CD4+ T cells from patients with human immunodeficiency virus (HIV) infection undergo apoptosis at an increased rate, which leads to their depletion during disease progression. Both the Fas-Receptor (Fas-R) and interleukin-1β (IL-1β)–converting enzyme (ICE; caspase 1) appear to play a role in the mechanism of apoptosis of CD4+ lymphocytes. Although Fas-R is upregulated on both CD4+ and CD8+ cells in HIV-infected patients, results from our laboratory and others indicate that, in patients with advanced disease, CD4+ cells preferentially express ICE. Protease inhibitors have successfully halted the progression of HIV disease and increased CD4+ T counts. In this study, we examined the effect of protease inhibitors on Fas-R (CD95), ICE (caspase 1) expression, apoptosis, and cell death in CD4+ T cells of (1) HIV-infected patients who were receiving protease inhibitors, and (2) normal and patient CD4+ T cells cultured with a protease inhibitor in vitro. Fifteen patients with advanced HIV disease on treatment showed dramatically decreased CD4+ T-cell ICE expression, diminished apoptosis, and increased numbers of CD4+ cells within 6 weeks of institution of protease inhibitor therapy, and before down-modulation of Fas-R (CD95) expression was evident. To determine the role of HIV infection, we studied the effect of ritonavir, a protease inhibitor, on normal and patient cells in vitro. Stimulated and unstimulated normal CD4+ T cells, cultured with protease inhibitor, demonstrated markedly decreased apoptosis and ICE expression (P = .01). While Fas-R expression was not significantly altered during short-term culture by such treatment, Fas-Ligand (Fas-L) membrane expression of phytohemagglutinin (PHA)-stimulated blood lymphocytes was decreased by protease inhibitor. In the presence of ritonavir, CD4+ T cells from HIV-infected patients showed similar changes in ICE intracellular levels without alteration of Fas expression. In conclusion, protease inhibitors appear to decrease CD4+ T-cell ICE expression and apoptosis before they affect Fas-R expression in HIV-infected patients. This action was independent of HIV infection, as similar effects were seen in CD4+ T cells from normal controls. Some of the benefit of protease inhibitors may be related to modification of programmed cell death, which increases CD4+ T-cell number. Whether this is due to directly to the changes effected in the caspase system remains to be determined.
INFECTION WITH THE human immunodeficiency virus type 1 (HIV-1) is associated with apoptosis and subsequent depletion of CD4+ cells, a process partially mediated by the Fas-Receptor (Fas-R; CD95) and by interleukin-1β (IL-1β)–converting enzyme (ICE; caspase 1).1-3 Apoptosis of CD4+ cells is responsible for depletion of CD4+ cells, independent of virally mediated cytolysis.4-6 Spontaneous and activation-induced programmed cell death leads to depletion of uninfected CD4+ cells in patients with HIV infection.2,4,6 Fas-mediated apoptosis may be responsible for a portion of CD4+ cell death. Fas-R (CD95) expression is increased on both CD4+ and CD8+ cells in HIV-infected individuals.7,8Apoptosis is mediated by cross-linking of the Fas-R by Fas-Ligand (Fas-L). Upregulation of the Fas-R, as well as Fas-L, is associated with HIV infection and may be induced by HIV-Tat and glycoprotein (gp)120.9,10 TH1 cells have a differential susceptibility to gp120-mediated apoptosis.11 However, not all apoptosis in HIV-infected individuals is mediated by Fas; for example, apoptosis induced by activation of the CD4+ cells by CD3 antibody is independent of Fas-R expression. Nef, a protein of HIV that binds to and induces apoptosis of uninfected CD4+ cells, also acts independently of Fas-R expression.12
ICE is integral to both Fas-R–independent and –dependent apoptosis, as ICE inhibitors block apoptosis in most CD4+cells.1-3 While Fas-R expression is upregulated in both CD4+ and CD8+ cells of HIV-infected patients,7,8 ICE protein is preferentially expressed in CD4+ T cells in this group, but not in normals.1 Uninfected CD4+ T cells express ICE mRNA, but they do not express active ICE protein.1Activation-induced cell death of CD4+ cells in HIV-infected individuals is Fas-independent and can be blocked by inhibitors of ICE.1-3 Because ICE protein is expressed in CD4+ T cells of HIV-infected patients and ICE inhibitors block apoptosis and cell death of these cells in culture, agents that modulate ICE protein expression and activity might potentially modify CD4+ T-cell depletion.
HIV-1 protease inhibitors cause substantial increases in CD4+ cell counts, halt disease progression, and decrease HIV viremia.13,14 The central mechanism of action of these protease inhibitors is to block HIV protease activity, which is necessary for the maturation of infectious virions. Previously, we reported that a small group of HIV-infected patients who were receiving protease inhibitors showed significantly reduced ICE expression in CD4+T cells, although they had advanced disease.1 Nucleoside analogs decrease Fas-L mRNA expression and Fas-R expression in HIV-1–infected patients,15 and one interpretation of this observation was that the increased CD4+ cell count and the favorable clinical response resulted from modulation of apoptosis in this lymphocyte population.
In the current study, we systematically examined the effects of HIV protease inhibitors on CD4+ T-cell apoptosis, cell death, and ICE expression in CD4+ T lymphocytes obtained from HIV-infected patients who had previously failed to respond to treatment with nucleoside analogs. We then determined if these effects were dependent on the presence of HIV by examining the in vitro actions of ritonavir, a protease inhibitor, on lymphocytes of both normal and HIV-infected persons. We also tested the influence of protease inhibitors on normal CD4+ cell expression of Fas-R and Fas-L to further assess the mechanism by which they alter susceptibility to apoptosis.
MATERIALS AND METHODS
Patient selection.
Peripheral blood (PB) samples were obtained from patients with AIDS and normal volunteers who provided informed consent according to protocols approved by the Institutional Review Boards of the National Heart, Lung, and Blood Institute (Bethesda, MD) and the Georgetown University Medical Center (Washington, DC). HIV-infected patients, who were not currently taking protease inhibitors, but for whom the clinical decision was already made by their primary care physician to place them on protease inhibitors, were chosen from the patient population of the HIV Clinic at Georgetown University Hospital. Patients received a combination of protease inhibitors and were examined weekly to biweekly intervals. Venous blood was obtained for studies immediately before initiation of treatment with protease inhibitors and then at 3 to 6 weeks and 4 to 6 months.
Cell separation and culture.
PB mononuclear cells (PBMC) were separated using density gradient centrifugation with lymphocyte separation media (Organon, Durham, NC). Thereafter, cells were washed twice with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 supplemented with fetal calf serum (FCS; both Life Technologies, Gaithersburg, MD). Cultures were performed at a cell density of 0.5 × 106 cells/mL. When appropriate, natural lymphocyte-derived IL-2 (Boehringer Mannheim, Indianapolis, IN) or phytohemagglutinin (PHA; Boehringer) was used for stimulation at concentrations of 10 U/mL or 5 μg/mL, respectively. Anti-Fas monoclonal antibody (MoAb) CH11 (Kamyia, San Francisco, CA), which mimics the Fas-L by cross-linking the Fas-R, was used in some cultures.
Flow cytometry.
For the measurement of Fas expression on CD4+ and CD8+ PBMC by flow cytometry, a whole-blood test was used. Briefly, 100 μL of cells were incubated with 20 μL phycoerythrin (PE)-conjugated anti-CD4 or -CD8 MoAb (Becton Dickinson, Mountain View, CA) combined with 20 μL of fluorescein isothiocyanate (FITC)-conjugated anti-CD95 MoAb (UB2; PharMingen, San Diego, CA) or anti–Fas-L (Immunotech, Miami, FL). After 15 minutes of incubation, cells were washed and fixed in 0.2% paraformaldehyde. Samples were analyzed using an Epics ELITE flow cytometer (Coulter, Miami, FL).
Intracellular staining.
Intracellular staining for ICE expression was performed using the PharMingen Intracellular Staining Kit.16 PBMC were stained with CD4 MoAb, fixed, and permeabilized and stained with anti-ICE antibody and FITC-labeled conjugated antirabbit antibody. Permeabilized isotypic controls were stained with secondary antibodies.
Apoptosis assays.
Annexin assay PBMC were prepared as described earlier, washed with PBS, and stained with annexin and propidium iodide (Tat assay) as previously described.17 Samples were analyzed using flow cytometry. An in situ thymidine deoxyneuclease (TdT) assay (Apotag; Oncor, Gaithersburg, MD) also was used to quantitate the number of cells that underwent apoptosis. Cells were harvested, fixed with 4% neutral buffered formalin, and centrifuged. Endogenous peroxidase was quenched with 0.5% hydrogen peroxide and the cells were permeabilized using company-supplied equilibration buffer. The 3′-OH ends of degraded DNA were reacted with TdT and digoxygenin-labeled adenosine triphosphate (ATP) for 30 minutes. After washing with PBS, cells were reacted with antidigoxygenin MoAb conjugated to peroxidase, washed, and exposed to 3,3′ diaminobenzidine tetrahydrochloride (DAB; Pierce, Rockford, IL) and the percent fluorescence quantitated by flow cytometry.
p24 enzyme-linked immunosorbent assay.
Viral replication was determined by p24 production. Tissue culture supernatants were harvested and stored at −70°C until analysis. p24 concentrations in tissue culture supernatants were determined in duplicate using a commercially available enzyme-linked immunosorbent assay (ELISA; Coulter) according to the manufacturer’s recommendations. To count for cell death, p24 production was expressed per 1 × 106 viable cells.
RESULTS
Effect of ritonavir on viability, apoptosis, and ICE expression in HIV-infected patients placed on protease inhibitors.
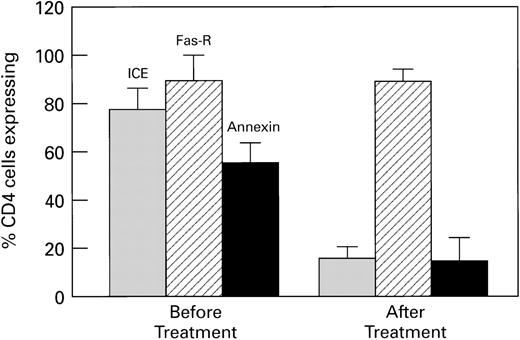
As an extension of our previous observations that HIV-infected patients who received protease inhibitors demonstrated decreased ICE expression in CD4+ T cells, we studied a group of patients before and following institution of protease inhibitor therapy. Fifteen patients with HIV disease, who had failed to respond to treatment with nucleoside analogs, were studied before and following initiation of treatment with a HIV protease inhibitor combination (Table1). Patients had no active bacterial infections, although 2 had stable cytomegalovirus (CMV) disease, for which they were receiving ganciclovir therapy. The average increase in CD4+ cell count during the period of evaluation was 20 cells/mL. When lymphocytes were stained with CD4+-PE, with annexin and propidium iodide (PI), or with ICE antibody, CD4+ cells from patients after a mean of 6 weeks of treatment demonstrated significantly decreased apoptosis, cell death, and ICE expression (Fig 1). Fas-R expression was not significantly changed after a mean of 6 weeks of treatment. Patients who received protease inhibitors for longer periods of time (3 to 4 months), with significant clinical responses and undetectable viral titers, demonstrated normal Fas-R and ICE expression (data now shown). In this group, apoptosis of CD4+ cells was still increased compared with normals (Fig2 and Table 2), which suggests that excessive apoptosis and CD4+ T-cell depletion continued to occur, despite optimal therapy, and that pathways that lead to apoptosis, which do not involve ICE and FAS, might be involved. TdT assay also demonstrated apoptosis (data not shown). Staining for other effectors of apoptosis, tumor necrosis factor receptor (TNF-R), bcl-2, and p53, was similar in normals and this group of HIV-infected patients (data not shown).
Patient Characteristics
| Patient No. . | Before . | After . | ||
|---|---|---|---|---|
| CD4 Count/μl . | Antiviral . | CD4 Count/μl . | Antiviral . | |
| 1 | 386 | AZT, 3TC | 517 | 3TC, D4T, saquinavir |
| 2 | 2 | D4T | 20 | Nelfinavir, 3TC, D4T |
| 3 | 2 | D4T, 3TC | 20 | D4T, 3TC, indinavir |
| 4 | 470 | AZT, 3TC | 562 | 3TC, saguinavir |
| 5 | 420 | AZT, 3TC | 500 | 3TC, saguinavir |
| 6 | 223 | D4T, 3TC | 227 | 3TC, crixivan |
| 7 | 10 | D4T, 3TC | 10 | D4T, 3TC, norvir |
| 8 | 21 | D4T, 3TC | 26 | D4T, 3TC, nelfinavir |
| 9 | 6 | D4T, 3TC | 70 | D4T, 3TC, nelfinavir |
| 10 | 2 | D4T, 3TC | 14 | D4T, 3TC, nelfinavir |
| 11 | 116 | AZT, 3TC | 135 | AZT, 3TC, nelfinavir |
| 12 | 135 | AZT, 3TC | 116 | AZT, 3TC, nelfinavir |
| 13 | 576 | None | 612 | Crixivan, D4T, 3TC |
| 14 | 16 | 3TC, AZT | 20 | 3TC, AZT, nelfinavir |
| 15 | 5 | D4T, 3TC | 120 | D4T, 3TC, nelfinavir |
| Patient No. . | Before . | After . | ||
|---|---|---|---|---|
| CD4 Count/μl . | Antiviral . | CD4 Count/μl . | Antiviral . | |
| 1 | 386 | AZT, 3TC | 517 | 3TC, D4T, saquinavir |
| 2 | 2 | D4T | 20 | Nelfinavir, 3TC, D4T |
| 3 | 2 | D4T, 3TC | 20 | D4T, 3TC, indinavir |
| 4 | 470 | AZT, 3TC | 562 | 3TC, saguinavir |
| 5 | 420 | AZT, 3TC | 500 | 3TC, saguinavir |
| 6 | 223 | D4T, 3TC | 227 | 3TC, crixivan |
| 7 | 10 | D4T, 3TC | 10 | D4T, 3TC, norvir |
| 8 | 21 | D4T, 3TC | 26 | D4T, 3TC, nelfinavir |
| 9 | 6 | D4T, 3TC | 70 | D4T, 3TC, nelfinavir |
| 10 | 2 | D4T, 3TC | 14 | D4T, 3TC, nelfinavir |
| 11 | 116 | AZT, 3TC | 135 | AZT, 3TC, nelfinavir |
| 12 | 135 | AZT, 3TC | 116 | AZT, 3TC, nelfinavir |
| 13 | 576 | None | 612 | Crixivan, D4T, 3TC |
| 14 | 16 | 3TC, AZT | 20 | 3TC, AZT, nelfinavir |
| 15 | 5 | D4T, 3TC | 120 | D4T, 3TC, nelfinavir |
Abbreviations: AZT, zidovudine; 3TC, lamidudine; D4T, stavudine.
Combination treatment with protease inhibitors decreases ICE expression and apoptosis in CD4 cells from HIV-infected patients. Fifteen patients who failed nucleoside analog treatment were placed on antiviral therapy that included a protease inhibitor. Blood was analyzed before and 6 to 8 weeks after treatment. Cells were analyzed for ICE and Fas-R expression, and apoptotic cells assessed by annexin V binding. There were significant decreases in ICE expression and apoptosis following treatment (P < .01), while there was no significant change in Fas-R expression. CD4 counts increased a mean of 20 cells/mL.
Combination treatment with protease inhibitors decreases ICE expression and apoptosis in CD4 cells from HIV-infected patients. Fifteen patients who failed nucleoside analog treatment were placed on antiviral therapy that included a protease inhibitor. Blood was analyzed before and 6 to 8 weeks after treatment. Cells were analyzed for ICE and Fas-R expression, and apoptotic cells assessed by annexin V binding. There were significant decreases in ICE expression and apoptosis following treatment (P < .01), while there was no significant change in Fas-R expression. CD4 counts increased a mean of 20 cells/mL.
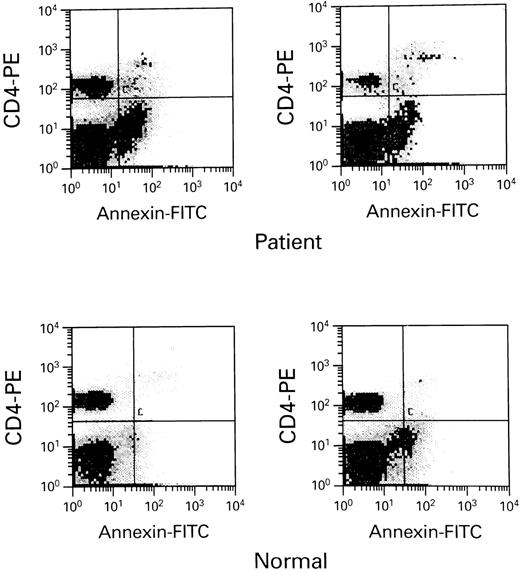
HIV-infected persons with undetectable viral loads still demonstrate excessive apoptosis when compared with normal controls. Ten HIV patients receiving combination antiviral therapy with a protease inhibitor combination demonstrated normal Fas-R and ICE expression, but excessive apoptosis when compared with 6 normal controls. Scattergrams of apoptotic cells are seen above form-selected HIV-infected patients and normals.
HIV-infected persons with undetectable viral loads still demonstrate excessive apoptosis when compared with normal controls. Ten HIV patients receiving combination antiviral therapy with a protease inhibitor combination demonstrated normal Fas-R and ICE expression, but excessive apoptosis when compared with 6 normal controls. Scattergrams of apoptotic cells are seen above form-selected HIV-infected patients and normals.
CD4+ T Cells From Normal and Patient (selected because of undetectable viral titers) Samples Stained for PI and Annexin
| Subject No. . | % PI+ . | % Pl−/Annexin+ . |
|---|---|---|
| Patients | ||
| 1 | 21 | 26 |
| 2 | 33 | 35 |
| 3 | 20 | 24 |
| 4 | 50 | 40 |
| 5 | 30 | 26 |
| 6 | 7 | 43 |
| 7 | 4 | 10 |
| 8 | 35 | 20 |
| 9 | 25 | 15 |
| 10 | 17 | 25 |
| Mean | 24.2 | 29.4 |
| Controls | ||
| 1 | 4 | 1 |
| 2 | 6 | 5 |
| 3 | 7 | 9 |
| 4 | 5 | 3 |
| 5 | 2 | 3 |
| 6 | 1 | 1 |
| Mean | 4.1 | 3.6 |
| Subject No. . | % PI+ . | % Pl−/Annexin+ . |
|---|---|---|
| Patients | ||
| 1 | 21 | 26 |
| 2 | 33 | 35 |
| 3 | 20 | 24 |
| 4 | 50 | 40 |
| 5 | 30 | 26 |
| 6 | 7 | 43 |
| 7 | 4 | 10 |
| 8 | 35 | 20 |
| 9 | 25 | 15 |
| 10 | 17 | 25 |
| Mean | 24.2 | 29.4 |
| Controls | ||
| 1 | 4 | 1 |
| 2 | 6 | 5 |
| 3 | 7 | 9 |
| 4 | 5 | 3 |
| 5 | 2 | 3 |
| 6 | 1 | 1 |
| Mean | 4.1 | 3.6 |
Ritonavir decreases ICE expression, apoptosis, and cell death in cultured normal CD4+ cells deprived of IL-2 or stimulated with Fas agonist.
While adequate control of viral replication appeared to be the most likely explanation of the beneficial effects and drug therapy on apoptosis, we could not exclude a direct effect of protease inhibitors on apoptotic pathways that were independent of the virus. To address this hypothesis, we performed in vitro experiments using both patient and normal uninfected PB lymphocytes (PBLs). When PBLs from normal controls (n = 10) deprived of IL-2 so as to induce programmed cell death were cultured with ritonavir for 72 hours, they demonstrated significantly deceased apoptosis and cell death when compared with cultures in the presence of protease inhibitor (P< .01; Fig 3). Similar results were obtained using the TdT assay (Fig 4). Fas-R expression was not significantly altered by drug exposure. High ritonavir concentrations (>20 nmol/L) resulted in increased cell death. Similar, but less pronounced changes were seen in CD8+ cells (data not shown). CPP32 expression was unaffected by ritonavir (data not shown).
Ritonavir decreases ICE expression and apoptosis in normal CD4+ cells deprived of IL-2. PBMC from normal donors were cultured without IL-2. After 72 hours, cells were analyzed by flow cytometry; ICE expression and apoptosis were significantly decreased in cells cocultivated with ritonavir (P < .01).
Ritonavir decreases ICE expression and apoptosis in normal CD4+ cells deprived of IL-2. PBMC from normal donors were cultured without IL-2. After 72 hours, cells were analyzed by flow cytometry; ICE expression and apoptosis were significantly decreased in cells cocultivated with ritonavir (P < .01).
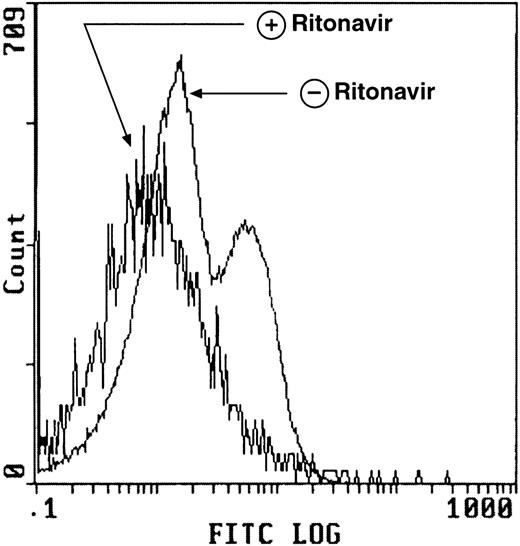
Ritonavir decreases apoptosis in normal CD4+ T cells deprived of IL-2 using the TdT assay. PBMC from normal donors prepared and cultured as described in Fig 3 demonstrate decreased apoptosis as measured by the TdT assay after exposure to ritonavir. The number of apoptotic cells was determined using 2-color flow cytometry. Cells were gated to include only CD4+ cells (stained with FITC-labeled CD4-PE MoAb) are seen in the histogram above.
Ritonavir decreases apoptosis in normal CD4+ T cells deprived of IL-2 using the TdT assay. PBMC from normal donors prepared and cultured as described in Fig 3 demonstrate decreased apoptosis as measured by the TdT assay after exposure to ritonavir. The number of apoptotic cells was determined using 2-color flow cytometry. Cells were gated to include only CD4+ cells (stained with FITC-labeled CD4-PE MoAb) are seen in the histogram above.
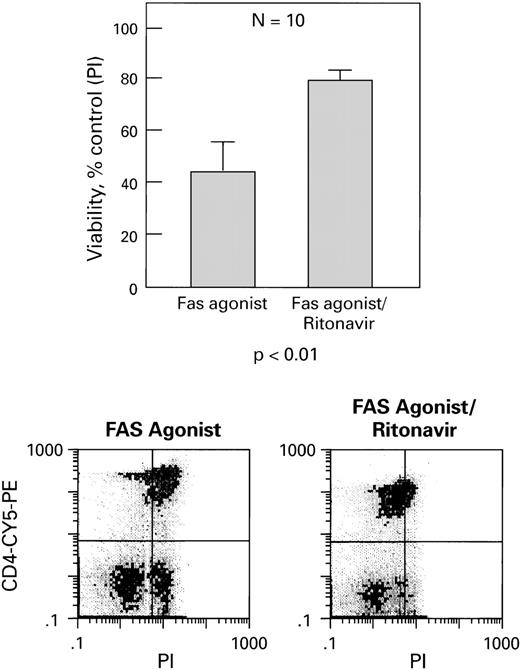
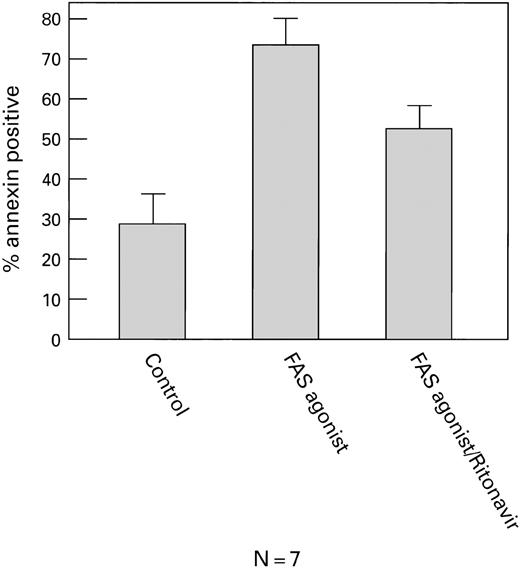
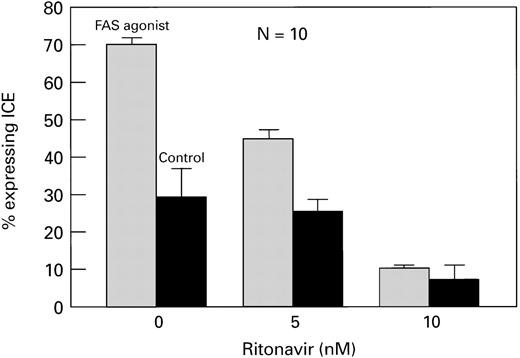
In a second set of experiments, when normal CD4+ cells were activated with IL-2 and PHA and then exposed to a Fas agonist (MoAb CH11). These cells also showed increased viability (Fig5), decreased apoptosis (Fig6), and decreased ICE expression (Fig7) in the presence of ritonavir (P< .01). All effects were dose-dependent, and most pronounced after stimulation with Fas agonist. Similar findings were obtained when CD4+ cells from HIV+ patients (n = 4) were examined (data not shown).
Ritonavir increases cell viability of CD4+cells treated with Fas agonist (CH11). PBMC from normal donors were cultured with Fas agonist with and without ritonavir (5 nmol/L). Viability is expressed as percentage of untreated (ie, without Fas agonist) control. Cell viability was determined by PI staining. Examples of scattergrams are shown.
Ritonavir increases cell viability of CD4+cells treated with Fas agonist (CH11). PBMC from normal donors were cultured with Fas agonist with and without ritonavir (5 nmol/L). Viability is expressed as percentage of untreated (ie, without Fas agonist) control. Cell viability was determined by PI staining. Examples of scattergrams are shown.
Ritonavir decreases apoptosis in CD4+cells cultured with Fas agonist. Normal PBMC were cultured with Fas agonist, with and without ritonavir (5 nmol/L). Cells were stained with CD4-PE and FITC-annexin V and analyzed by flow cytometry. There was a significant (P < .05) decrease in annexin V binding in cells treated with ritonavir.
Ritonavir decreases apoptosis in CD4+cells cultured with Fas agonist. Normal PBMC were cultured with Fas agonist, with and without ritonavir (5 nmol/L). Cells were stained with CD4-PE and FITC-annexin V and analyzed by flow cytometry. There was a significant (P < .05) decrease in annexin V binding in cells treated with ritonavir.
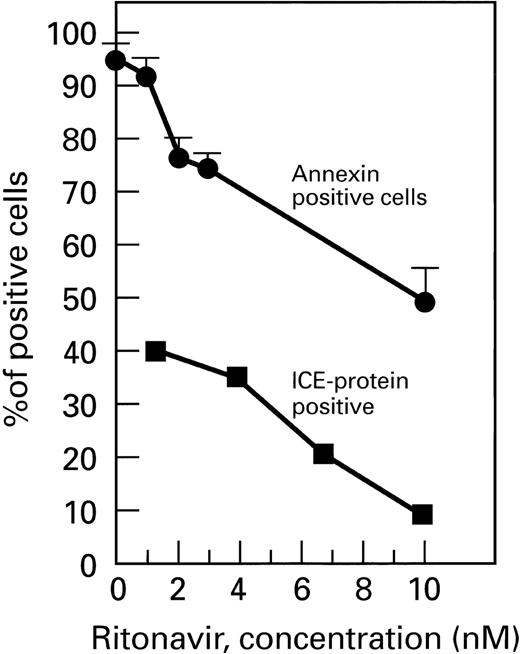
Ritonavir decreases ICE expression in CD4+cells cultured with Fas agonist. Normal PBMC were cultured with and without ritonavir. Cells were surface-stained with CD4-PE, permeabilized, and stained with ICE-FITC. CD4+ cells expressing ICE are shown. There was a significant, dose-dependent decrease in ICE expression (P < .01).
Ritonavir decreases ICE expression in CD4+cells cultured with Fas agonist. Normal PBMC were cultured with and without ritonavir. Cells were surface-stained with CD4-PE, permeabilized, and stained with ICE-FITC. CD4+ cells expressing ICE are shown. There was a significant, dose-dependent decrease in ICE expression (P < .01).
We also tested whether a nucleoside analog had a similar effect on CD4+ T cells from normal controls (n = 2) when cells were cultured under similar conditions. There was no difference in apoptosis, cell death, or ICE expression after 72 hours in culture with IL-2 and Fas agonist (data not shown).
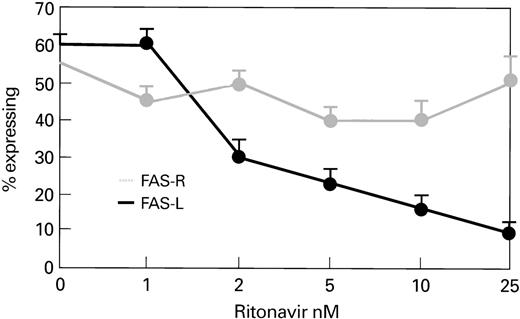
To assess whether protease inhibitors exerted their effect through Fas-L, we cocultivated normal lymphocytes (n = 3) with ritonavir and examined expression of Fas-L. When PBLs were cultured with IL-2 and PHA, ritonavir-treated CD4+ cells showed decreased expression of Fas-L, which was dose-dependent and independent of Fas-R expression (Fig 8).
Ritonavir decreases Fas-L expression, but does not alter Fas-R expression in CD4+ cells stimulated with PHA. Samples of normal PBMC were cultured with PHA for 72 hours at 37°C at different concentrations of ritonavir. There was a significant dose-dependent decrease in Fas-L expression with ritonavir, although there was no change in Fas-R expression.
Ritonavir decreases Fas-L expression, but does not alter Fas-R expression in CD4+ cells stimulated with PHA. Samples of normal PBMC were cultured with PHA for 72 hours at 37°C at different concentrations of ritonavir. There was a significant dose-dependent decrease in Fas-L expression with ritonavir, although there was no change in Fas-R expression.
DISCUSSION
Protease inhibitors have favorably altered the quality of life of HIV-infected patients. HIV protease inhibitors block cleavage of gag and gag-pol protein precursors, aborting virus infectivity.18
These drugs increase CD4+ T-cell counts, increase naive and memory cells, enhance lymphoproliferative responses, and reduce plasma concentrations of cytokines such as TNF-α that promote apoptosis.19 Protease inhibitors are presumed to exert their positive effect on CD4+ T-cell number and immune function by inhibiting viral replication. However, clinical data suggest that the beneficial consequences of protease inhibitors persist even after viral resistance to the drug has developed,20and these drugs may thus affect the immune system, independent of their inhibition of retroviral replication.
The current work demonstrates that protease inhibitor therapy is associated with decreased ICE (caspase 1) expression, apoptosis, and cell death of CD4+ T cells. We further show that these changes in ICE protein expression and in apoptotic cell death may not solely be related to changes in viremia, viral products, or cytokines. In vitro experiments, using CD4+ T cells from normal uninfected controls, showed similar significant declines in ICE expression, apoptosis, and cell death when lymphocytes exposed to Fas agonist or cultured in the absence of IL-2. Protease inhibitors may thus exert effects on apoptosis, independent of their effects on HIV, by affecting directly or indirectly the generation of active ICE protein in the apoptotic pathway.
Others have reported that effective treatment of HIV-infected patients with antiviral therapy lowered Fas-L and Fas-R expression with decreased apoptosis in the CD4+ T-cell population.15 Our study demonstrates that, in the HIV-infected patient, protease inhibitors block apoptosis and cell death, while they inhibit active ICE formation in CD4+cells. We confirm the observations of others that Fas-R expression declines after initiation of antiviral therapy, which is probably a result of lower levels of viremia and fewer viral products to promote Fas-R expression.13 However, findings due to changes in Fas-R expression temporally followed changes in apoptosis and ICE expression, and therefore decreases in Fas-R expression do not appear alone sufficient to account for decreased apoptosis in the lymphocytes observed with treatment. This observation is consistent with those of others who showed that HIV kills CD4+ cells by a Fas-independent mechanism.21
The pathophysiologic pathway responsible for inhibition of ICE expression by protease inhibitors is unknown. Expression of active ICE protein in mammalian cells is regulated posttranscriptionally by control and cleavage of an aspartate residue from the inactive pro-ICE protein. To determine if protease inhibitors could potentially bind to the inactive ICE precursor in a manner analogous to their binding to the HIV protease, we compared the sequences of HIV-1 protease and caspase 1 (ICE), but no significant similarity was found between them nor did HIV-1 protease sequence show similarity to either caspase 2 (ICH-1) or caspase 3 (CPP32). Further studies are needed to determine if protease inhibitors actually bind to caspase 1 or to pro-caspase 1.
The number of apoptotic and dead CD4+ T cells remained abnormally high in the protease inhibitor–treated patients, despite undetectable viral loads, even with apparently normal ICE and Fas-R expression. This suggests that an additional ICE- and Fas-independent pathway is responsible for apoptosis and cell death in this population. HIV infection is associated with an increased rate of activation-associated necrosis,22 although in this study, apoptosis was excessive and appeared to account for most of the lymphocyte death seen. Other caspases also are involved in apoptosis, including CPP32 and FLICE (caspase 8), and more recent studies suggest that these caspases may have more prominent roles in apoptosis than caspase 1.23 TNF-related apoptosis-inducing ligand (TRAIL)24 also has been shown to be responsible for apoptosis in HIV infection and is independent of ICE.
One implication of our findings is that inhibitors of ICE or CD4 apoptosis, without antiviral activity, may have potential therapeutic utility in the treatment of HIV infection, even in patients with low viral loads. However, caution is indicated, as increased viral replication in infected cells subjected to ICE blockade has been reported in vitro,25 possibly related to the role of apoptosis in controlling viral replication.26 27
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Elaine M. Sloand, MD, National Institutes of Health, National Heart, Lung, and Blood Institute, 31 Center Dr, MSC 2490, Building 31, Room 4A11, Bethesda, MD 20892-2490.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal