Follicular dendritic cells (FDCs) reside within germinal centers of secondary lymphoid tissue where they play a critical role in antigen-driven immune responses. FDCs express numerous adhesion molecules that facilitate cellular interactions with B and T cells within the germinal center microenvironment. Although human FDCs have been shown to influence B-cell development, very little is known about the ability of FDCs to regulate T-cell responses. To investigate this functional aspect of FDCs, highly enriched preparations were isolated by magnetic cell separation using the FDC-restricted monoclonal antibody HJ2. We found that isolated human FDCs inhibited proliferation of both autologous and allogeneic T cells, and were dependent on the number of FDCs present. Inhibition by FDCs was observed using two serologically distinct superantigens at multiple concentrations (Staphylococcus enterotoxin A and B). In contrast, B cells failed to inhibit, and often augmented superantigen-induced T-cell proliferation. Antibody-blocking studies showed that CD54 and CD106 were involved in the ability of FDC to inhibit T-cell proliferative responses. When FDCs and T cells were separated by a semipermeable membrane, the inhibitory effect was partially abrogated, demonstrating that in addition to cell-cell interactions, a soluble factor(s) was also involved in the process. The addition of indomethicin to cultures improved the proliferative response in the presence of FDCs, indicating that inhibition was mediated, in part, by prostaglandins. These results indicate that FDCs regulate T-cell proliferation by two molecular mechanisms and that FDC:T-cell interactions may play a pivotal role in germinal center development.
FOLLICULAR DENDRITIC CELLS (FDCs) are distinct antigen-presenting cells that reside within germinal centers (GCs) of secondary lymphoid tissue.1Although FDCs are a minor component of GCs, comprising only 1% to 2% of all cells, they exhibit numerous cytoplasmic extensions that form an extensive network throughout the GC.1 This enables FDCs to be in intimate contact with neighboring B cells (90% of GC cells) and T cells (5% to 10% of GC cells).1-3 FDCs express large amounts of the adhesion molecules CD54 (ICAM-1) and CD106 (VCAM-1) that facilitate cellular interactions.3,4 Indeed, in vitro binding studies have shown that adhesion between either B cells or T cells with FDCs is mediated by adhesion pathways involving interactions between CD54 with CD11a/CD18 (LFA-1 α/β) and CD106 with CD49d (VLA-4).5,6 The hallmark of FDCs is their ability to trap and retain antigens on their surface for long periods of time in the form of antigen-antibody complexes that contain complement (immune complexes).7 FDCs express receptors for numerous complement components (CD11b, CD21, and CD35) and the Fc portion of IgG (CD32) that readily bind immune complexes.4,8 Studies with immunized mice have shown that antigens rapidly associate with circulating antibodies to form complement-containing antigen-antibody complexes, and localize to regional lymph nodes where they are trapped and expressed on the surface of FDCs.9 These immune complexes, named iccosomes, are then transferred to neighboring B cells during cellular interactions, and are then processed for subsequent presentation to T cells.10,11 Thus, FDCs interact with B cells within the GC microenvironment and may regulate many aspects of B-cell development including antigen-driven B-cell proliferation,2 heavy-chain class switching,12formation of memory B cells,13 and selection of B-cell clones producing high-affinity antibodies.14 15
Because FDCs represent a minor population of cells within lymphoid tissue, functional studies have been hard to perform because of difficulties in obtaining adequate numbers of highly purified human FDCs. Several studies have been performed using FDC clusters containing approximately 1 FDC to 20 contaminating lymphocytes. FDC clusters have been shown to prolong survival,16,17 augment proliferation,16-18 and promote differentiation of B cells.18,19 Other groups have studied FDC function by generating FDC-like cell lines. These lines were produced by culturing FDC-enriched tonsil cell preparations with or without exogenous cytokines,20-22 by Epstein-Barr virus transformation,23 or by fusion and immortalization with a mouse myeloma cell line.24 Studies have shown that FDC-like cells promote B-cell survival,22,23differentiation,25 and either augment or inhibit B-cell proliferation.20-23 However, the origin of these FDC-like cell lines is questionable because of the impurity of the starting population and the observation that surface antigens associated with freshly isolated FDCs are often lost upon culture.
Monoclonal antibodies (MoAbs) that are relatively specific for human FDCs have been developed, such as HJ2,26 7D6,4DRC-1,27 and Ki-M4.28 These antibodies can be used to identify FDCs in situ and for isolating FDCs in single-cell suspension. 7D6, DRC-1, and Ki-M4 have recently been shown to recognize an isoform of CD21 (long form) that is expressed by human FDCs and not B cells.29 It is important to note that these FDC-associated antibodies do not react with other types of dendritic cells such as GC dendritic cells30 and interdigitating dendritic cells found in T-cell areas of lymphoid tissue.30,31 A limited number of studies have been performed using highly purified human FDCs. Interestingly, isolated FDCs were shown to either inhibit or augment B-cell proliferation, depending on the stimulating agent that was used.32,33 For instance, purified FDCs inhibited B-cell proliferation whenStaphylococcus aureus strain Cowan I was used as the activating agent,32 whereas B-cell proliferation was augmented using antibodies against CD40 or surface immunoglobulin.33Although further studies are needed to identify specific molecules produced by isolated FDCs that control B-cell responses, these data show that human FDCs regulate B-cell responses and, as a result, play a pivotal role in B-cell development within GCs.
Although FDC:B-cell interactions have been studied, the ability of human FDCs to regulate T-cell responses has not been examined. This is important because T cells are critical for GC formation34and provide B-cell help through cellular interactions and the production of cytokines.35 It is well known that FDCs do not process antigens.36 However, FDCs can acquire processed antigens consisting of major histocompatibility complex (MHC) class II-peptide complexes from neighboring B cells for presentation to T cells.37 Indeed, murine studies have shown that FDCs express processed antigens in a form capable of inducing antigen-specific T-cell proliferation.37 With the long-term goal of characterizing molecular mechanisms by which FDCs control T-cell responses, we examined the ability of highly purified human FDCs to regulate T-cell proliferative responses. We discovered that purified human FDCs inhibit superantigen (sAg)-induced T-cell proliferation by two distinct mechanisms: one is mediated by cellular interactions involving CD54 and CD106, and the other is mediated by prostaglandins (PGs).
MATERIALS AND METHODS
Antibodies and reagents.
Tissue-culture supernatants from hybridomas (purchased from American Type Culture Collection, Rockville, MD) producing the following mouse MoAbs were used: OKT3 (anti-CD3, IgG2a); Lym-1 (anti-DR, IgG2a); HB180 (anti-class II, IgG2a); HB45 (anti-kappa light chain, IgG1); CRL1763 (anti-lambda light chain, IgG2a); and OKM1 (anti-CD11b, IgG2b). HJ2, an IgM mouse MoAb that recognizes human FDCs has been previously described.26 Ascites containing Ki-M4 (IgG3) was kindly provided by Dr M. Parwaresch (University of Kiel, Kiel, Germany). R-phycoerythrin (PE)-conjugated mouse MoAbs against CD19, DR, CD3, CD4, CD13, and CD22 were obtained from Becton Dickinson (Mountain View, CA). Purified MoAbs against CD106 (VCAM-1, IgG1) and CD86 (B7-2, IgG1) were obtained from PharMingen (San Diego, CA), and MoAbs against CD54 (ICAM-1, IgG1) and CD80 (B7-1, IgG1) were obtained from Immunotech (Westbrook, ME). Indomethicin, and the bacterial sAgs, Staphylococcus enterotoxin A (SEA) and B (SEB), were obtained from Sigma Chemical Co (St Louis, MO).
FDC isolation.
Tonsils were obtained from Arkansas Children’s Hospital after routine tonsillectomies. Tonsil tissue was cut into small fragments and digested in 20 mL of RPMI 1640 containing 2 mg/mL collagenase (type IV; Worthington Biochemical Corp, Freehold, NJ), 0.1 mg/mL DNase (type I; Sigma Chemical Co), 10% fetal bovine serum (FBS; Atlanta Biologicals, Inc, Norcross, GA), and 5 mmol/L EDTA for 1 hour at 4°C, as described.3 Residual red blood cells and dead cells were eliminated by centrifugation over a Ficoll-Hypaque density gradient (Sigma Chemical Co). The resulting cells were resuspended at 107/mL and centrifuged at 250g for 4 minutes. The low-density cells in the supernatant contained the majority of FDCs (3% to 10% FDCs) and was used to obtain highly purified FDCs. Low-density cells were stained with HJ2 (25 μL of tissue-culture supernatant per 5 × 105 cells), followed by rat anti-mouse IgM-coated microbeads (15 μL beads per 107cells; Miltenyi Biotec Inc, Auburn, CA) and fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgM antibodies (μ-chain specific with no cross-reactivity with IgG antibody; Jackson ImmunoResearch Laboratories, West Grove, PA). HJ2+ cells (FDCs) were isolated by positive selection using the magnetic cell separation system from Miltenyi. Labeled cells were applied to a magnetic separation column (type WHM) equilibrated with column buffer according to the manufacturer’s instructions. Flow rates through the column were 10 to 15 drops per minute. The column was removed from the magnet and adherent cells were obtained (HJ2+ cells) by forcing the cells through the column using 1 mL of column buffer and the rubber plunger supplied by the manufacturer. Adherent cells were subsequently reapplied to a second column and collected as above. We typically obtained between 6 and 12 × 105 cells after application to two columns when starting with a cell suspension from an individual tonsil donor. The purity of the FDC preparations used in all the described experiments was always greater than 80%, based on HJ2+ cells.
T- and B-cell isolation.
T cells and B cells were isolated from the FDC-depleted cell fraction (cells in the pellet after low-speed centrifugation) by negative selection using the magnetic cell separation system described above. Single-cell suspensions were stained with MoAbs against class II molecules (Lym-1 and HB180), kappa light chain (HB45), lambda light chain (CRL1763), and monocytes (HB180), or antibodies against T cells (CD3) and monocytes (OKM1) to isolate T cells and B cells, respectively. After incubation with goat anti-mouse IgG-coated microbeads, cells were applied to a magnetic separation column (Miltenyi Biotec Inc) and cells passing through the column when attached to the magnet (nonadherent) were collected. The purity of T cells and B cells isolated by this method was always greater than 90%, based on reactivity with CD3 (T-cell purity) or CD19 (B-cell purity). For experiments requiring more highly purified T cells, the nonadherent cells were applied to a second magnetic separation column and the nonadherent cells collected. This resulted in a tonsil T-cell preparation (>97% CD3+) that failed to proliferate to sAg without adding APCs.
Flow cytometry.
Single-cell suspensions (0.5 to 1 × 105 cells) of HJ2+ cells (labeled with HJ2 and FITC-conjugated goat anti-mouse IgM antibodies) were stained with PE-conjugated MoAbs against CD19, DR, CD3, CD4, CD13, and CD22. Cells were incubated on ice with each antibody for 20 minutes followed by two washes with phosphate-buffered saline. HJ2+ cells were also stained with Ki-M4 followed by PE-conjugated goat anti-mouse IgG antibodies (Tago, Burlingame, CA). The anti-mouse IgG reagent was IgG-specific and exhibited no cross-reactivity with mouse IgM antibodies. Flow cytometry was performed on a FACScan (Becton Dickinson) using WinMDI software. The instrument was calibrated with CaliBRITE beads (Becton Dickinson) using AutoCOMP software. Dead cells were excluded from analysis and 10,000 cells were collected either ungated (HJ2 v Ki-M4), or gated based on HJ2 positivity.
Proliferation assays.
Varying numbers of FDCs or B cells (2 × 104 to 1.25 × 103; γ-irradiated 3,000 rads) were cultured with 105 T cells in proliferation media (RPMI-1640 containing 10% FBS, L-glutamine, and penicillin-streptomycin) for 3 days (autologous assays) or 5 days (allogeneic assays) at 37°C in 5% CO2. Each stimulation condition was performed in triplicate wells of a 96-well microtiter plate in a final volume of 200 μL. Indomethicin (100 μg/mL) and sAg (SEA or SEB at 100 ng/mL) were added at the initiation of each culture. Where indicated, FDCs and B cells (2 × 104 cells) were pretreated with 1 μg/mL of MoAb against various surface molecules for 30 minutes at 37°C. Cells were extensively washed to remove unbound antibody before their addition to T-cell cultures. One microcurie of tritiated thymidine (New England Nuclear, Boston, MA) was added during the last 24 hours of culture and the contents of each well was harvested onto glass fiber filters using a Skatron cell harvester (Sterling, VA), and counted for radioactivity after being placed in a aqueous scintillation cocktail. To normalize for the variability among individual experiments, background cpm (T cells alone) were subtracted from all experimental values. The means of triplicates from individual experiments were then pooled. Data points were expressed as the mean cpm + SEM or as a percentage of the maximum response, as indicated.
Boyden chamber assays.
To physically separate T cells from FDCs or B cells, some proliferation assays were performed using polyethylene terephtalate cell culture inserts with 3-μm pores (Becton Dickinson) that were placed into wells of a 24-well cluster plate. In preliminary experiments, we determined that optimal T-cell proliferation was obtained by culturing 4 × 105 T cells and SEB in cell culture inserts in a 500-μL final volume. FDCs or B cells (γ-irradiated 3,000 rads) at a 1:5 ratio were either placed in the cell insert along with the T cells or in the well of the cluster plate underneath the cell insert. Each stimulation condition was performed in duplicate and tritiated thymidine uptake measured on day 3. The means of duplicates from individual experiments were pooled and expressed as a percentage of the response obtained when T cells were cultured with only SEB.
Statistics.
Means from individual experiments were used and background cpm (T cells alone) were subtracted from experimental values for all statistical analyses. T-cell cultures stimulated with sAg in the presence of either FDC or B cells were compared to cultures containing T cells and sAg using a paired t-test. A one-way analysis of variance (ANOVA) test and Tukey’s post-hoc analysis were used for comparing multiple experimental groups. Experiments involving antibody treatment (blocking studies) or indomethicin were compared using an ANOVA and Tukey’s post-hoc analysis. A P value <.05 was considered statistically significant. Statistical analysis was performed using SigmaStat for Windows 2.0 software (Jandel Corp, San Rafael, CA).
RESULTS
FDCs downregulate autologous T-cell proliferation in response to sAgs.
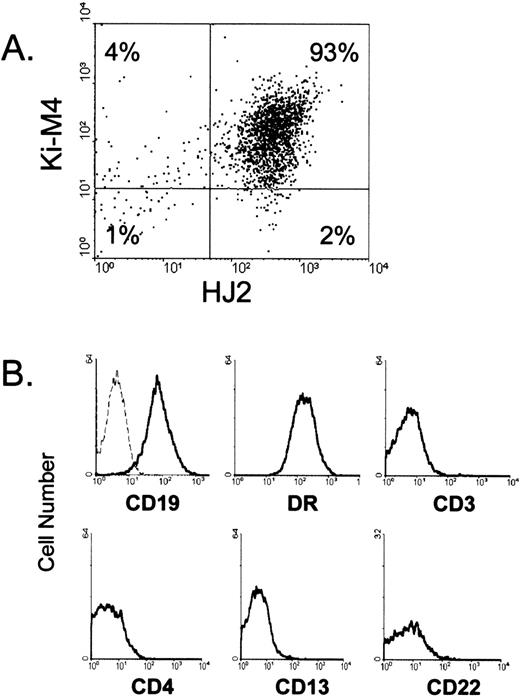
FDCs were isolated from human tonsil tissue using HJ2, a mouse MoAb that binds human FDCs both in tissue sections and in single-cell suspension.26 Low-density tonsil cells (enriched for FDCs) were stained with HJ2 followed by anti-mouse IgM microbeads, and HJ2+ cells were isolated by magnetic cell separation. Cells obtained by this method were always greater than 80% HJ2+, and virtually all of the HJ2+ cells were also Ki-M4+ (Fig 1A), confirming their identity as FDCs and distinguishing these cells from the interdigitating dendritic cells found in T-cell regions of tonsils that are Ki-M4− and CD19−.3,4,26,31 As expected, HJ2+ cells were positive for CD19 and DR, but failed to express markers associated with T cells (CD3 and CD4), monocytes/tingible body macrophages (CD13), and B cells (CD22) (Fig1B). Furthermore, the HJ2+ cells also differ from the recently identified dendritic cells found within GCs of lymphoid tissue that are CD4+, Ki-M4−, and CD19−, the opposite phenotype of the cells used in these studies.30
Phenotype and purity of FDCs isolated from human tonsils. In (A), isolated HJ2+ cells were stained with Ki-M4 followed by PE-conjugated goat anti-mouse IgG specific antibodies. In (B), isolated cells were stained with PE-conjugated primary antibodies, and HJ2+ cells were examined for red fluorescence. The dotted line represents the fluorescent profile of HJ2+cells stained with a PE-conjugated isotype-matched control antibody. Ten thousand cells are displayed in each profile.
Phenotype and purity of FDCs isolated from human tonsils. In (A), isolated HJ2+ cells were stained with Ki-M4 followed by PE-conjugated goat anti-mouse IgG specific antibodies. In (B), isolated cells were stained with PE-conjugated primary antibodies, and HJ2+ cells were examined for red fluorescence. The dotted line represents the fluorescent profile of HJ2+cells stained with a PE-conjugated isotype-matched control antibody. Ten thousand cells are displayed in each profile.
To investigate the ability of isolated FDCs to regulate Ag-driven proliferative responses by autologous T cells, we used the sAgs, SEA, and SEB. sAgs were chosen because they activate 5% to 15% of all T cells without regard for antigen specificity by binding specific TCR β chains.38 Superantigens are similar to conventional Ags because they require presentation by antigen-presenting cells (APCs) for optimal T-cell responses.39 Varying numbers of isolated FDCs were cultured with a constant number of autologous CD3+ tonsillar T cells in the presence of sAg. As shown in Fig 2A, human FDCs inhibited SEB-induced T-cell proliferation in an FDC dose-dependent manner when examining the results from seven independent experiments. For instance, at an FDC:T cell ratio of 1:5, T-cell proliferation was inhibited by 75% (P < .05), compared to T cells stimulated with SEB in the absence of FDCs. When the concentration of FDCs was reduced by a factor of 4 (1:20 FDC:T-cell ratio), T-cell proliferation increased by 44%, but was still significantly lower than the response observed in the absence of FDCs (P < .05). At FDC:T-cell ratios of 1:80, T-cell proliferation was not significantly altered, indicating that the inhibitory nature of FDCs was dose dependent (Fig 2A). In individual experiments, FDCs also significantly inhibited T-cell proliferation at FDC to T-cell ratios of 1:5 and 1:20, and occasionally inhibited proliferation at a 1:80 ratio. Similar results were obtained using SEB at concentrations ranging from 10 to 1,000 ng/mL, indicating that the inhibitory effect is not limited to a particular sAg concentration (data not shown). In contrast to FDCs, autologous B cells significantly augmented T-cell proliferation by 65% and 30% at ratios of 1:5 and 1:20 (n = 7, P < .05), respectively (Fig 2A). Similar results were obtained when B cells were isolated by positive selection using anti-CD22-microbeads (T-cell proliferation was augmented by 24% ± 3.3% for positively selected B cells v 21% ± 2.0% for negatively selected B cells), suggesting that differences in the function of FDC and B cells are not due to whether they are isolated using positive or negative selection methods. The finding that T cells proliferated to SEB in the absence of exogeneously added APC indicates that the T cells used in these experiments contained residual APCs because T-cell proliferation to sAg requires presentation by APCs.39 Nevertheless, these findings show that human FDCs downregulate SEB-induced T-cell proliferation in an FDC-dose-dependent manner.
The ability of human FDCs to control T-cell proliferation in response to sAg. Varying numbers of FDCs (A through D) or B cells (A) were cultured with 1 × 105 autologous tonsil T cells and sAg (100 ng/mL) for 3 days (A and B) or allogeneic tonsil T cells and sAg (100 ng/mL) for 5 days (C and D). Tritiated thymidine uptake was measured during the last 24 hours of culture. Each bar represents the mean cpm + SEM of seven (A) or five (B through D) independent experiments. Cpm of cultures containing only T cells ranged from 193 to 1,290 in (A), 519 to 1,846 in (B), 138 to 2,547 in (C), and 541 to 2,158 in (D). Cpm of cultures containing only irradiated FDCs or B cells were always less than background (T cells alone). FDC significantly inhibited T-cell proliferation at a 1:5 and 1:20 ratio in (A) and (C), and at a 1:5 ratio in (B) and (D). B cells significantly augmented T-cell proliferation at a 1:5 and 1:20 ratio in (A). Statistical differences were determined using a pairedt-test.
The ability of human FDCs to control T-cell proliferation in response to sAg. Varying numbers of FDCs (A through D) or B cells (A) were cultured with 1 × 105 autologous tonsil T cells and sAg (100 ng/mL) for 3 days (A and B) or allogeneic tonsil T cells and sAg (100 ng/mL) for 5 days (C and D). Tritiated thymidine uptake was measured during the last 24 hours of culture. Each bar represents the mean cpm + SEM of seven (A) or five (B through D) independent experiments. Cpm of cultures containing only T cells ranged from 193 to 1,290 in (A), 519 to 1,846 in (B), 138 to 2,547 in (C), and 541 to 2,158 in (D). Cpm of cultures containing only irradiated FDCs or B cells were always less than background (T cells alone). FDC significantly inhibited T-cell proliferation at a 1:5 and 1:20 ratio in (A) and (C), and at a 1:5 ratio in (B) and (D). B cells significantly augmented T-cell proliferation at a 1:5 and 1:20 ratio in (A). Statistical differences were determined using a pairedt-test.
To rule out the possibility that inhibition of T-cell proliferation by human FDCs was restricted to a unique TCR β-chain(s) recognized by SEB, similar studies were performed with SEA, an sAg that binds different TCR β chains than those recognized by SEB.40 As shown in Fig 2B, FDCs significantly inhibited T-cell proliferation to SEA by 44% at a 1:5 FDC to T-cell ratio, compared to T cells cultured with SEA in the absence of FDCs (n = 5, P < .05). Thus, the ability of FDCs to inhibit T-cell proliferation to sAgs is a general finding that is not restricted to a T-cell subset expressing certain families of TCR chains.
FDCs downregulate allogeneic T-cell proliferation.
We next examined the ability of human FDCs to regulate sAg-induced proliferation using allogeneic tonsil T cells, because sAg presentation by APC is not MHC class II-restricted.38 As shown in Fig2C, FDCs significantly inhibited SEB-induced allogeneic T-cell proliferation in an FDC dose-dependent manner by 54% and 21% at FDC to T-cell ratios of 1:5 and 1:20, respectively (n = 5, P< .05). Similar results were obtained using SEA to induce allogeneic T-cell proliferative responses (Fig 2D). FDCs also significantly downregulated tetanus toxoid–induced allogeneic T-cell proliferation (52% to 62% reduction at a 1:5 ratio at 2 tetanus toxoid concentrations), indicating that the inhibitory nature of FDCs is a general finding not restricted to sAg-induced T-cell proliferation (data not shown). Thus, inhibition of T-cell proliferation is a unique functional property associated with FDCs that is not dependent on the type or dose of stimulating agent used, and is active in both autologous and allogeneic culture systems.
FDCs can present sAgs to T cells.
The observation that FDCs inhibit rather than stimulate T-cell proliferation prompted us to examine whether FDCs can present sAg and stimulate T-cell proliferative responses in the absence of other APCs. For these experiments, highly purified tonsil T cells were isolated that were free from contamination with residual APCs (cells were passed over a second magnetic separation column). As shown in Fig 3 (n = 3), highly purified T cells were unable to proliferate to sAg, based on a proliferative response that was only 3% of the response observed when B cells were added at a 1:5 ratio (maximum proliferative response). The addition of FDCs to cultures containing T cells and SEB resulted in a modest increase in T-cell proliferation that was only 18% of the response observed using B cells at the same ratio. Interestingly, when smaller numbers of FDCs were used, T-cell proliferation dramatically increased (Fig 3). For instance, the addition of FDCs at a 1:20 and 1:80 ratio resulted in augmentation of the T-cell proliferative response by 41% and 73% (P < .05), respectively, compared with the response using FDCs at 1:5. T-cell proliferation using FDCs at a 1:80 ratio was comparable to the response obtained using B cells at a much higher ratio of 1:5. Thus, FDCs can present sAg and provide costimulatory signals that induce vigorous T-cell proliferation when FDCs are used in limiting numbers.
The ability of human FDCs to present sAg to T cells. Highly purified autologous tonsil T cells were obtained that failed to proliferate in response to sAg without adding APCs. Varying numbers of FDCs were cultured with 105 autologous tonsil T cells and SEB at 100 ng/mL for 3 days. Tritiated thymidine uptake was measured during the last 24 hours of culture. Each bar represents the mean cpm + SEM of three independent experiments, and is expressed as a percentage of the response generated when B cells were used at a 1:5 ratio (maximum proliferative response). Cpm from cultures containing only T cells ranged from 272 to 522. FDC significantly induced T-cell proliferation, compared to cultures without APC, at 1:20 and 1:80 ratios by ANOVA and Tukey’s analysis.
The ability of human FDCs to present sAg to T cells. Highly purified autologous tonsil T cells were obtained that failed to proliferate in response to sAg without adding APCs. Varying numbers of FDCs were cultured with 105 autologous tonsil T cells and SEB at 100 ng/mL for 3 days. Tritiated thymidine uptake was measured during the last 24 hours of culture. Each bar represents the mean cpm + SEM of three independent experiments, and is expressed as a percentage of the response generated when B cells were used at a 1:5 ratio (maximum proliferative response). Cpm from cultures containing only T cells ranged from 272 to 522. FDC significantly induced T-cell proliferation, compared to cultures without APC, at 1:20 and 1:80 ratios by ANOVA and Tukey’s analysis.
Inhibition of T-cell proliferation by FDCs is mediated by CD54 and CD106.
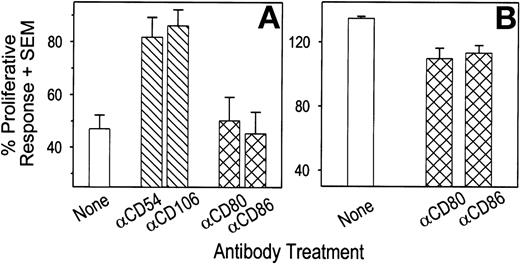
FDCs may alter T-cell proliferative responses by delivering inhibitory signals during cellular interactions and/or through the production of biologically active molecules. To determine the role of cellular contact in the ability of FDCs to downregulate T-cell proliferation, antibodies against molecules expressed on the surface of FDCs were used to block specific FDC:T-cell interactions. Because human FDCs express large quantities of the adhesion molecules CD54 and CD106,4 5 FDCs were treated with purified antibodies against these molecules before addition to T-cell cultures. For these studies, FDCs were used at an FDC to T-cell ratio of 1:5. Residual unbound antibody was removed by extensive washing. As shown in Fig 4A (n = 5), pretreatment of FDCs with either anti-CD54 or anti-CD106 antibodies resulted in an increase in T-cell proliferation from 47% in untreated cultures to 82% and 86%, respectively. Pretreatment of FDCs with an isotype-matched irrelevant control antibody failed to reverse the inhibitory effect of FDCs on T-cell proliferation (data not shown). Further increases in T-cell proliferation in the presence of FDCs were not observed using higher antibody concentrations (data not shown). In addition, pretreatment of T cells with antibodies against CD54 or CD106 failed to alter SEB-induced T-cell proliferation (data not shown), indicating that FDC function and not T-cell function was blocked by the antibody treatment. We also examined the regulatory role of the costimulatory molecules CD80 and CD86 and found that pretreatment of FDCs with antibodies against CD80 and CD86 failed to reverse the ability of FDC to inhibit T-cell proliferation in response to SEB (Fig 4A). In contrast, the ability of B cells to augment T-cell proliferation at a 1:5 ratio was abrogated by pretreating B cells with either anti-CD80 or anti-CD86 antibodies at a similar concentration (Fig 4B), showing that these antibodies were capable of blocking cellular interactions in our culture system.
The ability of antibodies against adhesion and costimulatory molecules to block the inhibitory effect of FDCs on sAg-induced autologous T-cell proliferation. Where indicated, 2 × 104 FDCs (A) or B cells (B) were incubated with 1 μg/mL of purified MoAb for 30 minutes and extensively washed before addition to cultures containing 105 autologous tonsil T cells and SEB at 100 ng/mL. Tritiated thymidine uptake was measured during the last 24 hours of a 3-day culture. Each bar represents the mean cpm + SEM of five (A) or three (B) independent experiments, and is expressed as a percentage of the response generated when T cells were cultured with only SEB. Cpm from cultures containing only T cells ranged from 535 to 1,591 in (A), and 1,004 to 3,155 in (B). Pretreatment of FDCs with antibodies against CD54 and CD106 significantly increased T-cell proliferation compared with untreated FDCs by ANOVA and Tukey’s analysis. Antibodies against CD80 and CD86 significantly reduced the ability of B cells to augment T-cell proliferation.
The ability of antibodies against adhesion and costimulatory molecules to block the inhibitory effect of FDCs on sAg-induced autologous T-cell proliferation. Where indicated, 2 × 104 FDCs (A) or B cells (B) were incubated with 1 μg/mL of purified MoAb for 30 minutes and extensively washed before addition to cultures containing 105 autologous tonsil T cells and SEB at 100 ng/mL. Tritiated thymidine uptake was measured during the last 24 hours of a 3-day culture. Each bar represents the mean cpm + SEM of five (A) or three (B) independent experiments, and is expressed as a percentage of the response generated when T cells were cultured with only SEB. Cpm from cultures containing only T cells ranged from 535 to 1,591 in (A), and 1,004 to 3,155 in (B). Pretreatment of FDCs with antibodies against CD54 and CD106 significantly increased T-cell proliferation compared with untreated FDCs by ANOVA and Tukey’s analysis. Antibodies against CD80 and CD86 significantly reduced the ability of B cells to augment T-cell proliferation.
Inhibition of T-cell proliferation by FDCs can be partially overcome by preventing cellular contact.
To further evaluate the role of cellular interactions on the ability of FDCs to inhibit T-cell proliferative responses, FDCs and T cells were cultured in chambers separated by a membrane that prevents cellular interactions while allowing free exchange of soluble molecules between chambers. As expected, addition of FDCs to the chamber containing T cells (insert) resulted in a significant reduction in SEB-induced T-cell proliferation of 63%, compared to T cells cultured only with SEB (Table 1, n = 3). When FDCs were separated from T cells by a membrane (FDC in the well and T cells in the insert), the T-cell proliferative response increased by 19% (P < .05). Although T-cell proliferation was not completely restored when FDCs were separated from T cells, these data indicate that FDCs produce a soluble factor(s) that has anti–T-cell proliferative activity. In contrast to FDCs, the ability of B cells to augment SEB-induced T-cell proliferation was compromised when B cells were placed in a separate chamber, indicating that B cells augment SEB-induced T-cell proliferation through cellular interactions (Table1).
The Ability of FDC to Inhibit T-Cell Proliferation in the Absence of Cellular Contact
| Cells Present in* . | % Proliferative Response (±SEM)† . | |
|---|---|---|
| Insert . | Well . | |
| T cells + FDC | — | 37 ± 3.7 |
| T cells | FDC | 56 ± 5.1‡ |
| T cells + B cells | — | 123 ± 22.1 |
| T cells | B cells | 103 ± 4.0 |
| Cells Present in* . | % Proliferative Response (±SEM)† . | |
|---|---|---|
| Insert . | Well . | |
| T cells + FDC | — | 37 ± 3.7 |
| T cells | FDC | 56 ± 5.1‡ |
| T cells + B cells | — | 123 ± 22.1 |
| T cells | B cells | 103 ± 4.0 |
Assays were performed in 24-well plates fitted with cell culture inserts containing a 3-μm pore size membrane.
T cells (4 × 105) and SEB at 100 ng/mL were placed in the insert. FDCs and B cells (8 × 104) were placed either in the insert or well, as indicated. The mean proliferative responses from three independent experiments are combined and expressed as a percentage (±SEM) of the response obtained when T cells were cultured with only SEB.
Tritiated thymidine was measured during the last 24 hours of a 3-day culture. Cpm for cultures containing only T cells ranged from 1,498 to 1,658.
P < .05 using a paired t-test when compared with cultures containing T cells and FDCs in the same chamber.
Inhibition of T-cell proliferation by FDCs is partially reversed by indomethicin.
It has previously been reported that FDCs within lymphocyte clusters are capable of producing prostaglandin E2(PGE2) .41 Because PGE2has been shown to inhibit peripheral blood T-cell proliferation,42 one possible mechanism by which FDCs inhibit sAg-induced T-cell proliferation is through the production of PGs. To examine the role of PGs in this process, indomethicin was used to block PG production by FDCs. Indomethicin acts on the cyclooxygenase pathway and blocks production of several PGs, including PGE2.43 The addition of indomethicin to cultures containing FDCs and T cells at a 1:5 ratio resulted in a significant increase in T-cell proliferation of 50%, compared to cultures containing FDC without indomethicin (Fig 5, n = 3). In the absence of FDCs, SEB-induced T-cell proliferation was not altered by indomethicin, indicating that the inhibitory effect of FDCs is mediated through the production of PGs by FDCs and not a direct effect of indomethicin on T cells (Fig 5).
The ability of indomethicin to reverse the inhibitory effect of FDCs on sAg-induced autologous T-cell proliferation. FDCs (2 × 104) were cultured with 105 autologous tonsil T cells and SEB (100 ng/mL) in the presence and absence of indomethicin (100 μg/mL) for 3 days. Tritiated thymidine uptake was measured during the last 24 hours of culture. Each bar represents the mean cpm + SEM of three independent experiments and is expressed as a percentage of the response generated when T cells were cultured with only SEB. Cpm from cultures containing only T cells ranged from 1,290 to 3,939. The addition of indomethicin to cultures containing FDCs resulted in a significant increase in T-cell proliferation compared to cultures containing FDCs without indomethicin using a pairedt-test.
The ability of indomethicin to reverse the inhibitory effect of FDCs on sAg-induced autologous T-cell proliferation. FDCs (2 × 104) were cultured with 105 autologous tonsil T cells and SEB (100 ng/mL) in the presence and absence of indomethicin (100 μg/mL) for 3 days. Tritiated thymidine uptake was measured during the last 24 hours of culture. Each bar represents the mean cpm + SEM of three independent experiments and is expressed as a percentage of the response generated when T cells were cultured with only SEB. Cpm from cultures containing only T cells ranged from 1,290 to 3,939. The addition of indomethicin to cultures containing FDCs resulted in a significant increase in T-cell proliferation compared to cultures containing FDCs without indomethicin using a pairedt-test.
DISCUSSION
Results from this study show that isolated human FDCs have the ability to downregulate sAg-induced T-cell proliferative responses. The ability of FDC to inhibit T-cell proliferation was observed when FDCs were isolated using either HJ2 or antibodies against CD14 (data not shown), suggesting that the inhibitory effect is not mediated through cross-linking the HJ2 antigen because HJ2 and anti-CD14 antibodies recognize different surface molecules (unpublished data). To our knowledge, this is the first report examining regulation of T-cell proliferative responses by human FDCs. Interestingly, T-cell proliferation was significantly inhibited at FDC concentrations that mimic the ratio of FDCs to T cells normally found within GCs (1:5 ratio), based on immunohistological studies of tonsil tissue. Although the majority of studies were performed with SEB, similar results were obtained with SEA, a serologically distinct sAg, with limited sequence homology to SEB.40 Because SEA activates T cells expressing TCR Vβ chains differing from those recognized by SEB,40 our findings reflect a general functional property of human FDCs that is not restricted to a certain serological type of sAg. Furthermore, the inhibitory nature of FDCs on T-cell proliferative responses was also observed using a different type of antigen (tetanus toxoid), illustrating that these findings are physiologically relevant and applicable to other antigen systems. This is of particular importance in view of the observation that human FDCs either induce or inhibit B-cell proliferation depending on the B-cell activating agent used.32 33
Interactions between FDCs and B cells have been extensively studied; however, the ability of FDCs to directly present antigens and elicit T-cell responses is poorly understood. Murine studies have shown that FDCs capture antigens in the form of complement containing antigen-antibody complexes and express these complexes on their surface in an unaltered form.9 These complexes (named iccosomes) are transferred to neighboring B cells for processing and subsequent presentation to T cells.10,11 Although FDCs do not process Ags,36 they can bind MHC class II-peptide complexes that are shed by neighboring B cells.37 FDCs expressing MHC class II-peptide complexes are capable of eliciting antigen-specific T-cell proliferative responses.37 Electron microscopy studies of human tonsil FDCs have shown that spherical structures similar to iccosomes are present on the surface of FDCs.4In an earlier study, we reported that human FDCs can present certain antigens to T cells, based on the ability of isolated FDCs to induce proliferation of unfractionated peripheral blood mononuclear cells in response to alloantigen.26 Our present study examined tonsil T-cell responses using sAgs and was designed to provide a superior model for examining FDC:T-cell interactions. The finding that small numbers of human FDCs (FDC to T-cell ratios of 1:80) can elicit T-cell proliferation in response to sAgs illustrates that FDCs can deliver stimulatory signals to T cells when presenting antigens not requiring cellular processing. This supports the conclusion that FDCs directly downregulate T-cell proliferation and that it is unlikely that this effect is mediated through third-party cells. A threshold number of FDCs may be required to provide adequate inhibitory signals to override the stimulatory nature of antigens being presented by FDCs. Based on these data, we propose that the degree of FDC:T-cell interactions within the GC microenvironment (based on a ratio of 1 FDC to 5 T cells that is normally present in GCs) regulates T-cell responsiveness to antigens during the generation of immune responses. This would explain why GC T cells do not normally proliferate, in situ, at certain times after antigen stimulation.44,45 Ratios of FDCs to B cells normally found within GCs (1:40 to 1:80) are extremely small and would favor antigen-driven B-cell proliferative responses, a theory supported by studies showing that FDCs can induce in vitro B-cell proliferation using antibodies against either CD40 or surface immunoglobulin.33 It is tempting to speculate that FDCs may inhibit T-cell proliferative responses in order to augment production of cytokines that favor B-cell development within GCs. Consistent with this possibility, we have found in initial studies that human FDCs downregulate interleukin-2 (IL-2) and interferon-γ mRNA production while stimulating production of IL-4 and IL-6, cytokines that promote B-cell differentiation (unpublished data, November 1997).
To explore molecular mechanisms, by which human FDCs regulate T-cell proliferative responses to sAgs, we examined molecules expressed on the surface of FDCs for their role in regulating T-cell proliferation. We found that adhesion pathways involving CD54 and CD106 are involved in the ability of FDCs to downregulate T-cell proliferation, as illustrated by antibody-blocking studies. Although it has been shown that interactions with integrin molecules can provide costimulatory signals that induce T-cell proliferation, engagement of certain integrin epitopes is not always costimulatory, and may result in negative T-cell signaling.46 In addition, CD54 and CD106 antigens on FDCs may differ structurally from those on other APCs, thereby providing unique regulatory signals during interactions with T-cell integrin molecules. Alternatively, engagement of CD54 and/or CD106 may trigger FDCs to produce soluble molecules with anti-proliferative properties. Further studies examining this possibility are clearly needed to better understand this aspect of FDC biology.
Other mechanisms besides cellular adhesion play a role in regulating T-cell responses since antibodies against CD54, CD106, or a combination of both antibodies (data not shown) did not always completely reverse the inhibitory effect of FDCs on T-cell proliferation. Interestingly, it has been shown that adhesion pathways involving CD54 and CD106 are also critical for B-cell binding to human FDCs.5,6 We also found that costimulation involving CD80 and CD86 was not involved in FDCs ability to inhibit T-cell proliferation. This is particularly interesting in view of studies indicating that interactions between CD80/86 and CD152 (CTLA-4) on activated T cells results in inhibition of T-cell proliferative responses.47 However, it is presently unclear whether FDCs express these costimulatory molecules because studies examining CD80 expression by isolated FDCs have yielded inconsistent results3 48 and the presence or absence of CD86 on FDC has not been reported. Consistent with our antibody blocking studies showing that CD80 and CD86 was not involved in regulating T cell proliferative responses, we have been unable to detect expression of either of these costimulatory molecules on isolated FDCs (unpublished data, December 1998).
Although inhibition of T-cell proliferative responses by FDCs is facilitated through cellular adhesion, we also found that inhibition occurred in the absence of cellular contact, indicating that a soluble mediator(s) was involved in the process. Supernatants from cultures containing FDCs were examined for their ability to inhibit T-cell proliferative responses and failed to produce consistent results. In addition, we found that supernatants from B-cell cultures sometimes inhibited T-cell proliferation to sAg, indicating that supernatants from cell cultures contain metabolic products that nonspecifically altered proliferative responses. To begin to characterize the active inhibitory molecule(s), additional studies were performed using indomethicin, a compound that blocks production of PGs of the D, E, and F series.43 PGs can modulate immune responses and PGE2 has been shown to inhibit mitogen-driven T-cell proliferative responses.42 The ability of indomethicin to partially restore T-cell proliferation in the presence of FDCs lends support to the hypothesis that FDC-mediated inhibition is facilitated by production of PGE2. This is further supported by an earlier study showing that FDC-containing lymphocyte clusters synthesize PGE2 in culture.41 Although T cells do not normally produce PGs,43 we cannot completely rule out the possibility that T cells are also producing PGs as a result of interactions with FDCs. Interestingly, PGs of the F series (F1 and F2) do not inhibit mitogen-induced T-cell proliferative responses43 and we found that both PGE1 and PGE2 can downregulate sAg-induced tonsil T-cell proliferation in a dose-dependent fashion (unpublished data, January 1998). Additional studies are underway to specifically identify the arachidonic acid metabolite(s) that is produced by FDCs with anti–T-cell proliferative activity and the mechanisms that trigger its release.
In summary, we have shown that human FDCs inhibit T-cell proliferation by two distinct, but possibly not mutually exclusive, mechanisms: one mechanism involves cellular interactions and is mediated by CD54 and CD106; the other mechanism involves FDC production of PGs. Because PGs, such as PGE2, have an extremely short half-life in vivo,43 both regulatory mechanisms may work in concert within the microenvironment of the GC. For instance, FDCs may trap and retain antigen-specific T cells in GCs by cellular interactions involving adhesion molecules. Signaling of T cells through adhesion molecule interactions, together with localized production of PGs by FDCs, may provide the necessary signals to block further expansion of T cells and induce production of cytokines that favor optimal antigen-driven B-cell responses. Alternatively, T-cell adhesion to FDCs may trigger the release of soluble molecules from FDCs with anti-proliferative properties similar to those exhibited by PGs. In addition, FDCs may be induced through interactions with T cells or B cells to produce chemokines, such as RANTES, that attract activated CD45RO+ T cells to migrate to GCs. Indeed, a recent study has shown that human FDCs have chemotactic activity for tonsil T cells.49 Insight into mechanisms used by FDCs that regulate cytokine production by T cells can lead to a better understanding of antigen-driven immune responses within the unique environment of GCs.
ACKNOWLEDGMENT
We thank Dr Moon H. Nahm for providing HJ2 antibody and for his continued encouragement, and Dan Ayers for statistical consultation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Anthony W. Butch, PhD, Department of Pathology, Slot 502, University of Arkansas for Medical Sciences, Little Rock, AR 72212; e-mail: twbutch@bloodbank.uams.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal