Abstract
The Wiskott-Aldrich syndrome (WAS) is an X-linked hereditary disease characterized by thrombocytopenia with small platelet size, eczema, and increased susceptibility to infections. The gene responsible for WAS was recently cloned. Although the precise function of WAS protein (WASP) is unknown, it appears to play a critical role in the regulation of cytoskeletal organization. The platelet defect, resulting in thombocytopenia and small platelet size, is a consistent finding in patients with mutations in the WASP gene. However, its exact mechanism is unknown. Regarding WASP function in cytoskeletal organization, we investigated whether these platelet abnormalities could be due to a defect in proplatelet formation or in megakaryocyte (MK) migration. CD34+ cells were isolated from blood and/or marrow of 14 WAS patients and five patients with hereditary X-linked thrombocytopenia (XLT) and cultured in serum-free liquid medium containing recombinant human Mpl-L (PEG-rHuMGDF) and stem-cell factor (SCF) to study in vitro megakaryocytopoiesis. In all cases, under an inverted microscope, normal MK differentiation and proplatelet formation were observed. At the ultrastructural level, there was also no abnormality in MK maturation, and normal filamentous MK were present. Moreover, the in vitro produced platelets had a normal size, while peripheral blood platelets of the same patients exhibited an abnormally small size. However, despite this normal platelet production, we observed that F-actin distribution was abnormal in MKs from WAS patients. Indeed, F-actin was regularly and linearly distributed under the cytoplasmic membrane in normal MKs, but it was found concentrated in the center of the WAS MKs. After adhesion, normal MKs extended very long filopodia in which WASP could be detected. In contrast, MKs from WAS patients showed shorter and less numerous filopodia. However, despite this abnormal filopodia formation, MKs from WAS patients normally migrated in response to stroma-derived factor-1 (SDF-1), and actin normally polymerized after SDF-1 or thrombin stimulation. These results suggest that the platelet defect in WAS patients is not due to abnormal platelet production, but instead to cytoskeletal changes occuring in platelets during circulation.
THE WISKOTT-ALDRICH syndrome (WAS) is an X-linked hereditary disease characterized by thrombocytopenia with small platelet size, eczema, and increased susceptibility to infections.1-3 A milder form, designated as hereditary X-linked thrombocytopenia (XLT), is characterized by isolated thrombocytopenia with small platelet size.4,5 The gene responsible for WAS was recently cloned.6 Sequence analysis identified mutations of the WAS gene in both WAS and XLT, suggesting that they are two different phenotypes of the same disease.6-9 The exact function of WAS protein (WASP) is still unknown, but through its GBD domain that binds to the small guanosine triphosphatase (GTPase) protein Cdc4210-12 and through its verprolin and cofilin homology domains located at its C-terminal region,13 WASP participates in cytoskeletal organization. Moreover, WASP has been described to interact with many SH3-containing proteins by its proline rich domain.14-16 Thus, WASP appears to play an important role in the regulation of cytoskeletal organization and in signal transduction.
The platelet defect, thrombocytopenia, and small platelet size is a consistent finding in patients with mutations in the WASP gene. The exact mechanism of this platelet defect is still unknown. Splenectomy is generally effective in elevating platelet counts toward normal,17,18 and the number and morphology of megakaryocytes (MKs) in WAS bone marrow are normal.3,19These data suggest peripheral destruction of platelets in WAS. However, the association of decreased platelet turnover and normal or increased MK mass suggests ineffective thrombocytopoiesis.3 20
Platelet release by MKs is related to a unique phenomenon called proplatelet formation. At the end of maturation, MKs extend long filamentous processes. Constriction areas delineate future platelets, which are detached after breaking of the processes. Proplatelet formation is related to a reorganization of the cytoskeleton involving microtubules and actin filaments. Therefore, it is possible that WASP is involved in the platelet formation process, ie, development of platelet demarcation membranes, proplatelet formation, and/or platelet release from MKs.20,21 In addition, as platelets are not actually released within the marrow, the transendothelial migration of MKs is absolutely required for release of platelets into the circulation.22 This process requires reorganization of actin filaments and formation of pseudopods. It is worth noting that Bernard Soulier syndrome, another disease involving changes in actin organization, is associated with thrombocytopenia, but with large platelets.23
To study the megakaryocytopoiesis of WAS patients and to examine if patient MKs demonstrate abnormal proplatelet formation and platelet release, we performed in vitro cultures of CD34+ cells isolated from blood and/or bone marrow of 19 patients with WAS or XLT under conditions that allowed the differentiation of CD34+cells into platelet producing MKs24 25 and compared them with CD34+ cells from normal controls. stroma-derived factor-1α (SDF-1α)–induced migration of MKs was also tested. It was found that WAS megakaryopoiesis and platelet production, as well as the SDF-1α–induced migration of MKs, were normal.
MATERIALS AND METHODS
Monoclonal antibodies.
Phycoerythrin (R-PE)-conjugated anti-CD41a monoclonal antibody (MoAb) (Pharmingen, San Diego, CA), R-PE–conjugated anti-CD34 MoAb (HPCA-2; Becton Dickinson, Mountain View, CA), and fluorescein isothyocyanate (FITC)-labeled phalloidin (Sigma Chemical Co, St Louis, MO) were used for flow cytometric analysis. Mouse anti-CD34 MoAb conjugated to magnetic beads (Miltenyi Biotech GmbH, Bergisch Gladbuch, Germany) was used for the Miltenyi technique of purification. Rabbit anti-von Willebrand factor (vWF) polyclonal antibody (Dako, Glostrup, Denmark), mouse anti-WASP MoAb,26 FITC-labeled donkey antimouse IgG (Jackson Immunoresearch, West Grove, PA), tetramethylrhodamine isothiocyanate (TRITC)-conjugated donkey antirabbit IgG, aminomethylcoumarine (AMCA)-conjugated donkey antirabbit IgG (Jackson Immunoresearch), FITC-labeled phalloidin (Sigma) were used for indirect immunofluorescence assays.
Purification of CD34+ cells and cell cultures.
Fresh blood samples (n = 19) and bone marrow aspirates (n = 5) were harvested from 19 patients with either WAS (n = 14) or XLT (n = 5) during medical evaluations (blood samples) or under general anesthesia for splenectomy (marrow aspirates). In 10 patients, the mutation in the WASP gene was characterized. They consisted of R211stop, Y83stop, R86H, and a mutation in intron 10 in one patient, respectively. S483FSstop494 was found in two related patients and V75L in four other related patients. Characterization of the mutation is currently being performed in the other nine patients.
Cells were separated over a Ficoll-metrizoate gradient (Lymphoprep, Nycomed Pharma, Oslo, Norway) to obtain an enriched fraction of mononuclear cells. CD34+ cells were then isolated by the Miltenyi immunomagnetic bead technique as previously reported.24 25 Cells were incubated for 30 minutes at 4°C with an anti-CD34 MoAb conjugated to magnetic beads. The CD34+ cells were retained on the column and were eluted by pressure using the plunger supplied with the column. Subsequently, cells were purified by cell sorting. Briefly, cells were labeled with a R-PE anti-CD34 MoAb and, after one wash, were sorted on a FACSvantage (Becton Dickinson) equipped with an argon ion laser (INNOVA 70-4, Coherent Radiation, Palo Alto, CA) tuned to 488 nm and operating at 500 mW. A “morphological” gate including 80% of the events and all of the CD34+ cells was determined on two-parameter histograms (side scatter [SSC] versus forward scatter [FSC]). Control CD34+ cells were purified by the same procedure from normal bone marrow of patients undergoing hip surgery or from the peripheral blood of patients after mobilization by chemotherapy and granulocyte colony-stimulating factor (G-CSF). Informed consent was obtained in all cases (patients and controls) in accordance with the institutional guidelines of the Committee on Human Investigation.
To obtain MKs, purified CD34+ cells were cultured in serum-free liquid medium containing a recombinant truncated form of Mpl-L (PEG-rHuMGDF, Amgen, Thousand Oaks, CA, 10 ng/mL) and recombinant human stem cell factor (SCF; Amgen, 50 ng/mL) as previously described.25
Cultures were observed daily under an inverted microscope to compare normal and WAS MKs.
Ultrastructural studies.
Cultured cells were studied by electron microscopy. They were washed twice in Hanks medium at 4°C, fixed in 1.25% glutaraldehyde in phosphate buffer (0.1 mol/L, pH = 7.4) for 1 hour, and washed twice. Cells were then fixed with osmium tetroxide, dehydrated, and embedded in Epon. Thin sections were examined with a Philips CM 10 electron microscope (Philips, Eindhoven, The Netherlands) after uranyl acetate and lead citrate staining. Immunolabelling for αIIbβ3 was performed on glycol-methacrylate-embedded MK cultures and platelets with polyclonal anti-αIIbβ3 antibody (a generous gift from D. Pidard, Institut Pasteur, Paris, France) followed by immunogold (goat antirabbit IgG coupled to 10 nm colloidal gold) (British Biocell, Cardiff, UK). The diameter of shed platelets was measured on electron micrographs.
MK immunofluorescence microscopy.
After 7 to 13 days, cultured cells were fixed in 2% paraformaldehyde (PFA) for 15 minutes, washed in phosphate-buffered saline (PBS), and resuspended in PBS at the concentration of 105 cells/100 μL. Cell suspensions (100 μL) were cytocentrifuged at 500 rpm for 2 minutes. Cells were then fixed once more in 2% PFA for 5 minutes, rehydrated in PBS, permeabilized with 0.1% Triton for 3 minutes, and washed with PBS before incubation for 30 minutes at room temperature with a rabbit anti-vWF polyclonal antibody. After three washes with PBS, cells were incubated at room temperature with TRITC-labeled donkey antirabbit IgG and FITC-labeled phalloidin. DNA was labeled by 7.5 ng/mL Hoechst 33258 (Hoechst 33528, Sigma) for 15 minutes in the dark.
Cell preparations were analyzed with a fluorescence microscope equipped with the appropriate filter combinations (Zeiss, Oberkochen, Germany).
For the immunofluorescence studies on adherent MKs, glass coverslips were coated with poly-L-lysine for 1 hour and gently washed with PBS. Cultured cells were resuspended in PBS without any fixation at the concentration of 105/100 μL. The unfixed cell suspension (100 μL) was pipetted onto the coverslips and allowed to adhere for 1, 5, 15, 30, or 60 minutes. After these different time points, cells were fixed in PFA and permeabilized with Triton, as described above, before incubation for 30 minutes at room temperature with a rabbit anti-vWF polyclonal antibody and, in some experiments, with a mouse anti-WASP MoAb. After three washes with PBS, cells were incubated with TRITC- or AMCA-labeled donkey antirabbit IgG, FITC-labeled phalloidin, and TRITC-labeled donkey antimouse IgG. When the AMCA-labeled donkey antirabbit antibody was not used as secondary antibody, DNA was labeled by Hoechst, as described above.
MK migration after SDF-1α stimulation.
To analyze MK migration, CD34+ cells were cultured for 7 days. Then, cultured cells were resuspended in serum-free medium at a final concentration of 2.5 × 106 cells/mL. Migration assays were performed using 5-μm pore filters (Transwell, 24-well cell clusters; Costar, Cambridge, MA). Cell suspensions (2.5 × 105 cells in a 100-μL volume) were placed into the upper chamber, whereas 600 μL of medium with or without recombinant human SDF-1α (300 ng/mL) (R & D Systems, Minneapolis, MN) was introduced in the lower chamber. The chambers were incubated for 1 hour at 37°C in 5% CO2 and 95% air. The cells in the upper and in the bottom chamber were recovered separately in the same volume for counting. The different cell fractions were then labeled with a PE-anti–CD41a MoAb and analyzed by flow cytometry. All assays were performed in triplicate. Data are presented as the percentage of migrating cells (number of cells migrating [lower chamber]/total number of cells [cells in the lower chamber + remaining cells in the upper chamber]).
MK actin polymerisation after thrombin and SDF-1α stimulation.
After 7 to 13 days, cultured cells were resuspended in three equal 0.1-mL aliquots. The first aliquot was incubated with thrombin (0.1 IU/mL) for 30 seconds with shaking at 37°C. The second aliquot was incubated with SDF-1α (300 ng/mL) for 30 seconds in the same conditions. The remaining tube was incubated in the same conditions without thrombin or SDF-1α to assess baseline activation. Cells were then fixed with 2% paraformaldehyde (PFA) for 15 minutes, washed in PBS, permeabilized in 0.1% Triton for 3 minutes, washed, and resuspended in PBS. Cells were then incubated at 4°C for 30 minutes with PE-labeled anti-CD41a MoAb and FITC-labeled phalloidin. Cell samples were analyzed on a FacSort (Becton Dickinson). For each sample, 10,000 cells were acquired in the list mode and analyzed with the Cellquest software package (Becton Dickinson).
RESULTS
MK maturation and differentiation under light and electron microscopy.
To study a large series of patients, we developed a purification technique that allowed the isolation of CD34+ cells from 10 mL of blood. CD34+ cells were first purified by the Miltenyi technique with one passage on the column. After this first separation, the purity was between 5% and 10%. Cells were then further purified by cell sorting, pemitting the recovery of 2,000 to 5,000 CD34+ cells per sample with a purity of over 95%. In five cases, in addition to blood CD34+ cells, marrow aspirates could be obtained from patients during general anesthesia for splenectomy, and the same procedure allowed the purification of 50,000 to 100,000 CD34+ cells per aspirate. The number of purified CD34+ cells obtained from blood and/or marrow of WAS patients was the same as obtained with blood and/or marrow from controls. As previously described, addition of PEG-rHuMGDF and SCF to a serum-free liquid culture of these cells led to the differentiation of CD34+ cells into MKs, and eventually to their maturation into platelet-producing cells. Under these culture conditions, the number of MKs obtained from cultured CD34+ cells was the same in WAS as in control cultures (four MKs per CD34+cell) when cultures were analyzed by flow cytometry after staining with a PE-CD41a MoAb. All of the steps of differentiation and maturation of MKs could be observed in the cultures of all patient CD34+cells (n = 19) and gave identical results, whatever the origin of CD34+ cells (blood or marrow).
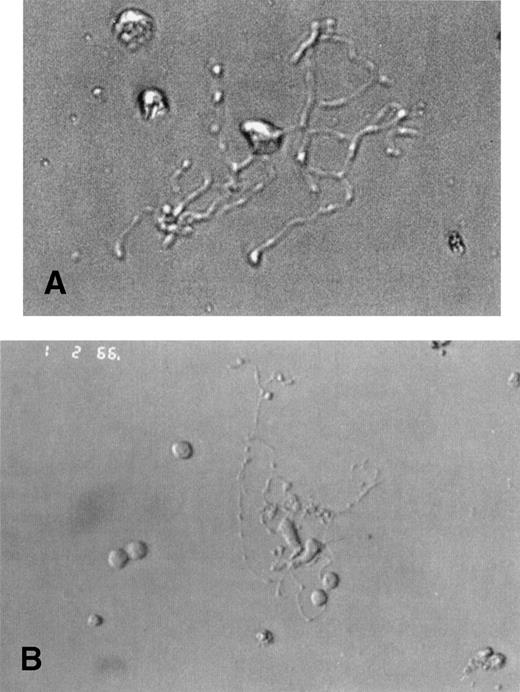
Unexpectedly, MKs from WAS and XLT patients were morphologically similar to normal MKs, with the same timing of differentiation. Under light microscopy, round MKs began to deform at day 7 of culture and formed a pseudopod that progressively elongated to become as long and thin as that of MKs from normal controls (Fig 1A and B), and that ultimately gave rise to detached (pro)platelets. These platelet-producing MKs were as numerous in patient as in normal cultures (about 10% to 20% of the MKs being filamentous), and no obvious abnormality could be detected in their morphologic appearance.
Platelet-producing normal and WAS MKs have the same morphological aspect. CD34+ cells of WAS patients (A) or normal controls (B) were purified from blood or bone marrow and were grown in the presence of PEG-rHuMGDF and SCF. Analysis of cultured cells under light microscopy by day 8 allowed the identification of platelet-producing MK characterized by the extension of very long and thin pseudopods. They give rise to proplatelets by breaking irregularly at several constriction sites. The frequency and appearance of these platelet shedding MKs were similar in WAS patients (A) and in normal controls (B).
Platelet-producing normal and WAS MKs have the same morphological aspect. CD34+ cells of WAS patients (A) or normal controls (B) were purified from blood or bone marrow and were grown in the presence of PEG-rHuMGDF and SCF. Analysis of cultured cells under light microscopy by day 8 allowed the identification of platelet-producing MK characterized by the extension of very long and thin pseudopods. They give rise to proplatelets by breaking irregularly at several constriction sites. The frequency and appearance of these platelet shedding MKs were similar in WAS patients (A) and in normal controls (B).
To study the polymerization and distribution of actin in MKs, cultured cells were costained with anti-vWF antibodies to precisely identify MKs and with phalloidin to observe actin filaments. F-actin was linearly located under the cytoplasmic membrane and regularly distributed in normal MKs (Fig 2A through C). In contrast, it was found concentrated near the nucleus in the center of 50% of the WAS MKs (about 60 MKs studied in each patient) (Fig 2D through F). A part of the F-actin was also distributed under the cytoplasmic membrane. However, in platelet-producing MKs from normals and WAS patients, the immunofluorescence appearance of F-actin was identical. Phalloidin staining was linearly distributed all along the pseudopods that give rise to platelets after breaking (Fig 3). It is worth emphasizing that WAS protein, as well as vWF, were present in pseudopods of normal MKs.
Abnormal F-actin redistribution in WAS MKs. Cultures were performed as described in Fig 1. At day 8, cells were fixed and, after cytocentrifugation, were incubated with anti-vWF (TRITC) to localize the MKs (B and E) and with phalloidin (FITC) to study F-actin distribution (C and F). DNA was stained by Hoechst dye (A and D). In normal MKs (A, B, and C), F-actin distribution was located linearly under the cytoplasmic membrane and regularly distributed (C), while in WAS MKs (D, E, and F), F-actin was localized in the center of the cell (F).
Abnormal F-actin redistribution in WAS MKs. Cultures were performed as described in Fig 1. At day 8, cells were fixed and, after cytocentrifugation, were incubated with anti-vWF (TRITC) to localize the MKs (B and E) and with phalloidin (FITC) to study F-actin distribution (C and F). DNA was stained by Hoechst dye (A and D). In normal MKs (A, B, and C), F-actin distribution was located linearly under the cytoplasmic membrane and regularly distributed (C), while in WAS MKs (D, E, and F), F-actin was localized in the center of the cell (F).
Immunofluorescent appearance of a platelet-producing WAS MK. Cultures were performed and labeled as described in Fig 2. Phalloidin staining (FITC) was distributed linearly all along the pseudopod that gives rise to platelets by breaking irregularly at several constriction sites (A). vWF (TRITC) was present in this pseudopod (B), while Hoechst staining determined the localization of the nucleus (C). This immunofluorescent appearance of platelet-producing WAS MK was similar to that observed in normal control platelet-producing MKs (not shown).
Immunofluorescent appearance of a platelet-producing WAS MK. Cultures were performed and labeled as described in Fig 2. Phalloidin staining (FITC) was distributed linearly all along the pseudopod that gives rise to platelets by breaking irregularly at several constriction sites (A). vWF (TRITC) was present in this pseudopod (B), while Hoechst staining determined the localization of the nucleus (C). This immunofluorescent appearance of platelet-producing WAS MK was similar to that observed in normal control platelet-producing MKs (not shown).
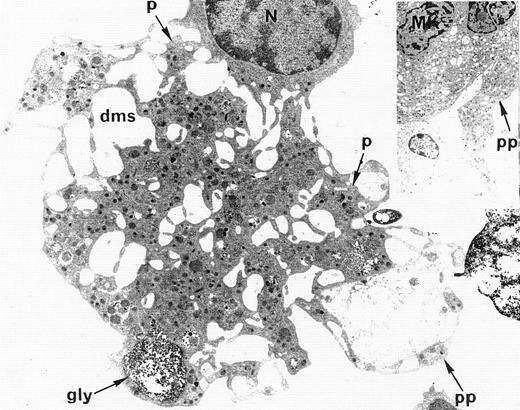
Ultrastructural analysis of patient MKs was performed in nine cases. In all cases, electron microscopic appearance of WAS MKs was similar to that of normal MKs. The demarcation membrane system was well developed and normal in distribution in WAS MKs (Fig4). In only one case, the membrane demarcation system seemed to be more developed than in normal MKs, but this observation was not confirmed in the other eight patients. The maturation of WAS MKs during platelet shedding, consisting of alignment and dilatation of the peripheral demarcation membranes, proplatelet formation (Fig 4), detachment of newly formed platelets, and presence of platelets in the supernatant of the culture medium was also normal in WAS MKs. Surprisingly, the diameter of in vitro shed platelets measured by electron micrographs in seven WAS patients (10 platelets in each case) was normal, as compared with the diameter of shed platelets from normal MKs (2.73 ± 0.9 μm and 2.87 ± 0.9 μm, respectively), whereas the mean in vivo platelet volume of these patients was abnormally low when measured in peripheral blood with an electrical impedance particule counter (5.5 ± 0.3 fL) as compared with mean platelet volume of normal controls (8.2 ± 1.9 fL).
Electron microscopic view of a cultured mature WAS MK. This MK is starting the process of platelet shedding. The nucleus (N) is excentric, the demarcation membrane system (dms) is widening, delimitating platelet territories (p) and, at the cell periphery, proplatelets (pp) start to extend. (A, -granules; gly, glycogen).M= × 7.375. Insert: Normal proplatelet (pp) extend from mature MK.M= × 2.145.
Electron microscopic view of a cultured mature WAS MK. This MK is starting the process of platelet shedding. The nucleus (N) is excentric, the demarcation membrane system (dms) is widening, delimitating platelet territories (p) and, at the cell periphery, proplatelets (pp) start to extend. (A, -granules; gly, glycogen).M= × 7.375. Insert: Normal proplatelet (pp) extend from mature MK.M= × 2.145.
Immunofluorescence studies of adherent MKs.
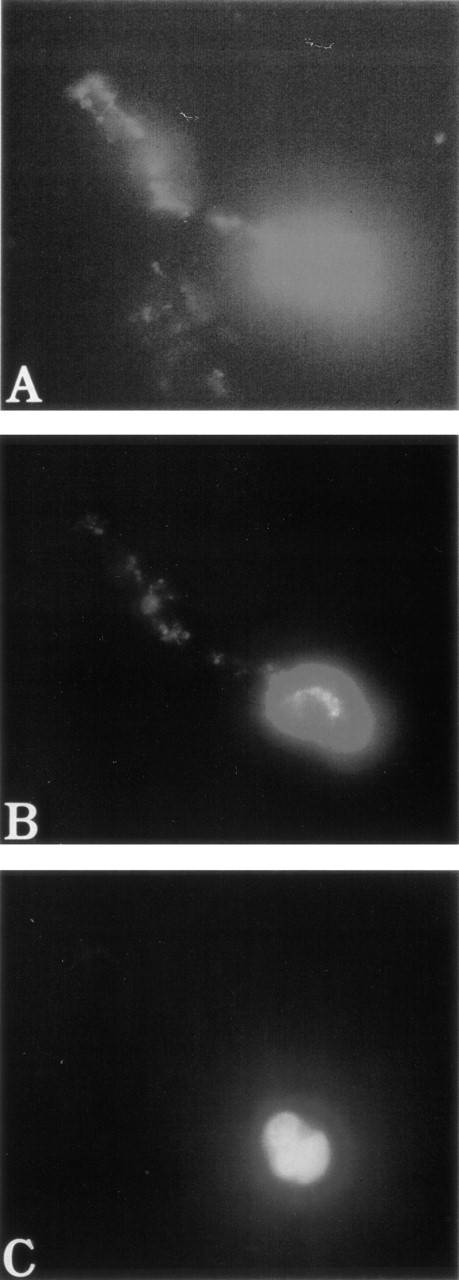
To test the distribution of F-actin during the adhesion process, unfixed MKs were pipetted onto poly-L-lysine–coated glass coverslips. Adhesion to poly-L-lysine emphasized the abnormal F-actin distribution in WAS MKs as compared with normal MKs (Fig5). After 5 minutes of adhesion, normal MKs began to extend long filopodia that reached their maximum extension after 30 to 60 minutes (Fig 5A and B). In the same conditions, WAS MKs could extend filopodia, which however were shorter and less numerous than in normal MKs, even after 60 minutes of adhesion (Fig 5C and D). In contrast to the proplatelet filaments, no vWF could be detected in the filopodia of both normal and WAS MKs. Lamellipodial formations were observed in normal and WAS MKs and did not seem different (not shown). The defects observed in F-actin distribution and filopodia formation were equally represented among WAS and XLT patients. By staining normal MKs with anti-WASP antibody, we could show that unlike vWF, WASP protein localized along the filopodia with the same topography as F-actin (Fig 6A and B). However, in the central part of the MKs, WASP did not exactly colocalize with F-actin, but was in a more central position (Fig 6B). WASP protein expression was studied in two patients with a missense mutation. In both cases, WASP protein was detected in cultured MKs, but in one of them in contrast to normal MKs, WASP did not colocalize with F-actin.
Abnormal filopodia formation of WAS MKs after adhesion to poly-L-lysine. Cultured cells were pipetted onto poly-L-lysine–coated coverslips. After 30 minutes of adhesion, cells were fixed, permeabilized, and labeled as described in Fig 3. (A and B) Examination of a normal MK with a combination of filters allowing simultaneous visualization of vWF (TRITC), F-actin (FITC), and DNA (Hoechst). The normal MK displays long, thin, and numerous filopodia that contain F-actin, but not vWF, which remains localized in the center of the cell. In contrast, WAS MKs fail to extend long filopodia (C) or extend very short, but less numerous filopodia (D) than do normal MK. Examination of WAS MKs with a combination of filters was not interpretable because of the central localization of F-actin that overlaps with that of vWF and therefore was not shown in this figure.
Abnormal filopodia formation of WAS MKs after adhesion to poly-L-lysine. Cultured cells were pipetted onto poly-L-lysine–coated coverslips. After 30 minutes of adhesion, cells were fixed, permeabilized, and labeled as described in Fig 3. (A and B) Examination of a normal MK with a combination of filters allowing simultaneous visualization of vWF (TRITC), F-actin (FITC), and DNA (Hoechst). The normal MK displays long, thin, and numerous filopodia that contain F-actin, but not vWF, which remains localized in the center of the cell. In contrast, WAS MKs fail to extend long filopodia (C) or extend very short, but less numerous filopodia (D) than do normal MK. Examination of WAS MKs with a combination of filters was not interpretable because of the central localization of F-actin that overlaps with that of vWF and therefore was not shown in this figure.
Localization of WASP in normal MKs after adhesion on Poly-L-lysine. Examination of normal MKs was performed as described in Fig 5. MKs were labeled with anti-vWF polyclonal antibody (AMCA), phalloidin (FITC), and anti-WASP MoAb (TRITC) after 30 minutes of adhesion to poly-L-lysine–coated coverslips. Anti-vWF labeling (AMCA) allowed the localization of MKs. Unlike vWF, WASP is localized in filopodia (A) with the same topography as F-actin (B).
Localization of WASP in normal MKs after adhesion on Poly-L-lysine. Examination of normal MKs was performed as described in Fig 5. MKs were labeled with anti-vWF polyclonal antibody (AMCA), phalloidin (FITC), and anti-WASP MoAb (TRITC) after 30 minutes of adhesion to poly-L-lysine–coated coverslips. Anti-vWF labeling (AMCA) allowed the localization of MKs. Unlike vWF, WASP is localized in filopodia (A) with the same topography as F-actin (B).
Migration of MKs stimulated by SDF-1α.
Because it has been shown that SDF-1α can induce MK migration, we tested migration of MKs to SDF1-α in WAS. The baseline percentage of migrating MKs (no SDF-1α in the lower chamber) was invariably under 0.1% for normal and WAS MKs. After SDF-1α stimulation, the mean percentage of migrating cells was 21% (± 8%) for WAS MKs and 19% (± 8%) for normal MKs (P, not significant [NS]).
Polymerization of actin after stimulation of MKs by thrombin and/or by SDF-1α.
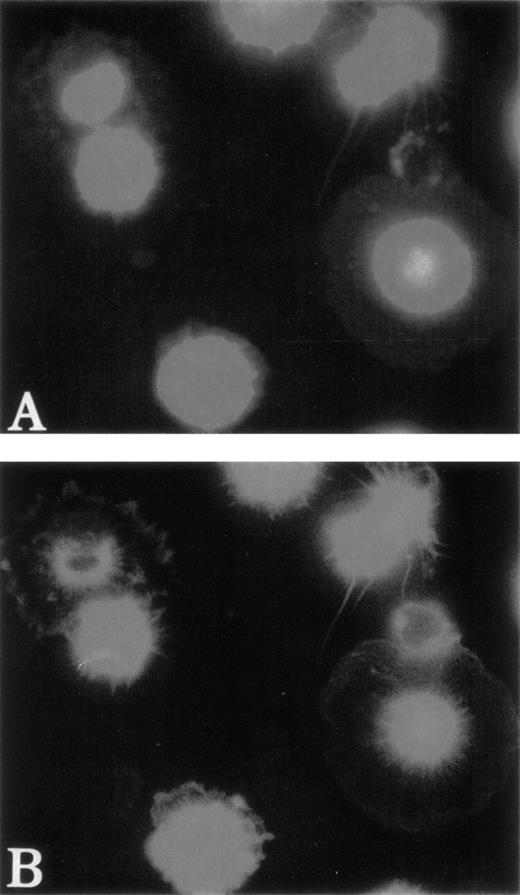
Finally, we investigated whether an abnormality in actin polymerization was present in WAS MKs after thrombin or SDF-1α stimulation. For this purpose, the intensity of phalloidin staining was studied by flow cytometry after double-staining with FITC-phalloidin and R-PE anti-CD41a MoAb. As illustrated in the representative examples (Fig 7), the same significant increase in F-actin content after stimulation by thrombin (0.1 IU/mL) or SDF-1α (300 ng/mL) was found in MKs (CD41a+ cells) from normal and WAS patients. Moreover, kinetic analysis of actin polymerization by the same method did not show any difference between normal and WAS MKs after stimulation by thrombin or SDF-1α (data not shown). Baseline F-actin content was also similar in normal and WAS MKs.
Normal actin polymerization of WAS MKs after stimulation by SDF-1 and thrombin. After 7 to 13 days, cultured cells were resuspended and stimulated with thrombin (0.1 IU/mL) or SDF-1 (300 ng/mL) for 30 seconds with shaking at 37°C. Cells were then fixed, permeabilized, and incubated with PE-labeled anti-CD41a and FITC-labeled phalloidin. Cell samples were analyzed on a FacSort (Becton Dickinson). MKs were selected by gating the CD41+cells (A). Analysis of FITC intensity on CD41+ cells showed that the baseline content of F-actin and the increase of actin polymerization after stimulation of MKs by SDF-1 (B and D) and thrombin (C and E) were the same in normal MKs (B and C) and in WAS MKs (D and E).
Normal actin polymerization of WAS MKs after stimulation by SDF-1 and thrombin. After 7 to 13 days, cultured cells were resuspended and stimulated with thrombin (0.1 IU/mL) or SDF-1 (300 ng/mL) for 30 seconds with shaking at 37°C. Cells were then fixed, permeabilized, and incubated with PE-labeled anti-CD41a and FITC-labeled phalloidin. Cell samples were analyzed on a FacSort (Becton Dickinson). MKs were selected by gating the CD41+cells (A). Analysis of FITC intensity on CD41+ cells showed that the baseline content of F-actin and the increase of actin polymerization after stimulation of MKs by SDF-1 (B and D) and thrombin (C and E) were the same in normal MKs (B and C) and in WAS MKs (D and E).
DISCUSSION
Thrombocytopenia and small platelet size are two consistent findings in WAS and XLT. Their precise mechanisms are unknown. In addition to increased peripheral platelet destruction that probably contributes to the thrombocytopenia, platelets of WAS patients have several intrinsic abnormalities in morphology, phenotype, and function.19,27-29 In addition, defects in the cytoskeletal architecture and organelle organization have been reported.19,28 By studying autologous platelet survival, platelet function, and megakaryocyte histology in four WAS patients, Ochs et al3 demonstrated a decrease in platelet turnover, which was 30% of the normal value in all four cases. In contrast, marrow megakaryocyte mass was normal or increased. This discrepancy between the normal or increased megakaryocyte mass and the decreased platelet turnover suggests that ineffective thombocytopoiesis does occur in WAS and may be responsible for thrombocytopenia.20In some patients, we studied the thrombopoietin (TPO) level in the plasma by enzyme-linked immunosorbent assay (ELISA) and found that despite the thrombocytopenia, the TPO level was normal or slightly elevated (data not shown), as described in immune thrombocytopenia patients (ITP). This clearly reinforces the contention that the MK mass is normal or increased in WAS patients, but platelet numbers are limited by dysmegakaryopoiesis or platelet destruction. The late stages of megakaryocytopoiesis are accompanied by profound modifications of the cell structure leading to proplatelet formation.24,30,31 Thus, we hypothesized that the platelet abnormalities in WAS could be due to a defect in proplatelet production as a consequence of an abnormal interaction between WASP and the cytoskeleton. In favor of this hypothesis, Miki et al21reported that in MEG-01 cells, a megakaryoblastic cell line, WASP interaction with actin filaments was indispensable for microvesicle formation, a process hypothesized to be similar to MK proplatelet formation.
To test this hypothesis, we performed in vitro cultures of CD34+ cells from 19 patients in conditions supporting the differentiation of CD34+ cells into platelet-producing MKs.24 By analyzing F-actin distribution after phalloidin staining, we confirmed that F-actin distribution was abnormal in WAS MKs and that this phenomenon was exacerbated during adhesion to poly-L-lysine. Indeed, we found that WAS MK F-actin was not linearly distributed at the periphery of the cell, but was concentrated in the center of the cell, as described recently in WAS patient lymphocytes.32 After adhesion to poly-L-lysine, WAS MKs could extend some filopodia, but in general, they were shorter and less numerous than in normal MKs. One possible explanation for this defect is the interaction of WASP with Cdc42 through its GBD domain.10-12 Cdc 42 is a member of the Rho family of proteins that plays a critical role in cytoskeletal organization33-35 and is particularly involved in filopodia formation. Thus, absent or abnormal interaction between WASP and Cdc42 may explain abnormal filopodia formation in WAS MKs. Because this phenomena was observed in all cases tested except for one (n = 7), this would mean that WASP interaction with Cdc42 was impaired in all or most cases. However, of all the mutations reported so far,20,36,37 none was located in the exon encoding the GBD domain. This implies that direct interaction of WASP with Cdc42 may be necesssary, but is not sufficient to allow filopodia formation. Alternatively it has been reported that the majority of the mutations alter the quantitative level of WASP expression.38 It is also possible that the central localization of F-actin does not allow normal extension of filopodia despite an eventual normal interaction between WASP and Cdc42.
Despite this abnormal F-actin distribution and filipodia formation, all steps of normal MK differentiation could be observed in vitro in these patients. Extension of long pseudopods, development and distribution of the demarcation membrane system, proplatelet formation and detachment of newly formed platelets, phenomenons that involve profound reorganization of cytoplasmic structure, were all normal under light and electron microscopy. Unexpectedly, in vitro produced platelets had a normal apparent diameter, while platelets from the same patients were abnormally small in the blood. Recently, it has been shown that Wistar Furth rat platelets have defective filopodia formation.39It is noteworthy that in both diseases, characterized by a defective filopodia formation, a thrombocytopenia is present, but with major differences in the platelet volume. Indeed, Wistar Furth rats have thrombocytopenia with large platelets, whereas platelets of WAS patients are small. This suggests that proplatelet formation and filopodia formation result from two independent mechanisms.
If one accepts that these in vitro observations are applicable to normal in vivo platelet production, our results would suggest that diminution of platelet size occurs after platelet production. It can be hypothesized that as a consequence of abnormal cytoskeletal organization, platelet deformation occurs in the blood circulation, especially in spleen sinusoids, and leads to diminution of size and spleen destruction. Alternatively, due to abnormal membrane deformability, WAS platelets of normal size would be preferentially destroyed in the spleen, whereas small platelets would be submitted to less prononced deformation in the vessels and would thus escape destruction. The fact that the half-life of reinfused, autologous, small-sized platelets was only moderately reduced, ie, 5 ± 1.3 days compared with 9.5 ± 0.6 days in normal subjects3 argues for the second hypothesis. This may also explain why platelet size increases after splenectomy.18 In five patients who underwent splenectomy in the course of this study, platelet volume also increased, but slightly without reaching normal values. It is worth emphasizing that WASP-deficient mice have only modest alterations in their platelet numbers (five of seven wild-type mice), with normal platelet size.40 The absence of a marked thrombocytopenia in mice may be related to the much smaller platelet size in comparison to human platelets, allowing normal passage through the spleen.
In vitro chemotaxis of WAS neutrophils and monocytes has been shown to be deficient.3,41,42 It was recently reported that SDF-1α could induce the migration of MKs through endothelial cells43 or directly through a 5-μm pore size transwell.44,45 Because migration involves a profound reorganization of the cytoskeleton with polarization of actin filaments, we tested to see if SDF-1α–induced migration of MKs was altered in WAS. However, in all cases, normal migration through transwells was observed. Because SDF-1α has been described to induce actin polymerization in lymphocytes46 and MKs,44 we also tested the effects of SDF-1α on induction of actin polymerization in WAS. No differences were found between normal and WAS MKs in the induction of actin polymerization by SDF-1α or thrombin. These observations demonstrate that the abnormal F-actin distribution in WAS MKs does not impair migration and actin polymerization in vitro. These results also demonstrate that the machinery necessary for migration is functional in cultured MKs. This normal in vitro MK migration does not definitively exclude a defect in in vivo transmigration of WAS MKs through the endothelial barrier. Indeed, the in vivo process also requires that MKs normally adhere to the extracellular matrix and to endothelial cells. Hamada et al43 demonstrated that a neutralizing MoAb against E-selectin blocked the SDF-1–induced transmigration of MKs by 50%, suggesting that cellular interaction of MKs with bone marrow endothelial cells was critical for MK migration. Furthermore, it was recently reported that, in human platelets, WASP is tyrosine-phosphorylated after stimulation by collagen,47 a molecule that may be involved in the interaction between MKs and endothelial cells. Thus, one cannot exclude that abnormal interaction between WAS MKs and endothelial cells through adhesion signals other than SDF-1 may have a direct impact on platelet formation. Further analyses investigating the interactions between bone marrow endothelial cells, collagen, and WAS MKs will be necessary to resolve this issue.
Taken together, these results confirm that the abnormalities of cytoskeletal reorganization are probably very complex in WAS and may vary among the different hematopoietic lineages, as already observed for B and T lymphocytes. The fact that all of the steps of maturation of MKs, including the size of shed platelets, are normal in vitro suggest that the platelet defect is probably related to a peripheral mechanism. However, these studies are limited by the number of MKs that can be isolated from a small volume of blood and/or marrow of patients. Moreover, all of the tests were performed on MKs cultured in vitro under stimulation by Mpl-L and SCF. One cannot completely exclude that in vitro stimulation by these cytokines does not change the behavior of MKs. The recent description of WASP-deficient mice should make it possible to obtain enough material to study platelets and MKs in vitro and in vivo and to determine the precise mechanisms of cytoskeletal reorganization regulated by WASP during adhesion and MK migration.
ACKNOWLEDGMENT
The authors are grateful to J.-L. Nichol (Amgen, Thousand Oaks, CA) and C. Caillot (Amgen, Neuilly, France) for providing SCF and PEG-rHuMGDF. We thank Pr G. Tchernia (Hôpital Kremlin Bicêtre, Bicêtre, France), Pr J.P. Fermand (Hôpital St Louis, Paris, France), and Pr B. Varet, Pr S. Blanche (Hôpital Necker-Enfants Malades, Paris, France), Dr M. Michaux (Hôpital Pellegrin, Bordeaux, France), and Dr Mazingue (Hôpital Claude Huriez, Lille, France). We thank Klaus Schwarz (Ulm, Germany) for having performed mutation analysis. We thank J.-M. Massé for photographic assistance and Pr B. Forget for improving the English of this manuscript.
Supported by grants from the INSERM, Institut Gustave Roussy, and the Association pour la Recherche contre le Cancer (to N.D.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Elie Haddad, MD, INSERM U 362, PR1, Institut Gustave Roussy, Villejuif 94805, France.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal