Abstract
In the present study, we attempted to clarify the effects of interleukin-6 (IL-6) on the growth and properties of human mast cells using cultured mast cells selectively generated by stem cell factor (SCF) from CD34+ cord blood cells. The addition of IL-6 to cultures containing mast cells resulted in a substantial reduction of the number of progenies grown by SCF in the liquid culture. This IL-6–mediated inhibition of mast cell growth may be due in part to the suppression at the precursor level, according to the results of a clonal cell culture assay. Moreover, a flow cytometric analysis showed that the cultured mast cells grown in the presence of SCF+IL-6 had decreased c-kit expression. The exposure of cultured mast cells to SCF+IL-6 also caused substantial increases in the cell size, frequency of chymase-positive cells, and intracellular histamine level compared with the values obtained with SCF alone. The flow cytometric analysis showed low but significant levels of expression of IL-6 receptor (IL-6R) and gp130 on the cultured mast cells grown with SCF. The addition of either anti–IL-6R antibody or anti-gp130 antibody abrogated the biological functions of IL-6. Although IL-4 exerted an effect similar to that of IL-6 on the cultured mast cells under stimulation with SCF, the results of comparative experiments suggest that the two cytokines use different regulatory mechanisms. Taken together, the present findings suggest that IL-6 modulates SCF-dependent human mast cell development directly via an IL-6R-gp130 system.
MAST CELLS PLAY an important role as the primary effector cells in immediate-type hypersensitivity reactions. There are at least two phenotypically distinct subpopulations of mast cells in rodents,1,2 ie, mucosal mast cells and connective tissue mast cells. In humans, two types of mast cells have been identified on the basis of protease expression: one phenotype is positive only for tryptase, and the other is positive for both tryptase and chymase.3,4 To elucidate the physiological and pathological characteristics of human mast cells, numerous investigators5-10 have attempted to establish human mast cell cultures, because only limited numbers of mast cells can be obtained from human tissues. Stem cell factor (SCF) has been demonstrated to be a pivotal growth factor that promotes the development of mast cells6-10 as well as the proliferation and differentiation of various hematopoietic progenitors. However, the purity of cultured mast cells grown by SCF alone has ranged from approximately 40% to 85%.6,8 9
Interleukin-4 (IL-4) has been demonstrated to diminish the number of mast cells that develop in response to SCF.11,12 In addition, IL-4 has various biological effects on human cultured mast cells, including the upregulation of the expressions of functional high-affinity IgE receptor (FcεRI), intercellular adhesion molecule-1, and lymphocyte function-associated antigen-1.13-15 Toru et al16 recently reported that IL-4 promotes the morphologic maturation of human cultured mast cells in accordance with the increase of chymase expression.
In contrast to the relative abundance of information regarding the role of IL-4 in the regulation of development and function of human mast cells, the effects of IL-6 remain unclear. In the present study, we attempted to clarify the effects of IL-6 on the development and properties of human mast cells by using cultured mast cells generated by SCF from CD34+ cord blood cells. Because fetal bovine serum (FBS) is a potential endogenous source of hematopoietic growth factors,17 we used serum-deprived cultures in this study.
MATERIALS AND METHODS
Factors and antibodies.
Human recombinant SCF and IL-3 were generously provided by Kirin Brewery Co Ltd (Takasaki, Japan). Human recombinant IL-4 and recombinant transforming growth factor-β1 (TGF-β1) were purchased from R&D Systems (Minneapolis, MN). Human recombinant IL-6 was kindly provided by Ajinomoto Co (Kawasaki, Japan).
For the experiments of neutralization of IL-6 activity at the receptors, we used antihuman IL-6 receptor antibody (anti–IL-6R Ab; R&D Systems) and antihuman gp130 Ab (B-R3; Biosource International, Camarillo, CA). The polyclonal anti–IL-6R Ab was made by immunizing goats with recombinant human IL-6 soluble R derived from the insect cell line Sf21. The monoclonal antibody (MoAb) against gp130 was made by immunizing mice with natural soluble gp130. These Abs recognize IL-6R and gp130, respectively, and can neutralize IL-6 activity. The neutralization dose50 (ND50) of the anti–IL-6R Ab was determined to be approximately 1 to 4 μg/mL in the presence of 20 ng/mL of IL-6 using the M1 cell line. Approximately 25 ng of the anti-gp130 Ab neutralized 0.2 ng of IL-6 activity by 50% in an XG-1 cell proliferation bioassay. The polyclonal rabbit antihuman IL-6 Ab (1.2 mg/mL) was a gift from Ajinomoto Co. It was made by immunizing rabbits with recombinant IL-6 in complete Freund’s adjuvant.18 One microgram of this Ab neutralized the activity of 3 ng IL-6, as determined with the use of the cell line, SKW6-CL4. A polyclonal sheep antihuman IL-4 Ab was purchased from Genzyme Co (Cambridge, MA). The Ab at 0.1 to 1 μg/mL neutralized the bioactivity of a 0.25 ng/mL solution of IL-4. The mouse MoAb against human granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Oncogene Science Inc (Uniondale, NY). This azide-free antibody at 2 μg/mL reduced the growth of granulocyte-macrophage colonies supported by 10 ng/mL of GM-CSF to 37%.19 The neutralizing antihuman TGF-β antibody was obtained from R&D Systems. The ND50 of the antibody was determined to be 0.2 to 0.6 μg/mL in the presence of 0.25 ng/mL of TGF-β1, using TGF-β–responsive HT-2 cells.
For immunocytochemical staining, purified MoAbs for tryptase (MAB1222) and chymase (3D5) were purchased from Chemicon International Inc (Temecula, CA) and Biogenesis Inc (Sandown, NH), respectively. The MoAb for CD2 (T11) was from Coulter (Miami, FL); the MoAbs for CD11b (2LPM19c), CD15 (C3D-1), CD19 (HD37), and glycophorin A (JC159) were from Dako (Glostrup, Denmark). The MoAb for eosinophil peroxidase (MAB1087) was obtained from Chemicon International Inc. Control isotype mouse MoAbs were purchased from Dako.
For the flow cytometric analysis, the MoAbs for c-kit (95C3, phycoerythrin [PE]) and CD9 (ALB6, fluorescein isothiocyanate [FITC]) were purchased from Immunotech S.A. (Marseilles, France); the MoAbs for CD34 (HPCA-2 FITC), CD33 (LeuM9 PE), CD11a (LFA1α FITC), and CD11b (Leu15 PE) were from Becton Dickinson Immunocytometry Systems (Mountain View, CA); and the MoAbs for CD45 (T29/33 FITC), CD61 (Y2/51 FITC), and CD68 (KP1, FITC) were from Dako. The MoAb for human FcεRI (CRA-1) was obtained from Kyokuto Pharmaceutical Industria Co (Takahagi, Japan). For the analysis of IL-6R and gp130 expressions on the cultured mast cells grown with SCF, we used the PE-conjugated MoAb against human IL-6R (M91; Immunotech) and the anti-gp130 MoAb described above, respectively.
Cell preparation.
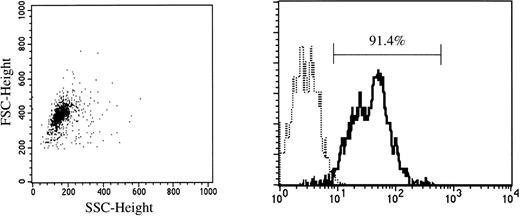
Cord blood samples were aspirated in heparinized plastic syringes from the umbilical vein at normal delivery. Fully informed consent was obtained from the mothers of all neonates before harvesting the specimens. Mononuclear cells were separated by density centrifugation over Ficoll-Paque (Pharmacia Fine Chemicals, Piscataway, NJ), washed twice, and suspended in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 1 mmol/L EDTA-2Na and 2.5% FBS (Hyclone, Logan, UT). After treatment with Silica (Immuno-Biological Laboratories, Fujioka, Japan) for 30 minutes at 37°C, CD34+ cells were enriched using a Dynal CD34 Progenitor Cell Selection System (Dynal A.S., Oslo, Norway). Briefly, 2 to 4 × 107 cells were mixed with the same number of polystyrene beads coated with an MoAb specific for CD34 (Dynabeads M-450 CD34) and incubated for 30 minutes at 4°C. Bead-rosetted cells were separated by a magnet. For the detachment of the beads from the cells, affinity-purified polyclonal antibodies against the Fab portion of anti-CD34 Ab (Detach-a-Bead CD34) were added, and incubation was performed for 45 minutes at room temperature. The detached beads were removed by the magnet, and the cells were collected as CD34+ cells. As shown in Fig 1, greater than 90% of the isolated cells were CD34+, as determined by FACScan flow cytometry (Becton Dickinson).
CD34 expression on cord blood cells separated by immunomagnetic beads coated with anti-CD34 monoclonal antibody. (—) Labeled with FITC-conjugated anti-CD34 MoAb. (···) Labeled with FITC-conjugated mouse IgG.
CD34 expression on cord blood cells separated by immunomagnetic beads coated with anti-CD34 monoclonal antibody. (—) Labeled with FITC-conjugated anti-CD34 MoAb. (···) Labeled with FITC-conjugated mouse IgG.
Suspension cultures.
Serum-deprived liquid cultures were performed in 6-well culture plates (#3046; Becton Dickinson) using a modification of the technique described previously.20 CD34+ cells (1 × 105) were cultured in each well containing 10 mL of α-medium (Flow Laboratories Inc, Rockville, MD) supplemented with 1% deionized bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), 300 μg/mL fully iron-saturated human transferrin (∼98% pure; Sigma), 16 μg/mL soybean lecithin (Sigma), 9.6 μg/mL cholesterol (Nakalai Chemicals Ltd, Tokyo, Japan), 10 ng/mL of SCF, 100 U/mL of IL-3, and 50 ng/mL of IL-6, alone or in combination. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. Half of the cells and culture medium was replaced weekly with fresh medium containing the cytokine(s). The number of viable cells was determined by a trypan-blue exclusion test using a hemocytometer. For the examination of the effects of IL-6, IL-4, and IL-3 on the SCF-dependent development of the cultured mast cells, 1 to 2 × 104 cells at various culture stages were incubated for 1 to 2 weeks in 24-well culture plates (#3047; Becton Dickinson) containing 10 or 100 ng/mL of SCF, 50 ng/mL of IL-6, 20 ng/mL of IL-4, and 100 U/mL of IL-3, alone or in combination.
Serum-containing cultures contained 10% FBS or 10% pooled cord blood sera instead of a combination of BSA, transferrin, lecithin, and cholesterol. To examine the effects of FBS on SCF-dependent or SCF+IL-6–dependent mast cell growth, we used different sources of FBS, ie, Hyclone, GIBCO BRL (Grand Island, NY), Sigma, Stem Cell Technologies (Vancouver, British Columbia, Canada).
Serum-deprived single-cell culture.
Single-cell sorting was performed by two-step sorting. Cord blood mononuclear cells (2 × 106) were stained with 20 μL of FITC-conjugated anti-CD34 MoAb. After two washes, CD34+cord blood cells were collected in 5-mL tubes and were resorted into the individual wells of a 96-well U-bottomed tissue culture plate (#3077; Becton Dickinson) containing 100 μL of α-medium supplemented with 1% BSA, 300 μg/mL of fully iron-saturated human transferrin, 16 μg/mL of soybean lecithin, 9.6 μg/mL of cholesterol, and 10 ng/mL of SCF, using the FACStarplusflow cytometer equipped with an automatic cell deposition unit (Becton Dickinson), as described previously.21 22 Ninety-nine percent of the wells contained a single cell on the first day of culture. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. The number of cells in each well was serially counted until 4 weeks under direct microscopic visualization. Then, colonies consisting of more than 50 cells were individually lifted with an Eppendorf micropipet, and the constituent cells of colonies were stained with antitryptase MoAb.
Clonal cell cultures.
The mast cell colony assay was performed in 35-mm Lux suspension culture dishes (#171099; Nunc, Naperville, IL) using a modification of the technique described previously.23 The culture consisted of 5,000/mL 10-week-old cultured cells grown by 10 ng/mL of SCF, α-medium, 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 1% BSA, 300 μg/mL of fully iron-saturated human transferrin, 16 μg/mL of soybean lecithin, 9.6 μg/mL of cholesterol, and 100 ng/mL of SCF with or without 50 ng/mL of IL-6. Dishes were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. On days 7 and 14, aggregates consisting of 30 or more cells were scored as mast cell colonies, and those consisting of 10 to 29 cells were scored as mast cell clusters. To confirm the in situ identification of mast cells, 60 individual colonies and clusters were lifted with a 3-μL Eppendorf micropipette, spread on glass slides using a Cytospin II (Shandon Southern, Sewickly, PA), and stained with the antitryptase MoAb or mouse IgG1 using the alkaline phosphatase-antialkaline phosphatase (APAAP) technique. Almost all of the constituent cells were positive for tryptase.
Cytochemical staining.
Cultured cells (5 × 103) were spread on glass slides using a Cytospin II and stained with May-Grünwald-Giemsa, toluidine blue, and Bieblich scarlet. Cytochemical reactions with peroxidase and α-naphthyl butyrate esterase were performed by the conventional methods.
Immunocytochemical staining.
Reactions with mouse MoAbs against tryptase, chymase, eosinophil peroxidase, and CD11b were detected using the APAAP method (Dako APAAP Kit System; Dako Corp, Carpinteria, CA), as described previously.24 The isotype mouse MoAb was also used as a control. Briefly, cytocentrifuged samples were fixed with Carnoy’s fluid, washed with PBS, and preincubated with normal rabbit serum to saturate the Fc receptors on the cell surface. After being washed with PBS three times, the samples were reacted with a mouse MoAb for 30 minutes at room temperature in a humidified chamber. After three more washes with PBS, the samples were incubated with rabbit antimouse IgG antibody, washed three times, and successively reacted with the calf intestinal alkaline phosphatase mouse monoclonal antialkaline phosphatase complex. Finally, alkaline phosphatase activity was detected with naphthol AS-MX phosphate, Fast Red TR, and levamisole to inhibit nonspecific alkaline phosphatase activity. The specimens were counterstained with hematoxylin. Five hundred cells were examined.
The diameter of the mast cells was measured by calculating the average of two perpendicular diameters of tryptase+ cells on glass slides, using a microscope equipped with an ocular micrometer.
Flow cytometric analysis.
For the analysis of surface markers on the cultured cells, 1 to 2 × 105 cells were collected in plastic tubes and incubated with an appropriately diluted FITC- or PE-conjugated MoAb, as described previously.21 22 After the cells were washed twice, their surface markers were analyzed with a FACScan flow cytometer using the Lysis 2 software program. Based on our preliminary results obtained by the incubation of nonfixed mast cells with propidium iodine, viable cells were gated according to their forward scatter characteristics and side scatter characteristics. The proportion of positive cells was determined by comparison to cells stained with an FITC- or PE-conjugated mouse isotype-matched IgG (Dako).
For the analysis of FcεRI and gp130 on the cultured mast cells, 1 × 105 cells were incubated with 20 μL anti-FcεRI MoAb or anti-gp130 MoAb for 30 minutes at 4°C. The mouse isotype MoAb was used as a control. The cells were washed three times and stained with FITC-conjugated goat antimouse Ig (GAM; Becton Dickinson) for 15 minutes.
For the assay of the intracellular CD68 expression of the cultured cells grown with SCF, the cells were treated with 2 mL of ORTHO PermeaFix (Ortho Diagnostic Systems, Raritan, NJ) for 40 minutes at room temperature and then incubated with the FITC-conjugated anti-CD68 MoAb, as described previously.20 An FITC-conjugated isotype MoAb was used as a control.
Ultrastructural study.
For the ultrastructural examination, the cells were fixed with 1.25% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.2) for 2 hours and postfixed in 1% osmium tetroxide, as described previously.24 The specimens were then dehydrated in alcohol and embedded in Araldite (Nissin Co, Tokyo, Japan). Ultrathin sections were stained with uranyl acetate and lead citrate. These sections were then examined with an electron microscope (H-300; Hitachi, Tokyo, Japan).
Assay of histamine and cytokine levels.
Histamine concentrations in cell lysates obtained by the treatment of the cultured cells with 0.5% Nonidet P-40 and in supernatant were measured by a radioimmunoassay (RIA; Immunotech). The GM-CSF concentrations in the supernatant of the cultured cells were measured by an enzyme-linked immunosorbent assay (Amersham International, Buckinghamshire, UK). We also measured the concentrations of GM-CSF, IL-4, IL-6, and TGF-β1 in pooled cord blood sera using this assay. All assays were conducted in triplicate.
Statistical analysis.
All experiments were performed at least two times and were shown to be reproducible. Values are expressed as the means ± SD. The Student’s t-test was used to determine the significance of differences between two independent groups. One-way analysis of variance, followed by post hoc contrasts with Bonferroni limitation, was used for more than three independent groups.
RESULTS
SCF alone stimulates the selective growth of mast cells from CD34+ cord blood cells.
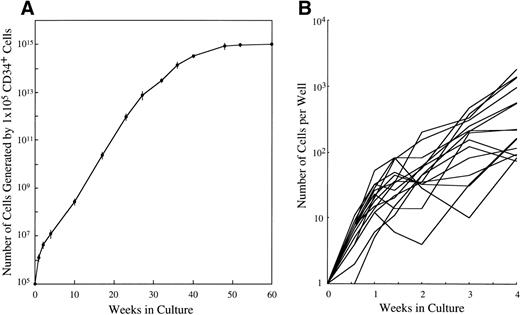
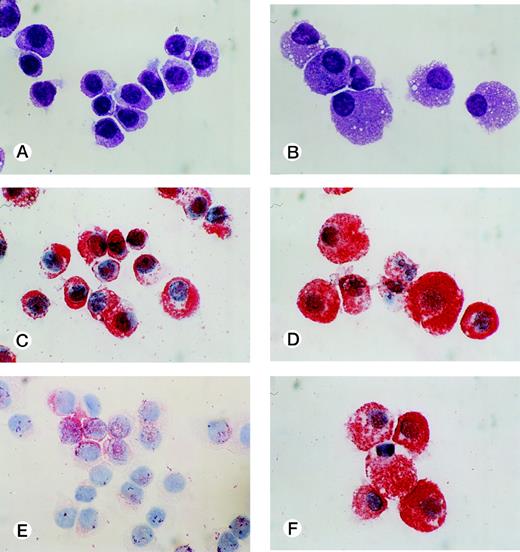
CD34+ cord blood cells (1 × 105) separated by immunomagnetic beads were plated in wells containing 10 mL of serum-deprived culture medium supplemented with 10 ng/mL of SCF. Half of the cultured cells and culture medium was replaced weekly with fresh culture medium containing 10 ng/mL of SCF. Cultured cells were nonadherent throughout the culture period. As shown in Fig 2A, a progressive, steady increase of cell production was achieved, but the cell growth abated after 26 weeks. The cumulative cell number reached 1010-fold the input quantity at 50 weeks of culture. There was no cell growth in the absence of SCF. The May-Grünwald-Giemsa staining showed that almost all of the cultured cells generated after 4 weeks had a round or oval nucleus and contained prominent granules in the cytoplasm, as shown in Fig 3A. The cells became larger and contained more granules in the cytoplasm with prolongation of the culture period (Fig 3B). The cultured cells grown beyond 4 weeks were negative for peroxidase, α-naphthyl butyrate esterase, and Biebrich scarlet stainings. The cells with other lineage-specific markers (CD2, CD19, CD11b, CD15, or glycophorin A) were at negligible levels according to the immunocytochemical staining. The granules showed metachromasia, as determined by toluidine-blue staining. As shown in Table 1, tryptase-positive cells appeared at 1 week of culture. After 4 weeks, almost all of the cultured cells reacted with antitryptase MoAb, but were negative for chymase. At 10 weeks of culture, a part of the cultured cells weakly reacted with antichymase MoAb (Fig 3E). After 36 weeks, a vast majority of the cells were positive for both types of protease, as shown in Fig 3D and F. When 1 × 104 CD34+ cord blood cells sorted by the FACStarplus flow cytometer were used as target cells, significant cell production was also observed: 3.5 ± 0.3 × 104 cells at 1 week, 9.5 ± 0.3 × 104 cells at 2 weeks, 23.3 ± 2.6 × 104 cells at 3 weeks, and 59.5 ± 2.6 × 104 cells at 4 weeks. Almost all of the 4-week cultured cells were positive for tryptase.
Time course of mast cell development from CD34+ cord blood cells in serum-deprived liquid cultures. (A) CD34+ cord blood cells (1 × 105) were cultured in each well containing 10 mL of serum-deprived liquid culture medium supplemented with 10 ng/mL of SCF. The number of viable cells was serially counted, and the results presented are corrected for demipopulation. Similar results were obtained in the other two experiments. Values are expressed as the mean ± SD. (B) The single CD34+ cord blood cells were sorted into the individual wells of a 96-well culture plate containing 10 ng/mL of SCF, as described in Materials and Methods. The number of cells in each well was serially counted until 4 weeks. The cell growth of a total of 15 colonies that generated more than 50 cells positive for tryptase at 4 week is shown.
Time course of mast cell development from CD34+ cord blood cells in serum-deprived liquid cultures. (A) CD34+ cord blood cells (1 × 105) were cultured in each well containing 10 mL of serum-deprived liquid culture medium supplemented with 10 ng/mL of SCF. The number of viable cells was serially counted, and the results presented are corrected for demipopulation. Similar results were obtained in the other two experiments. Values are expressed as the mean ± SD. (B) The single CD34+ cord blood cells were sorted into the individual wells of a 96-well culture plate containing 10 ng/mL of SCF, as described in Materials and Methods. The number of cells in each well was serially counted until 4 weeks. The cell growth of a total of 15 colonies that generated more than 50 cells positive for tryptase at 4 week is shown.
Cytological characteristics of 10-week and 40-week cultured cells grown by SCF. Cytochemical and immunologic stainings of the cultured cells grown by 10 ng/mL of SCF were performed on cytocentrifuged samples. Staining of 10-week cultured cells (A) and 40-week cultured cells (B) with May-Grünwald-Giemsa. Staining of 10-week cultured cells (C) and 40-week cultured cells (D) with an MoAb for tryptase. Staining of 10-week cultured cells (E) and 40-week cultured cells (F) with an MoAb for chymase. (Original magnification × 1,000.)
Cytological characteristics of 10-week and 40-week cultured cells grown by SCF. Cytochemical and immunologic stainings of the cultured cells grown by 10 ng/mL of SCF were performed on cytocentrifuged samples. Staining of 10-week cultured cells (A) and 40-week cultured cells (B) with May-Grünwald-Giemsa. Staining of 10-week cultured cells (C) and 40-week cultured cells (D) with an MoAb for tryptase. Staining of 10-week cultured cells (E) and 40-week cultured cells (F) with an MoAb for chymase. (Original magnification × 1,000.)
Appearance of Tryptase+ Cells and Chymase+ Cells in Culture With CD34+ Cord Blood Cells and SCF
| Weeks in Culture . | Tryptase+ Cells (%) . | Chymase+ Cells (%) . |
|---|---|---|
| 1 | 7 ± 2 | 0 |
| 2 | 78 ± 4 | 0 |
| 3 | 96 ± 3 | 0 |
| 4 | 100 | 0 |
| 7 | 100 | 0 |
| 10 | 100 | 2 ± 1 |
| 14 | 100 | 8 ± 1 |
| 22 | 100 | 40 ± 3 |
| 32 | 100 | 91 ± 4 |
| 36 | 100 | 100 |
| 40 | 100 | 100 |
| 48 | 100 | 100 |
| 60 | 100 | 100 |
| Weeks in Culture . | Tryptase+ Cells (%) . | Chymase+ Cells (%) . |
|---|---|---|
| 1 | 7 ± 2 | 0 |
| 2 | 78 ± 4 | 0 |
| 3 | 96 ± 3 | 0 |
| 4 | 100 | 0 |
| 7 | 100 | 0 |
| 10 | 100 | 2 ± 1 |
| 14 | 100 | 8 ± 1 |
| 22 | 100 | 40 ± 3 |
| 32 | 100 | 91 ± 4 |
| 36 | 100 | 100 |
| 40 | 100 | 100 |
| 48 | 100 | 100 |
| 60 | 100 | 100 |
The cultured cells shown in Fig 2A were processed for immunocytochemical staining with an MoAb against tryptase or chymase. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. Values are expressed as the mean ± SD.
To elucidate the cellular growth in the early phase of culture, we performed single CD34+ cell culture studies. The cell growth of a total of 15 colonies that generated more than 50 cells at 4 week is shown in Fig 2B. The number of cells in each well was 21 ± 12 (range, 5 to 52) cells at 1 week, 59 ± 53 (range, 4 to 202) cells at 2 weeks, 189 ± 140 (range, 10 to 467) cells at 3 weeks, and 525 ± 578 (range, 74 to 1821) cells at 4 weeks. Almost all of the 4-week cultured cells in the individual wells reacted with antitryptase MoAb. On the other hand, 29% of CD34+ cells showed an apparent, but transient progeny generation and eventually degenerated. The remaining CD34+ cells underwent no cell division.
The electron microscopic analysis showed that the 40-week cultured cells had more granules in their cytoplasm compared with the 10-week cultured cells. The complete scrolls reported for lung tryptase+ mast cells by Craig et al25 26 were not observed in the cells at either timepoint. Instead, the granules were similar to electron-dense cores, as described for immature tryptase+ chymase+ mast cells in tissues.
The cultured cells had a significant amount of intracellular histamine. The histamine levels in the cell lysates increased with the culture period: 1.05 ± 0.08 pg/cell at 4 weeks, 1.53 ± 0.11 pg/cell at 10 weeks, 2.57 ± 0.13 pg/cell at 30 weeks, and 5.05 ± 0.1 pg/cell at 40 weeks. The flow cytometric analysis showed that, whereas both the 10-week and 40-week cultured cells displayed high c-kit antigen density, the mean intensity was substantially lower in the 40-week cells. Both of the cells were positive for CD33 and CD45 antigens and weakly positive for FcεRI. The percentages of CD9 and CD61 were low and those of CD11a and CD11b were virtually negative. Moreover, an intracellular expression but not surface expression of CD68 was observed. Thus, the present results indicated that SCF alone could stimulate the selective growth of mast cells from CD34+ human cord blood cells in serum-deprived culture conditions.
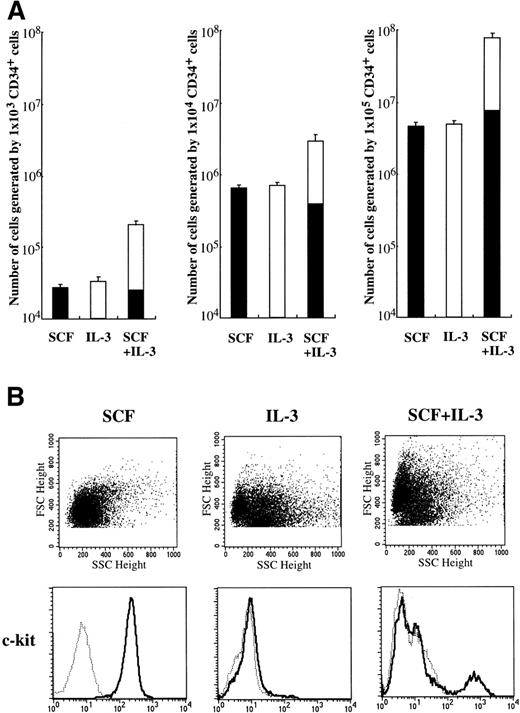
Since Durand et al10 reported that a cocktail of SCF and IL-3 is necessary for human mast cell differentiation from CD34+ cord blood cells, we examined whether IL-3 influenced SCF-dependent mast cell development from CD34+ cord blood cells in our culture condition. As shown in Fig 4A, IL-3 alone could not support the generation of tryptase+ cells. The frequency of tryptase+ cells grown under stimulation with SCF+IL-3 was 10.5% to 13.5% at 4 weeks. Consequently, there was no difference in the absolute numbers of tryptase+ cells between SCF and SCF+IL-3. These results were confirmed by the reaction with anti–c-kit MoAb (Fig 4B). Significant numbers of the cultured cells grown by IL-3 or SCF+IL-3 were identified to be eosinophils and basophils by toluidine blue staining and immunocytochemical staining. Next, we examined the effects of IL-3 on the development of 10-week-old cultured mast cells grown by SCF. After 2 weeks, the 10-week cultured cells did not grow/survive in the presence of IL-3 alone. In addition, IL-3 failed to influence SCF-dependent progeny generation by 1 × 104 10-week-old cultured cells: the numbers of progenies after 2 weeks were 1.08 ± 0.16 × 105 in SCF alone and 1.11 ± 0.15 × 105 in SCF+IL-3. No difference in the percentages of chymase+ cells was observed between the two groups.
Effects of IL-3 on mast cell growth by CD34+ cord blood cells. (A) CD34+ cord blood cells (1 × 103 to 1 × 105) were cultured with 10 ng/mL of SCF and/or 100 U/mL of IL-3. The number of viable cells was counted at 4 weeks, and then the percentage of tryptase+ cells (▪) was determined by immunocytochemical staining. Values are expressed as the mean ± SD. (B) Expression of c-kit on the 4-week cultured cells was analyzed by flow cytometry. The percentage of c-kit+ cells in the cultured cells grown by SCF+IL-3 was 12.7%. (—) Labeled with PE-conjugated anti–c-kit MoAb. (···) Labeled with PE-conjugated mouse IgG.
Effects of IL-3 on mast cell growth by CD34+ cord blood cells. (A) CD34+ cord blood cells (1 × 103 to 1 × 105) were cultured with 10 ng/mL of SCF and/or 100 U/mL of IL-3. The number of viable cells was counted at 4 weeks, and then the percentage of tryptase+ cells (▪) was determined by immunocytochemical staining. Values are expressed as the mean ± SD. (B) Expression of c-kit on the 4-week cultured cells was analyzed by flow cytometry. The percentage of c-kit+ cells in the cultured cells grown by SCF+IL-3 was 12.7%. (—) Labeled with PE-conjugated anti–c-kit MoAb. (···) Labeled with PE-conjugated mouse IgG.
Effects of IL-6 on the growth and properties of cultured mast cells supported by SCF.
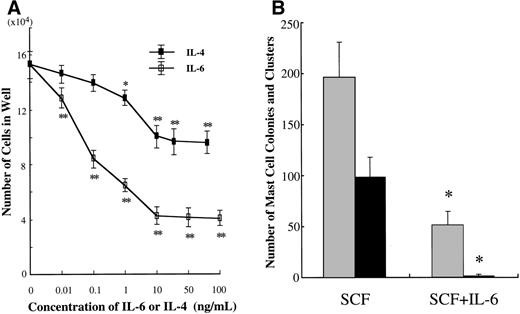
We examined the effects of IL-6 on the growth and properties of cultured mast cells generated by SCF from CD34+ cord blood cells. Ten-week-old cultured mast cells (1 × 104) were incubated for 2 weeks in wells containing IL-6 at concentrations ranging from 0.01 to 100 ng/mL, with SCF at 100 ng/mL. The results are shown in Fig 5A. The addition of IL-6 to the culture with SCF gave rise to a significant decrease in the numbers of progenies. Maximum inhibition was seen with greater than 10 ng/mL of IL-6. In subsequent experiments, IL-6 at 50 ng/mL was used. To examine whether IL-6 exerted an inhibitory effect at the mast cell precursor level, 10-week cultured cells were plated at 5,000 cells per dish containing serum-deprived methylcellulose culture medium supplemented with SCF or SCF+IL-6. As shown in Fig 5B, SCF alone supported the formation of 98.0 ± 20.0 mast cell colonies and 195.5 ± 35.1 clusters. The addition of IL-6 resulted in a marked reduction of SCF-dependent mast cell colony growth (1.5 ± 1.3 colonies and 51.0 ± 14.4 clusters). The ratio of colonies to clusters changed with time in culture; at 1 week, 1.0 ± 0.8 colonies and 69.0 ± 8.3 clusters were grown in the culture with SCF alone, whereas no colonies and 0.8 ± 0.5 clusters were formed in the culture with SCF+IL-6.
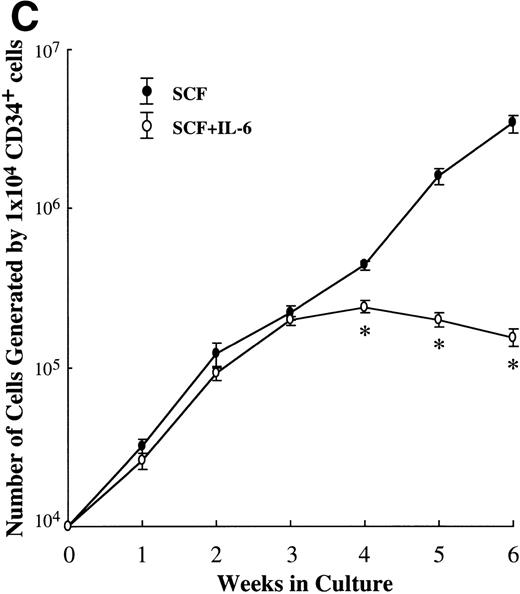
Effects of IL-6 on SCF-dependent mast cell development. (A) Dose response to IL-6 or IL-4 of mast cell growth supported by SCF. Ten-week cultured mast cells (1 × 104) were incubated in wells containing either IL-6 at 0.01 to 100 ng/mL or IL-4 at 0.01 to 80 ng/mL with SCF at 100 ng/mL. After 2 weeks, the number of viable cells was determined. Significantly different from SCF alone (*P < .0005, **P < .0001). (B) Effects of IL-6 on the formation of mast cell colonies and clusters supported by SCF. Five thousand 10-week cultured mast cells were plated per dish containing serum-deprived methylcellulose culture medium supplemented with SCF or SCF+IL-6. After 14 days, aggregates consisting of 30 or more cells were scored as mast cell colonies and those of 10 to 29 cells were scored as mast cell clusters. SCF, 100 ng/mL; IL-6, 50 ng/mL. Numbers of mast cell colonies (▪) and mast cell clusters (▩). Results shown are the mean ± SD of three experiments. Significantly different from SCF alone (*P < .0001). (C) Effects of IL-6 on the cell growth by CD34+ cord blood cells under stimulation with SCF. CD34+ cord blood cells (1 × 104) were cultured with 10 ng/mL of SCF or 10 ng/mL of SCF + 50 ng/mL of IL-6. The number of viable cells was determined every week. Significantly different from SCF alone (*P < .0001).
Effects of IL-6 on SCF-dependent mast cell development. (A) Dose response to IL-6 or IL-4 of mast cell growth supported by SCF. Ten-week cultured mast cells (1 × 104) were incubated in wells containing either IL-6 at 0.01 to 100 ng/mL or IL-4 at 0.01 to 80 ng/mL with SCF at 100 ng/mL. After 2 weeks, the number of viable cells was determined. Significantly different from SCF alone (*P < .0005, **P < .0001). (B) Effects of IL-6 on the formation of mast cell colonies and clusters supported by SCF. Five thousand 10-week cultured mast cells were plated per dish containing serum-deprived methylcellulose culture medium supplemented with SCF or SCF+IL-6. After 14 days, aggregates consisting of 30 or more cells were scored as mast cell colonies and those of 10 to 29 cells were scored as mast cell clusters. SCF, 100 ng/mL; IL-6, 50 ng/mL. Numbers of mast cell colonies (▪) and mast cell clusters (▩). Results shown are the mean ± SD of three experiments. Significantly different from SCF alone (*P < .0001). (C) Effects of IL-6 on the cell growth by CD34+ cord blood cells under stimulation with SCF. CD34+ cord blood cells (1 × 104) were cultured with 10 ng/mL of SCF or 10 ng/mL of SCF + 50 ng/mL of IL-6. The number of viable cells was determined every week. Significantly different from SCF alone (*P < .0001).
Next, we examined the stage of differentiation of hematopoietic progenitors into mast cell lineage on which IL-6 exerted the inhibitory action. IL-6 was added together with CD34+ cord blood cells and SCF on day 0. As shown in Fig 5C, the number of viable cells generated by SCF+IL-6 was comparable to the value obtained by SCF alone until 3 weeks. However, significant differences were observed after 4 weeks. Even when SCF was added on day 1 to the culture initiated with IL-6, SCF-dependent cell growth was not affected during the early culture period. There was no difference in the frequency of tryptase+ cells in the 2-week cultured cells between SCF alone and SCF+IL-6 (70% v 74%).
Nakahata et al27 reported that IL-6 is a requisite for the growth of sufficient numbers of pure mast cells from cord blood cells in a serum-containing liquid culture medium supplemented with SCF. Thus, we compared the SCF-dependent (10 ng/mL) and SCF (10 ng/mL)+IL-6 (50 ng/mL) –dependent progeny production by 1 × 104cultured mast cells at the age of 10 weeks in the two different liquid culture conditions. In the serum (Hyclone’s FBS)-containing cultures, SCF alone generated 0.19 ± 0.03 × 104 cells and SCF+IL-6 generated 1.59 ± 0.27 × 104 cells after 2 weeks. In the serum-deprived cultures, SCF alone yielded 3.13 ± 0.18 × 104 cells and SCF+IL-6 yielded 1.99 ± 0.15 × 104 cells. Because similar results were obtained by the other sources of FBS, we attempted to identify a negative regulator(s) in FBS using the cord blood sera of normal human neonates because of the unavailability of antibovine cytokine antibodies. The addition of 10% pooled cord blood sera to the culture containing the 10-week cultured cells and 100 ng/mL of SCF decreased the number of progenies by 35% after 1 week. According to the conventional enzyme immunoassay, the concentrations of GM-CSF, IL-4, IL-6, and TGF-β1 in pooled cord blood sera were less than 2 pg/mL, less than 0.5 pg/mL, 8.46 pg/mL, and 55.4 ng/mL, respectively. None of neutralizing anti–GM-CSF antibody at 2 μg/mL, anti–IL-4 antibody at 10 μg/mL, and anti–IL-6 antibody at 10 μg/mL influenced the progeny generation in the presence of SCF plus cord blood sera, whereas the addition of anti–TGF-β antibody at 200 μg/mL partially but significantly restored the cell production. Furthermore, 5 to 10 ng/mL of TGF-β1 substantially inhibited SCF-dependent mast cell growth.
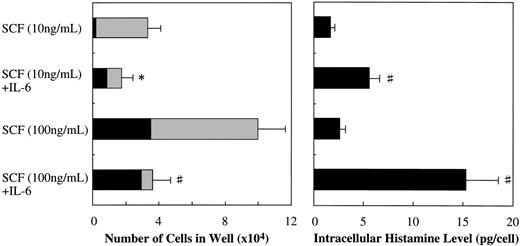
Along with the cell growth retardation, SCF+IL-6 caused a significant increment in the mean diameter of cultured mast cells: 15.3 ± 2.2 μm (range, 11.5 to 20 μm; n = 100) in 10 ng/mL of SCF alone versus 22.4 ± 4.6 μm (range, 12 to 32 μm; n = 100) in 10 ng/mL of SCF + 50 ng/mL of IL-6 (P < .0001), and 21.0 ± 3.6 μm (range, 14 to 41 μm; n = 100) in 100 ng/mL of SCF alone versus 28.9 ± 5.4 μm (range, 18 to 50 μm; n = 100) in 100 ng/mL of SCF + 50 ng/mL of IL-6 (P < .0001). Furthermore, IL-6 substantially increased the frequency of chymase+ cells in the cultured mast cells grown by SCF, as shown in Fig 6. At the initiation of culture, almost 100% of the 10-week cultured cells were positive for tryptase, but only 4.0% ± 1.4% of them reacted with antichymase MoAb. After 2 weeks, in the case of 10 ng/mL of SCF, the percentage of chymase+ cells increased to 5.0% ± 1.2% in SCF alone and 49.7% ± 8.1% in SCF+IL-6. In the case of 100 ng/mL of SCF, the percentages of chymase+cells were 34.7% ± 8.6% in SCF alone and 81.8% ± 10.4% in SCF+IL-6. Next, we examined the effect of IL-6 on the intracellular histamine levels of the cultured mast cells. As shown in Fig 6, the addition of IL-6 to the culture containing 10 ng/mL or 100 ng/mL of SCF substantially increased the histamine content compared with SCF alone. Similar results were obtained in cultures with 25-week cultured mast cells (data not shown).
Effects of IL-6 on SCF-dependent development and intracellular histamine levels of cultured mast cells. Ten-week cultured mast cells (1 × 104) were plated in wells containing SCF or SCF+IL-6 for 2 weeks. After the numbers of viable cells were counted, the percentages of tryptase+cells and chymase+ cells were determined by immunocytochemical staining. At the same time, the intracellular histamine concentrations were measured by the RIA. Results shown are the mean ± SD of three experiments. SCF, 10 or 100 ng/mL; IL-6, 50 ng/mL. Tryptase+chymase− cells (▩), tryptase+chymase+ cells (▪). Significantly different from SCF alone (*P < .05, #P< .001).
Effects of IL-6 on SCF-dependent development and intracellular histamine levels of cultured mast cells. Ten-week cultured mast cells (1 × 104) were plated in wells containing SCF or SCF+IL-6 for 2 weeks. After the numbers of viable cells were counted, the percentages of tryptase+cells and chymase+ cells were determined by immunocytochemical staining. At the same time, the intracellular histamine concentrations were measured by the RIA. Results shown are the mean ± SD of three experiments. SCF, 10 or 100 ng/mL; IL-6, 50 ng/mL. Tryptase+chymase− cells (▩), tryptase+chymase+ cells (▪). Significantly different from SCF alone (*P < .05, #P< .001).
Expressions of IL-6 receptor and gp130 on cultured mast cells grown by SCF.
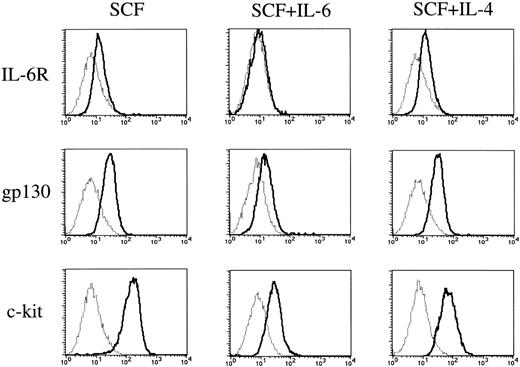
To elucidate whether IL-6 exerted its effect through the receptor, we examined the expressions of IL-6R and gp130 on cultured mast cells grown by SCF using flow cytometry. The cultured mast cells showed low but significant expressions of IL-6R and gp130 (Fig 7). The addition of IL-6 to the cultures containing SCF resulted in a decrease in the expressions of the two receptors on cultured mast cells.
Expressions of IL-6 receptor, gp130, and c-kit on cultured mast cells grown by SCF. Expressions of IL-6R, gp130, and c-kit on 10-week mast cells exposed to SCF, SCF+IL-6, or SCF+IL-4 for 2 weeks were analyzed by flow cytometry according to the procedure described in Materials and Methods. (—) Labeled with FITC-conjugated anti–IL-6R MoAb, anti-gp130 MoAb followed by FITC-conjugated GAM, or PE-conjugated anti–c-kit MoAb. (···) Labeled with FITC-conjugated mouse IgG, mouse IgG followed by FITC-conjugated GAM, or PE-conjugated mouse IgG. SCF, 100 ng/mL; IL-6, 50 ng/mL; IL-4, 20 ng/mL.
Expressions of IL-6 receptor, gp130, and c-kit on cultured mast cells grown by SCF. Expressions of IL-6R, gp130, and c-kit on 10-week mast cells exposed to SCF, SCF+IL-6, or SCF+IL-4 for 2 weeks were analyzed by flow cytometry according to the procedure described in Materials and Methods. (—) Labeled with FITC-conjugated anti–IL-6R MoAb, anti-gp130 MoAb followed by FITC-conjugated GAM, or PE-conjugated anti–c-kit MoAb. (···) Labeled with FITC-conjugated mouse IgG, mouse IgG followed by FITC-conjugated GAM, or PE-conjugated mouse IgG. SCF, 100 ng/mL; IL-6, 50 ng/mL; IL-4, 20 ng/mL.
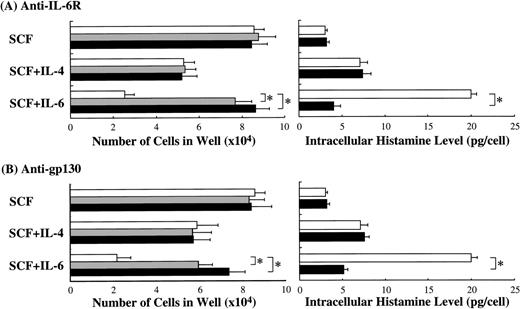
Next, we examined whether anti–IL-6R Ab or anti-gp130 Ab could counteract the biological activity of IL-6. The results presented in Fig 8 are from one representative experiment of three. Similar results were obtained in the other two experiments. Anti–IL-6R Ab or anti-gp130 Ab at 5 or 10 μg/mL was added to the cultures containing SCF or SCF+IL-6. Anti–IL-6R Ab neutralized the inhibitory effect of IL-6 on mast cell growth, although the numbers of mast cells grown by SCF alone were not influenced. Conversely, the anti–IL-6R Ab abrogated the stimulatory effect of IL-6 on the intracellular histamine content. Like anti–IL-6R Ab, anti-gp130 Ab neutralized both of these effects of IL-6.
Neutralization of IL-6 activity by anti–IL-6R Ab or anti-gp130 Ab. To examine whether anti–IL-6R Ab or anti-gp130 Ab counteracted the biological effects of IL-6 on the cultured mast cells supported by SCF, each Ab was added at 5 μg/mL (▩) or 10 μg/mL (▪) to wells containing 1 × 104 10-week cultured cells with SCF, SCF+IL-6, or SCF+IL-4. SCF, 100 ng/mL; IL-6, 50 ng/mL; IL-4, 20 ng/mL. Significantly different from no Ab (*P< .0001).
Neutralization of IL-6 activity by anti–IL-6R Ab or anti-gp130 Ab. To examine whether anti–IL-6R Ab or anti-gp130 Ab counteracted the biological effects of IL-6 on the cultured mast cells supported by SCF, each Ab was added at 5 μg/mL (▩) or 10 μg/mL (▪) to wells containing 1 × 104 10-week cultured cells with SCF, SCF+IL-6, or SCF+IL-4. SCF, 100 ng/mL; IL-6, 50 ng/mL; IL-4, 20 ng/mL. Significantly different from no Ab (*P< .0001).
Comparison of effects of IL-6 with those of IL-4 on mast cell development supported by SCF.
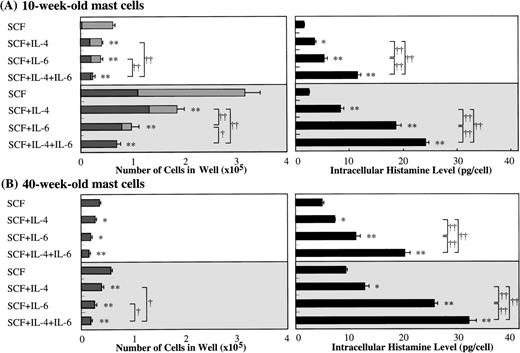
Because several investigators11-16 have reported the effect of IL-4 on the growth, c-kit expression, and functions of mast cells using serum-containing liquid cultures, we compared the effects of IL-6 with those of IL-4 on the development of cultured mast cells supported by SCF. The addition of IL-4 decreased the numbers of mast cells grown by SCF, and maximum inhibition was seen with greater than 10 ng/mL of IL-4 (Fig 5A). Moreover, the exposure of 10-week cultured mast cells to SCF at 10 or 100 ng/mL plus IL-4 at 20 ng/mL caused an increase in the histamine concentrations in the cell lysates compared with the values obtained by SCF alone, as shown in Fig 9. Similar results were obtained in the case of 40-week cultured mast cells. It is of interest that IL-6 was a more potent stimulator of the intracellular histamine content of cultured mast cells under stimulation with SCF than was IL-4. In the cultures containing 10-week cultured mast cells and 100 ng/mL of SCF, IL-6 more intensely inhibited the mast cell growth compared with IL-4, which was consistent with the results shown in Fig 5A. When SCF, IL-6, and IL-4 were used together, both the inhibition of mast cell growth and the elevation of intracellular histamine levels were further amplified, except for the cultures containing 40-week cells and 10 ng/mL of SCF. We then examined whether neutralizing anti–IL-4 antibody and anti–IL-6 antibody influenced the action of IL-6 and IL-4 on SCF-dependent mast cell growth, respectively. In the experiments using anti–IL-4 antibody at 100 μg/mL, the numbers of viable cells grown by 1 × 104 10-week-old cultured cells after 2 weeks were: 8.1 ± 0.7 × 104 cells without the antibody and 8.3 ± 0.8 × 104 cells with the antibody in SCF (100 ng/mL) alone; 2.2 ± 0.6 × 104 cells without the antibody and 2.1 ± 0.3 × 104 cells with the antibody in SCF (100 ng/mL) +IL-6 (50 ng/mL); and 5.4 ± 0.7 × 104 cells without the antibody and 8.0 ± 0.8 × 104 cells with the antibody in SCF (100 ng/mL) +IL-4 (20 ng/mL). On the other hand, in the experiments using anti–IL-6 antibody at 60 μg/mL, the numbers of progenies were as follows: 8.6 ± 0.5 × 104 cells without the antibody and 8.8 ± 0.7 × 104 cells with the antibody in SCF alone; 2.2 ± 0.6 × 104 cells without the antibody and 8.6 ± 0.7 × 104 cells with the antibody in SCF+IL-6; and 5.9 ± 1.0 × 104 cells without the antibody and 5.8 ± 0.7 × 104 cells with the antibody in SCF+IL-4.
Comparison of effects of IL-6 with those of IL-4 on mast cell development supported by SCF. Ten-week or 40-week cultured mast cells (2 × 104) were incubated in culture wells containing 10 or 100 ng/mL of SCF, 50 ng/mL of IL-6, or 20 ng/mL of IL-4, alone or in combination. After 2 weeks, the number of viable cells was determined, and the cells were then processed for immunocytochemical staining with an antitryptase or antichymase MoAb. At the same time, histamine amounts in cell lysates were analyzed by the RIA. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. Experiment groups using 10 ng/mL of SCF (□) experiment groups using 100 ng/mL of SCF (▩); tryptase+ chymase−cells (▩); tryptase+chymase+ cells (▪). Significantly different from SCF alone (*P < .0005, **P < .0001). Significantly different among the two- or three-factor combinations (†P < .0005, ††P < .0001).
Comparison of effects of IL-6 with those of IL-4 on mast cell development supported by SCF. Ten-week or 40-week cultured mast cells (2 × 104) were incubated in culture wells containing 10 or 100 ng/mL of SCF, 50 ng/mL of IL-6, or 20 ng/mL of IL-4, alone or in combination. After 2 weeks, the number of viable cells was determined, and the cells were then processed for immunocytochemical staining with an antitryptase or antichymase MoAb. At the same time, histamine amounts in cell lysates were analyzed by the RIA. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. Experiment groups using 10 ng/mL of SCF (□) experiment groups using 100 ng/mL of SCF (▩); tryptase+ chymase−cells (▩); tryptase+chymase+ cells (▪). Significantly different from SCF alone (*P < .0005, **P < .0001). Significantly different among the two- or three-factor combinations (†P < .0005, ††P < .0001).
Finally, we compared the effects of IL-6 with those of IL-4 on the c-kit expression of cultured mast cells grown by SCF. As shown in Fig7, IL-6 reduced the c-kit expression to a greater extent than did IL-4.
DISCUSSION
The present study clearly showed that, even when SCF was used alone, the selective growth of a large number of mast cells from CD34+ cord blood cells could be supported in long-term serum-deprived cultures. The possibility of basophil lineage of the cultured cells was ruled out, because the cells were positive for c-kit, tryptase, and CD68, but negative for CD11b. Single CD34+ cell culture studies imply that SCF acts as a proliferative rather than survival factor in mast cell development from cord blood hematopoietic progenitors. Whereas SCF and IL-3 are required for murine mast cell development,28 the present study showed that IL-3 failed to stimulate the growth of mast cells from CD34+ cord blood cells in the presence or absence of SCF. These results may represent a species-specific growth factor requirement.
Nakahata et al27 demonstrated that IL-6 is a requisite for the growth of sufficient numbers of almost 100% pure mast cells from cord blood cells in a serum-containing liquid culture medium supplemented with SCF. Based on the present experiments in which the effects of SCF or SCF+IL-6 on the growth of cultured mast cells were compared between serum-containing and serum-deprived cultures, the discrepancy in the results was due to the distinct liquid culture systems. The results obtained by the addition of neutralizing anticytokine antibodies suggest a possible role of TGF-β as a negative regulator present in serum.
The addition of IL-6 resulted in a substantial reduction of the number of cultured mast cells grown by SCF. In the clonal cell culture, the numbers of mast cell colony-forming cells and cluster-forming cells were markedly lower in the cultures with SCF+IL-6 than in the cultures with SCF. These results suggest that the decreased number of mast cells by the addition of IL-6 to the culture containing SCF in the liquid culture is attributable in part to the inhibition at the mast cell precursor level. However, IL-6 failed to alter SCF-dependent cell growth by CD34+ cord blood cells until 3 weeks. In addition, there was no difference in the frequency of tryptase+ cells in the 2-week cultured cells between SCF alone and SCF+IL-6. Thus, IL-6 does not appear to affect the differentiation process into mast cell lineage. The flow cytometric analysis showed that the exposure of the cultured mast cells to SCF+IL-6 attenuated the c-kit expression. The addition of IL-4 to the culture with 10-week cultured mast cells and 100 ng/mL of SCF also caused the retardation of the cell growth and the reduction of c-kit expression, but both of these parameters were at lower magnitudes compared with the values obtained by the addition of IL-6 (Figs 5A, 7, and 9). Furthermore, the 40-week cultured mast cells generated the progenies in response to SCF to a lesser extent compared with the 10-week cultured mast cells (Fig 9). The mean intensity of c-kit antigen density was substantially shifted to lower values on the late-appearing mast cells. These results imply the association of the proliferative potential to SCF of cultured mast cells with the c-kit expression. Therefore, one possible explanation is that the IL-6–mediated reduction of c-kit expression causes the growth retardation of mast cells. However, we cannot rule out the possibility that the decreased levels of c-kit are a consequence, but not a cause of an intrinsically decreased cell proliferation potential.
The cultured mast cells exposed to SCF+IL-6 for 2 weeks also showed a more substantial increase in the intracellular histamine levels than those exposed to SCF alone. The stimulation of the 10-week cultured mast cells with SCF+IL-6 for 24 hours did not influence the cell number, but increased the histamine concentrations of both the cell lysate and the supernatant compared with the values obtained with SCF alone (unpublished data). Thus, the increased level of intracellular histamine may reflect an upregulation of histamine production rather than an increment in histamine storage.
Because GM-CSF was reported to suppress SCF-dependent mast cell growth,29 30 it is possible that the effect of IL-6 on mast cell development is mediated by GM-CSF. In the present study, the levels of GM-CSF in the supernatant of the 10-week-old mast cells cultured with 100 ng/mL of SCF and with 100 ng/mL of SCF + 50 ng/mL of IL-6 for 2 weeks were 3.1 ± 0.3 pg/mL and 2.8 ± 0.1 pg/mL, respectively. Moreover, the addition of neutralizing anti–GM-CSF Ab did not influence the SCF-dependent or SCF+IL-6–dependent mast cell growth (unpublished data). The present flow cytometric analysis showed low but significant expressions of IL-6R and gp130 on the cultured mast cells grown by SCF. The addition of anti–IL-6R Ab abrogated both the inhibition of mast cell growth and the elevation of the intracellular histamine content induced by SCF+IL-6. The anti-gp130 Ab also neutralized the biological activity of IL-6. These results imply that the effects of IL-6 on the cultured mast cells are exerted directly via an IL-6R-gp130 system.
Both IL-6 and IL-4 decreased the numbers of cultured mast cells but also increased intracellular histamine levels under stimulation with SCF. Comparative assays of the effects of IL-6 with those of IL-4 on SCF-dependent mast cell development showed that significantly higher concentrations of histamine in cell lysates were obtained in the cultures with SCF+IL-6. Thus, IL-6 may be a more potent stimulator of the intracellular histamine content of mast cells supported by SCF than is IL-4. When SCF, IL-6, and IL-4 were used together, both the inhibition of the cell growth and the elevation of intracellular histamine levels were further amplified compared with the values obtained by the two-factor combinations except for the cultures containing 40-week cultured mast cells and 10 ng/mL of SCF. In addition to the lack of effects of the anti–IL-6R Ab and anti-gp130 Ab on the biological activity of IL-4 (Fig 8), the neutralizing anti–IL-6 Ab did not influence the numbers of cultured mast cells grown by SCF+IL-4. On the other hand, the numbers of cultured mast cells grown by SCF+IL-6 were not affected by the addition of neutralizing anti–IL-4 Ab. Therefore, IL-6 appears to modulate SCF-dependent human mast cell development by a mechanism different from that of IL-4.
ACKNOWLEDGMENT
The authors are deeply indebted to Prof A. Komiyama (Department of Pediatrics, Shinshu University School of Medicine, Matsumoto, Japan) for helpful comments and to Prof T. Katsuyama (Department of Laboratory Medicine, Shinshu University School of Medicine and Central Clinical Laboratory, Shinshu University Hospital) for valuable discussion on all aspects of electron microscopy. We also thank Dr S. Ito (Blood Transfusion Service, Shinshu University Hospital) for his excellent technical assistance.
Supported by Grants-in Aid No. 09670796 and 09770537 from the Ministry of Education of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenichi Koike, MD, Department of Pediatrics, Shinshu University School of Medicine, 3-1-1, Asahi, Matsumoto, 390-8621, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal