Abstract
To explore the role of chemokines in mast cell chemotaxis and accumulation at sites of inflammation, we first investigated the response of human mast cells to 18 different chemokines by induction of intracellular calcium mobilization in the human mast cell line, HMC-1. Only a subgroup of CXC chemokines defined by the conserved sequence motif glutamic acid-leucine-arginine (ELR) tripeptide motif, which included interleukin-8 (IL-8), growth-regulated oncogene (GRO), neutrophil-activating peptide-2 (NAP-2), and epithelial cell–derived neutrophil activating peptide-78 (ENA-78), induced calcium flux in the cells. These observations suggested that the receptor CXCR2 (IL-8RB) should be expressed on the surface of these cells. Using the RNAse protection assay, CXCR2 mRNA, but not CXCR1 (IL-8RA) mRNA expression was detected in HMC-1 cells. Flow cytometry analysis documented the surface expression of CXCR2. A binding analysis performed with125I-IL-8 determined that there were approximately 3,600 high affinity IL-8 binding sites per HMC-1 cell, with a calculated kd of 1.2 to 2 nmol/L. The activity of this receptor was further explored using IL-8, which was found to induce dose-dependent chemotactic and haptotactic responses in both HMC-1 cells and in vitro cultured human cord blood–derived mast cells. These results show the expression of functional CXCR2 receptors on the surface of human mast cells, which may play an important role in mast cell recruitment during the genesis of an inflammatory response.
MAST CELLS ARE multifunctional effector cells of the immune system.1,2 A local accumulation of mast cells has been described in such diverse pathologic conditions as allergic inflammation, parasitic infections, scleroderma, rheumatoid arthritis, interstitial cystitis, and in transplanted tissues undergoing rejection.3 Directional migration of inflammatory cells is presumably based on the local production of chemotactic factors. Reports that stem cell factor (SCF), transforming growth factor-β (TGF-β), and the anaphylatoxins, C3a and C5a, are mast cell chemoattractants4-8 have provided support for this hypothesis.
The chemokines are a superfamily of proinflammatory cytokines associated with inflammatory pathology. They play a critical role in the selective recruitment of leukocytes by acting as chemotaxins.9 The chemokines are classified into the four subfamilies C, CC, CXC, and C(X)3C, based on the number and arrangement of conserved cysteine residues.10 The prototypic CXC chemokine is interleukin-8 (IL-8), which was originally described as a monocyte-derived factor that attracts neutrophils.11 12 Several other CXC chemokines are also potent neutrophil chemoattractants. These include growth-regulated oncogene α (GROα), epithelial cell–derived neutrophil activating peptide-78 (ENA-78), and neutrophil-activating peptide-2 (NAP-2). In contrast, the CC chemokines preferentially act on monocytes, lymphocytes, natural killer (NK) cells, basophils, and eosinophils.
Chemokines mediate their effects by binding to seven transmembrane-spanning, G protein-coupled receptors.13,14Five CXC-receptors (CXCR) and nine CCR have been described to date. Cells of hematopoietic origin express unique, but overlapping, subsets of these chemokine receptors. Chemokine receptor subtypes in turn are selective for unique, but overlapping, subsets of chemokines. CXCR1 and CXCR2 have been shown to bind IL-8 with high affinity.15-17CXCR1 is specific for IL-8, while CXCR2 binds IL-8, as well as NAP-2, GRO, and ENA-78.18-20 IL-8R is expressed on neutrophils, monocytes, NK cells, and T lymphocytes.21-23
In the current study, we initially screened the response of the human mast cell line HMC-1 to 18 different CXC, CC, and C chemokines. The results led us to characterize the expression of CXCR2 on the surface of HMC-1 cells and the migratory response of both HMC-1 cells and in vitro developed human cord blood–derived mast cells to IL-8.
MATERIALS AND METHODS
Cell cultures.
The human mast cell line, HMC-1 (kindly provided by Dr J.H. Butterfield, Mayo Clinic, Rochester, MN)24,25was cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% heat inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 IU/mL penicillin, 50 μg/mL streptomycin, and 1.2 mmol/L α-thioglycerol. The cells were passaged every 3 to 4 days. Human cultured mast cells were obtained by placing umbilical cord blood cells in complete RPMI 1640 supplemented with 10% heat-inactivated FCS and 100 ng/mL SCF (R & D Systems, Minneapolis, MN) as described.26
Intracellular [Ca2+] measurements.
HMC-1 cells (5 × 106/mL) were incubated in RPMI medium with 1% FCS, containing 2.5 μmol/L FURA-2 AM for 60 minutes at 30°C. The cells were washed and resuspended at 1 × 106/mL RPMI 1640 with Ca2+ and Mg2+and 1% FCS. A total of 2 mL of the cell suspension was placed in a continuously stirred cuvette at 37°C in a fluorimeter (Photon Technology Inc, South Brunswick, NJ). Fluorescence was monitored at λex1 = 340 nm, λex2 = 380 nm, and λem = 510 nm, and the data presented as the relative ratio of fluorescence excited at 340 and 380 nm. Data were collected every 500 ms. The following chemokines were tested: Lymphotactin, IL-8, monocyte chemotactic protein (MCP)-3, GROα, NAP-2, ENA-78, and interferon-inducible protein (IP)-10 (Peprotech, Rocky Hill, NJ); RANTES, macrophage inflammatory protein (MIP)-1α, MIP-1β, and MCP-1 (Genzyme, Cambridge, MA); HCC-1 and I-309 (R & D Systems, Minneapolis, MN); and platelet factor-4 (PF-4) (Sigma Chemical Co, St Louis, MO). Eotaxin was a kind gift from Dr O. Yoshi (Shionogi Institute for Medical Science, Osaka, Japan), MCP-2 was a kind gift from Dr J. Van Damme (University of Leuven, Leuven, Belgium), T-cell activation (TCA)3 was a kind gift from Dr M.E. Dorf (Harvard Medical School, Boston, MA) and MIG (monokine induced by interferon-γ) was a kind gift from Dr J.M. Farber (NIAID, NIH, Bethesda, MD).
RNAse protection analysis.
Detection of human chemokine receptor message expression was performed with an RNAse protection analysis system (RiboQuant; Pharmingen, San Diego, CA). Two multiprobe template sets (hCR5 and hCR6) were used for in vitro transcription reactions using T7 polymerase to direct synthesis of high specific activity [32P]-labeled antisense RNAs that hybridize with human RNAs encoding CXCR1, CXCR2, CXCR3, CXCR4, BLR-1, BLR-2, V28, and two housekeeping control gene products L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH); and CCR1, CCR3, CCR4, CCR5, TERI, CCR2a, CCR2b, L32, and GAPDH, respectively. Templates were transcribed using a Maxiscript Kit (Ambion, Austin, TX) in the presence of a32P-uridine triphosphate (UTP) (800 Ci/mmol; NEN, Beverly, MA). Total RNA was isolated using Trizol (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instructions. RNAse protection analysis of 20 μg total RNA was performed after overnight hybridization at 60°C with 1.5 × 106 cpm of 32P-hCR6 using the RPAII Kit (Ambion) according to the manufacturer’s instructions. Protected fragments were precipitated and electrophoresed on 6% sequencing gels. Gels were dried and scanned on a phosphorimager (Molecular Dynamics, Palo Alto, CA). Dried gels were photographed on XAR-5 film (Kodak, KEBO Lab, Spånga, Sweden) at −70°C.
Flow cytometric analysis.
HMC-1 cells were stained with monoclonal antibodies against CXCR1 or CXCR2 using antibodies purchased from R & D Systems conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE). Flow cytometric analysis was performed on a FACStar plus (Becton Dickinson, Mountain View, CA).
Chemokine binding studies.
For binding assays, 5 × 105 cells per mL were incubated in phosphate-buffered saline (PBS) with125I-labeled IL-8 ligand (0.2 nmol/L) (specific activity, 2,200 Ci/mmol; New England Nuclear, Boston, MA) and varying concentrations of unlabeled ligands at 4°C for 1 hour. The incubation was terminated by removing aliquots from the cell suspension and separating cells from buffer by centrifugation through a silicone/paraffin oil mixture as described.23 Nonspecific binding was determined in the presence of 1 μmol/L unlabeled ligand. The binding data were curve fit with the computer program LIGAND (Biosoft, St Louis, MO) to determine the affinity (KD), number of sites, and nonspecific binding.
Chemotaxis assay.
Mast cell migration was examined using a 48-well microchemotaxis assay as described.27 Briefly, various concentrations of chemokine were placed in the lower compartment of a 48-well microchemotaxis chamber. Mast cells (2 to 5 × 106cells/mL) were then placed in the upper compartment. The upper and lower compartments of the chamber were separated by a 5 μm polycarbonate filter coated with fibronectin (Sigma Chemical Co).5 The chambers were incubated for 4 hours at 37°C, a time period over which chemokine equilibrium between the upper and lower chambers is optimally achieved. Filters were then scraped, washed, fixed with methanol, and stained with Diff-Quik. Cell migration was measured by counting the number of cells attached to the lower surface of the filter in three high-power fields (HPF). Each concentration of chemokine was tested in either triplicate or sets of six wells. The results were expressed as the average of the number of migrating cells per three HPF (±standard error of the mean [SEM]). A checkerboard analysis of mast cell motility was conducted according to the method of Zigmond and Hirsch.28
For determination of haptotactic response of HMC-1 cells to IL-8, polycarbonate filters were precoated with Matrigel (Collaborative Biomedical Products, Bedford, MA), washed, dried, and incubated with either medium, IL-8, or PF-4 at the designated concentrations. After a 24-hour incubation, the filters were gently washed, dried, and tested in microchemotaxis chambers with HMC-1 cells.
Treatment with pertussis toxin.
Pertussis toxin (Sigma Chemical Co) treatment was performed by incubating 2.5 × 106 cells/mL for 90 minutes at 37°C with 0.1 or 1 μg/mL of the toxin in complete medium. After incubation, the cells were washed and resuspended in fresh medium before use.
RESULTS
Analysis of chemokine-induced [Ca2+]i in HMC-1 cells.
Changes in [Ca2+]i are classically associated with chemokine activation of cells and provide a mechanism by which receptor engagement and response specificity may be examined.14 HMC-1 cells were therefore tested for [Ca2+]i after treatment with chemokines of the C, CC, and CXC families (Table 1). Of the chemokines tested, only CXC chemokines defined by the conserved sequence glutamic acidleucine-arginine (ELR) tripeptide motif, namely IL-8, GROα, NAP-2, and ENA-78, induced an intracellular calcium flux in the HMC-1 cells. The kinetics of the responses to IL-8, ENA-78, GROα, and NAP-2 was almost indistinguishable (Fig 1A). The magnitude of the peak of [Ca2+]i was dependent on the ligand concentration tested (Fig 1B). The 50% effective dose (ED50) for IL-8, ENA-78, GROα, and NAP-2 was approximately 5 ng/mL, 10 ng/mL, 10 ng/mL, and 25 ng/mL, respectively.
Effect of Chemokines on Calcium Mobilization in FURA-2–Loaded HMC-1 Cells
| Chemokine . | Chemokine Family . | [Ca2+]i . |
|---|---|---|
| Lymphotactin | C | − |
| Eotaxin | C-C | − |
| RANTES | C-C | − |
| MIP-1α | C-C | − |
| MIP-1β | C-C | − |
| MIP-2 | C-C | − |
| MCP-1/MCAF | C-C | − |
| MCP-2 | C-C | − |
| MCP-3 | C-C | − |
| TCA3/I-309 | C-C | − |
| HCC-1 | C-C | − |
| IL-8 | C-X-C | + |
| GROα | C-X-C | + |
| NAP-2 | C-X-C | + |
| ENA-78 | C-X-C | + |
| IP-10 | C-X-C | − |
| SDF-1 | C-X-C | − |
| MIG | C-X-C | − |
| Chemokine . | Chemokine Family . | [Ca2+]i . |
|---|---|---|
| Lymphotactin | C | − |
| Eotaxin | C-C | − |
| RANTES | C-C | − |
| MIP-1α | C-C | − |
| MIP-1β | C-C | − |
| MIP-2 | C-C | − |
| MCP-1/MCAF | C-C | − |
| MCP-2 | C-C | − |
| MCP-3 | C-C | − |
| TCA3/I-309 | C-C | − |
| HCC-1 | C-C | − |
| IL-8 | C-X-C | + |
| GROα | C-X-C | + |
| NAP-2 | C-X-C | + |
| ENA-78 | C-X-C | + |
| IP-10 | C-X-C | − |
| SDF-1 | C-X-C | − |
| MIG | C-X-C | − |
−, no effect; +, effect.
Calcium mobilization by IL-8, GRO, NAP-2, and ENA-78 in HMC-1 cells. (A) Kinetics: agonists were added at the time indicated by the arrows. The identity and concentration are indicated to the right of each arrow. (B) Concentration dependence: calcium mobilization was measured in the cells stimulated as indicated. The amplitude of the peak change in fluorescence is shown as a function of the chemokine concentration. Results from one cell experiment are shown. Similar results were obtained in three independent experiments.
Calcium mobilization by IL-8, GRO, NAP-2, and ENA-78 in HMC-1 cells. (A) Kinetics: agonists were added at the time indicated by the arrows. The identity and concentration are indicated to the right of each arrow. (B) Concentration dependence: calcium mobilization was measured in the cells stimulated as indicated. The amplitude of the peak change in fluorescence is shown as a function of the chemokine concentration. Results from one cell experiment are shown. Similar results were obtained in three independent experiments.
Activation of G protein–coupled receptors typically induces a refractory period during which the receptor cannot transduce signals when stimulated a second time with same or other agonists, a phenomenon known as “desensitization.” IL-8, ENA-78, GROα, and NAP-2 all induce homologous desensitization in HMC-1 cells (Fig 2 and data not shown). Furthermore, IL-8 caused heterologous desensitization of ENA-78–, GROα–, and NAP-2–induced activation of HMC-1 cells (Fig 2). In contrast, ENA-78, GROα, or NAP-2, when added first, reduced, but did not abolish, the calcium signal induced by IL-8 (Fig 2). No cross-desensitization of the response to IL-8 was detected when RANTES or MIG was used as the first agonist (data not shown).
Desensitization of calcium transients in HMC-1 cells. Relative fluorescence was monitored from FURA-2–loaded cells before and during sequential addition of CXC chemokines at the times indicated by the arrows. The identity of each stimulus is indicated to the right of each arrow. The concentrations used were 10 ng/mL IL-8 and GRO and 25 ng/mL NAP-2 and ENA-78. Each tracing shown is from a single experiment and is representative of at least two separate experiments.
Desensitization of calcium transients in HMC-1 cells. Relative fluorescence was monitored from FURA-2–loaded cells before and during sequential addition of CXC chemokines at the times indicated by the arrows. The identity of each stimulus is indicated to the right of each arrow. The concentrations used were 10 ng/mL IL-8 and GRO and 25 ng/mL NAP-2 and ENA-78. Each tracing shown is from a single experiment and is representative of at least two separate experiments.
Detection of mRNA and surface expression of CXCR2 on HMC-1 cells.
The results from these studies on chemokine-induced calcium flux suggested the expression of IL-8 receptors on HMC-1 cells. Two IL-8R have been described, CXCR1 and CXCR2. Thus, CXC receptor expression in HMC-1 cells was first analyzed at the level of message using Pharmingen hCR6 multiprobes in an RNAse protection assay (RPA). In addition to the housekeeping gene GAPDH (96), a protected fragment of 321 was detected, reflecting the presence of the CXCR2 RNA (Fig 3A). In contrast, CXCR1 mRNA was not detected. None of the CCRs could be detected using the hCR5 multiprobe (data not shown). While not shown, HMC-1 cells also expressed RNA for CXCR4 and hv28.
Expression of IL-8 receptors by HMC-1 cells. (A) CXC receptor expression was measured at the level of message by RPA. In addition to the housekeeping gene product GAPDH (96), a protected fragment for CXCR2 (321) was detected. The two lanes represent two preparations of HMC-1. (B) CXCR2 expression on the surface of HMC-1 cells analyzed by flow cytometry. The bold histogram shows the expression of CXCR2; the thin histogram represents isotype control MoAb. This result is representative of four independent experiments.
Expression of IL-8 receptors by HMC-1 cells. (A) CXC receptor expression was measured at the level of message by RPA. In addition to the housekeeping gene product GAPDH (96), a protected fragment for CXCR2 (321) was detected. The two lanes represent two preparations of HMC-1. (B) CXCR2 expression on the surface of HMC-1 cells analyzed by flow cytometry. The bold histogram shows the expression of CXCR2; the thin histogram represents isotype control MoAb. This result is representative of four independent experiments.
Surface expression of IL-8 receptors was next analyzed by flow cytometry using monoclonal antibodies (MoAbs) directed against CXCR1 and CXCR2. Expression of CXCR2 could be detected on the surface of HMC-1 cells, consistent with the previous data (Fig 3B). CXCR1 could not be detected (data not shown).
Characterization of 125I-IL-8 binding sites on HMC-1 cells.
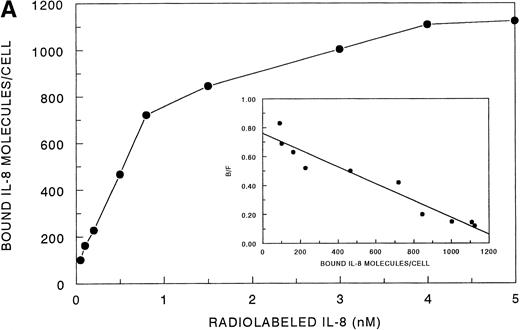
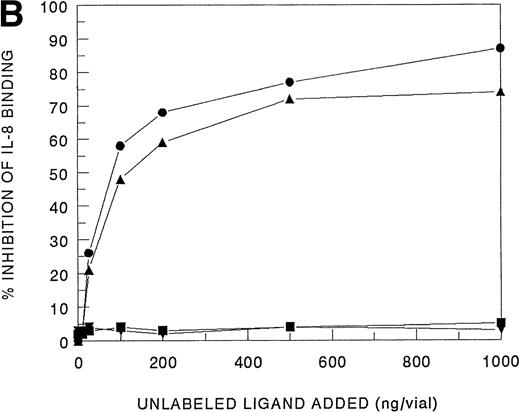
Receptor binding experiments using 125I-IL-8 and HMC-1 cells showed specific binding at 4°C (Fig 4A). A Scatchard plot analysis of binding of 125I-IL-8 to HMC-1 cells demonstrated a single class of approximately 3,600 high-affinity IL-8 binding sites with a calculated kd of 1.2 to 2 nmol/L (Fig 4A, insert). Bound125I-IL-8 was displaced by unlabeled IL-8 or GROα, but not by unlabeled IP-10 or PF-4 (Fig 4B).
Analysis of 125I-IL-8 binding to HMC-1 cells. (A) A representative equilibrium-binding analysis is shown, and Scatchard plot transformation of the binding data is presented in the insert. (B) Concentration-dependent displacement of125I-IL-8 binding to HMC-1 cells by IL-8 (•), GRO (▴), IP-10 (▪), and MIG (▾). HMC-1 cells (5 × 105 cells in each tube) were incubated with125I-IL-8 (0.2 nmol/L) in the presence or absence of increasing concentrations of unlabeled ligand for 60 minutes at 4°C.
Analysis of 125I-IL-8 binding to HMC-1 cells. (A) A representative equilibrium-binding analysis is shown, and Scatchard plot transformation of the binding data is presented in the insert. (B) Concentration-dependent displacement of125I-IL-8 binding to HMC-1 cells by IL-8 (•), GRO (▴), IP-10 (▪), and MIG (▾). HMC-1 cells (5 × 105 cells in each tube) were incubated with125I-IL-8 (0.2 nmol/L) in the presence or absence of increasing concentrations of unlabeled ligand for 60 minutes at 4°C.
IL-8–mediated migration of human mast cells.
The migratory response of human mast cells was determined in a microchemotaxis chamber. HMC-1 cells migrated in response to IL-8 and GROα in a dose-related manner, but not to PF-4 (Fig 5A). Similarly, IL-8, but not PF-4, induced migration of in vitro cultured human mast cells (Fig 5B). IL-8 induced maximal migration at 100 ng/mL, with an ED50 of approximately 1 ng/mL. The ability of IL-8 to stimulate directional migration (chemotaxis) versus random migration (chemokinesis) was analyzed using a checkerboard analysis. As shown in Table 2, IL-8 was found to be chemotactic, but not chemokinetic for mast cells. Chemokinesis was calculated to be less than 5% of the total migration observed.
Chemotactic effect of IL-8 on HMC-1 cells (A) and in vitro cultured human mast cells (B). Cells were exposed to various concentrations of IL-8, GRO, or PF-4, as indicated for 4 hours. The results are expressed as the number of countable cells per 3HPF (mean ± SEM) (n ≥ 5). *P < .05, **P < .01.
Chemotactic effect of IL-8 on HMC-1 cells (A) and in vitro cultured human mast cells (B). Cells were exposed to various concentrations of IL-8, GRO, or PF-4, as indicated for 4 hours. The results are expressed as the number of countable cells per 3HPF (mean ± SEM) (n ≥ 5). *P < .05, **P < .01.
Checkerboard Analysis of Mast Cell Chemotaxis to IL-8
| IL-8 ng/mL . | 0 . | 1 . | 10 . | 100 . |
|---|---|---|---|---|
| 0 | 11 (2) | 19 (5) | 9 (4) | 7 (6) |
| 1 | 36 (3) | 16 (4) | 8 (3) | 11 (5) |
| 10 | 65 (7) | 26 (3) | 13 (2) | 9 (5) |
| 100 | 86 (4) | 45 (3) | 15 (5) | 13 (3) |
| IL-8 ng/mL . | 0 . | 1 . | 10 . | 100 . |
|---|---|---|---|---|
| 0 | 11 (2) | 19 (5) | 9 (4) | 7 (6) |
| 1 | 36 (3) | 16 (4) | 8 (3) | 11 (5) |
| 10 | 65 (7) | 26 (3) | 13 (2) | 9 (5) |
| 100 | 86 (4) | 45 (3) | 15 (5) | 13 (3) |
Column under IL-8 represents increasing concentrations of IL-8 in lower wells, and the concentrations given next to IL-8, line, represent the concentration in upper wells. The results are expressed as the number of cells per 3 HPF (±SEM), n = 3.
In contrast to chemotaxis, which is defined as migration towards a gradient of soluble factor, haptotaxis is migration to surface-bound gradients of the chemoattractant. IL-8 is known to easily bind to surfaces and has been shown to induce haptotaxis in neutrophils.29 We therefore investigated whether IL-8 could mediate haptotaxis in mast cells. IL-8 was incubated in different concentrations with polycarbonate filters precoated with extracellular matrix proteins. The filters were then used in a microchemotaxis chamber. IL-8 was found to induce haptotactic migration of HMC-1 cells in a dose-related manner (Fig 6). PF-4–coated filters did not promote mast cell migration.
Haptotactic effect of IL-8, but not PF-4, on HMC-1 cells. The migration of cells was tested on precoated filters exposed to medium alone or to various concentrations of IL-8 or PF-4. The results are expressed as the number of countable cells per 3HPF (mean ± SEM) (n = 3). *P < .05, **P < .01.
Haptotactic effect of IL-8, but not PF-4, on HMC-1 cells. The migration of cells was tested on precoated filters exposed to medium alone or to various concentrations of IL-8 or PF-4. The results are expressed as the number of countable cells per 3HPF (mean ± SEM) (n = 3). *P < .05, **P < .01.
IL-8–induced migration was inhibited by prior treatment of HMC-1 cells with 100 ng/mL or 1,000 ng/mL of pertussis toxin for 90 minutes (Table 3). SCF-induced migration, which is mediated through receptor tyrosine kinase, was not inhibited by similar treatment with the pertussis toxin. This data is consistent with the conclusion that CXCR2 expressed on mast cells is coupled to Gi protein, as reported for CXCR2 expressed on neutrophils.30
Pertussis Toxin Treatment Blocks IL-8–Induced Migration of Mast Cells
| Chemokine . | PTx (ng/mL) . | Cells/3HPF (±SEM) . |
|---|---|---|
| IL-8 | — | 108 (6) |
| 100 | 43 (5)3-150 | |
| 1,000 | 26 (5)3-151 | |
| PF-4 | — | 19 (6) |
| 100 | 26 (4) | |
| 1,000 | 12 (7) | |
| SCF | — | 187 (14) |
| 100 | 174 (9) | |
| 1,000 | 178 (9) |
| Chemokine . | PTx (ng/mL) . | Cells/3HPF (±SEM) . |
|---|---|---|
| IL-8 | — | 108 (6) |
| 100 | 43 (5)3-150 | |
| 1,000 | 26 (5)3-151 | |
| PF-4 | — | 19 (6) |
| 100 | 26 (4) | |
| 1,000 | 12 (7) | |
| SCF | — | 187 (14) |
| 100 | 174 (9) | |
| 1,000 | 178 (9) |
HMC-1 cells were incubated for 90 minutes at 37°C before the chemotaxis experiment with either 0, 100, or 1,000 ng/mL pertussis toxin. The treated cells were tested for their ability to respond to IL-8 (100 ng/mL), PF-4 (100 ng/mL), or SCF (100 ng/mL).
P < .05.
P < .01 (n = 3).
DISCUSSION
The data presented show that human mast cells express CXCR2 (Figs 3 and4) and that the interaction of CXCR2 with its natural ligands, IL-8 and GROα, induces calcium mobilization (Fig 1) and cell migration (Fig 5A and B). Although IL-8 is an inflammatory cytokine that is known to function as a neutrophil chemoattractant and activating factor, monocytes, NK cells, and T lymphocytes also respond chemotactically to IL-8.13,21-23 31 Similarly, mast cells are thus also capable of responding to IL-8–induced signals.
Mast cells accumulate during both acute and chronic inflammation. This local increase in mast cells is due, at least in part, to the redistribution and recruitment of neighboring mast cells. One example of the rapid accumulation of mast cells is the increase in these cells observed within the intraepithelial cell layer of the nasal mucosa after local allergen provocation.32 Several factors capable of attracting human mast cells have been reported. These include SCF, TGF-β, and the anaphylatoxins.5,7,8 However, the only chemokine reported to induce migration of human in vitro developed mast cells and pulmonary mast cells was RANTES,5,33 which did not induce HMC-1 cell migration or calcium influx5 (and this study). Furthermore, no transcripts for any of the receptors interacting with RANTES were detected in HMC-1 cells, ie, CCR1, CCR3, CCR4, and CCR5. These results are in agreement with the results of Hartmann et al,8 who have also reported that CC chemokines do not promote chemotaxis of HMC-1 cells. Although unresponsive to CC chemokines, human mast cells, as noted in the current study, do respond to multiple ELR+ CXC chemokines, ie, IL-8, ENA-78, GROα, and NAP-2.
Chemokines act via G protein–coupled receptors. With these receptors, there is typically a refractory period after initial stimulation during which the receptor cannot transduce signals when stimulated a second time with the same or other agonists. All of the ELR+ CXC chemokines tested in this study showed this homologous desensitization. IL-8 also desensitized the calcium response to GROα, ENA-78, and NAP-2 in HMC-1 cells (heterologous desensitization). In contrast, GROα, ENA-78, or NAP-2 given as the first stimuli, reduced, but not abolished the calcium flux induced by IL-8. This is well described for the CXCR2 receptor, although the precise mechanism for this effect is unknown.20 Thus, mast cells and neutrophils respond similarly to CXCR2 agonist treatment.
Surface CXCR2 appears to represent all IL-8 binding sites on HMC-1 cells. CXCR2, but not CXCR1, message was detected in HMC-1 cells, and CXCR2 protein expression was shown at the single cell level with specific antibodies and flow cytometry. A Scatchard plot analysis showed fewer IL-8 binding sites on HMC-1 cells (3,600 binding sites/cell) than on neutrophils (60,000 binding sites/cell).18 This IL-8 binding site number on mast cells is similar to the number estimated on T lymphocytes.23
Both HMC-1 cells and in vitro cultured human mast cells migrated in response to IL-8. The number of mast cells of both types migrating to 100 ng/mL of IL-8 (approximately 75/3HPF) is fewer than the number of mast cells migrating in response to platelet-activating factor or C5a (approximately 100 to 140/3HPF) (Nilsson et al, submitted). However, the migration of mast cells to IL-8 (and GROα for HMC-1 cells) was significant and specific. No migration could be determined to PF-4 used as a chemokine control.
In summary, CXCR2 is expressed on HMC-1 cells and IL-8 is a potent chemotaxin for human mast cells. These observations may be relevant to the understanding of mast cell migration in vivo, as IL-8 is known to be elevated in several inflammatory diseases, where an increase in mast cells have been described, including rheumatoid arthritis, inflammatory bowel diseases, and psoriasis.13 34 Mast cell migration towards IL-8 appears thus to represent an important mechanism in the recruitment of mast cells to sites of tissue inflammation.
ACKNOWLEDGMENT
We thank Dr P.M. Murphy (NIH, Bethesda, MD) for advice on the intracellular calcium measurements.
Supported in part by grants from the Swedish Cancer Society, the Swedish Foundation for Health Care Sciences and Allergy Research, the Swedish Heart Lung Foundation, King Gustav V:s 80 Year Foundation, Konsul Th C Bergh Foundation, and Ollie and Elof Ericssons Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Gunnar Nilsson, PhD, Unit of Pathology, Department of Genetics and Pathology, Uppsala University, S-751 85 Uppsala, Sweden; e-mail: Gunnar.Nilsson@patologi.uu.se.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal