Abstract
The molecular mechanisms by which multiple myeloma (MM) cells evade glucocorticoid-induced apoptosis have not been delineated. Using a human IgAκ MM cell line (ARP-1), we found that dexamethasone (Dex)-induced apoptosis is associated with decreased NF-κB DNA binding and κB-dependent transcription. Both nuclear p50:p50 and p50:p65 NF-κB complexes are detected in ARP-1 cells by supershift electrophoretic mobility shift assay (EMSA). Dex-mediated inhibition of NF-κB DNA binding precedes a notable increase in annexin V binding, thereby indicating that diminished NF-κB activity is an early event in Dex-induced apoptosis. Overexpression of bcl-2 in ARP-1 cells prevents Dex-mediated repression of NF-κB activity and apoptosis. Sustained NF-κB DNA binding is also observed in two previously characterized Dex-resistant MM cell lines (RPMI8226 and ARH-77) that express moderate levels of endogenous bcl-2 and IκB proteins. In addition, enforced bcl-2 expression in ARP-1 cells did not prevent the augmentation of IκB protein by Dex. We also noted a possible association between Dex-mediated downregulation of NF-κB in freshly obtained primary myeloma cells and the patients’ responsiveness to glucocorticoid-based chemotherapy. Collectively, our data suggest that the protective effects of bcl-2 in MM cells act upstream in the NF-κB activation–signaling pathway and the potential use of NF-κB as a biomarker in progressive MM.
MYELOMA CELLS are terminally differentiated neoplastic B cells with a low proliferative index and a long life span. Glucocorticoids such as dexamethasone (Dex) and alkylating agents such as melphalan (Mel), all potent inducers of apoptosis, are frequently used to treat multiple myeloma (MM). Using standard doses of these agents, this malignancy remains incurable, with a complete remission (CR) rate of 5% and a median survival of 30 to 36 months. Drug resistance increases with prolonged treatment, suggesting that primary drug resistance is a major underlying factor in the resilience of myeloma cells to apoptotic signals and therapeutic interventions.
Like in other chronic B-cell malignancies such as chronic lymphocytic leukemia, chemoresistance has been attributed to bcl-2. Moderate to high levels of bcl-2 protein have been reported in numerous human MM cell lines and freshly isolated MM cells.1,2 In diverse experimental systems, bcl-2 acts as an antioxidant and alters intracellular calcium levels, plasma membrane asymmetry, mitochondrial permeability, cytochrome c release, and caspase activation.3 The characterization of bcl-2 and its antiapoptotic homologs, such as bcl-XL as ion channel proteins and docking proteins,3,4 indicates that bcl-2 interacts with proteins that are directly or indirectly involved in the apoptotic pathway. Several examples are calcineurin,5Raf1,6 GTPases R-, and H-Ras.7 This cascade of events ultimately modulates the expression of genes critical for apoptosis, including the transcription factors (TFs) NF-AT,5,8 p53-BP2,9 and NF-κB.10-14
The NF-κB family of dimeric TFs is a critical regulator of genes expressed during acute phase and inflammatory responses, Ig class switching, cellular differentiation, and apoptosis. Members of this family of TFs can form homodimers or heterodimers that bind to a specific decameric DNA sequence termed the κB site.15,16The activated NF-κB prototype is composed of the p50 and p65 (relA) subunits. Other members of this family include relB, c-rel, v-rel, and p52. With the exception of mature B lymphocytes and plasmacytomas, a latent form of NF-κB is sequestered in the cytoplasm of most cell types by a family of inhibitor proteins (IκB) that mask the nuclear localization domain of Rel proteins. To date, seven IκB proteins (IκBα, IκBβ, IκBε, IκBγ, Bcl3, p100, and p105) have been described: the roles of only IκBα and IκBβ have been investigated in great detail.17 Activators of NF-κB, such as tumor necrosis factor (TNF)-α and phorbol esters, cause site-specific phosphorylation of IκBα (serines 32 and 36) and IκBβ (serines 19 and 23), leading to their degradation via the ubiquitin/proteasome pathway.18,19 The direct consequence is the release of active NF-κB complex, its nuclear translocation, and transcriptional activation of the κB site in promoters of numerous target genes. Activation of NF-κB signaling pathway by TNF-α and interleukin (IL)-1 requires the NF-κB–inducing kinase (NIK), a member of the MAP kinase kinase kinase (MAP3K) family.20 Although the molecular mechanisms for its activation are not yet delineated, NIK has been shown to interact with and activate two downstream targets, IκB kinase (IKK)α and IKKβ that phosphorylate IκBα and IκBβ, respectively.21-23
Recent studies implicated NF-κB as a critical regulator of apoptosis. NF-κB activation can promote apoptosis or survival, depending on the cellular context. Because glucocorticoids have been shown to act as potent inhibitors of NF-κB activity24 25 and susceptibility to glucocorticoid-induced apoptosis is inversely related to bcl-2 expression, it was of interest to examine the interplay between Dex-mediated resistance in MM cells, bcl-2, and NF-κB activation. We report that maintenance of NF-κB activation is associated with the ability of both endogenous or enforced bcl-2 to render MM cell lines resistant to Dex-induced apoptosis. Furthermore, our findings suggest that NF-κB activation is a central factor in the development of resistance to glucocorticoid therapy and may be useful as a predictive factor for assessing the efficacy of glucocorticoid-based therapy in MM patients.
MATERIALS AND METHODS
Cell culture and treatments.
The ARP-1 cell line was established from a bone marrow aspirate of a patient with an IgA/κ secreting myeloma.26 The ARH77 and RPMI 8226 human MM-derived cell lines were obtained from American Type Culture Collection (Rockville, MD). Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, and 100 mg/mL of penicillin/streptomycin. For apoptosis assays, 2.5 × 105 cells/mL (>95% viable cells by trypan blue exclusion) were treated with and without 0.1 to 10 μmol/L of Dex (Sigma Chemical Company, St Louis, MO) for different times. For electrophoretic mobility shift assay (EMSA) and western blot analysis, 5 to 10 × 106 cells/treatment were used for the preparation of nuclear extracts (NEs), cytoplasmic extracts (CytEs), and whole cell extracts (WCEs).
Transfections and luciferase assays.
For transient transfections, ARP-1 cells were electroporated (BTX; 1,300 μF, 175 V) with 10 μg of reporter DNA. The thymidine-kinase–driven luciferase (TK-Luc) reporter plasmid and the TK-Luc reporter plasmid containing three copies of the consensus κB site were kindly provided by John L. Cleveland (St Judes Children’s Hospital, Memphis, TN). After transfection, cells were allowed to recover for 6 to 8 hours and were then treated with Dex (0.1 μmol/L) for 18 hours. Cells were harvested, lysed, and assayed for luciferase activity (Luciferase Assay System; Promega, Madison, WI) by using a Turner TD-20e luminometer (Promega). Luciferase activity was normalized according to protein content. Protein concentrations of cell lysates were determined according to the protocol for the Pierce Micro BCA (Pierce, Rockford, IL). For stable transfections, ARP-1 cells were transfected with 10 μg of either human bcl-2 pSFFV-neo or pSFFV-neo empty-containing expression plasmids (kindly provided by Stanley J. Korsemeyer, Washington University School of Medicine, St Louis, MO) by electroporation at 275 V and 1,180 μF, using a Cell-Porator (Life Technologies, Grand Island, NY). After 48 hours, bulk selection was performed in 800 μg/mL G418 sulfate (Life Technologies) for 2 weeks. Individual clones were then isolated by cell sorting using flow cytometry. Bcl-2 expression was confirmed by Western blotting analysis using a mouse anti–bcl-2 monoclonal antibody (Dako Corporation, Carpinteria, CA).
Nuclear, cytoplasmic, and whole cell extracts.
Cells were collected, centrifuged, and washed twice in ice-cold phosphate-buffered saline. Crude CytEs and NEs were prepared as described by Schreiber et al27 with the following modifications: buffers A and C contain 1 μg/mL of aprotinin, leupeptin, and pepstatin; 50 mmol/L β-glycerophosphate; 10 mmol/L NaF; and 1 mmol/L sodium vanadate. For preparation of WCEs, cells were lysed in buffer containing 1% (vol/vol) Nonidet P-40; 240 mmol/L NaCl; 50 mmol/L HEPES (pH 7.9); 10% glycerol; 0.1 mmol/L EDTA; 1 mmol/L dithiothreitol; 0.5 mmol/L phenylmethylsulfonyl fluoride; 1 μg/mL of aprotinin, leupeptin, and pepstatin; 50 mmol/L β-glyceraldehyde phosphate; and 1 mmol/L sodium vanadate. Protein content was determined according to the Bradford method using the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA).
Electrophoretic mobility shift assay.
Assays were performed as described28 using 5 to 10 μg of NE or WCE, 0.2 to 0.5 ng of probe, 20 mmol/L Tris (pH 7.5), 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 10% glycerol, and 2 μg poly(dI-dC) (poly(dI-dC); Pharmacia Biotech, Piscataway, NJ). The κB oligonucleotide from the IL-6 promoter (5′-agctTCAAATGTGGGATTTTCCCATGAG-3′, IL-6 κB),29 and the OCT-1 oligonucleotide based on consensus sequence of OCT-1 (Santa Cruz Biotechnology, Santa Cruz, CA) were end-labeled by T4 polynucleotide kinase with 32P-γATP (Dupont NEN, Boston, MA). The binding reaction (25 μL) is incubated at room temperature for 20 minutes, and then electrophoresis is performed on 4% nondenaturing polyacrylamide gel. In our competition studies, 10 and 50 molar excess of unlabeled oligonucleotide competitor was added to the binding reaction for 10 minutes before EMSA with radiolabeled oligonucleotide probe. Competitor oligonucleotides were: IL-6 κB, IL-6 mutant κB (5′-agctTCAAATGTTACATTTTCCCATGAG-3′),29and Ig κB (5′-CAGAGGGGACTTTCCGAGA-3′). For supershifts, 5 μg of unrelated or specific antibody to putative binding proteins is added to the binding reaction for 30 minutes before EMSA with radiolabeled oligonucleotide probe. Rabbit or goat polyclonal antibodies specific for the p50 subunit, p65 subunit, c-rel subunit, and unrelated goat anti-p107 and rabbit anti-USF were obtained from Santa Cruz.
Western blot analysis.
Equal amounts of protein (10 to 50 μg) in CytEs or WCEs were electrophoresed in a reducing 10% to 12.5% sodium-dodecyl sulfate polyacrylamide gel. Protein was transferred to nitrocellulose membranes (Amersham Life Sciences, Cleveland, OH) and stained with either mouse anti–bcl-2 monoclonal antibody or rabbit polyclonal IκBα antibody (Santa Cruz Biotechnology) followed by goat antimouse or donkey antirabbit IgG horseradish peroxidase conjugate (Amersham). For detection of proteins, an enhanced chemiluminescence system (Amersham) was used. Equal protein loading was confirmed by Fast Green staining of the membrane (Sigma Chemical Co).
Apoptosis assays.
For in situ end labeling (ISEL), cytospins from untreated and Dex-treated cells were subsequently fixed in ethanol overnight at −20°C and air dried. The KLENOW FragEL DNA fragmentation detection assay was used according to the manufacturer’s instructions (Oncogene Research Products, Cambridge, MA). For annexin V binding, 0.5 to 1 × 106 cells, before and after treatment, are collected, washed twice with phosphate-buffered saline (PBS) and resuspended in 0.5 mL of annexin V binding buffer (2.5 mmol/L CaCl2, 20 mmol/L HEPES [pH 7.4], and 140 mmol/L NaCl) containing fluorescein isothiocyanate (FITC)-conjugated annexin V (Caltag Laboratories, Burlingame, CA) for 15 minutes in ice. After the addition of propidium iodide (10 μg/mL), annexin V staining is determined by flow cytometry on a FACScan (Becton-Dickinson, San Jose, CA).
Myeloma cell purification.
Heparinized bone marrow aspirates were obtained from patients with myeloma during scheduled clinic visits, as prescribed in the Investigational Review Board (IRB)-approved protocol. Signed informed consents were obtained and are kept on record. Myeloma plasma cells were purified from bone marrow aspirates as previously described.30 Briefly, light density (=1.077 g/cm3) cells were reacted with monoclonal antibodies to CD38 (phycoerythrin [PE]-conjugated) and CD45 (FITC-conjugated) and separated on the FACStar Plus cell sorter (Becton-Dickinson). MM cells were identified by high CD38 fluorescence, low-intermediate CD45 fluorescence, and by their light scatter properties. MM plasma cells were then sorted at a flow rate of 4,000 to 6,000 events per second. The purity of MM cells in the sorted cell preparation was confirmed immunohistochemically by monotypic cytoplasmic immunoglobulin content and was equal to 97%.
Clinical criteria for patient’s response to glucocorticoid-based therapy.
The definition of clinical responsiveness to the most recent application of glucocorticoid-based therapy was based on a 50% reduction in the patient’s serum or urine paraprotein.
RESULTS
Ectopic expression of bcl-2 blocks Dex-induced apoptosis in ARP-1 cells.
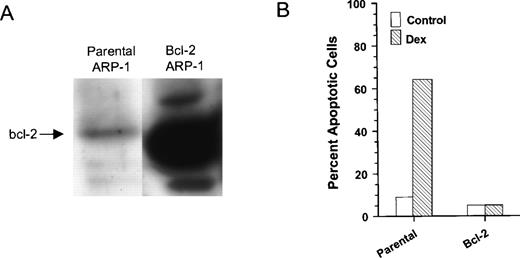
The human IgA MM cell line, ARP-1, serves as an excellent model to examine the role of bcl-2 in Dex-induced apoptosis of myeloma cells. ARP-1 cells undergo extensive apoptosis upon treatment with a clinically relevant dose of Dex (0.1 μmol/L). In addition, ARP-1 cells express low endogenous levels of bcl-2 protein. We therefore transfected ARP-1 cells with either bcl-2 or empty parental vectors, and several stably transfected clones were isolated and characterized. The degree of bcl-2 expression varied among the clones (data not shown), and a high bcl-2 expressing clone (B491) confirmed by Western blot analysis (Fig 1A) was further characterized. Parental and bcl-2 ARP-1 transfectants were treated for 48 hours with and without Dex, and the percentage of apoptotic cells was determined by ISEL. The addition of Dex resulted in a sevenfold increase in the percentage of apoptotic cells in our parental ARP-1 transfectants (Fig 1B). The ectopic expression of bcl-2 protected ARP-1 cells from both spontaneous and Dex-induced apoptosis. This result is consistent with earlier studies that showed an inverse correlation between glucocorticoid susceptibility and bcl-2 expression.31 It is also noteworthy that induction of apoptosis by Dex and resistance to Dex-induced apoptosis mediated by bcl-2 were not caused by clonal variation because we obtained similar results with bulk-selected ARP-1 transfectants.
Overexpression of bcl-2 protects Dex-induced apoptosis in ARP-1 cells. (A) WCEs prepared from parental and bcl-2 ARP-1 transfectants were Western blotted with antibody specific for bcl-2 protein. (B) Parental and bcl-2 ARP-1 transfectants were treated with and without Dex (0.1 μmol/L) for 48 hours. Percentage of apoptosis was measured by ISEL.
Overexpression of bcl-2 protects Dex-induced apoptosis in ARP-1 cells. (A) WCEs prepared from parental and bcl-2 ARP-1 transfectants were Western blotted with antibody specific for bcl-2 protein. (B) Parental and bcl-2 ARP-1 transfectants were treated with and without Dex (0.1 μmol/L) for 48 hours. Percentage of apoptosis was measured by ISEL.
Dex-induced apoptosis in ARP-1 cells is associated with inhibition of NF-κB.
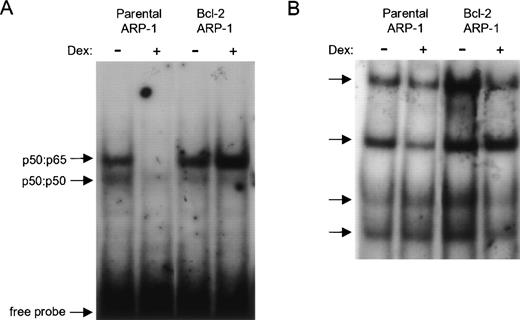
To investigate whether Dex-induced apoptosis correlated with inhibition of NF-κB activity in the ARP-1 cell line, NF-κB activity was tested in NEs prepared from parental and bcl-2 ARP-1 transfectants. Two constitutive nuclear NF-κB complexes were detected. The slower migrating constitutive NF-κB complex was dramatically inhibited by a 24-hour exposure to Dex (Fig 2A). In contrast, overexpression of bcl-2 prevented downregulation of NF-κB activity by Dex. To show that Dex selectively downmodulated NF-κB DNA binding, the same 24-hour NEs used in the κB DNA binding assay (Fig 2A) were tested for binding to the consensus binding site for the octamer binding protein (Oct-1). As shown in Fig 2B, Oct-1 DNA binding was not markedly inhibited by Dex. Collectively, our data indicate that the selective inhibition of NF-κB nucleoprotein complexes that accompanies Dex-induced apoptosis is prevented by enforced bcl-2 expression.
Enforced bcl-2 prevents the selective downmodulation of NF-κB DNA binding by Dex. Effect of Dex on (A) NF-κB and (B) octamer binding protein (Oct-1) DNA binding activities in parental and bcl-2 ARP-1 transfectants. (A) NEs from parental and bcl-2 ARP-1 transfectants treated with and without Dex (0.1 μmol/L) for 24 hours were incubated with a 32P-labeled probe containing the κB binding site of the IL-6 promoter and assayed by EMSA. (B) The same NEs used in (A) were tested for Oct-1 DNA binding by EMSA using a radiolabeled Oct-1 probe containing a consensus binding site for Oct family homeodomain TFs. Arrows indicate two distinct NF-κB complexes in (A) and four Oct-1 complexes in (B).
Enforced bcl-2 prevents the selective downmodulation of NF-κB DNA binding by Dex. Effect of Dex on (A) NF-κB and (B) octamer binding protein (Oct-1) DNA binding activities in parental and bcl-2 ARP-1 transfectants. (A) NEs from parental and bcl-2 ARP-1 transfectants treated with and without Dex (0.1 μmol/L) for 24 hours were incubated with a 32P-labeled probe containing the κB binding site of the IL-6 promoter and assayed by EMSA. (B) The same NEs used in (A) were tested for Oct-1 DNA binding by EMSA using a radiolabeled Oct-1 probe containing a consensus binding site for Oct family homeodomain TFs. Arrows indicate two distinct NF-κB complexes in (A) and four Oct-1 complexes in (B).
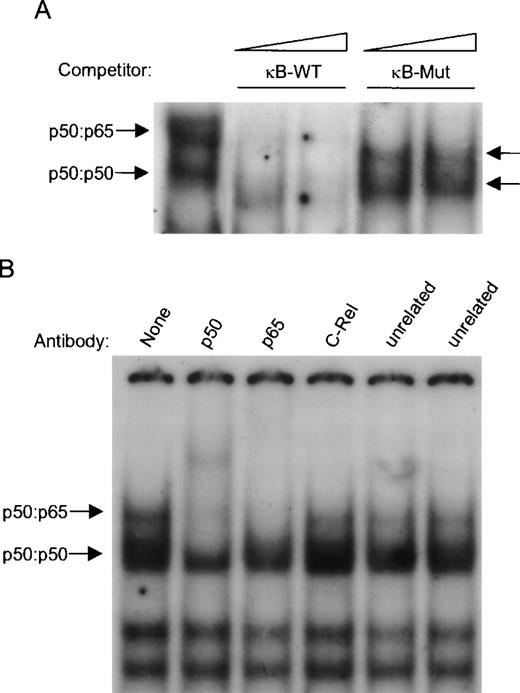
The specificity of these two protein-DNA complexes was confirmed by competition analysis (Fig 3A). Excess unlabeled κB oligonucleotide competed with NF-κB complex formation, whereas a mutated version of the κB site failed to compete for NF-κB binding. An EMSA supershift was employed to identify the components of NF-κB subunits bound to the κB site. Anti-p50 antibody caused the loss of both NF-κB complexes and yielded a supershifted complex (Fig 3B). The addition of anti-p65 antibody abrogated complex formation of the slower migrating complex, and a faint supershifted complex was detected. Unrelated and anti–c-Rel antibodies did not react with the NF-κB complex. Hence, the slower and faster migrating NF-κB complexes contained the classical p50:p65 heterodimer and p50:p50 homodimers, respectively (Fig 3B).
Characterization of constitutive nuclear NF-κB complexes in ARP-1 cells. (A) For competitive EMSA, NEs prepared from untreated parental ARP-1 transfectants were incubated with either a 10- or 50-fold molar excess of unlabeled wild-type or mutated κB oligonucleotides and assayed for their ability to compete with radiolabeled wild-type κB probe in a standard EMSA. Arrows indicate two specific NF-κB complexes. (B) p50:p50 and p50:p65 dimers bind to the κB site. Supershift EMSA was performed using whole cell extracts prepared from parental ARP-1 transfectants. When marked, antibodies specific for p50, p65, c-Rel, and USF and E2F-1 (two control unrelated antibodies) were incubated in an EMSA reaction. The positions of p50:p50 and p50:p65 NF-κB complexes are indicated by arrows.
Characterization of constitutive nuclear NF-κB complexes in ARP-1 cells. (A) For competitive EMSA, NEs prepared from untreated parental ARP-1 transfectants were incubated with either a 10- or 50-fold molar excess of unlabeled wild-type or mutated κB oligonucleotides and assayed for their ability to compete with radiolabeled wild-type κB probe in a standard EMSA. Arrows indicate two specific NF-κB complexes. (B) p50:p50 and p50:p65 dimers bind to the κB site. Supershift EMSA was performed using whole cell extracts prepared from parental ARP-1 transfectants. When marked, antibodies specific for p50, p65, c-Rel, and USF and E2F-1 (two control unrelated antibodies) were incubated in an EMSA reaction. The positions of p50:p50 and p50:p65 NF-κB complexes are indicated by arrows.
Loss of NF-κB DNA binding is an early event in drug-induced apoptosis.
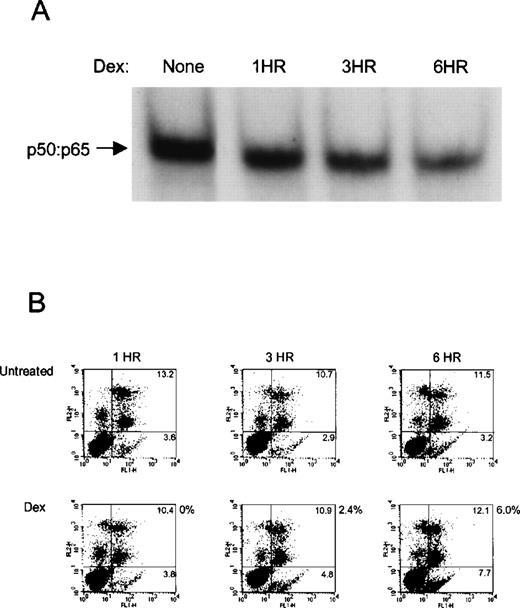
To test whether the inhibition of NF-κB DNA binding was an early event in the commitment phase of apoptosis or a late event in the execution phase of Dex-induced apoptosis, we compared the kinetics of Dex-mediated downregulation of NF-κB DNA binding with the kinetics of annexin V binding, a landmark analysis for early and late apoptosis. As compared with untreated control, treatment with Dex inhibited NF-κB DNA binding by 5.4% after 1 hour (Fig4A). At 3 and 6 hours Dex significantly diminished NF-κB DNA binding by 30% and 56%, respectively. The percentage of inhibition of NF-κB DNA binding by Dex (relative to untreated control) was quantitated by a Phospholmager (Molecular Dynamics, Sunnyvale, CA). In comparison, no marked increase in annexin V binding was detected after 1 and 3 hours with Dex with only a minimal increase after 6 hours of Dex treatment (Fig 4B). A significant increase in annexin V binding begins after 12 hours of Dex and further increases with prolonged treatment of Dex, 24 and 48 hours (data not shown). In bcl-2 ARP-1 transfectants, Dex induced only negligible changes in annexin V binding and in situ end labeling that were associated with lack of Dex-mediated inhibition of NF-κB DNA binding. Thus, the progressive decline of NF-κB DNA binding in parental ARP-1 transfectants after different exposure times with Dex preceded changes in annexin V binding, indicating that loss of NF-κB DNA binding is an early event in the commitment of cell death.
Loss of NF-κB DNA binding precedes annexin V binding. (A) For NF-κB DNA binding, NEs prepared from parental ARP-1 transfectants treated with and without Dex (0.1 μmol/L) for the indicated times were examined by EMSA. (B) Parental ARP-1 cells treated as described above were double stained with annexin-V-FITC and propidium iodide and analyzed for annexin-V binding by flow cytometry. Numbers within dot plots represent percentages of cells in early apoptosis (annexin V+/PI−; lower right) and in late apoptosis and necrosis (annexin V+/PI+; upper left). Percentage of apoptotic cells (next to each lower dot plot) represents the fraction of cells undergoing apoptosis with Dex.
Loss of NF-κB DNA binding precedes annexin V binding. (A) For NF-κB DNA binding, NEs prepared from parental ARP-1 transfectants treated with and without Dex (0.1 μmol/L) for the indicated times were examined by EMSA. (B) Parental ARP-1 cells treated as described above were double stained with annexin-V-FITC and propidium iodide and analyzed for annexin-V binding by flow cytometry. Numbers within dot plots represent percentages of cells in early apoptosis (annexin V+/PI−; lower right) and in late apoptosis and necrosis (annexin V+/PI+; upper left). Percentage of apoptotic cells (next to each lower dot plot) represents the fraction of cells undergoing apoptosis with Dex.
Bcl-2 prevents Dex-mediated downregulation of κB-dependent transcription.
The above findings prompted us to determine whether the modulation of NF-κB DNA binding in Dex-treated ARP-1 cells correlated with κB-dependent transcriptional activity in vivo. Parental and bcl-2 ARP-1 transfectants were transfected with either a TK-Luc reporter construct containing three repeats of the κB site (3κB) or a control TK-Luc reporter plasmid, and the effects of Dex on luciferase activity were examined. Negligible luciferase activity was detected in both parental and bcl-2 ARP-1 cells transfected with TK-Luc reporter, and treatment with Dex for 18 hours did not affect TK promoter activity (Fig 5). The level of κB transcriptional activity is indicated as percentage of control (relative light units [RLU]/μg of protein) and was normalized to protein content. In untreated parental and bcl-2 ARP-1 cells, transfected 3κB reporter displayed 164.8-fold and 94.9-fold higher luciferase activity, respectively, than TK-Luc reporter. The addition of Dex significantly reduced κB-dependent luciferase activity in parental ARP-1 cells by 22% (P = 0.019), but only had a marginal effect (6.5%;P = 0.165) in the bcl-2 overexpressing cells. According to these functional studies, comparable high levels of constitutive NF-κB activity were detected in both parental and bcl-2 ARP-1 cells. More importantly, the protective function of bcl-2 in ARP-1 cells seems to be attributed to the preservation of κB-dependent transcription.
Effect of bcl-2 on Dex-mediated downregulation of κB-dependent reporter gene activity. Parental and bcl-2 ARP-1 transfectants were transiently transfected with either a TK-Luc reporter plasmid or TK-Luc reporter plasmid harboring three κB sites and subsequently cultured in the presence or absence of Dex (0.1 μmol/L) for 20 hours. The level of κB transcriptional activity is indicated as percentage control (relative light units [RLU]/μg of protein) and normalized to protein content. The average of 13 (parental ARP-1) and 12 (bcl-2 ARP-1) individual experiments is represented.
Effect of bcl-2 on Dex-mediated downregulation of κB-dependent reporter gene activity. Parental and bcl-2 ARP-1 transfectants were transiently transfected with either a TK-Luc reporter plasmid or TK-Luc reporter plasmid harboring three κB sites and subsequently cultured in the presence or absence of Dex (0.1 μmol/L) for 20 hours. The level of κB transcriptional activity is indicated as percentage control (relative light units [RLU]/μg of protein) and normalized to protein content. The average of 13 (parental ARP-1) and 12 (bcl-2 ARP-1) individual experiments is represented.
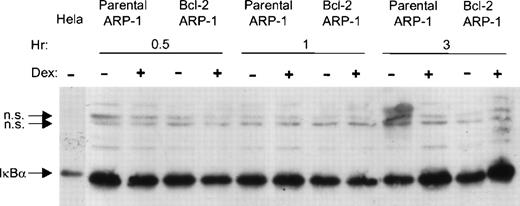
Bcl-2 does not affect Dex-mediated augmentation of IκBα protein.
Because transrepression of NF-κB activity by glucocorticoids could be caused, in part by the transcriptional induction of IκBα synthesis,25 32 we asked whether enforced bcl-2 expression prevented increased IκBα expression by Dex. Whole cell extracts were prepared from parental and bcl-2 ARP-1 transfectants with and without Dex for 0.5, 1, and 3 hours. Steady state levels of IκBα protein were analyzed by Western blotting. Dex profoundly increased IκBα protein expression after 3 hours, even in the bcl-2 overexpressing ARP-1 cells (Fig 6). Thus, Dex-mediated upregulation of steady state levels of IκBα protein is not sufficient for Dex-induced apoptosis.
Bcl-2 does not prevent upregulation of IκB protein by Dex. Parental and bcl-2 ARP-1 transfectants were treated with and without Dex (0.1 μmol/L) for different times. WCEs were prepared and assayed for IκB protein expression by Western blotting. WCE from HeLa cell line was used as a positive control for IκB protein expression. IκB and NS, a nonspecific cross-reacting protein, are indicated by arrows.
Bcl-2 does not prevent upregulation of IκB protein by Dex. Parental and bcl-2 ARP-1 transfectants were treated with and without Dex (0.1 μmol/L) for different times. WCEs were prepared and assayed for IκB protein expression by Western blotting. WCE from HeLa cell line was used as a positive control for IκB protein expression. IκB and NS, a nonspecific cross-reacting protein, are indicated by arrows.
It is also noteworthy that although enforced bcl-2 sustained NF-κB activity, IκBα degradation was not evident at any of the time points analyzed. In addition, the relative abundance of IκBα protein is higher in the bcl-2 ARP-1 transfectants. These observations suggest that the ability of bcl-2 to maintain NF-κB activity is independent of IκBα.
Correlation between Dex-resistance, bcl-2 levels, and constitutive NF-κB activation.
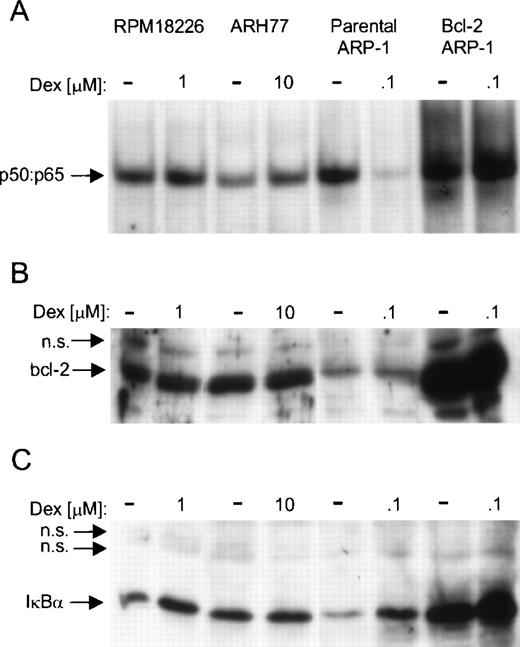
To further show the relationship between persistent NF-κB activity, bcl-2 expression, and resistance to Dex-induced apoptosis, we analyzed NF-κB DNA binding and endogenous bcl-2 in two Dex-resistant human MM cell lines, RPMI8226 and ARH77. Relative to the low bcl-2 overexpressing parental ARP-1 transfectants, both cell lines expressed moderate endogenous levels of bcl-2 (Fig7A). In addition, treatment with Dex did not alter bcl-2 protein levels in the Dex-sensitive (parental ARP-1 transfectants) and the Dex-resistant (bcl-2 ARP-1 transfectants, RPMI8226 and ARH77) cells. Both RPMI8226 and ARH77 MM cells had constitutive NF-κB DNA binding, and by 20 hours, 10 μmol/L of Dex did not inhibit NF-κB DNA binding (Fig 7B). Thus, resistance to Dex-induced and spontaneous apoptosis seems to be linked to bcl-2 expression and maintenance of constitutive NF-κB activation.
Correlation between Dex-resistance, constitutive NF-κB DNA binding, and endogenous bcl-2 protein levels. RPMI8226, ARH77, parental, and bcl-2 ARP-1 transfectants were cultured with or without the indicated concentration of Dex for 20 hours (A) WCEs were prepared and analyzed for NF-κB DNA binding by EMSA with a radiolabeled κB probe (B) and (C) WCEs were blotted with antibody specific for (B) bcl-2 and (C) Iκ.
Correlation between Dex-resistance, constitutive NF-κB DNA binding, and endogenous bcl-2 protein levels. RPMI8226, ARH77, parental, and bcl-2 ARP-1 transfectants were cultured with or without the indicated concentration of Dex for 20 hours (A) WCEs were prepared and analyzed for NF-κB DNA binding by EMSA with a radiolabeled κB probe (B) and (C) WCEs were blotted with antibody specific for (B) bcl-2 and (C) Iκ.
Because IκBα has been shown to regulate transient and persistent activation of NF-κB, we examined the overall level of IκBα protein in these three MM cell lines. There was no apparent difference in the levels of IκBα protein in RPMI8226, ARH77, and parental ARP-1 transfectants (Fig 7C). Exposure to Dex increased the level of IκBα protein in parental and bcl-2 ARP-1 transfectants, as well as in RPMI8226 cells. Dex did not, however, increase IκBα protein levels in ARH77 cells. Taken together, our findings suggest that constitutive NF-κB activation in these three MM cell lines cannot be explained by low or negligible steady state levels of IκBα.
Dex-mediated downregulation of NF-κB DNA binding activity is associated with patients’ clinical response to Dex.
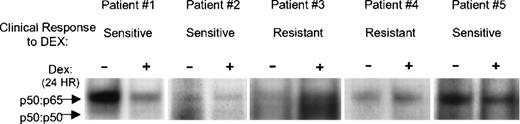
To investigate whether primary myeloma cells respond to Dex in a similar fashion, we examined NF-κB DNA binding in myeloma cells from five MM patients. WCEs were prepared from sort-purified myeloma plasma cells (≥95%) from bone marrow aspirates of MM patients. The cells were cultured in vitro for 24 hours in the presence and absence of Dex, and NF-κB DNA binding was determined by EMSA. Dex reduced DNA binding in three patient samples. No effect was noticed in the other two (Fig8). Interestingly, the three patients whose myeloma cells responded to Dex in vitro, but not those whose myeloma cells showed no response, were responsive to Dex-based treatment. Patient’s responsiveness to Dex was defined as more than 50% decline in their serum or urine paraprotein/tumor-specific Ig levels. These results may suggest an association between Dex-mediated decreased NF-κB activity and favorable clinical response to Dex.
Dex-mediated downregulation of NF-κB DNA binding activity correlates with patients’ clinical response to Dex. FACS-sorted–purified myeloma plasma cells from bone marrow aspirates of MM patients were treated with and without Dex (0.1 μmol/L) for 24 hours. WCEs were prepared and subjected to EMSA for binding to the κB oligonucleotide probe.
Dex-mediated downregulation of NF-κB DNA binding activity correlates with patients’ clinical response to Dex. FACS-sorted–purified myeloma plasma cells from bone marrow aspirates of MM patients were treated with and without Dex (0.1 μmol/L) for 24 hours. WCEs were prepared and subjected to EMSA for binding to the κB oligonucleotide probe.
DISCUSSION
It is widely accepted that dysregulation of genes that control apoptosis contributes to the pathogenesis of numerous diseases, including MM. Although bcl-2 has been implicated in primary drug resistance in MM, the molecular mechanisms by which MM cells evade chemotherapy-induced apoptosis remain unclear. In this study, we present evidence that Dex-induced apoptosis is associated with the selective downmodulation of NF-κB activity in our preclinical myeloma model (ARP-1 cell line). Enforced bcl-2 expression in ARP-1 cells prevents Dex-induced apoptosis by maintaining NF-κB activity. A striking association between Dex-mediated resistance, endogenous bcl-2 protein levels, and constitutive nuclear NF-κB is observed in two other MM cell lines that fail to undergo Dex-induced apoptosis. Moreover, persistent NF-κB activation in MM cell lines is not regulated by differential IκBα expression. Most intriguing is the apparent association between Dex-mediated inhibition of NF-κB DNA binding in patients’ MM cells and the patients’ response to treatment with glucocorticoid-based therapy.
Persistent NF-κB activation is observed in several MM cell lines and primary patient samples. Earlier studies have shown that mature B cell lines and plasmacytomas manifest constitutively nuclear NF-κB activity.33 In our model system, the NF-κB complex is composed of a heterodimer of p50 and p65 subunits. Recent studies have also shown the involvement of the p50:p65 NF-κB heterodimer in promoting survival of Hodgkin/Reed-Sternberg cells,34estrogen-independent human breast cancer cell lines,35carcinogen-induced primary rat mammary tumors,36 and thyroid carcinomas.37 The underlying factors responsible for constitutive NF-κB activation in MM cells are presently unclear. It is possible that autocrine and paracrine TNF-α and IL-1β and stimulation of CD30 and CD40 ligands in MM cells may be responsible for constitutive NF-κB activation in MM cells. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis revealed the presence of TNF-α and IL-1β mRNA in ARP-1 cells (data not shown).
Our data clearly show that NF-κB plays a critical role in Dex-mediated resistance in MM cells. We found that Dex-induced apoptosis in ARP-1 cells is associated with the inhibition of NF-κB DNA binding and κB-dependent transcription. These observations are in agreement with earlier reports showing that activation of NF-κB prevents TNF-α, ionizing radiation–, and daunorubicin-induced apoptosis.38-40 Similarly, inhibition of p50/65 NF-κB complexes is also involved in IgM-induced apoptosis in the WEHI 231 cell line,41 and overexpression of a super IκBα repressor mutant in Hodgkin’s cells increased their susceptibility to stress-induced apoptosis.34
To further explore the role of NF-κB as a survival factor in MM cells, we show in this report a relationship between bcl-2 and NF-κB in conferring resistance to Dex-induced apoptosis in MM cells. Bcl-2 is able to protect ARP-1 cells from Dex-induced apoptosis, and its antiapoptotic effects are mediated by NF-κB. More specifically, enforced bcl-2 prevented the downmodulation of NF-κB DNA binding and κB-dependent transcription by Dex in ARP-1 cells. Consistent with this observation was the correlation between resistance to Dex-mediated apoptosis in two MM cell lines (RPMI8226 and ARH77), endogenous bcl-2 protein levels, and constitutive NF-κB DNA binding activity. Several studies have shown that bcl-2 can either positively or negatively modulate NF-κB activation, depending on the cellular environment. Restoration of NF-κB activation by enforced bcl-2 has been reported in HeLa10 and Jurkat42 cells that failed to undergo CD95(fas)-induced apoptosis. Contrary to our system, bcl-2 has been shown to attenuate the transactivation domain of p65 during serum-deprived apoptosis in 293 cells11 and prevent NF-κB DNA binding in alphavirus-induced apoptosis in a prostate carcinoma line.14 With the exception of one report showing that the protective effects of bcl-2 on TNF-α–mediated cytotoxicity in prostate carcinoma cells are not linked to NF-κB signaling,43 the above studies suggest that bcl-2 acts upstream in the NF-κB activation signaling pathway.
A possible mechanism for maintenance of NF-κB activation by bcl-2 in MM cells is caused by the ability of bcl-2 to block proteolysis. A recent study has shown that inhibition of NF-κB activity in Jurkat cells that undergo apoptosis when triggered with CD95 is a consequence of the proteolytic cleavage of both p50 and p65 by caspase-3–related proteases.42 Although we cannot exclude this possibility, the onset of Dex-mediated inhibition of NF-κB DNA binding (3 hours) in ARP-1 cells was detected before any significant changes in annexin V binding (6 hours) were seen. Because loss of phospholipid asymmetry (annexin V binding) is a prominent feature in both early and late events during apoptosis, the disappearance of NF-κB DNA binding in ARP-1 cells undergoing apoptosis does not seem to reflect proteolysis. We conclude that inhibition of NF-κB may play a critical and causative role in the execution of apoptosis.
Earlier studies have shown that transcriptional repression domain of the activated glucocorticoid-receptor is responsible for glucocorticoid-induced apoptosis of human leukemic cells.44Two independent mechanisms are involved in the inhibition of NF-κB activity by glucocorticoids: induction of IκBα synthesis and direct interference between the activated glucocorticoid receptor and the transactivation domain of p65.24,45,46 Our present studies only addressed the effects of Dex and overexpressed bcl-2 on IκBα protein expression. In other studies, Dex has been shown to markedly increase IκB-α expression25,32 and facilitate the nuclear translocation of IκB-α in which it inhibits NF-κB DNA binding by causing the nuclear export of activated DNA-bound NF-κB complexes. In our system, Dex augmented steady state levels of IκBα protein expression in both parental and bcl-2 ARP-1 transfectants within 3 hours, thereby indicating that bcl-2 does not interfere with transcriptional induction of IκBα by Dex. Furthermore, there were no major differences in the amount of endogenous IκBα protein in low (parental ARP-1) or high (RPMI8226, ARH77) bcl-2 expressing MM cell lines. Recent studies have reported that transrepression of NF-κB activity by glucocorticoids is independent of IκBα expression.45,47 In contrast to our findings, low steady state levels of IκBα protein have been shown to account for constitutive NF-κB activation in Hodgkin/Reed-Sternberg cells that express activated p65.48 In a related study, the inability of CD40 ligand to further activate κB-dependent transcription in three Hodgkin cell lines is possibly caused by aberrant IκBα protein expression.49 Although we did not examine inducible IκBα degradation in MM cells, our data suggests that continuous basal IκBα degradation is not responsible for constitutive NF-κB activation and for the maintenance of NF-κB activation by bcl-2 in MM cells. Nevertheless, from the data herein, we cannot exclude the possibility that IκBα protein may be defective in its regulation of NF-κB in MM.
Studies exploring the mechanisms responsible for constitutive NF-κB activity showed that a hypophosphorylated form of IκBβ is found to shield NF-κB complex from IκBα-mediated inhibition,50,51 and it is conceivable that IκBβ plays an important role in the persistent activation of NF-κB. The phosphorylated form of IκBβ has been shown to have a higher affinity for p50:p65 complexes than for p50-RelB and p50:c-Rel complexes. A newly identified IκBε protein has also been recently characterized to inhibit the late, transient activation of a subset of genes that are primarily regulated by p65 and c-Rel.52 53Further studies investigating the roles of IκBα, IκBβ, and IκBε in the regulation of constitutive NF-κB activation are warranted.
Although preliminary, the observed relationship between Dex-mediated inhibition of NF-κB DNA binding activity in purified myeloma cells and the patient’s clinical response to glucocorticoid-based treatment, if confirmed on a larger patient population, is intriguing. It is tempting to speculate, on the basis of these observations, that the ability of drugs to inhibit NF-κB activity could serve as a predictive factor for treatment outcome. Indeed, drugs that selectively inhibit NF-κB activity could be used in patients with progressive disease. The search for new biologically relevant predictive factors, such as NF-κB, to guide therapy and the identification and characterization of molecular targets involved in the bcl-2 antiapoptotic signaling pathway in MM will hopefully improve the overall median survival of MM patients.
ACKNOWLEDGMENT
The authors thank Dr Victoria Richon for comments on the manuscript and helpful discussions, and Julian A. Terry and Jeff Woodliff for their expert technical assistance.
Supported in part by grant CA-55819 from the National Cancer Institute. R.F. is a recipient of the 1996 Brian D. Novis Research Grant for Multiple Myeloma from the International Myeloma Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Rena Feinman, PhD, Department of Surgery MSB G-519, UMDNJ-New Jersey Medical School, 185 South Orange Ave, Newark, NJ 07103.

![Fig. 5. Effect of bcl-2 on Dex-mediated downregulation of κB-dependent reporter gene activity. Parental and bcl-2 ARP-1 transfectants were transiently transfected with either a TK-Luc reporter plasmid or TK-Luc reporter plasmid harboring three κB sites and subsequently cultured in the presence or absence of Dex (0.1 μmol/L) for 20 hours. The level of κB transcriptional activity is indicated as percentage control (relative light units [RLU]/μg of protein) and normalized to protein content. The average of 13 (parental ARP-1) and 12 (bcl-2 ARP-1) individual experiments is represented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/9/10.1182_blood.v93.9.3044/4/m_blod40913005x.jpeg?Expires=1769289539&Signature=TAGiHtNHjxeBcDyD9smvxyQz71ejXGnNlq--j9QiJ-Fw9iFwAFt3o8iTHIlTTiwqbV-BO-GIquFGq10MorI0JLU88x-5APbbDl6TQz3qSRlxFITPLY7Ayvx7-6L94VKPvNYJIUBDzpvD7VYzdsTZrQ8uzL6bphJmP-rtpM1NRUezN5HDBJ3TMz0kN-NhV1aspenHWFZ4mGyvT10yOVly~4v~nGD1VgDrcaEa37XW~MHdFrJtCCiIacSaZl9Iy4fgCWDbRJXKuh5iNL8CltXSxyLCl7WgcvCo7GpaMO4SzQOVQwYvZKuCgAN7sLosl00-qahBYWMOLvAkTx3X1qo0QA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal