procedure and treatment with enoxaparine, skin necrosis continued at the stump. Pulse therapy of 1G methylprednisolone per day for 5 days finally resulted in cessation of the necrotic process. Two months later, while on 40 mg enoxaparine daily, the patient developed left cavernous sinus thrombosis resulting in blindness of the left eye. Consequently, the dose of enoxaparine was increased to 40 mg every 12 hours. Nine months later, while on this regimen, the patient presented with right hemiparesis and motor aphasia. Computerized tomography of the brain showed infarctions in the left internal capsule, caudate, and right parietal lobes. After this additional thrombotic event, the daily dose of enoxaparine was further increased to 80 mg every 12 hours and aspirin 100 mg/d was added. Since September 1993, the patient has been doing well. The patient’s mother, maternal grandmother, and uncle died of ischemic stroke at ages 46, 40, and 67, respectively. They all had morbid obesity and hypertension.
RESULTS
Search for a Possible Thrombophilia
PT, APTT, platelet count, fibrinogen level, thrombin time, and atroxin time were all within normal limits. Serum protein electrophoresis performed on several occasions was normal. Laboratory evaluation of the patient in 1992 disclosed normal values of antithrombin activity in a chromogenic assay, protein C amidolytic activity, and free protein S antigen (Table 1). Circulating anticoagulant and an increased titer of antiphospholipid antibodies were not found. The patient bore the normal factor V gene sequences G1691 and G1091, factor II G20210, and was heterozygous for MTHFR C677T.
Coagulation Tests of the Patient at Presentation
| . | Protein C Activity (%) . | Free Protein S Antigen (%) . | AT III Activity (%) . | CAC . | FV:C (%) . | FVIII:C (%) . | FVIIIAg (%) . |
|---|---|---|---|---|---|---|---|
| Patient | 100 | 95 | 89 | Negative | 109 | 109 | 160 |
| Normal range | 70-130 | 60-130 | 75-120 | Negative | 50-150 | 50-150 | 50-150 |
| . | Protein C Activity (%) . | Free Protein S Antigen (%) . | AT III Activity (%) . | CAC . | FV:C (%) . | FVIII:C (%) . | FVIIIAg (%) . |
|---|---|---|---|---|---|---|---|
| Patient | 100 | 95 | 89 | Negative | 109 | 109 | 160 |
| Normal range | 70-130 | 60-130 | 75-120 | Negative | 50-150 | 50-150 | 50-150 |
Abbreviations: CAC, circulating anticoagulant; Ag, antigen.
A plasma sample taken in 1993 showed an APCR ratio of 1.4 by an APTT-based assay (Coatest Chromogenix) as compared with values of >2.0 of normal individuals. The APCR ratio of patient’s plasma diluted with 9 parts factor VIII–deficient plasma was 3.3 versus an APCR ratio of 3.6 in normal plasma similarly diluted. This made a mutation in factor VIII gene an unlikely cause of the APCR phenotype. In contrast, the APCR ratio of the patient’s plasma was 1.8 (control value, 2.2) when diluted with 9 parts factor V–deficient plasma, suggesting a defect in the factor V gene responsible for the APCR phenotype. In retrospect, however, this was a misinterpretation, as the normalized APCR was 0.82 (1.8:2.2), which was in the low normal range (see below).
Search for a Defect in the Factor V Gene
In view of the central role of factor V defects giving rise to APCR, we sequenced the factor V cDNA. The analysis disclosed no remarkable mutations, and we did not find a previously identified APCR-related factor V HR2 haplotype.9 The only findings were four polymorphisms, all in the heterozygous state. These alterations, confirmed by restriction analysis of genomic DNA, were: A327G and G495A, (previously described28), and two new polymorphisms G642T in exon 4 (fully linked with G495A) and C4300T in exon 13. The frequency of the C4300 allele was 0.89 in 26 subjects examined. These analyses were inconsistent with a significant defect in the factor V gene.
Retesting of APCR Normalized Ratios
In 1998, we renewed our attempts to understand the nature of the thrombotic tendency exhibited by the patient. The patient’s plasma obtained on different occasions over the 5 years of follow-up was tested using four available commercial assays for APCR. APCR normalized ratios of the patient’s undiluted plasma were significantly decreased throughout the 5 years. In each assay the results were similar to those obtained with plasma from a homozygote for factor V Q506 (Table 2). However, 1:10 dilution of patient’s plasma with factor V–deficient plasma in samples obtained in 1993 and 1995 and 1:5 dilution of sample obtained in 1998 yielded normal normalized APCR ratios (Table 2). The assays performed on diluted plasma obtained in 1998 showed a higher APCR normalized ratio compared with the values for the plasma sample of 1995, ie, 0.80 versus 0.56 by an APTT-based test (Coatest, Chromogenix) and 0.75 versus 0.50 by an RVV-based test (PC Impedence, Gradipore) (Table 2). These results suggested that the patient had an inhibitory activity against the protein C pathway, which declined between 1995 and 1998.
Normalized Ratio of APCR Measured by Different Assays
| Assay . | Patient’s Samples* . | Homozygous FV Q506 Individual . | Normal Samples . | ||
|---|---|---|---|---|---|
| (1993) . | (1995) . | (1998) . | |||
| Chromogenix-Coatest | |||||
| No dilution | 0.50 | ND | 0.52 | 0.50 | >0.72 |
| 1:5 dilution in Vdp | ND | 0.56 | 0.80 | 0.44 | >0.70 |
| 1:10 dilution in Vdp | 0.82 | 0.77 | ND | ND | >0.72 |
| Gradipore-PCl | |||||
| No dilution | ND | ND | 0.52 | 0.43 | >0.73 |
| 1:5 dilution in Vdp | ND | 0.50 | 0.75 | 0.45 | >0.80 |
| Gradipore-FV Leiden | |||||
| No dilution | ND | ND | 0.34 | 0.26 | ND |
| 1:5 dilution in Vdp | ND | ND | 0.79 | 0.33 | ND |
| 1:10 dilution in Vdp | ND | ND | 0.84 | 0.33 | ND |
| Behring-ProC Global | |||||
| No dilution | ND | 0.50 | 0.41 | 0.38 | ND |
| 1:5 dilution in Vdp | ND | ND | 0.81 | 0.44 | ND |
| 1:10 dilution in Vdp | ND | ND | 0.87 | 0.50 | ND |
| Assay . | Patient’s Samples* . | Homozygous FV Q506 Individual . | Normal Samples . | ||
|---|---|---|---|---|---|
| (1993) . | (1995) . | (1998) . | |||
| Chromogenix-Coatest | |||||
| No dilution | 0.50 | ND | 0.52 | 0.50 | >0.72 |
| 1:5 dilution in Vdp | ND | 0.56 | 0.80 | 0.44 | >0.70 |
| 1:10 dilution in Vdp | 0.82 | 0.77 | ND | ND | >0.72 |
| Gradipore-PCl | |||||
| No dilution | ND | ND | 0.52 | 0.43 | >0.73 |
| 1:5 dilution in Vdp | ND | 0.50 | 0.75 | 0.45 | >0.80 |
| Gradipore-FV Leiden | |||||
| No dilution | ND | ND | 0.34 | 0.26 | ND |
| 1:5 dilution in Vdp | ND | ND | 0.79 | 0.33 | ND |
| 1:10 dilution in Vdp | ND | ND | 0.84 | 0.33 | ND |
| Behring-ProC Global | |||||
| No dilution | ND | 0.50 | 0.41 | 0.38 | ND |
| 1:5 dilution in Vdp | ND | ND | 0.81 | 0.44 | ND |
| 1:10 dilution in Vdp | ND | ND | 0.87 | 0.50 | ND |
Abbreviations: ND, not done; Vdp, Factor V deficient plasma.
All samples were taken when patient received enoxaparine. Baseline clotting times (without APC) were within normal range in all assay systems.
Demonstration of an Inhibitor of APC
The APCR performed by an APTT-based assay (ProC-Global, Behring) showed that addition of the patient’s IgG (final concentration 4.7 mg/mL) to normal plasma yielded a ratio of 1.6, whereas comparable amounts of control IgG or IgG obtained from an individual homozygous for factor V Q506 yielded APCR ratios of 2.4 and 2.6, respectively (Table 3). These results were similar to those obtained when the patient’s plasma was mixed with normal plasma, suggesting that the patient’s IgG was responsible for the inhibitory effect in the APCR assay.
Effects of Patient and Control IgG on APCR Ratios
| Sample Tested . | APCR Ratio . | APCR Normalized Ratio . |
|---|---|---|
| Normal plasma | 2.9 | 1.0 |
| Patient’s plasma | 1.2 | 0.41 |
| Homozygous FV Q506 plasma | 1.1 | 0.38 |
| 2 parts NP + 1 part N IgG | 2.4 | 1 |
| 2 parts NP + 1 part P IgG | 1.6 | 0.67 |
| 2 parts NP + 1 part H IgG | 2.6 | 1.08 |
| 4 parts NP + 1 part PP | 1.9 | 0.66 |
| 4 parts NP + 1 part HP | 2.3 | 0.79 |
| Sample Tested . | APCR Ratio . | APCR Normalized Ratio . |
|---|---|---|
| Normal plasma | 2.9 | 1.0 |
| Patient’s plasma | 1.2 | 0.41 |
| Homozygous FV Q506 plasma | 1.1 | 0.38 |
| 2 parts NP + 1 part N IgG | 2.4 | 1 |
| 2 parts NP + 1 part P IgG | 1.6 | 0.67 |
| 2 parts NP + 1 part H IgG | 2.6 | 1.08 |
| 4 parts NP + 1 part PP | 1.9 | 0.66 |
| 4 parts NP + 1 part HP | 2.3 | 0.79 |
Abbreviations: NP, normal plasma; PP, patient’s plasma obtained in 1998; HP, homozygote for factor V Q506; N IgG, normal IgG; P IgG, patient’s IgG; H IgG, IgG of a homozygote for factor V Q506.
To elucidate against which protein in the protein C system the inhibitor was directed, we examined the ability of the patient’s IgG, immobilized on a protein A agarose column, to bind factor Va or APC. The amounts of factor Va applied and recovered from the column were 24 and 27 U, respectively, for the patient’s IgG, and 20 and 23 U for the normal IgG, respectively. Thus, purified factor Va did not bind to the patient’s or control IgG. In contrast, as shown by the elution profiles of APC (Fig 1), there was significant binding of APC to the patient’s immobilized IgG in comparison to the control IgG. Amidolytic activity of APC in the pooled eluate indicated that 60% of the applied APC bound to the patient’s IgG, whereas no binding was observed by control IgG (96% recovery). These data strongly suggested that the inhibitor was an IgG antibody directed against APC.
Retention of APC on protein A columns to which control IgG (▪) or patient’s IgG (◂) were adsorbed. A total of 200 μL of APC (200 ng) in TBS-BSA was applied to the respective protein A-IgG columns and fractions of 250 μL were collected and assayed for APC activity as outlined in Materials and Methods. The results are expressed as percent of the APC concentration applied to the column.
Retention of APC on protein A columns to which control IgG (▪) or patient’s IgG (◂) were adsorbed. A total of 200 μL of APC (200 ng) in TBS-BSA was applied to the respective protein A-IgG columns and fractions of 250 μL were collected and assayed for APC activity as outlined in Materials and Methods. The results are expressed as percent of the APC concentration applied to the column.
Direct evidence for the binding of the patient’s IgG to APC was obtained by crossed immunoelectrophoresis (Fig 2). Addition of patient’s IgG to purified APC yielded a shoulder in the precipitin line representing immune complexes between the two (Fig 2A). No such shoulder was observed with control IgG. When normal plasma was used as a source of native protein C, the patient’s IgG had no effect on the electrophoretic pattern (Fig 2B). Therefore, the patient’s IgG had an affinity only for the activated form of protein C. Notably, the patient’s antibodies against APC did not react with denatured APC transferred onto a membrane as examined by Western immunoblotting analysis (data not shown).
Crossed immunoelectrophoresis of purified APC (A) or protein C in plasma (B) in the presence of control or patient’s IgG. Polyclonal rabbit antihuman protein C antiserum was used for detection of either APC or protein C as described in Materials and Methods. (A) Electrophoresis of 20 μL TBS-BSA containing 150 ng APC and 200 μg of control IgG (1) or patient’s IgG (2). (B) Electrophoresis of 10 μL of patient’s plasma (1), a mixture of 10 μL of normal plasma (40 ng protein C), and 10 μL of normal IgG (140 μg) (2) or 10 μL of normal plasma and 10 μL (140 μg) patient’s IgG (3).
Crossed immunoelectrophoresis of purified APC (A) or protein C in plasma (B) in the presence of control or patient’s IgG. Polyclonal rabbit antihuman protein C antiserum was used for detection of either APC or protein C as described in Materials and Methods. (A) Electrophoresis of 20 μL TBS-BSA containing 150 ng APC and 200 μg of control IgG (1) or patient’s IgG (2). (B) Electrophoresis of 10 μL of patient’s plasma (1), a mixture of 10 μL of normal plasma (40 ng protein C), and 10 μL of normal IgG (140 μg) (2) or 10 μL of normal plasma and 10 μL (140 μg) patient’s IgG (3).
Mechanism of the APC Inhibitory Activity
In experiments on the effect of patient’s and control IgG on APC activity as measured by hydrolysis of a chromogenic substrate, the patient’s IgG did not exert an inhibitory activity. Thus, the relative amidolytic activity expressed as ΔΑ/min of 2.5 nmol/L APC was 36 with patient’s IgG and 38 with control IgG. These results explain the finding of normal patient’s protein C amidolytic activity obtained using a chromogenic assay. This finding also indicates that the patient’s IgG apparently did not interfere with activation of protein C to APC.
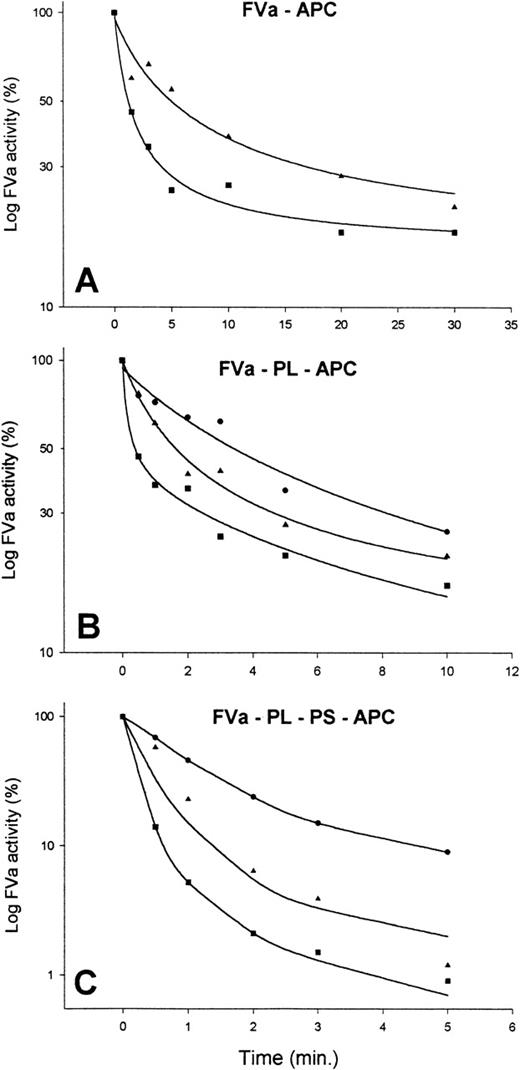
To clarify the mechanism by which the patient’s IgG inhibited APC activity, studies with purified factor Va and APC in the presence of control or patient’s IgG were undertaken (Fig 3). Factor Va inactivation by APC in the presence of phospholipids was decreased when patient’s IgG was added in comparison to normal IgG (Fig 3B). Similar results were obtained in the absence of phospholipids, although increased concentrations of APC were required to obtain the same extent of inactivation (Fig 3A). Addition of protein S, as well as phospholipids, significantly enhanced the ability of APC to inactivate factor Va in the presence of normal IgG, yielding 95% inactivation after 1 minute (Fig 3C). In contrast, in the presence of patient’s IgG at a concentration of 3.7 mg/mL, only a 20% inactivation of factor Va was observed, and using patient’s IgG at a concentration of 0.2 mg/mL resulted only in 50% inactivation (Fig 3C). In the presence of 3 mg/mL patient’s IgG, the extent of inhibition of APC-induced factor Va inactivation was similar whether or not APC was preincubated with the IgG (data not shown). These data were consistent with fast-acting antibodies. All experiments were performed with patient’s IgG obtained from plasma samples drawn in 1995. Patient’s IgG obtained in 1998 displayed a 20% to 50% less pronounced inhibitory activity indicating a decrease in the antibody titer (data not shown).
Inactivation of factor Va activity by APC in the presence of control or patient’s IgG. Factor Va was incubated with APC under various conditions. At different time points, samples were removed from the various incubation mixtures, kept at 37°C, and residual factor Va measured. Each point represents the mean of two experiments. (A) To TBS-BSA containing 5 mmol/L CaCl2 and 3 mg/mL of either control IgG (▪) or patient’s IgG (◂) factor Va at a final concentration of 12 nmol/L was added. This was followed by immediate addition of APC at a final concentration of 40 nmol/L. (B) Incubation mixtures of TBS-BSA containing 5 mmol/L CaCl2, 5% vol/vol phospholipid (PL), control (▪) or patient’s IgG at concentrations of 0.2 mg/mL (◂) or 3.7 mg/mL (•), 3 nmol/L factor Va, and 0.2 nmol/L APC. The curves for the two concentrations of control IgG were almost identical, and the curve shown represents the average of the data points. (C) Shows experiments as in (B) except for addition of protein S at a final concentration of 40 nmol/L immediately before the addition of factor Va and APC.
Inactivation of factor Va activity by APC in the presence of control or patient’s IgG. Factor Va was incubated with APC under various conditions. At different time points, samples were removed from the various incubation mixtures, kept at 37°C, and residual factor Va measured. Each point represents the mean of two experiments. (A) To TBS-BSA containing 5 mmol/L CaCl2 and 3 mg/mL of either control IgG (▪) or patient’s IgG (◂) factor Va at a final concentration of 12 nmol/L was added. This was followed by immediate addition of APC at a final concentration of 40 nmol/L. (B) Incubation mixtures of TBS-BSA containing 5 mmol/L CaCl2, 5% vol/vol phospholipid (PL), control (▪) or patient’s IgG at concentrations of 0.2 mg/mL (◂) or 3.7 mg/mL (•), 3 nmol/L factor Va, and 0.2 nmol/L APC. The curves for the two concentrations of control IgG were almost identical, and the curve shown represents the average of the data points. (C) Shows experiments as in (B) except for addition of protein S at a final concentration of 40 nmol/L immediately before the addition of factor Va and APC.
DISCUSSION
The patient described had a remarkable history of venous, arterial, and capillary thromboses spanning over 9 years until abated by use of high doses of low-molecular-weight heparin and aspirin. During none of the thrombotic episodes were provoking circumstances apparent and several events occurred despite heparin treatment. The only abnormality identified in the patient was a profound decrease in the APCR ratio, which was neither due to the common Arg506Gln prothrombotic polymorphism in factor V nor to a rare Arg306Thr change recently reported.8 Because dilution of the patient’s plasma in factor VIII–deficient plasma yielded a normal APCR ratio and dilution in factor V–deficient plasma only partially corrected the APCR ratio, we initially hypothesized that an unknown defect in factor V was responsible for the patient’s resistance to APC. However, an extensive analysis of the patient’s factor V cDNA failed to disclose significant alterations.
The data presented showed that the patient’s resistance to APC stemmed from an IgG inhibitor reacting with APC, but not with protein C. The evidence included: (1) substantial decrease of APCR ratio of normal plasma by the patient’s IgG (Table 3); (2) significant binding of purified normal APC to patient’s IgG immobilized on a protein A column (Fig 1); (3) abnormal migration in crossed immunoelectrophoresis of purified normal APC incubated with patient’s IgG, but normal migration of normal plasma protein C that was incubated with patient’s IgG (Fig2); and (4) inhibition of factor Va inactivation by APC in the presence of patient’s IgG (Fig 3). Additional evidence showed that the inhibitor failed to interfere with the hydrolytic activity of APC on a specific chromogenic substrate, which explained why the patient had normal plasma protein C in amidolytic activity assays. Taken together, it is conceivable that the patient’s IgG does not affect the active site of APC (the amidolytic activity is preserved), but does interfere with an APC exosite required for binding and degradation of factor Va.
Small amounts of APC circulate in blood of normal individuals,29 and primates infused with small amounts of thrombin exhibit increased levels of APC.30 The circulating APC has been suggested to operate as a physiological anticoagulant.29 Conceivably, the extensive thrombotic events experienced by our patient were related to partial or complete abolishment of the natural anticoagulant effects of circulating APC by the acquired inhibitor. A previously reported patient had an inhibitor directed against the functional activity of APC, which was associated with extensive thromboses.16 There are several differences between our patient and the one reported earlier.16 First, the reported patient had two IgG lambda paraproteins, whereas our patient had no gammopathy. Second, in the reported case, the antigen reacting with the antibodies was not defined, whereas direct interference of the IgG with APC was shown in the plasma from our patient. Third, in the reported patient, the anti-APC activity was only demonstrated in the presence of phospholipids, whereas, the anti-APC activity of our patient’s IgG was also demonstrable in the absence of phospholipids. A third patient with recurrent thrombotic events had an IgG lambda gammopathy, which was shown to bind protein C and inhibit its activity.31 Taken together, these patients illustrate that inhibitors of the protein C pathway can be associated with severe venous, capillary, and arterial thromboses.
The conspicuous laboratory finding in our patient was a profound decrease in the APCR ratio. Similar, although less pronounced, decreases in APCR ratio were observed in subjects using oral contraceptives,11 during normal pregnancy,10,32and in patients with antiphospholipid antibodies12,13 or ischemic stroke.14,15 Whereas increased factor VIII levels were suggested to be in part responsible for the diminished APCR ratios during normal pregnancy,32 no cause for the low APCR ratio in patients with ischemic stroke has been delineated. Similarly, many patients with venous thrombosis were recently shown to have a subnormal APCR ratio with a normal factor V genotype.33 It is speculated that autoantibodies causing mild acquired APCR are associated with at least some of these cases and may be far more common than heretofore appreciated.
Our observations imply that a search for anti-APC antibodies in such patients is warranted. However, such a search should not rely on currently used methods of APCR ratio determinations that include 1:5 or 1:10 dilution of patient’s plasma in factor V–deficient plasmas. Such a procedure may dilute out the effects of an antibody against APC. Screening for an APC inhibitor might consist of APCR ratio determination of 1:1 mixture of patient’s plasma and normal plasma. Alternatively, various protocols that involve mixing of patient’s IgG with APC before assaying APC activity would allow detection of anti-APC antibodies.
Supported in part by Grants No. HL21544 and HL52256 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Uri Seligsohn, MD, Institute of Thrombosis and Hemostasis, Department of Hematology, Sheba Medical Center, Tel-Hashomer 52621, Israel; e-mail: zeligson@post.tau.ac.il.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal