Abstract
Lineage-specific growth factors mobilize peripheral blood progenitor cells (PBPC) and accelerate hematopoietic recovery after high-dose chemotherapy. Recombinant human thrombopoietin (rhTPO) may further increase the progenitor-cell content and regenerating potential of PBPC products. We evaluated the safety and activity of rhTPO as a PBPC mobilizer in combination with granulocyte colony-stimulating factor (G-CSF) in 29 breast cancer patients treated with high-dose chemotherapy followed by PBPC reinfusion. Initially, patients received escalating single doses of rhTPO intravenously (IV) at 0.6, 1.2, or 2.4 μg/kg, on day 1. Subsequent patients received rhTPO 0.6 or 0.3 μg/kg on days −3, −1, and 1, or 0.6 μg/kg on days −1 and 1. G-CSF, 5 μg/kg IV or subcutaneously (SC) twice daily, was started on day 3 and continued through aphereses. Twenty comparable, concurrently and identically treated patients (who were eligible and would have been treated on protocol but for the lack of study opening) mobilized with G-CSF alone served as comparisons. CD34+ cell yields were substantially higher with the first apheresis following rhTPO and G-CSF versus G-CSF alone: 4.1 × 106/kg (range, 1.3 to 17.6) versus 0.8 × 106/ kg (range, 0.3 to 4.2), P = .0003. The targeted minimum yield of 3 × 106CD34+ cells/kg was procured following a single apheresis procedure in 61% of the rhTPO and G-CSF–mobilized group versus 10% of G-CSF–mobilized patients (P = .001). In rhTPO and G-CSF mobilized patients, granulocyte (day 8 v 9, P= .0001) and platelet recovery (day 9 v 10, P= .07) were accelerated, and fewer erythrocyte (3 v 4,P = .02) and platelet (4 v 5, P = .02) transfusions were needed compared with G-CSF–mobilized patients. Peripheral blood platelet counts, following rhTPO and G-CSF, were increased by greater than 100% and the platelet content of PBPC products by 60% to 110% on the first and second days of aphereses (P < .0001) with the greatest effect seen with repeated dosing of rhTPO at 0.6 μg/kg. rhTPO is safe and well tolerated as a mobilizing agent before PBPC collection. Mobilization with rhTPO and G-CSF, in comparison to a comparable, nonrandomized G-CSF–mobilized group of patients, decreases the number of apheresis procedures required, may accelerate hematopoietic recovery, and may reduce the number of transfusions required following high-dose chemotherapy for breast cancer.
THROMBOPOIETIN, the primary regulator of megakaryocyte development and the ligand for the cytokine receptor C-Mpl, has been cloned by several groups of investigators.1-5 C-Mpl is expressed on the surface of cells of megakaryocytic lineage and other hematopoietic progenitors; once activated, it can promote proliferation and maturation of megakaryocytes,6 and progenitors committed to the myelomonocytic7 and erythroid lineage.8
High-dose chemotherapy and stem-cell rescue has been increasingly used for the treatment of a variety of solid tumors and hematologic malignancies.9,10 Administration of colony-stimulating factors such as granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the setting of peripheral blood progenitor cell (PBPC) mobilization and apheresis has shortened the duration of potentially life-threatening pancytopenia following high-dose chemotherapy.11,12However, some patients will mobilize PBPC poorly with G-CSF or GM-CSF. In addition, a fraction of patients will become refractory to platelet transfusions despite the fact that the use of filtered platelets and changes in the quantitative indication for transfusion have diminished exposure to potentially immunogenic antigens.13 14
In the preclinical setting, a single injection of recombinant human thrombopoietin (rhTPO) into Rhesus monkeys decreased radiation-induced thrombocytopenia, while coadministration of rhTPO and G-CSF or GM-CSF resulted in accelerated neutrophil recovery.15Adenovirus-mediated transfer of the TPO gene given intraperitoneally into mice before exposure to radiation and carboplatin ameliorated thrombocytopenia.16 In the clinical setting, a single dose of rhTPO before chemotherapy resulted in expansion of all progenitor lines and a dose-related increase in the megakaryocyte pool; peripheral blood platelet counts increased 4 days after rhTPO injection with a peak between days 10 and 15.17
Based on favorable preclinical and clinical safety data, we set out to test the safety and efficacy of escalating single and multiple doses of rhTPO as a mobilizing agent before the procurement of PBPC.
MATERIALS AND METHODS
Patients.
All patients gave their written, voluntary informed consent. This study was approved by the Institutional Review Board of the City of Hope National Medical Center (Duarte, CA).
Between July 1996 and November 1997, 29 patients with responding stage IV or high-risk stage II/III primary breast carcinoma were accrued. Patients with a Karnofsky performance status greater than 80%, adequate organ function, left ventricular cardiac ejection fraction ≥55%, and a measured creatinine clearance ≥80 mL/min were eligible. The use of aspirin, nonsteroidal antiinflammatory agents, and anticoagulant drugs was not allowed (except to maintain line patency). Chemotherapy or radiation therapy within 3 weeks before mobilization, or the use of growth factors other than G-CSF, was prohibited.
Study design.
Patients had a double-lumen Hickman catheter (Bard Access Systems, Salt Lake City, UT) inserted before mobilization of PBPC. Safety of rhTPO, at any given dose level, had to be documented in three patients before accrual began at the next dose level. Initially, groups of patients received a single intravenous (IV) injection of rhTPO on day 1, escalating the dose through 0.6, 1.2, or 2.4 μg/kg (arm A). Completion of accrual on arm A was required before accrual to the subsequent cohort. Subsequent patients received rhTPO on days −3, −1, and 1 at doses of 0.6 μg/kg or 0.3 μg/kg (arm B) or rhTPO 0.6 μg/kg on days −1 and 1 (arm C). G-CSF (Neupogen; Amgen, Thousand Oaks, CA) 5 μg/kg twice daily, subcutaneously (SC) or IV was started on day 3 and continued through aphereses. rhTPO (Genentech Inc, South San Francisco, CA), a full-length glycosylated molecule, was reconstituted in preservative-free normal saline before injection.
Aphereses were started on the fourth day of G-CSF administration (day 6) using a Spectra (Cobe Laboratories, Lakewood, CO), or Fenwall CS-3000 Plus cell separator (Baxter, Deerfield, IL); approximately 10 L of blood was processed during each collection. The mobilization schema is illustrated in Fig 1. A minimum target of 3 × 106 CD34+ cells/kg (and ≥7 × 108 mononuclear cells/kg) was procured. A cohort of 20 concurrently treated patients were mobilized with G-CSF 5 μg/kg twice per day, SC or IV, daily. These patients were eligible and otherwise similar to those on-study and would have been treated on protocol but for the lack of study openings at the time of their evaluation. Starting on the fourth day of G-CSF administration, the duration and procedure of apheresis were identical to those of the rhTPO study group; ≥7 × 108 mononuclear cells/kg were collected. PBPC were cryopreserved by simple immersion in a −130°C freezer.18
High-dose chemotherapy.
Patients received both cisplatin 125 mg/m2 and etoposide 30 mg/kg on days −12 and −5 followed by cyclophosphamide 100 mg/kg on day −3. PBPC were reinfused on a divided schedule: 25% of PBPC were infused on day −2 following high-dose chemotherapy and 75% were infused on day 0. All patients received G-CSF 5 μg/kg twice daily IV from day −2 through absolute granulocyte recovery to ≥1,500/μL.18 Blood products were filtered and irradiated at 2,500 cGy and were transfused to maintain a platelet count of greater than 20 × 109/L and a hematocrit of ≥25 or as clinically indicated.19
Patient monitoring.
Patients were monitored for adverse reactions with each injection of rhTPO. Total WBC, differential, and platelet counts were measured on the days of rhTPO and G-CSF administration and during aphereses. Serum chemistries, coagulation parameters, and samples for anti-rhTPO antibodies were monitored during the mobilization, and following high-dose chemotherapy and PBPC reinfusion (transplant) phase. No more than two episodes of grade 3 toxicity were allowed at any dose level; any grade 4 toxicity related to the administration of rhTPO defined the maximum-tolerated dose of rhTPO. Development of neutralizing antibodies to rhTPO or a platelet count greater than 600,000/μL persisting beyond 24 hours were also considered dose-limiting toxicities.
Hematologic end points.
The number of days needed to reach an absolute granulocyte count ≥0.5 × 109/L and platelet independence (defined as the day after the last platelet transfusion) were calculated from day 0 of the “transplant phase.” The number of erythrocyte and platelet transfusions required were tabulated. Single apheresis and pooled platelet products were each counted as one product.
Flow cytometry studies.
PBPC apheresis or peripheral blood samples were stained with either phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody and dual stained with anti–CD45-fluorescein isothiocyanate (FITC) or, for assessment of the presence of megakaryocytic lineage samples, were stained with anti–CD34-PE/anti–CD41-FITC (Becton Dickinson, San Jose, CA). Samples of 300 μL containing approximately 1 million cells were incubated with antibody for 30 minutes at 4°C. After the addition of 2 mL erythrocyte lysing solution (Ortho-mune; Ortho Diagnostic Systems, Raritan, NJ), samples were incubated for 10 minutes at room temperature. Cells were then washed twice in phosphate-buffered saline (PBS) Buffer Reagent (Coulter, Miami, FL) and resuspended in 500 μL PBS. Analysis was performed using a FACSCalibur or FACSort flow cytometer equipped with argon-ion laser, tuned at 488 nm (Becton Dickinson), and analyzed with Cellquest 3.1 software (Becton Dickinson); 100,000 cells were acquired for each analysis. CD34/CD45 analysis was performed as described earlier.18 For the CD34/CD41 analysis, the primary gate was set up using forward scatter versus side scatter. All CD34+ cells were analyzed for side-scatter properties and expression of CD41; subsequently, CD34+ cells within the low side-scatter population were further analyzed for CD41 expression.
Progenitor-cell assays.
Peripheral blood was collected in heparin before each injection of rhTPO and on the days of apheresis. PBPC were diluted 1:5 with Iscove’s modified Dulbecco’s medium (IMDM) in heparin. Samples were shipped at room temperature overnight to the Stem Cell Laboratory (Mayo Clinic Cancer Center, Rochester, MN).
Mononuclear cells were isolated by density gradient centrifugation in Lymphocyte Separation Medium (LSM; Cappel, ICN Biomedical, Aurora, OH; 1.077 g/mL) and diluted with IMDM to no more than 5 × 106 cells/mL. Eight milliliters of cell suspension was centrifuged at 400g for 30 minutes at 22°C. Cells at the interface were collected and washed twice with 3 vol of IMDM.
Colony-forming unit–granulocyte, macrophage (CFU-GM), colony-forming unit–granulocyte, erythrocyte, megakaryocyte, macrophage (CFU-GEMM) and burst-forming unit–erythroid (BFU-E) were assayed in a methylcellulose medium (MethoCult GF H4434; StemCell Technologies, Vancouver, Canada). Cells were plated at a final cell density of 1 or 2 × 105/mL (peripheral blood) and 1 or 3 × 104/mL (PBPC). After 14 days at 37°C in 5% CO2, the colonies were identified by morphology and scored. Colony-forming unit–megakaryocyte (CFU-MK) was assayed in an agarose medium (MegaCult; StemCell Technologies); cells were plated at a final density of 4 × 105/mL (peripheral blood) or 1 × 105/mL (PBPC). After 18 days at 37°C in 5% CO2, the colonies were fixed with methanol-acetone and immunohistochemically detected by the anti-GPIIb/IIIa antibody and alkaline phosphatase detection system supplied by the manufacturer.
Statistical analysis.
The study was primarily designed to identify tolerable doses and schedules of rhTPO. Comparison of rhTPO doses and schedules, and comparison to patients not receiving rhTPO was planned to evaluate hematologic response in the context of a phase I study, and not to achieve any specified power. The G-CSF–only mobilized group consisted of comparable, concurrently treated patients who were eligible to enroll on study but did not for lack of an opening on the trial. Nonevaluable patients were excluded from analysis as described in the Results. Patients receiving rhTPO were combined in a single group for comparison to G-CSF–only patients using rank-sum tests; an F test was applied for overall comparison between these two groups after stratifying for prior exposure to radiation therapy. Comparisons among rhTPO regimens were evaluated by Kruskal-Wallis tests20; 95% confidence intervals (CIs) for percent (fold) differences were based on t statistics for log-percent difference.21The effects of dose and schedule were evaluated by fitting multiple regression models, using logarithms of responses as described in the Results. Separate regressions were fit to the first and second days of cell collections to evaluate the relationship between platelet content for stem cell products and peripheral blood platelet counts. Correlations and coefficients of determination are provided as rough summaries. An F test was applied to verify the presence of significant correlation.
RESULTS
The effects of rhTPO and G-CSF on PBPC mobilization were assessable in 26 of 29 patients (two patients were removed from the study due to bacteremia associated with Hickman catheter infection; one patient who had experienced prolonged leukocytopenia after prior standard chemotherapy mobilized so poorly that she was taken off study). Twenty-three of the 26 patients were assessable both during the mobilization and the “transplant” phase; three patients were not evaluable during the transplant phase due to delay of their high-dose chemotherapy (progression, n = 1; superior vena cava occlusion [likely due to a previous catheter infection], n = 1; bacteremia after apheresis, n = 1).
Characteristics of the 26 assessable rhTPO- and G-CSF-treated patients and 20 concurrently and identically treated G-CSF–only mobilized patients are listed in Table 1. Most characteristics, such as age, stage, prior chemotherapeutic exposure, and number of days between apheresis and PBPC reinfusion were similar except for a higher percentage of G-CSF-only–mobilized group (control group) having been exposed to prior radiation treatment.
Patient Characteristics
| Characteristic . | TPO Study Group (n = 26) . | Control Group (n = 20) . | ||
|---|---|---|---|---|
| Median . | Range . | Median . | Range . | |
| Age (yr) | 44 | 33-60 | 48 | 27-60 |
| No. of prior chemotherapy regimens | 1 | 1-4 | 1 | 1-3 |
| No. of prior chemotherapy cycles | 6 | 3-23 | 6 | 1-18 |
| Days from apheresis to reinfusion | 30 | 29-45 | 30.5 | 23-44 |
| No.% | No.% | |||
| Stage II/III disease | 16 | 62 | 12 | 60 |
| Stage IV | 10 | 38 | 8 | 40 |
| Prior radiation | 4 | 15 | 6 | 30 |
| Characteristic . | TPO Study Group (n = 26) . | Control Group (n = 20) . | ||
|---|---|---|---|---|
| Median . | Range . | Median . | Range . | |
| Age (yr) | 44 | 33-60 | 48 | 27-60 |
| No. of prior chemotherapy regimens | 1 | 1-4 | 1 | 1-3 |
| No. of prior chemotherapy cycles | 6 | 3-23 | 6 | 1-18 |
| Days from apheresis to reinfusion | 30 | 29-45 | 30.5 | 23-44 |
| No.% | No.% | |||
| Stage II/III disease | 16 | 62 | 12 | 60 |
| Stage IV | 10 | 38 | 8 | 40 |
| Prior radiation | 4 | 15 | 6 | 30 |
Table 2 demonstrates the CD34+cell (CD34+/CD45+) yield procured during the first apheresis, the number of aphereses needed to obtain ≥3 × 106 CD34+ cells/kg, and transfusion requirements in the combined cohorts of patients mobilized with rhTPO and G-CSF versus the control group mobilized with G-CSF alone and stratified by prior exposure to radiation therapy. The number of apheresis procedures needed to procure ≥3 × 106/kg CD34+ cells was lower in the group mobilized with rhTPO and G-CSF (2 [range, 1 to 3] v 3 [range, 1 to ≥5];P < .0001). The cumulative CD34+ cell yield never reached 3 × 106 /kg in 7 of 20 patients mobilized with G-CSF alone, although the required mononuclear-cell target for patients in the control group was reached by all. The number of actual aphereses performed (targeted originally to procure ≥3 × 106/kg CD34+ cells in the rhTPO/G-CSF group and >7 × 108 mononuclear cells/kg in the G-CSF–mobilized group) was fewer in the rhTPO-mobilized group (median, 2 [range, 2 to 3] v 3 [range, 2 to 6]; P < .0001, data not shown).
PBPC Yield and Transfusion Requirements
| Variable . | Prior Radiation . | rhTPO Group . | Control Group . | P Value* . | ||||
|---|---|---|---|---|---|---|---|---|
| Median . | Range . | N . | Median . | Range . | N . | |||
| No. of aphereses to procure | No | 1 | 1-3 | 22 | 2 | 1-5+ | 14 | .0005 |
| >3 × 106CD34+ cells/kg | Yes | 1 | 1-2 | 4 | 3 | 3-4+ | 6 | .001 |
| All patients | 1 | 1-3 | 26 | 3 | 1-5+ | 20 | <.0001 | |
| CD34+ × 106/kg/first apheresis | No | 3.6 | 1.3-18 | 22 | 1.3 | 0.3-4.2 | 14 | .0005 |
| Yes | 5.1 | 2.4-15 | 4 | 0.7 | 0.6-0.9 | 4 | .014 | |
| All patients | 4.1 | 1.3-17.6 | 26 | 0.8 | 0.3-4.2 | 20 | .0003 | |
| No. of RBC transfusions† | No | 3 | 2-7 | 21 | 4.5 | 2-10 | 14 | .07 |
| Yes | 3.5 | 3-4 | 2 | 4 | 3-8 | 6 | .37 | |
| All patients | 3 | 2-7 | 23 | 4 | 2-10 | 20 | .02 | |
| No. of platelet transfusions† | No | 4 | 0-10 | 21 | 5 | 2-19 | 14 | .09 |
| Yes | 3 | 2-4 | 2 | 6.5 | 3-11 | 6 | .2 | |
| All patients | 4 | 0-10 | 23 | 5 | 2-19 | 20 | .02 | |
| Variable . | Prior Radiation . | rhTPO Group . | Control Group . | P Value* . | ||||
|---|---|---|---|---|---|---|---|---|
| Median . | Range . | N . | Median . | Range . | N . | |||
| No. of aphereses to procure | No | 1 | 1-3 | 22 | 2 | 1-5+ | 14 | .0005 |
| >3 × 106CD34+ cells/kg | Yes | 1 | 1-2 | 4 | 3 | 3-4+ | 6 | .001 |
| All patients | 1 | 1-3 | 26 | 3 | 1-5+ | 20 | <.0001 | |
| CD34+ × 106/kg/first apheresis | No | 3.6 | 1.3-18 | 22 | 1.3 | 0.3-4.2 | 14 | .0005 |
| Yes | 5.1 | 2.4-15 | 4 | 0.7 | 0.6-0.9 | 4 | .014 | |
| All patients | 4.1 | 1.3-17.6 | 26 | 0.8 | 0.3-4.2 | 20 | .0003 | |
| No. of RBC transfusions† | No | 3 | 2-7 | 21 | 4.5 | 2-10 | 14 | .07 |
| Yes | 3.5 | 3-4 | 2 | 4 | 3-8 | 6 | .37 | |
| All patients | 3 | 2-7 | 23 | 4 | 2-10 | 20 | .02 | |
| No. of platelet transfusions† | No | 4 | 0-10 | 21 | 5 | 2-19 | 14 | .09 |
| Yes | 3 | 2-4 | 2 | 6.5 | 3-11 | 6 | .2 | |
| All patients | 4 | 0-10 | 23 | 5 | 2-19 | 20 | .02 | |
Comparisons between the rhTPO v control groups were performed using the Wilcoxon rank-sum test. F test, adjusting for prior radiation, was used to combine strata in the overall analysis.
Transfusion requirements were evaluated in 23 assessable patients.
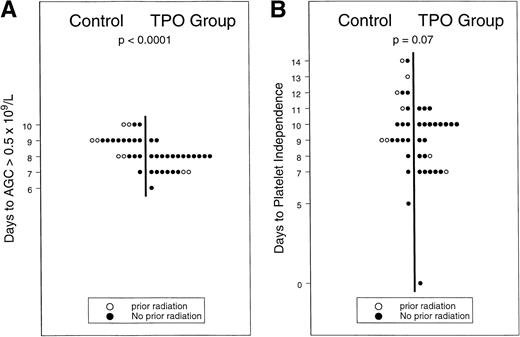
In 16 of 26 patients in the rhTPO-mobilized group, the targeted yield of ≥3 × 106 CD34+ cells/kg was reached with the first apheresis procedure versus in only 2 of 20 G-CSF mobilized patients (χ2, P < .001), apheresis from three additional patients in the G-CSF–only group resulted in ≥3 × 106 CD34+ cell yield/kg on days 5 (n = 2) and 6 (n = 1), while no patient in the control group yielded ≥3 × 106 CD34+ cells/kg beyond day 6 (data not shown). In the G-CSF–mobilized group, the highest numbers of CD34+ cells were procured during the first apheresis (day 4 of G-CSF) in 8 and during the second procedure (day 5 of G-CSF) in 5 patients; CD34+ cell yield from 7 patients peaked evenly between days 6 and 8. The median CD34+ yield was 4.1 × 106/kg (range, 1.3 to 17.6 × 106/kg) in products collected on the first scheduled day of apheresis from patients in the rhTPO-mobilized group versus 0.8 × 106/kg (range, 0.3 to 4.2 × 106/kg) in the concurrently treated and G-CSF–mobilized control group. A 3.5-fold increase in CD34+ cell content per apheresis (95% CI, 2.7-to 6.6-fold increase in geometric mean) following administration of rhTPO was observed: the median number of (range) CD34+cells per kilogram per apheresis was 3.8 × 106 (1.1 to 12.7) in the rhTPO-mobilized group versus 1.1 × 106 (0.5 to 4.7) in the control group (P = .014). Higher yields of mononuclear cells were procured following administration of rhTPO: 3.7 × 108 mononuclear cells/kg/apheresis (range, 0.5 to 6.5) versus 2.8 × 108 mononuclear cells/kg/apheresis (range, 1.6 to 4.5). We observed an approximate 30% increase in the mononuclear-cell yield per apheresis (95% CI, 16% to 59% increase in geometric mean), with a statistically significant difference seen even after stratification for the possible effects of prior radiation treatment (P = .04 data not shown). Both RBC (3 v 4, P = .02) and platelet (4v 5, P = .02) transfusion requirements were decreased in the rhTPO-mobilized group compared with G-CSF–mobilized controls. Hematopoietic recovery was also faster in the rhTPO group: granulocyte and platelet recoveries were accelerated, as illustrated in Fig2A and B (8 v 9 days [P = .0001] and 9 v 10 days [P = .07], respectively). Progenitor-cell yield and hematopoietic recovery were improved following mobilization with the rhTPO/G-CSF combination versus G-CSF alone, in patients with or without prior exposure to radiation therapy.
(A and B) Hematopoietic recovery after mobilization with rhTPO and G-CSF v G-CSF.
(A and B) Hematopoietic recovery after mobilization with rhTPO and G-CSF v G-CSF.
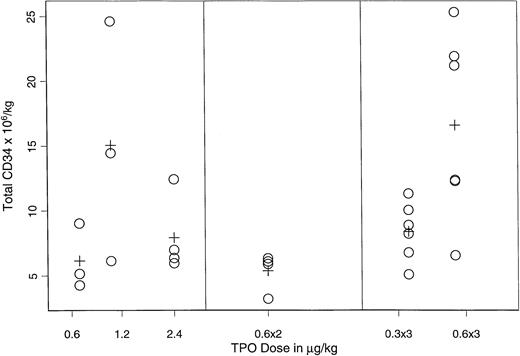
Figure 3 displays the mean CD34+ yield for cohorts of patients receiving different doses and/or schedules of rhTPO on arms A, B, and C. The Kruskal-Wallis test suggested a dose/schedule dependent effect (P = .03); the highest yield (15.1 × 106/kg v 7.5 × 106/kg) was observed when three doses of rhTPO 0.6 μg/kg were administered every other day (P = .004, F test).
CD34+ progenitor-cell yield in the PBPC products of rhTPO– and G-CSF–mobilized patients.
CD34+ progenitor-cell yield in the PBPC products of rhTPO– and G-CSF–mobilized patients.
Table 3 compares the effects of rhTPO/G-CSF mobilization versus G-CSF alone on peripheral blood platelet counts on the days of aphereses and depicts the platelet content of each apheresis product. We observed an approximately 100% and 130% increase in peripheral blood platelet counts on the first and second days of apheresis and 60% and 110% increase in platelet yields in the apheresis products after mobilization with rhTPO and G-CSF, in comparison to mobilization with G-CSF only.
Platelet Content of PBPC Products and Peripheral Blood Platelet Concentration on the First and Second Days of Apheresis by Mobilizing Regimen
| Variable . | . | rhTPO 0.6-2.4 μg/kg and G-CSF 5 μg/kg, Twice Daily . | G-CSF 5 μg/kg, Twice Daily . | P Value . | ||
|---|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | |||
| PBd1 | PLTs × 109/L | 456 | 218-995 | 224 | 130-369 | <.0001 |
| PBd2 | PLTs × 109/L | 351 | 142-890 | 153 | 77-236 | <.0001 |
| PBPCd1 | PLTs × 1010 | 52 | 24-124 | 31 | 16-48 | <.0001 |
| PBPCd2 | PLTs × 1010 | 42 | 18-73 | 20 | 7.4-36 | <.0001 |
| Variable . | . | rhTPO 0.6-2.4 μg/kg and G-CSF 5 μg/kg, Twice Daily . | G-CSF 5 μg/kg, Twice Daily . | P Value . | ||
|---|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | |||
| PBd1 | PLTs × 109/L | 456 | 218-995 | 224 | 130-369 | <.0001 |
| PBd2 | PLTs × 109/L | 351 | 142-890 | 153 | 77-236 | <.0001 |
| PBPCd1 | PLTs × 1010 | 52 | 24-124 | 31 | 16-48 | <.0001 |
| PBPCd2 | PLTs × 1010 | 42 | 18-73 | 20 | 7.4-36 | <.0001 |
Abbreviations: PB, peripheral blood; d1, day 1; d2, day 2; PLTs, platelets.
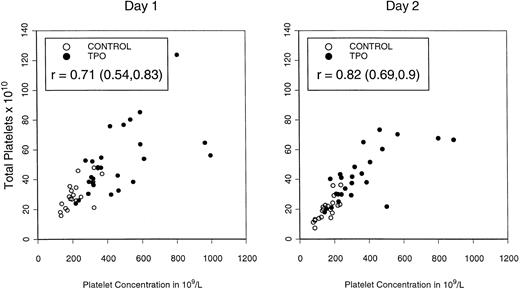
Table 4 depicts the mean platelet concentrations in peripheral blood on the first 2 days of apheresis (5 and 6 days after the last dose of rhTPO) and illustrates the total platelet content of the combined apheresis products sorted by the different doses/ schedules of rhTPO. Both peripheral blood platelet counts (P = .01) and total platelet content (P = .02) were highest in patients mobilized with three doses of rhTPO at 0.6 μg/kg, consistent with schedule- and dose-dependent effects. Platelet content of apheresis products was directly related to peripheral blood platelet concentration, as shown in Fig4.
Peripheral Blood Platelet Concentration and Platelet Content of PBPC Products by rhTPO Dose
| rhTPO Dose (μg/kg) . | Peripheral Blood Platelet Count (×109/L) . | Total Platelet Content/PBPC (×1010) . | ||
|---|---|---|---|---|
| Mean . | Range . | Mean . | Range . | |
| Arm A | ||||
| 0.6 × 1 | 214 | 180-270 | 51 | 42-66 |
| 1.2 × 1 | 288 | 254-332 | 92 | 85-96 |
| 2.4 × 1 | 328 | 236-482 | 66 | 51-83 |
| Arm B | ||||
| 0.3 × 3 | 407 | 268-544 | 123 | 78-158 |
| 0.6 × 3 | 635 | 358-942 | 124 | 59-194 |
| Arm C | ||||
| 0.6 × 2 | 309 | 252-381 | 90 | 63-119 |
| rhTPO Dose (μg/kg) . | Peripheral Blood Platelet Count (×109/L) . | Total Platelet Content/PBPC (×1010) . | ||
|---|---|---|---|---|
| Mean . | Range . | Mean . | Range . | |
| Arm A | ||||
| 0.6 × 1 | 214 | 180-270 | 51 | 42-66 |
| 1.2 × 1 | 288 | 254-332 | 92 | 85-96 |
| 2.4 × 1 | 328 | 236-482 | 66 | 51-83 |
| Arm B | ||||
| 0.3 × 3 | 407 | 268-544 | 123 | 78-158 |
| 0.6 × 3 | 635 | 358-942 | 124 | 59-194 |
| Arm C | ||||
| 0.6 × 2 | 309 | 252-381 | 90 | 63-119 |
Total platelet content of PBPC products in the PBPC products collected on the first and second days of apheresis in relationship to peripheral blood platelet concentration.
Total platelet content of PBPC products in the PBPC products collected on the first and second days of apheresis in relationship to peripheral blood platelet concentration.
Table 5 describes the percentage of CD41+cells within the CD34+ cell population: increased percentages of CD41+ cells were seen following administration of either single or repeated injections at the higher (1.2 to 2.4 μg/kg) rhTPO doses. We observed the highest percentage of CD41+ cells within the CD34+ population following sequential administration of rhTPO at 0.6 μg/kg (P= not significant [NS]).
Percentage of CD41+ Cells Within the CD34+ Low Side-Scatter Population From PBPC Products by rhTPO Dose Level
| rhTPO (μg/kg) . | PBPCd1 . | PBPCd2 . | ||||||
|---|---|---|---|---|---|---|---|---|
| CD41+ Cells (%) . | CD34+ 106/kg (%) . | CD41+ Cells (%) . | CD34+ 106/kg (%) . | |||||
| Median . | Range . | Median . | Range . | Median . | Range . | Median . | Range . | |
| Arm A | ||||||||
| 0.6 × 15-150 | 9 | 6.1 | 19 | 7.9 | ||||
| 1.2 × 1 | 48 | 29-74 | 3.4 | 2.8-6.1 | 64 | 14-79 | 5.9 | 2.4-7.1 |
| 2.4 × 1 | 58 | 19-65 | 3.9 | 1.7-6.8 | 67.5 | 10-68 | 5.0 | 2.2-7.2 |
| Arm B | ||||||||
| 0.3 × 3 | 22 | 9-64 | 4.9 | 1.7-6.6 | 51 | 18-78 | 5.3 | 1.8-7.6 |
| 0.6 × 3 | 68 | 23-89 | 8.1 | 3.4-18.1 | 70.5 | 50-97 | 4.6 | 3-7.9 |
| Arm C | ||||||||
| 0.6 × 2 | 24 | 4-31 | 2.5 | 2.2-4 | 26 | 9-40 | 2.7 | 1.1-4.2 |
| rhTPO (μg/kg) . | PBPCd1 . | PBPCd2 . | ||||||
|---|---|---|---|---|---|---|---|---|
| CD41+ Cells (%) . | CD34+ 106/kg (%) . | CD41+ Cells (%) . | CD34+ 106/kg (%) . | |||||
| Median . | Range . | Median . | Range . | Median . | Range . | Median . | Range . | |
| Arm A | ||||||||
| 0.6 × 15-150 | 9 | 6.1 | 19 | 7.9 | ||||
| 1.2 × 1 | 48 | 29-74 | 3.4 | 2.8-6.1 | 64 | 14-79 | 5.9 | 2.4-7.1 |
| 2.4 × 1 | 58 | 19-65 | 3.9 | 1.7-6.8 | 67.5 | 10-68 | 5.0 | 2.2-7.2 |
| Arm B | ||||||||
| 0.3 × 3 | 22 | 9-64 | 4.9 | 1.7-6.6 | 51 | 18-78 | 5.3 | 1.8-7.6 |
| 0.6 × 3 | 68 | 23-89 | 8.1 | 3.4-18.1 | 70.5 | 50-97 | 4.6 | 3-7.9 |
| Arm C | ||||||||
| 0.6 × 2 | 24 | 4-31 | 2.5 | 2.2-4 | 26 | 9-40 | 2.7 | 1.1-4.2 |
Available only from 1 patient at this dose level.
We analyzed the effect of different rhTPO doses and schedules on CFU-GM, CFU-GEMM, CFU-MK, and BFU-E formation from peripheral blood samples. We found a significant increase over baseline in the number of CFU-GM (5.15-fold mean increase; range, 0.9 to 31; 95% CI, 3.6 to 7.5), CFU-GEMM (2.5-fold mean increase; range, 0.4 to 15; 95% CI, 1.7 to 3.6), and CFU-MK (2.5-fold increase; range, 0.2 to 37; 95% CI, 1.4 to 4.4) and BFU-E (2.5-fold mean increase; range, 0.4 to 15; 95% CI, 0.8 to 3.7) following administration of rhTPO and G-CSF; for all CFUs, the largest number of colonies was observed on the days of apheresis (day 5 and 6 after the last dose of rhTPO). rhTPO dose and schedule had no significant effect on the rate of CFU formation from baseline to postmobilization. There was no statistically significant trend favoring any particular rhTPO dosing schedule when we assessed the amount of CFU-MK, BFU-E, CFU-GEMM, and CFU-GM in the stem-cell products, although the highest yields were seen following sequential administration of rhTPO 0.6 μg/kg, as illustrated in Table 6.
CFU-GM, CFU-GEMM, CFU-MK and BFU-E Growth by rhTPO Dose in Patients Mobilized by rhTPO in Combination With G-CSF
| rhTPO (μg/kg) . | CFU-GM6-150 . | CFU-GEMM6-150 . | CFU-MK6-150 . | BFU-E6-150 . | ||||
|---|---|---|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | Median . | Range . | Median . | Range . | |
| Arm A | ||||||||
| 0.6 × 1 | 0.9 | 0.7-2.5 | 0.11 | 0.07-0.11 | 0.9 | 0.9-1.2 | 1.6 | 1.2-3.0 |
| 1.2 × 1 | 2.5 | 0.9-6.5 | 0.22 | 0.09-0.68 | 0.8 | 0.3-2.0 | 2.9 | 1.5-8.2 |
| 2.4 × 1 | 1.2 | 0.7-1.6 | 0.12 | 0.03-0.26 | 0.6 | 0.4-1.6 | 2.2 | 1.3-3.5 |
| Arm B | ||||||||
| 0.3 × 3 | 2.2 | 0.6-2.8 | 0.26 | 0.05-0.42 | 0.9 | 0.4-1.6 | 3.0 | 1.1-3.7 |
| 0.6 × 3 | 2.9 | 0.8-10.1 | 0.67 | 0.05-1.04 | 1.8 | 0.6-3.5 | 4.7 | 1.7-10.3 |
| Arm C | ||||||||
| 0.6 × 2 | 2.5 | 1.1-5.2 | 0.58 | 0.12-1.46 | 0.8 | 0.6-2.6 | 4.3 | 2.3-5.0 |
| rhTPO (μg/kg) . | CFU-GM6-150 . | CFU-GEMM6-150 . | CFU-MK6-150 . | BFU-E6-150 . | ||||
|---|---|---|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | Median . | Range . | Median . | Range . | |
| Arm A | ||||||||
| 0.6 × 1 | 0.9 | 0.7-2.5 | 0.11 | 0.07-0.11 | 0.9 | 0.9-1.2 | 1.6 | 1.2-3.0 |
| 1.2 × 1 | 2.5 | 0.9-6.5 | 0.22 | 0.09-0.68 | 0.8 | 0.3-2.0 | 2.9 | 1.5-8.2 |
| 2.4 × 1 | 1.2 | 0.7-1.6 | 0.12 | 0.03-0.26 | 0.6 | 0.4-1.6 | 2.2 | 1.3-3.5 |
| Arm B | ||||||||
| 0.3 × 3 | 2.2 | 0.6-2.8 | 0.26 | 0.05-0.42 | 0.9 | 0.4-1.6 | 3.0 | 1.1-3.7 |
| 0.6 × 3 | 2.9 | 0.8-10.1 | 0.67 | 0.05-1.04 | 1.8 | 0.6-3.5 | 4.7 | 1.7-10.3 |
| Arm C | ||||||||
| 0.6 × 2 | 2.5 | 1.1-5.2 | 0.58 | 0.12-1.46 | 0.8 | 0.6-2.6 | 4.3 | 2.3-5.0 |
Units are expressed × 106/kg.
Toxicities.
We observed no allergic reactions associated with the administration of rhTPO. The superior vena cava occlusion in one patient assigned to receive a single injection of rhTPO 0.6 μg/kg preceded the administration of rhTPO and was most likely due to a previous catheter infection; her platelet counts remained within the normal range (234 × 109/L and 151 × 109/L) on the days of apheresis. She underwent high-dose therapy 6 months later without any thromboembolic complications.
We observed platelet count elevations greater than 600 × 109/L lasting for 24 hours in two of six patients (peaks of 995 × 109/L and 964 × 109/L) treated with rhTPO 0.6 μg/kg on days −3, −1, and 1 (arm B). Both peaks occurred on the first day of apheresis, and platelet counts decreased by the second day of apheresis. To assess further the possibility of excessive thrombocytosis following repeated administration, an additional rhTPO dose level was added, and six patients received three doses of rhTPO 0.3 μg/kg every other day. Platelet counts in these patients never reached 600 × 109/L.
Serially tested serum prior and subsequent to the administration of rhTPO and post–high-dose chemotherapy remained negative for the presence of any neutralizing antibodies in all subjects.
DISCUSSION
C-Mpl ligand, by expanding, and possibly by protecting, the progenitor-cell pools of megakaryocytic, myelomonocytic, and erythroid lineage, can prevent or ameliorate hematopoietic toxicity inflicted by cytotoxic therapy.15-17 Thrombopoietin may also accelerate hematopoietic recovery following chemotherapy. When administered after carboplatin and cyclophosphamide a truncated, pegylated form of the C-Mpl ligand, PEG-rHuMGDF (MGDF) enhanced platelet recovery in a dose-dependent fashion when given together with G-CSF. CD34+ cell subsets, in patients treated at higher doses of MGDF (0.3 to 5 μg/kg), were increased and platelet counts increased to greater than 1,000,000/μL in 11 of 31 patients.22 MGDF (0.03 to 5 μg/kg), when administered after carboplatin and paclitaxel, resulted in less pronounced and shorter median platelet nadirs compared with controls.23 Similarly, single injections of rhTPO can attenuate thrombocytopenia when administered before high-dose carboplatin; rhTPO 1.2 μg/kg given after high-dose carboplatin may prevent the need for platelet transfusions.24
Both the full-length and pegylated, truncated molecules are capable of expanding the progenitor pool. rhTPO increased the number of multilineage progenitors in the bone marrow while mobilizing CD34+ cells into the peripheral blood in cancer patients. MGDF in combination with a variety of growth factors has been shown to expand the number of megakaryocyte progenitors ex vivo.25 26
We have found a substantial increase in the CD34+progenitor-cell content of apheresis products when rhTPO, in combination with G-CSF, was administered several days before apheresis. While one can argue that prolonged administration of 10 μg/kg G-CSF alone for up to 9 days (rather than 3 days) before initiating apheresis may increase the CD34+ yield, there are data to the contrary. In a study conducted in normal volunteers, administration of 10 μg/kg G-CSF resulted in peak peripheral blood CD34+cell counts on day 4 in two of five healthy subjects, while a broader distribution of multiple peak CD34+ cell counts (between days 3 and 7) was observed in three other volunteers.27Since the peripheral blood CD34+ cell count predicts for progenitor-cell yield in the apheresis product, we applied such short G-CSF course before apheresis in our 20 control patients. Indeed, the highest CD34+ yields were procured during the first and second apheresis in 65% of patients; yields in the other 35% of patients were highest between days 6 and 8. No patient in the control group yielded ≥3 × 106 CD34+ cells/kg beyond the sixth day of administering G-CSF, making it unlikely that prolonged administration of G-CSF would lead to a decrease in the number of aphereses needed to reach our target.
Patients in the rhTPO– and G-CSF–mobilized group and the control group were comparable in their exposure to the number of prior chemotherapy regimens and cycles; there were, however, half as many patients in the rhTPO-treated cohort with a history of prior radiation exposure (15% v 30%). Since we procured the targeted numbers of CD34+ cells/kg in 61% of patients in the rhTPO-mobilized group versus in 10% of controls with only one apheresis procedure, it is likely that this sixfold difference was the consequence of exposure to rhTPO and not due to any pretreatment imbalance between the study cohort and controls. Indeed, when we analyzed our data after stratifying for exposure for prior radiation therapy, the beneficial effect of rhTPO on procurement and engraftment had been confirmed. Although the number of patients with prior radiation exposure was small, a trend suggesting favorable effects of rhTPO on CD34+ cell yield and hematopoietic recovery was observed in all groups. Should our findings hold true in larger cohorts of patients (assuming that the CD34+ yield can be calculated expeditiously) the majority of high-dose chemotherapy candidates would require only a single apheresis procedure following rhTPO/G-CSF mobilization.
A large increase in the numbers of CFU-GEMM, CFU-GM, CFU-MK, and BFU-E over baseline was seen in the peripheral blood 5 to 6 days following the last dose of rhTPO. The observed rise in peripheral blood platelet concentration during the same period was probably due to both accelerated proliferation/increased mitotic activity and terminal differentiation since receptors for thrombopoietin are present on the surface of both megakaryocytes and platelets.28-30
Although a single injection of rhTPO or MGDF effectively stimulated platelet production,16 we have found that sequential administration of rhTPO at 0.6 μg/kg for three doses resulted in the highest CD34+ cell yield and peripheral blood platelet concentration and generated the highest percentage of cells of the megakaryocyte lineage as documented by CD41+ marking within the CD34+ progenitor-cell subset and by measuring CFU-MK growth. This schedule also produced the highest PBPC platelet content. While these findings need further confirmation in larger numbers of patients, repeated stimulation of the rhTPO receptor (C-Mpl) may be the most efficient way to expand both multipotential and megakaryocytic progenitor-cell pools in addition to generating the largest number of platelets in the peripheral blood.31
Accelerated recovery of the erythroid lineage and decreased erythrocyte and platelet transfusion requirements in comparison to our control group, which was closely matched for clinical and pretreatment characteristics, suggested a clinically broader benefit of rhTPO in patients undergoing high-dose chemotherapy.32-35 The observed decrease in erythrocyte transfusion requirements are likely to be due to stimulatory effects of rhTPO on stem and early progenitor cells, although it is possible that these effects are due to partial sequence homology between thrombopoietin and erythropoietin.8 Enhanced CFU-MK formation and an increase in the number of CD41+ cells within the CD34+population possibly accelerated differentiation. The resulting increase in peripheral blood platelet concentration and platelet yield of PBPC products might have helped to reduce platelet transfusion requirements; indeed, one patient in the rhTPO-mobilized group required no platelet transfusions.
rhTPO can be used to generate large numbers of both progenitor cells and platelets. Investigators have tested the effects of MGDF in healthy donors and found substantially increased platelet yields in the platelet apheresis products.36 37 We observed a strong correlation between peripheral blood platelet concentration and platelet content of PBPC products; hence, confirmation of our most effective sequential schedule of delivery has clinical relevance. However, one has to be careful not to overstimulate CFU-MK production so as to avoid persistently elevated peripheral blood platelet counts. Platelet and PBPC apheresis procedures need to be timed based on careful daily monitoring of peripheral blood platelet counts to optimize platelet and PBPC yield and prevent iatrogenic thrombocytosis. In addition, although we have not seen development of neutralizing antibodies and secondary thrombocytopenia in any of our patients, vigilant follow-up evaluation is necessary.
In summary, administration of rhTPO in combination with G-CSF was safe and well tolerated. rhTPO mobilization in combination with G-CSF increased the CD34+ progenitor-cell and mononuclear yield, peripheral blood platelet count, and platelet content of PBPC products. We observed an increase in CFU-MK, CFU-GEMM, CFU-GM, and BFU-E formation and platelet generation 5 to 6 days following the last dose of rhTPO compared to baseline. Of the doses tested, rhTPO 0.6 μg/kg given three times every other day produced the highest CD34+ progenitor yield, platelet count, and the highest percentage of CD41+ cells within the CD34+progenitor-cell subset; the largest numbers of CFU-MK, CFU-GM, CFU-GEMM, and BFU-E were also associated with this schedule. rhTPO in combination with G-CSF decreased the number of aphereses and has the potential to necessitate only a single apheresis procedure in the majority of patients. rhTPO accelerated granulocyte and platelet recovery, and decreased erythrocyte transfusion requirements in breast cancer patients undergoing high-dose chemotherapy with stem-cell support versus a comparable and identically treated nonrandomized control group. A prospective, randomized study to further clarify the optimal dose, schedule, and benefits resulting from the administration of rhTPO and G-CSF versus G-CSF alone is planned.
ACKNOWLEDGMENT
We thank Judy Brent, Debra Rader, and Debbie Reardon for their assistance with the clinical aspects and data management of this study, Barbara Nowicki for the flow cytometry analysis, and Yolanda Tamayo and Melinda Kirk for secretarial assistance.
Supported by National Cancer Institute Grants No. CA33572, CA62505, and CA63265, Genentech Inc, and the Mrs Adelyn Luther and the Ladish Family Foundations, and the Levien Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
The most active dose and schedule of rhTPO and G-CSF that were identified in our study have been tested in a randomized, placebo-controlled, phase II study. Preliminary results suggest that this regimen has significant activity in mobilizing PBPC compared with G-CSF alone.38
Author notes
Address reprint requests to George Somlo, MD, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010-3000.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal