Abstract
The factor-independent Dami/HEL and Meg-01 and factor-dependent Mo7e leukemic cell lines were used as models to investigate JAK/STAT signal transduction pathways in leukemic cell proliferation. Although Dami/HEL and Meg-01 cell proliferation in vitro was independent of and unresponsive to exogenous cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), IL-6, thrombopoietin (TPO), and tumor necrosis factor- (TNF-), the growth of Mo7e cells was dependent on hematopoietic growth factors. When these cell lines were cultured in medium without cytokines, a constitutively activated STAT-like DNA-binding factor was detected in nuclear extracts from both Dami/HEL and Meg-01 cells. However, the STAT-like factor was not detectable in untreated Mo7e cells, but was activated transiently in Mo7e cells in response to cytokine treatments. The constitutively activated and cytokine-induced STAT-like DNA-binding factor in these three cell lines was identified as STAT5 by oligonucleotide competition gel mobility assays and by specific anti-STAT antibody gel supershift assays. Constitutive activation of JAK2 also was detected in the factor-independent cell lines, but not in Mo7e cells without cytokine exposure. Meg-01 cells express a p185 BCR/ABL oncogene, which may be responsible for the constitutive activation of STAT5. Dami/HEL cells do not express the BCR/ABL oncogene, but increased constitutive phosphorylation of Raf-1 oncoprotein was detected. In cytokine bioassays using growth factor-dependent Mo7e and TF-1 cells as targets, conditioned media from Dami/HEL and Meg-01 cells did not show stimulatory effects on cell proliferation. Our results indicate that the constitutive activation of JAK2/STAT5 correlates with the factor-independent growth of Dami/HEL and Meg-01 cells. The constitutive activation of JAK2/STAT5 in Dami/HEL cells is triggered by a mechanism other than autocrine cytokines or the BCR/ABL oncoprotein.
SURVIVAL, PROLIFERATION, and differentiation of normal hematopoietic cells are controlled by a number of growth factors that interact with members of the hematopoietic growth factor receptor family or with one of several receptor tyrosine kinases. In contrast, leukemic cells or leukemic cell lines frequently grow in a factor-independent manner. The mechanisms that result in the conversion of factor-dependent cells to factor-independent cells are poorly understood. Some investigators have reported that mutations of growth factor receptor(s) or signal transduction proteins cause constitutive activation of signal transduction pathways and induce growth factor-independent cell proliferation.1-4 Autocrine growth factor-stimulated growth of human leukemic cells or cell lines due to aberrant cytokine production by the malignant cells has been detected infrequently.5-7
The signal transduction pathways triggered by growth factor ligands binding to cell surface receptors are involved in the reversible phosphorylation of receptors, cytosolic nonreceptor kinases, and other cellular proteins. Among the many known signal transduction pathways, the JAK (Janus kinase)/STAT (signal transducer and activator of transcription) cascade and the MAPK (mitogen-activated protein kinase) cascade are activated after exposure of megakaryocytic cells to cytokines.8-12 Although the significance of cytokine-induced activation of these signaling pathways remains under active investigation, several reports have shown that phosphorylation and activation of JAK and STAT proteins appears to play a critical role in thrombopoietin-induced signal transduction.8,13,14 JAK proteins are members of a family of cytoplasmic kinases, which is known to have four members: TYK2, JAK1, JAK2, and JAK3. JAK2 is activated in normal megakaryocyte precursors and megakaryocytic cell lines by several specific hematopoietic cytokines and has been reported to be required for megakaryocytic cell proliferation.14,15 The STATs, of which at least seven distinct family members and several alternative RNA splicing-derived variants have been described so far, are cytosolic transcription factors, which are tyrosine-phosphorylated after ligand activation.16 They dimerize and translocate into the nucleus, where they bind to specific DNA sequences and activate gene transcription. Originally, STAT5, which was described as a transcription factor activated by prolactin and required for the induction of transcription of milk caseins, now has been identified as two closely related proteins, STAT5a and STAT5b.17 Recent studies indicate that they are widely expressed in many tissues.18 STAT5 is known to be activated by the JAKs and Bmx tyrosine kinases,19,20 and the activated STAT5 dimers recognize and bind to a specific palindromic DNA sequence, TTCNNNGAA.16 This oligonucleotide sequence is found not only in the β-casein promoter, but also in a number of other genes, such as those encoding interferon regulatory factor-1 (IRF-1), interferon consensus sequence binding protein (ICSBP), rat serine protease inhibitor, human interstitial cell adhesion molecule, high affinity immunoglobulin G receptor, Oncostatin M, and cytokine-induced SH2 protein (CIS).21-24 All of these findings suggest that STAT5 is important in a broad variety of cellular functions.
In this study, we have investigated the activation of the JAK2/STAT5 signal transduction pathway in growth factor-dependent and factor-independent megakaryocytic cell lines. We found that the JAK2/STAT5 signaling pathway is activated constitutively in factor-independent Dami/HEL and Meg-01 cells, but is transiently cytokine-induced in factor-dependent Mo7e cells. The constitutive activation of the JAK2/STAT5 signaling pathway appears to correlate with the factor-independence of Dami/HEL and Meg-01 cell lines. Although BCR/ABL oncogene transcripts are detected in Meg-01 cells, Dami/HEL cells do not express the BCR/ABL oncogene. Constitutive phosphorylation of Raf-1 occurs in Dami/HEL, but not Meg-01 cells. Thus, constitutive activation of JAK/STAT5 and proliferation may be triggered by different mechanisms in Dami/HEL and Meg-01 cells.
MATERIALS AND METHODS
Reagents.
Recombinant human thrombopoietin (TPO), interleukin-3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and epidermal growth factor (EGF) were purchased from PeproTech (Rocky Hill, NJ). Anti–IL-3 and anti-TPO neutralizing antibodies were purchased from R & D Systems (Minneapolis, MN). Antiphosphotyrosine antibody-agarose and anti-JAK2 antiserum were purchased from Upstate Biotechnology (Lake Placid, NY). Phosphotyrosine Western blotting kit (chemiluminescence) was purchased from Boehringer Mannheim Biochemicals (Indianapolis, IN). Antigoat IgG and antimouse IgG antibodies labeled with fluorescein isothiocyanate were purchased from Zymed (South San Francisco, CA). Anti-STAT1, -STAT2, -STAT3, -STAT4, -STAT5 -STAT6, and anti–Raf-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PhosphoPlus p44/p42 MAPK antibody kit containing antitotal and anti-T202/Y204 phosphorylated p42/p44 MAPK antibody were purchased from New England Biolabs (Beverly, MA). [Methyl-3H]-thymidine ([3H]-TdR; specific activity, 70 to 86 Ci/mmol) and [32P]-deoxyadenosine triphosphate (dATP) (specific activity, >3,000 μCi/mmol) were purchased from Amersham Life Science (Arlington Heights, IL).
Cell lines.
Human megakaryocytic leukemic cell line, Dami, originally described by Greenberg et al,25 was obtained from American Type Culture Collection (ATCC, Rockville, MD) and was maintained in Iscove’s Modified Dulbecco’s Medium (IMDM; GIBCO-BRL, Grand Island, NY) containing 10% horse serum. Recently, ATCC and DSMZ (German Collection of Microorganisms and Cell Cultures) have determined that all samples of the Dami cell line that were available for them to analyze were genetically and karyotypically identical to the human erythroleukemia cell line (HEL) described previously,26which has both erythroid and megakaryocytic characteristics.27 For clarity and for comparison to prior reports, we are designating this cell line as Dami/HEL in this report. Human megakaryoblastic leukemic cell line, Meg-01, originally described by Ogura et al,28 was obtained from ATCC and was maintained in RPMI 1640 medium (GIBCO-BRL) with 10% heat-inactivated fetal bovine serum (FBS). Human factor-dependent megakaryoblastic leukemic cell line, Mo7e, originally described by Avanzi et al,29 was obtained from Genetics Institute (Boston, MA) and was maintained in IMDM with 10% FBS, 1% L-glutamine and 5 ng/mL GM-CSF. All of the cells were incubated at 37°C in a fully humidified atmosphere with 5% CO2 in medium alone or in the presence of various concentrations of cytokines. In experiments to detect the effects of known cytokines or potential cytokines in Dami/HEL or Meg-01 conditioned media, Mo7e and TF-1 cells were prepared by washing cells three times with medium and starved for 18 hours in medium without growth factors before cytokine or conditioned medium treatments, as described by Dusanter-Fourt et al.30 Dami/HEL cells were cultured in IMDM without serum, but with 1X Nutridoma-HU (Boehringer Mannheim Biochemicals), and Meg-01 cells in RPMI 1640 with 1X Nutridoma-HU in experiments in which cytokine production and specific cytokine effects were being studied.
Effects of cytokines on cell growth.
To determine the proliferative response of cells to cytokines, DNA synthesis was measured by [3H]-thymidine (TdR) incorporation. The assays were performed in triplicate, using 4 × 105 cells for Mo7e and Meg-01 or 2 × 105cells for Dami/HEL cells. Mo7e cells in IMDM with 10% FBS and Dami/HEL and Meg-01 cells in medium with 1X Nutridoma-HU were cultured for 72 hours without or with cytokines. Subsequently, the cells were labeled with 2 μCi/mL of [3H]-TdR for an additional 4 hours. Incorporation of [3H]-TdR into newly synthesized DNA (counts per minute; cpm) was determined by liquid scintillation counting, according to a previously described protocol.31
For the analysis of cell ploidy, cytokine-starved Mo7e cells (1 × 106 cells/mL) were cultured in IMDM with 10% FBS plus growth factors. Dami/HEL and Meg-01 cells (1 × 106/mL) were cultured in medium with 1X Nutridoma-HU plus growth factors, at 37°C for 1 to 10 days. The treated cells were harvested on days 3, 5, 7, and 10. The DNA content of the cells was quantitated with a FACScan flow cytometer (Becton Dickinson, Rutherford, NJ), following a published protocol.32 Isolated human lymphocytes were used as a diploid control.
Immunoprecipitation and Western blotting.
Unstimulated or cytokine-stimulated cells were lysed in modified radioimmunoprotection assay (RIPA) buffer with 1% NP-40 and extracts were immunoprecipitated as described.30 The detergent-soluble proteins were incubated with antiphosphotyrosine agarose for 3 hours at 4°C with shaking. The immunoprecipitates were washed four times with modified RIPA buffer, lysed in sodium dodecyl sulfate (SDS) buffer and separated by 7.5% nonreducing SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were then transferred to nitrocellulose membrane and probed sequentially with rabbit anti-JAK2 antibody and antirabbit IgG antibody labeled with peroxidase. Phosphorylated JAK2 was visualized with enhanced chemiluminescence techniques according to the manufacturer’s recommended procedure. For the detection of phosphorylation of Raf-1, a band shift assay was used in Western blot using an anti–Raf-1 antibody (Santa Cruz Biotechnology) following a previously reported procedure.33 For detecting the phosphorylation of cellular proteins, cells treated with or without cytokines were lysed in SDS buffer, and 40 to 80 μg of cellular proteins was loaded in each sample lane, separated by 10.5% SDS-PAGE. For detecting total and phosphorylated MAPK, the SDS-PAGE–separated proteins were transferred to polyvinylide difluoride (PVDF) membranes and probed with antitotal and antiphosphorylated p42/p44 MAPK, with antiphosphorylated STAT3 antibody (New England Biolabs), or with antiphosphotyrosine antibody (Boehringer Mannheim).
Preparation of nuclear extracts and gel mobility shift assays.
Preparation of nuclear extracts and electrophoretic gel mobility shift assay (EMSA) was performed according to methods described previously.34 Briefly, equal amounts of nuclear proteins (5 to 10 μg) for each sample were incubated for 30 minutes at 30°C with 10,000 dpm of [32P]-labeled double-strand DNA fragment (IRF-1 GAS; 5′-GCCTGATTTCCCCGAAATGACGGCA) containing the interferon-γ activation site (GAS) that binds to interferon regulatory factor (IRF-1).35 IRF-1 GAS contains an identical sequence to that of Bovine mammary gland factor element (MGFe), TTCCCCGAA. For competition assays, unlabeled c-Fos intragenic regulatory element (FIRE; 5′-AGCGCCTCCCCGGCCGGGGAG), interferon-stimulated response element (ISG15 ISRE; 5′-GATCGGGAAAGGGAAACCGAAACTGAAGCC), and sis-inducible element (SIE, 5′-AGCTTCATTTCCCGTAAATCCCTAAGC) were used as potential DNA-protein binding competitors. The competition was performed by adding 50X molar excess of each unlabeled DNA fragment along with the [32P]-labeled IRF-1 GAS oligonucleotide probe. Unlabeled MGFe was also used as a specific competitor for STAT5 binding to IRF-1 GAS. For gel mobility supershift assays, nuclear extracts were coincubated with the indicated specific anti-STAT antibodies and the [32P]-labeled oligonucleotide probes. The DNA-protein complexes and unbound probe were separated electrophoretically on 5% native polyacrylamide gels in 0.5X TBE buffer (44.5 mmol/L Tris, pH 8.0, 1 mmol/L EDTA, and 44.5 mmol/L boric acid) for 3 hours at constant 140 V. The gels were fixed and dried, and the DNA-protein complexes were visualized by autoradiography at −70°C with Kodak X-OMAT film (Eastman Kodak, Rochester, NY) and a DuPont Cranex lightning-plus intensifying screen (DuPont, Wilmington, DE).
Reverse transcription-polymerase chain reaction (RT-PCR).
Cellular mRNA was prepared with RNAzol (Cinna/Biotecx Lab, Houston, TX) following the protocol suggested by the manufacturer. A commercially available primer set (TransPrimers, Calbiochem, CA) was used to assess the presence of BCR/ABL transcripts. RT-PCR for normal c-ABL also was performed to check RNA quality. A RNA PCR Core kit (GeneAmp; Perkin Elmer, Branchburg, NJ) was used to perform RT-PCR following the manufacturer’s protocol. A 1.8% agarose gel was used for electrophoretic analysis of the PCR products. RT-PCR was performed at least twice to confirm the findings.
Bioassays of stimulatory cytokines.
Factor-independent Dami/HEL and Meg-0l cells were examined for the capacity to produce autocrine cytokines. Mo7e and TF-1 cell lines, the survival of which depend on the presence of any of several growth factors, including GM-CSF, IL-3, IL-6, IL-9, TPO, steel factor/c-kit ligand, tumor necrosis factor-α (TNF-α), and EPO etc, were used as bioassay systems to detect possible stimulatory factors that might be produced by Dami/HEL or Meg-01 cells. Conditioned medium was prepared by collecting supernatants from 3-day cultures of Dami/HEL or Meg-01 cells grown in serum-free conditions. The conditioned medium was incubated with freshly prepared Mo7e or TF-1 cells for 72 hours and cell proliferation was determined by [3H]-TdR incorporation into DNA, following the procedure described in the prior section.
RESULTS
Dami/HEL and Meg-01 cells grow in a factor-independent manner.
To determine effects of cytokines on DNA synthesis of the proliferating cells, various concentrations of cytokines were incubated with Dami/HEL, Meg-01, and Mo7e cells for 3 days, and the cells were labeled subsequently with [3H]-TdR for the final 4 hours of culture. When 0 to 200 ng/mL of TPO was incubated with the cytokine-independent cell lines, Dami/HEL and Meg-01, no significant stimulation of their basal [3H]-TdR incorporation was observed (Fig 1, Dami/HEL and Meg-01). The cells also were tested for their response to other cytokines, including GM-CSF, IL-3, IL-6 and TNF-α, and neither Dami/HEL nor Meg-01 cell proliferation was affected by any of these cytokines (data not shown). Addition of anti–IL-3 or anti-TPO neutralizing antibodies did not inhibit [3H]-TdR incorporation or cell proliferation (data not shown). However, when factor-dependent Mo7e cells were incubated with various concentrations of TPO, incorporation of [3H]-TdR was stimulated significantly, in a dose-dependent manner (Fig 1, Mo7e). At the concentration of 50 ng/mL, TPO regularly stimulated the incorporation of [3H]-thymidine 4.5-fold over that of control Mo7e cells at 3 days (Fig 1, Mo7e). IL-3, GM-CSF, IL-6, and TNF-α also stimulated Mo7e cell proliferation (data not shown).
Effects of TPO on DNA synthesis in human leukemic cell lines. Dami/HEL, Meg-01, and Mo7e cells were incubated with the indicated concentrations of TPO for 72 hours and labeled with 2 μCi/mL [3H]-TdR for 4 hours. The cpm of [3H]-TdR incorporation into newly synthesized DNA was determined by counting the radioactivity from triplicate samples and expressed as the mean cpm. This figure is representative of the data from three separate experiments.
Effects of TPO on DNA synthesis in human leukemic cell lines. Dami/HEL, Meg-01, and Mo7e cells were incubated with the indicated concentrations of TPO for 72 hours and labeled with 2 μCi/mL [3H]-TdR for 4 hours. The cpm of [3H]-TdR incorporation into newly synthesized DNA was determined by counting the radioactivity from triplicate samples and expressed as the mean cpm. This figure is representative of the data from three separate experiments.
To investigate effects of cytokines on the maturation of these megakaryocytic leukemic cell lines, the cells were fixed and stained with propidium iodide, and cell ploidy was analyzed by a FACscan flow cytometer. In the untreated Dami/HEL, Meg-01, or Mo7e cells, 95% of cells were diploid. When the cells were incubated with 100 to 400 ng/mL of TPO for 1 to 10 days, no significant increase of cell ploidy or morphologic signs of differentiation were observed in any of these cell lines (data not shown).
STAT-like DNA-binding factors are activated constitutively in Dami/HEL and Meg-01 cells.
The fact that the Dami/HEL and Meg-01 cell lines grow in a factor-independent manner led us to investigate whether an aberrant signal transduction pathway might be involved. Because JAK and STAT proteins have been reported to have a role in cytokine-induced proliferation of megakaryocytic and leukemic cells,8 we analyzed STAT DNA-binding factor activation by EMSA with a [32P]-labeled oligonucleotide containing the IRF-1 GAS consensus STAT binding site, as the probe. DNA-binding protein(s) were detected in the nuclear extracts from Dami/HEL and Meg-01 cells in the absence of cytokine exposure (Fig 2). Addition of as much as 400 ng/mL of TPO or 40 ng/mL IL-3 did not have any further effect on the constitutive DNA-binding protein activity in these growth factor-independent cell lines (Fig 2). Exposure of these cells to GM-CSF, IL-6, erythropoietin (EPO), or TNF-α also did not result in significant enhancing or inhibitory effects on the constitutive DNA-binding factor activity (data not shown). In contrast, no STAT-like DNA-binding factor was detectable in untreated control Mo7e cells; however, addition of 400 ng/mL TPO (Fig 2) or 40 ng/mL of GM-CSF or IL-3 (Fig 3) significantly induced a STAT-like transcriptional factor binding to IRF-1 GAS.
Constitutive activation of a STAT-like DNA-binding factor in Dami/HEL and Meg-01 cells. Nuclear extracts from each cell line cultured in the absence of cytokine (CTL) or in the presence of as much as 400 ng/mL TPO or 40 ng/mL IL-3, were incubated with [32P]-labeled, double-stranded IRF-1 GAS oligonucleotide. The DNA-binding complex and unbound probes were separated electrophoretically on 5% nondenaturing polyacrylamide gels. The autoradiograph shows the STAT-like DNA-binding factor (DBF) and the nonspecific bands (NS).
Constitutive activation of a STAT-like DNA-binding factor in Dami/HEL and Meg-01 cells. Nuclear extracts from each cell line cultured in the absence of cytokine (CTL) or in the presence of as much as 400 ng/mL TPO or 40 ng/mL IL-3, were incubated with [32P]-labeled, double-stranded IRF-1 GAS oligonucleotide. The DNA-binding complex and unbound probes were separated electrophoretically on 5% nondenaturing polyacrylamide gels. The autoradiograph shows the STAT-like DNA-binding factor (DBF) and the nonspecific bands (NS).
Effects of cytokines on activation of a STAT-like DNA-binding factor in Mo7e cells. Nuclear extracts from Mo7e cells grown in the absence of cytokine (CTL), or in the presence of 40 ng/mL EPO, 400 ng/mL TPO, 40 ng/mL GM-CSF or 40 ng/mL IL-3, were incubated with [32P]-labeled IRF-1 GAS oligonucleotide. The STAT-like DNA-binding complex and unbound probe were separated on 5% native polyacrylamide gel. The autoradiograph shows the DBF, NS, and free probes (P).
Effects of cytokines on activation of a STAT-like DNA-binding factor in Mo7e cells. Nuclear extracts from Mo7e cells grown in the absence of cytokine (CTL), or in the presence of 40 ng/mL EPO, 400 ng/mL TPO, 40 ng/mL GM-CSF or 40 ng/mL IL-3, were incubated with [32P]-labeled IRF-1 GAS oligonucleotide. The STAT-like DNA-binding complex and unbound probe were separated on 5% native polyacrylamide gel. The autoradiograph shows the DBF, NS, and free probes (P).
The constitutive and cytokine-activated STAT-like factor binds specifically to DNA containing the IRF-1 GAS sequence.
The question remained whether the constitutively activated STAT-like DNA-binding factor in Dami/HEL or Meg-01 cells is the same one as that activated by cytokines in Mo7e cells. We performed experiments to identify the specific STAT protein activated in these leukemic cell lines. First, we used a set of oligonucleotides to attempt to inhibit competitively the binding of the STAT-like factor to [32P]-labeled IRF-1 GAS. When Dami/HEL nuclear extracts were coincubated with the labeled probe plus a 50-fold molar excess of unlabeled FIRE, ISRE, or SIE oligonucleotides, which do not contain TTCNNNGAA sequence, no competitive inhibition of the formation of DNA-protein complexes was observed (Fig 4, Dami). However, a 50-fold molar excess of the unlabeled MGFe, which contains the same TTCCCCGAA STAT-binding sequence as IRF-1 GAS, completely abolished the formation of the labeled DNA-protein complexes. Similar results were found with Meg-01 cells (data not shown).
Specificity of binding of STAT-like DNA-binding factors in Dami/HEL and Mo7e cells to DNA containing the IRF-1 GAS sequence. (A) Dami/HEL cell nuclear extracts were incubated with [32P]-labeled IRF-I GAS probe (lane 1) or with [32P]-labeled probe plus a 50-fold excess of unlabeled oligonucleotide FIRE, ISRE, SIE, or MGFe, respectively (lanes 2 to 5). The DNA-binding complexes were separated in a 5% nondenaturing polyacrylamide gel. (B) Mo7e cell nuclear extracts from untreated control cells (lane 1) or cells treated with 400 ng/mL TPO (lane 2) were incubated with [32P]-labeled IRF-1 GAS probe or with [32P]-labeled probe plus a 50-fold excess of unlabeled oligonucleotide FIRE, ISRE, SIE, or MGFe, respectively (lanes 3 to 6). The DNA-binding complexes were separated in a 5% nondenaturing polyacrylamide gel. The autoradiograph shows the STAT-like DBF and NS.
Specificity of binding of STAT-like DNA-binding factors in Dami/HEL and Mo7e cells to DNA containing the IRF-1 GAS sequence. (A) Dami/HEL cell nuclear extracts were incubated with [32P]-labeled IRF-I GAS probe (lane 1) or with [32P]-labeled probe plus a 50-fold excess of unlabeled oligonucleotide FIRE, ISRE, SIE, or MGFe, respectively (lanes 2 to 5). The DNA-binding complexes were separated in a 5% nondenaturing polyacrylamide gel. (B) Mo7e cell nuclear extracts from untreated control cells (lane 1) or cells treated with 400 ng/mL TPO (lane 2) were incubated with [32P]-labeled IRF-1 GAS probe or with [32P]-labeled probe plus a 50-fold excess of unlabeled oligonucleotide FIRE, ISRE, SIE, or MGFe, respectively (lanes 3 to 6). The DNA-binding complexes were separated in a 5% nondenaturing polyacrylamide gel. The autoradiograph shows the STAT-like DBF and NS.
We then investigated whether the cytokine-induced DNA-binding factor in Mo7e cells has similar features as that in Dami/HEL cells. When the nuclear extracts from TPO-treated Mo7e cells were incubated with [32P]-labeled IRF-1 GAS probe or the [32P]-labeled probe plus a 50-fold molar excess of unlabeled FIRE, ISRE, SIE, or MGFe oligonucleotides, the cytokine-induced DNA-binding factor in Mo7e cells had the same features as the constitutively activated STAT-like factor in Dami/HEL cells. Both of the factors were able to bind to the IRF-1 GAS probe, and their binding activity could be abolished completely by unlabeled MGFe, but not by oligonucleotides that do not contain the TTCNNNGAA sequence.
The GAS-binding factor activated constitutively in Dami/HEL and Meg-01 cells and induced transiently by cytokine in Mo7e cells is STAT5.
Because different STAT transcription factors may share the same binding site to some of the DNA probes, the data from the gel mobility shift assays with IRF-1 GAS as the probe may not confirm specifically what STAT protein is activated, although the pattern is most consistent with activated STAT5. To identify the specific DNA-binding factor(s), gel mobility supershift assays with antibodies to specific STAT proteins were used to examine the type of STAT(s) involved in the DNA-protein complexes. When anti-STAT5 antiserum was used, supershift of the DNA-protein complexes was observed with nuclear extracts from Dami/HEL and Meg-01 cells cultured in medium without cytokines or from TPO-treated Mo7e cells (Fig 5), but not in untreated Mo7e cells (data not shown). When anti-STAT3 antiserum was used, no supershift of DNA-protein complexes occurred in TPO-treated Mo7e or unstimulated Dami/HEL or Meg-01 cells (Fig 5). However, anti-STAT1, anti-STAT3, and anti-STAT5 antibodies induced supershift of the DNA-protein complex in nuclear extracts from EGF-treated SK-BR-3 cells (Fig 5), in which STAT1, STAT3, and STAT5 have been shown previously to be activated.36 Reactions of the DNA-protein complexes with anti-STAT1, anti-STAT2, anti-STAT4 and anti-STAT6 antibodies also were investigated, and no supershifted DNA-protein complexes were observed (data not shown). These results indicate that the predominant or exclusive constitutively activated (in Dami/HEL and Meg-01 cells) or TPO– or GM-CSF–induced (in Mo7e cells) STAT DNA-binding factor in these megakaryocytic leukemic cell lines is STAT5.
Identification of the constitutively activated DNA-binding factor in Dami/HEL and Meg-01 cells and the cytokine-induced DNA-binding factor in Mo7e cells by gel electrophoretic mobility supershift assays. Nuclear extracts prepared from Dami/HEL cells without cytokine exposure were incubated with [32P]-labeled IRF-1 GAS plus anti-STAT3 or anti-STAT5 antibodies (lanes 1 and 2). Nuclear extracts from Meg-01 cells without cytokine exposure were incubated with the [32P]-labeled probe plus anti-STAT3 or anti-STAT5 antibodies (lanes 3 and 4). Nuclear extracts from Mo7e cells without cytokine exposure (lane 5) or treated with 400 ng/mL TPO (lanes 6 to 8), were incubated with [32P]-labeled IRF-1 GAS probe (lanes 5 and 6), or with [32P]-labeled probe plus anti-STAT3 or anti-STAT5 antibodies (lanes 7 and 8). Nuclear extracts, from SK-BR–3 cells incubated without cytokine exposure (lane 9) or with 20 ng/mL EGF (lanes 10 to 14), were incubated with [32P]-labeled IRF-1 GAS probe alone (lanes 9, 10, and 13), or with [32P]-labeled probe plus anti-STAT1, anti-STAT3, or anti-STAT5 antibodies (lanes 11, 12, and 14). The EGF-treated SK-BR–3 cells, which were reported by our group to have activated STAT1, STAT3, and STAT5,36 were used to show the antibody-induced supershift of STAT1, STAT3, and STAT5. The autoradiograph shows the STAT-like DBF, NS, and free IRF-1 GAS probe (P). The arrowheads indicate the supershifted complexes. These results are representative of three separate experiments.
Identification of the constitutively activated DNA-binding factor in Dami/HEL and Meg-01 cells and the cytokine-induced DNA-binding factor in Mo7e cells by gel electrophoretic mobility supershift assays. Nuclear extracts prepared from Dami/HEL cells without cytokine exposure were incubated with [32P]-labeled IRF-1 GAS plus anti-STAT3 or anti-STAT5 antibodies (lanes 1 and 2). Nuclear extracts from Meg-01 cells without cytokine exposure were incubated with the [32P]-labeled probe plus anti-STAT3 or anti-STAT5 antibodies (lanes 3 and 4). Nuclear extracts from Mo7e cells without cytokine exposure (lane 5) or treated with 400 ng/mL TPO (lanes 6 to 8), were incubated with [32P]-labeled IRF-1 GAS probe (lanes 5 and 6), or with [32P]-labeled probe plus anti-STAT3 or anti-STAT5 antibodies (lanes 7 and 8). Nuclear extracts, from SK-BR–3 cells incubated without cytokine exposure (lane 9) or with 20 ng/mL EGF (lanes 10 to 14), were incubated with [32P]-labeled IRF-1 GAS probe alone (lanes 9, 10, and 13), or with [32P]-labeled probe plus anti-STAT1, anti-STAT3, or anti-STAT5 antibodies (lanes 11, 12, and 14). The EGF-treated SK-BR–3 cells, which were reported by our group to have activated STAT1, STAT3, and STAT5,36 were used to show the antibody-induced supershift of STAT1, STAT3, and STAT5. The autoradiograph shows the STAT-like DBF, NS, and free IRF-1 GAS probe (P). The arrowheads indicate the supershifted complexes. These results are representative of three separate experiments.
JAK2 is activated constitutively in Dami/HEL and Meg-01 cells.
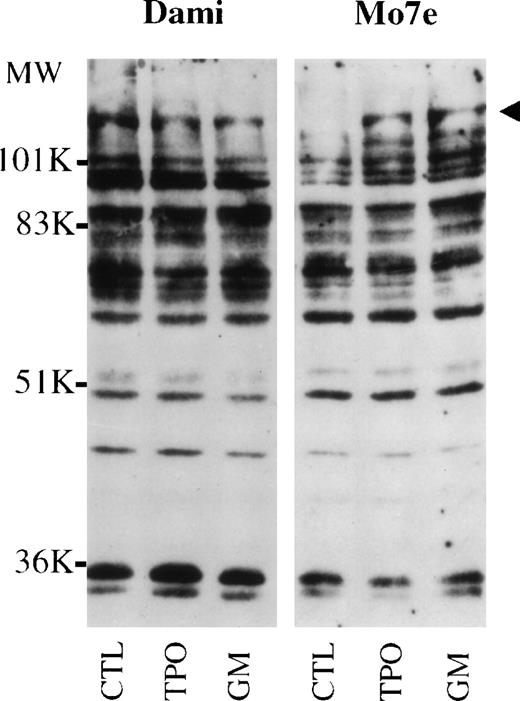
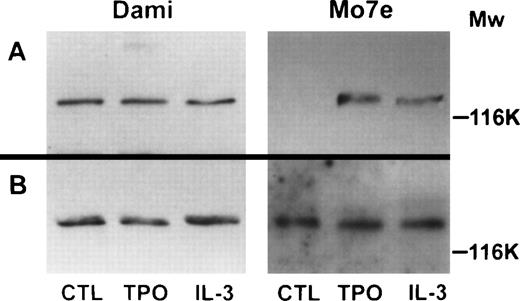
To examine protein phosphorylation patterns and to identify the mechanism for constitutive STAT5 activation, Dami/HEL and Meg-01 cells were incubated in medium without serum, and Mo7e cells were deprived of growth factors in medium with 10% FBS for 18 hours, and cells were then exposed to TPO or GM-CSF for 10 minutes. Two growth factor-induced tyrosine-phosphorylated protein bands of approximately 130 kD and 116 kD were observed in Mo7e cells (Fig 6, lanes 2 and 3, Mo7e). When Dami/HEL cells were examined, there was no detectable difference in the protein phosphorylation patterns in control Dami/HEL cells or cells treated with cytokines (Fig 6, lanes 1 through 3, Dami). Interestingly, the pattern of tyrosine phosphorylation in untreated Dami/HEL cells was very similar to that of the cytokine-treated Mo7e cells (compare Fig 6, lane 1, Dami and lanes 2 and 3, Mo7e). The results show that phosphorylated protein bands, which are undetectable or barely detectable in control Mo7e cells, but are induced by TPO or GM-CSF, are phosphorylated constitutively in untreated control Meg-01 (data not shown) and Dami/HEL cells. Because it has been reported that JAK2 is activated by TPO or other cytokines in megakaryocytic cells,14 15 we examined the tyrosine phosphorylation of JAK2 in Dami/HEL, Meg-01, and Mo7e cells. Lysates from cells treated with or without cytokines were immunoprecipitated with antiphosphotyrosine agarose. The results show that JAK2 is phosphorylated constitutively in control Dami/HEL (Fig 7, lane 1, Dami) and Meg-01 cells (data not shown). However, JAK2 was unphosphorylated in control Mo7e cells (Fig 7, lane 1, Mo7e) and phosphorylated in cytokine-treated Mo7e cells (Fig 7, lanes 2 and 3, Mo7e). No further effect on tyrosine-phosphorylation of JAK2 was observed when Dami/HEL cells were exposed to TPO or IL-3 (Fig 7, lanes 2 and 3, Dami). These results show that JAK2 is phosphorylated constitutively in the two growth factor-independent megakaryocytic leukemic cell lines and that its phosphorylation is induced by TPO and IL-3 in factor-dependent Mo7e cells.
Tyrosine phosphorylation of cellular proteins in Dami/HEL and Mo7e cells. Cell lysates from Dami/HEL cells (A) and Mo7e cells (B) were cultured in medium without cytokines (CTL), or with 400 ng/mL TPO or 40 ng/mL GM-CSF (GM). The cellular proteins were lysed in SDS buffer, separated on SDS-PAGE, transferred onto PVDF membrane, and probed with antiphosphotyrosine antibodies. The arrowheads indicate cytokine-induced tyrosine-phosphorylated protein bands in Mo7e cells, which are constitutively expressed in unstimulated control Dami/HEL cells.
Tyrosine phosphorylation of cellular proteins in Dami/HEL and Mo7e cells. Cell lysates from Dami/HEL cells (A) and Mo7e cells (B) were cultured in medium without cytokines (CTL), or with 400 ng/mL TPO or 40 ng/mL GM-CSF (GM). The cellular proteins were lysed in SDS buffer, separated on SDS-PAGE, transferred onto PVDF membrane, and probed with antiphosphotyrosine antibodies. The arrowheads indicate cytokine-induced tyrosine-phosphorylated protein bands in Mo7e cells, which are constitutively expressed in unstimulated control Dami/HEL cells.
Phosphorylation of JAK2 in Dami/HEL and Mo7e cells. (A) Cellular proteins (500 μg/mL) from untreated Dami/HEL or Mo7e cells (CTL), or from cells treated with 400 ng/mL TPO or 40 ng/mL IL-3, were precipitated with antiphosphotyrosine agarose. The immunoprecipitates were subjected to SDS-PAGE, transferred to PVDF membrane, and probed with antihuman JAK2 antibody to detect tyrosine-phosphorylated JAK2. (B) Cellular proteins (20 μg) from untreated Dami/HEL or Mo7e cells (CTL), or cells treated with 400 ng/mL TPO or 40 ng/mL IL-3, were subjected directly to 7.5% SDS-PAGE and the blotted PVDF membrane was probed with antihuman JAK2 antiserum to detect total JAK2 protein as a control. Molecular mass markers are indicated in kilodaltons.
Phosphorylation of JAK2 in Dami/HEL and Mo7e cells. (A) Cellular proteins (500 μg/mL) from untreated Dami/HEL or Mo7e cells (CTL), or from cells treated with 400 ng/mL TPO or 40 ng/mL IL-3, were precipitated with antiphosphotyrosine agarose. The immunoprecipitates were subjected to SDS-PAGE, transferred to PVDF membrane, and probed with antihuman JAK2 antibody to detect tyrosine-phosphorylated JAK2. (B) Cellular proteins (20 μg) from untreated Dami/HEL or Mo7e cells (CTL), or cells treated with 400 ng/mL TPO or 40 ng/mL IL-3, were subjected directly to 7.5% SDS-PAGE and the blotted PVDF membrane was probed with antihuman JAK2 antiserum to detect total JAK2 protein as a control. Molecular mass markers are indicated in kilodaltons.
Proto-oncogene Raf-1 is constitutively phosphorylated in Dami/HEL cells.
To examine the phosphorylation of Raf-1, Western blot with a specific anti–Raf-1 antibody was used to examine the band shift of Raf-1. The band shift of Raf-1 in Western blot has been identified to be induced by the phosphorylation of Raf-1.33,37 When cell lysates were prepared from cells treated with or without GM-CSF, and equal amounts of cellular proteins were subjected to SDS-PAGE, band shift of Raf-1 was detected by Western blot in Mo7e cells in response to GM-CSF treatment (Fig 8). In the cell lysates prepared from Dami/HEL and U937 cells without cytokine exposure, phosphorylation (band shift) of Raf-1 was detected (Fig 8, Dami and U937, CTL). Treating Dami/HEL cells with GM-CSF had no further effects on the phosphorylation of Raf-1 (Fig 8, Dami, GM). In Meg-01 cells without GM-CSF exposure, no significant band shift was observed (Fig 8, Meg-01, CTL). GM-CSF treatment also failed to induce any significant phosphorylation of Raf-1 in Meg-01 cells (Fig 8, Meg-01, GM). U937 cells, which have been shown previously to have increased phosphorylation of Raf-1 in cells without cytokine exposure,37 were used as the control to locate the phosphorylated Raf-1.
Phosphorylation of proto-oncogene Raf-1 and activation of MEK in Mo7e, Meg-01, and Dami/HEL cells. (A) Phosphorylation of Raf-1 was examined by band shift on Western blot. Equal amounts of cellular proteins (40 μg) from Mo7e, Dami/HEL, Meg-01, and U937 cells incubated with no cytokine (lanes marked as C) or 40 ng/mL GM-CSF (lanes, GM) were subjected directly to 10.5% SDS-PAGE. The separated proteins were transferred to a PVDF membrane and probed with anti–Raf-1 antibody. The arrow indicates the location of unphosphorylated Raf-1 and the arrowhead indicates the band shift of phosphorylated Raf-1. (B) Activation of MEK was detected by the kinase-induced phosphorylation of a kinase-defective p42mapk (K52R) labeled with [32P]-ATP. Equal amounts of cellular proteins (30 μg) from Mo7e, Meg-01, and Dami/HEL cells incubated with no cytokine (lanes marked as C) or 40 ng/mL GM-CSF (lanes, GM) were used to detect MEK activity. Phosphorylation of p42 is used as the readout to detect MEK activity.
Phosphorylation of proto-oncogene Raf-1 and activation of MEK in Mo7e, Meg-01, and Dami/HEL cells. (A) Phosphorylation of Raf-1 was examined by band shift on Western blot. Equal amounts of cellular proteins (40 μg) from Mo7e, Dami/HEL, Meg-01, and U937 cells incubated with no cytokine (lanes marked as C) or 40 ng/mL GM-CSF (lanes, GM) were subjected directly to 10.5% SDS-PAGE. The separated proteins were transferred to a PVDF membrane and probed with anti–Raf-1 antibody. The arrow indicates the location of unphosphorylated Raf-1 and the arrowhead indicates the band shift of phosphorylated Raf-1. (B) Activation of MEK was detected by the kinase-induced phosphorylation of a kinase-defective p42mapk (K52R) labeled with [32P]-ATP. Equal amounts of cellular proteins (30 μg) from Mo7e, Meg-01, and Dami/HEL cells incubated with no cytokine (lanes marked as C) or 40 ng/mL GM-CSF (lanes, GM) were used to detect MEK activity. Phosphorylation of p42 is used as the readout to detect MEK activity.
No increased activity of MAP kinase kinase (MEK) is detected in Meg-01 or Dami/HEL cells.
It has been reported that TPO and other cytokines stimulate both the MAPK and JAK/STAT signaling pathways in megakaryocytic cells. Because we observed the constitutive activation of the JAK2/STAT5 signaling pathway in Dami/HEL and Meg-01 cells and the increased Raf-1 phosphorylation in Dami/HEL cells, it was of interest to see whether the MEK also was activated constitutively in these cells. Our data show that all of the cells had a low level of MEK functional activity and no difference of the MEK activity could be distinguished among Mo7e, Meg-01, and Dami cells without any cytokine exposure (Fig 8B, lanes 1, 3, and 5). However, a significant increased level of MEK activity was detected transiently in all three cell lines after treatment with GM-CSF (lanes 2, 4, and 6), IL-3, TPO, or IL-6 (data not shown). Furthermore, when phosphorylation of p44/p42 MAPK was investigated in control and cytokine-treated cells to confirm the cytokine-induced MEK activation, the data showed that there was minimal phosphorylated MAPK detectable in the absence of any cytokine, and strong cytokine-induced phosphorylation of MAPK was observed in all three cell lines (data not shown).
No BCR/ABL oncogene product is detected in Mo7e or Dami/HEL cells.
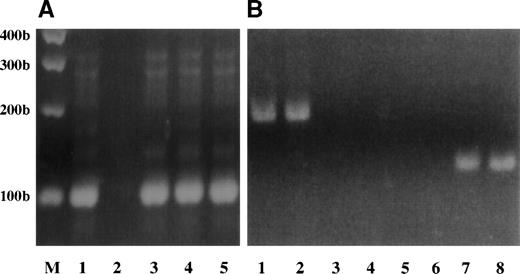
There are several reports showing that the BCR/ABL chimeric oncoprotein activates STATs and usually JAKs and induces cytokine-independent growth in cells transfected with the BCR/ABL oncogene.38-40Our data show no BCR/ABL transcripts detectable in factor-dependent Mo7e cells with or without cytokine exposure (Fig 9A, lanes 3 and 4). Although constitutive activation of STAT5 and phosphorylation of JAK2 are observed in Dami/HEL cells without cytokine exposure, no BCR/ABL transcript was detected in this cell line, either without (Fig 9, lane 5) or with (Fig 9, lane 6) TPO treatment. BCR/ABL transcripts were observed in Meg-01 and K562 cell lines (Fig 9, lanes 1, 2, 7, and 8), both of which are cell lines that were derived from patients with chronic myelogenous leukemia (CML). In Meg-01 cells, a smaller BCR/ABL PCR product was detected (consistent with p185 BCR/ABL), compared with that of K562 (consistent with p210 BCR/ABL) (compare Fig 9, lanes 7, 8 and 1, 2).
RT-PCR analysis of BCR/ABL and C-ABL transcripts in Dami/HEL, Meg-01, and K562 leukemic cell lines. (A) C-ABL expression was analyzed by RT-PCR using 1 μg total RNA from K562 (lane 1), Mo7e (lane 3), Dami/HEL (lane 4), and Meg-01 (lane 5) cell lines. RT-PCR was performed without cellular RNA as a negative control (lane 2). (B) BCR/ABL expression was analyzed by RT-PCR using 0.01 μg or 1 μg total RNA from K562 cells as positive controls (lanes 1 and 2), or using 1 μg of total RNA from Mo7e cells treated without (lane 3) or with 100 ng/mL TPO (lane 4), from Dami/HEL cells treated without (lane 5) or with 100 ng/mL TPO (lane 6), and from Meg-01 cells treated without (lane 7) or with 100 ng/mL TPO (lane 8). The sizes of PCR products are indicated in base pairs.
RT-PCR analysis of BCR/ABL and C-ABL transcripts in Dami/HEL, Meg-01, and K562 leukemic cell lines. (A) C-ABL expression was analyzed by RT-PCR using 1 μg total RNA from K562 (lane 1), Mo7e (lane 3), Dami/HEL (lane 4), and Meg-01 (lane 5) cell lines. RT-PCR was performed without cellular RNA as a negative control (lane 2). (B) BCR/ABL expression was analyzed by RT-PCR using 0.01 μg or 1 μg total RNA from K562 cells as positive controls (lanes 1 and 2), or using 1 μg of total RNA from Mo7e cells treated without (lane 3) or with 100 ng/mL TPO (lane 4), from Dami/HEL cells treated without (lane 5) or with 100 ng/mL TPO (lane 6), and from Meg-01 cells treated without (lane 7) or with 100 ng/mL TPO (lane 8). The sizes of PCR products are indicated in base pairs.
No detectable stimulatory factors are secreted by Dami/HEL or Meg-01 cells.
To investigate further the mechanism for the constitutive activation of JAK2 and STAT5 in Dami/HEL and Meg-01 cells, we performed experiments to examine whether the cytokine-independent Dami/HEL and Meg-01 cell lines acquired the capacity to produce cytokines that may result in autocrine stimulation of cell proliferation and JAK2/STAT5 activation. The Mo7e cell line, the survival of which is dependent on the presence of at least one of several growth factors, including GM-CSF, IL-3, IL-6, IL-9, steel factor/C-kit ligand, TNF-α, and TPO, was used as a bioassay system to detect the presence of possible stimulatory factor(s) released into the conditioned medium of Dami/HEL or Meg-01 cells. The results show that the conditioned medium from either Dami/HEL or Meg-01 cells did not stimulate [3H]-TdR incorporation in freshly prepared Mo7e cells, but addition of 2 ng/mL GM-CSF significantly increased the [3H]-TdR incorporation (Fig 10). TF-1 cells, which grow in a growth factor-dependent manner and have EPO receptors and are EPO-responsive, also were used as a bioassay to examine the conditioned media from Dami and Meg-01 cells, and no significant effects of the conditional medium from Dami/HEL or Meg-01 cells on [3H]-TdR incorporation were observed (data not shown).
Bioassays of possible stimulatory factors in Dami/HEL and Meg-01 conditioned medium. Mo7e cells, pretreated for 18 hours in medium without cytokines, were incubated for 72 hours with control or conditioned medium from cultures of Dami/HEL cells (A) or Meg-01 cells (B), using either (1) culture medium not exposed to Dami/HEL or Meg-01 cells (CTL), (2) conditioned medium collected from the 3-day culture of Dami/HEL or Meg-01 cells (CM), or (3) conditioned medium plus 2 ng/mL GM-CSF (CM + GM-CSF). The cells then were labeled with [3H]-TdR for an additional 4 hours. CPM of [3H]-TdR incorporation was determined from triplicate samples and expressed as the mean. These results are representative of three separate experiments.
Bioassays of possible stimulatory factors in Dami/HEL and Meg-01 conditioned medium. Mo7e cells, pretreated for 18 hours in medium without cytokines, were incubated for 72 hours with control or conditioned medium from cultures of Dami/HEL cells (A) or Meg-01 cells (B), using either (1) culture medium not exposed to Dami/HEL or Meg-01 cells (CTL), (2) conditioned medium collected from the 3-day culture of Dami/HEL or Meg-01 cells (CM), or (3) conditioned medium plus 2 ng/mL GM-CSF (CM + GM-CSF). The cells then were labeled with [3H]-TdR for an additional 4 hours. CPM of [3H]-TdR incorporation was determined from triplicate samples and expressed as the mean. These results are representative of three separate experiments.
DISCUSSION
Our studies show that the JAK2/STAT5 signal transduction pathway is activated constitutively in the growth factor-independent leukemic cell lines, Dami/HEL and Meg-01. In contrast, the activation of this signaling pathway is strictly cytokine-induced transiently in the growth factor-dependent leukemic cell line, Mo7e. Others also have reported transient cytokine-dependent activation of the JAK2/STAT signaling pathway in several growth factor-dependent cell lines.8-12,15 Although our present study is focused on two growth factor-independent megakaryocytic leukemic cell lines (Dami/HEL cells also have erythroid characteristics), the aberrant signaling pathway could also exist in other factor-independent leukemic cell lines. Based on the published observation that STAT-related transcription factors are activated constitutively in primary cells from acute myeloid and lymphoid and chronic myeloid leukemia patients41-44 and given the close relationship between cell proliferation and the activation of JAK2/STAT5, constitutive activation of the JAK2/STAT5 pathway may be one of the important mechanisms of leukemogenesis and of maintaining the leukemic phenotype.
Mechanisms for the growth factor-independent growth of leukemic cells are not well understood. The BCR/ABL oncoprotein is implicated in the pathogenesis of CML, as well as some acute leukemias, especially a subset of acute lymphoblastic leukemias. BCR/ABL exhibits protein kinase activity, which activates RAS, JAKs, STATs, and other signaling pathways.38-40,45 When cytokine-dependent cells are transfected with the BCR/ABL oncogene, constitutive activation of STAT5 and loss of a cytokine requirement for continued cell growth have been reported.39 In cells transformed with the BCR/ABL oncogene, the BCR/ABL oncoprotein may activate STAT5 directly, bypassing the need for activation of JAK2.46 Others, however, reported that the activation of STAT5 is still via the JAK kinase.40,41,45 47 We observed that the Meg-01 cell line, which is derived from a patient with CML, contains BCR/ABL oncogene transcripts, which is a likely explanation for the constitutive activation of STAT5 in this cell line. However, no BCR/ABL transcripts were detected in either Dami/HEL or Mo7e cells, indicating that the constitutive activation of JAK2/STAT5 in Dami/HEL cells is triggered by a mechanism other than the BCR/ABL oncogene.
The proto-oncogene, Raf-1, has been reported to have a role in the factor-independent growth of human myeloid leukemia cell lines.37 In our study, we did observe increased phosphorylation of Raf-1 in Dami/HEL cells and U937 cells without cytokine exposure, and cytokine treatment had no significant further effects on Raf-1 phosphorylation. Although the constitutive phosphorylation of Raf-1 may be responsible for the factor-independence of Dami/HEL and U937 cells, the relationship of the constitutive activation of Raf-1 and STAT5 is not clear at the present time. On the other hand, no significant increased phosphorylation of Raf-1 or MAPK, and no increased MEK activity were observed in Meg-01 cells without cytokine exposure. This result was unexpected, as Meg-01 cells express BCR/ABL gene transcripts, which are reported to have the capacity to induce the activation of the Ras-Raf-MAPK signal transduction pathway.38 40 Whether the lack of constitutive phosphorylation of Raf-1 and MAPK is related to the p185 BCR/ABL expression in Meg-01 cells would be interesting to investigate.
STAT signaling pathways, particularly STAT3 and STAT5, are frequently activated during oncogenesis.34,36,40,48 The initial report of STAT activation by a specific oncoprotein showed that one STAT family member, STAT3, is constitutively activated in rodent fibroblast cell lines stably transformed by the oncogenic Src tyrosine kinase.48 Although STAT3 seems to be the predominant family member activated by Src, STAT5 was reported to be activated by various oncoproteins in human leukemias.38-47 Recently, the fusion of the human TEL gene on chromosome 12p13 to the JAK2 gene on chromosome 9p24 has been reported in human leukemias.43,44The TEL gene was initially described as a “promiscuous” gene with a role in both myeloid and lymphoid leukemias. Three variants have been identified that fuse the TEL pointed (PNT) domain to the JAK2 JH1-kinase domain, part of, and all of the JH2 pseudokinase domain. More interestingly, all of the human TEL/JAK2 fusion variants are oncoproteins in vitro that strongly activate STAT5 and transform the IL-3–dependent murine hematopoietic cell line Ba/F3 to IL-3–independent growth.43 It will be important in future studies to determine whether an oncogenic mutation involving JAK2 is responsible for the constitutive activation of JAK2/STAT5 signaling pathway in at least Dami/HEL cells.
In addition to oncoproteins, there are accumulated data indicating that aberrant expression or mutational activation of cytokine receptors or their downstream signal transduction pathways may have an important role in abnormally, constitutively activated signal transduction pathways and in factor-independent cell growth. Transfecting growth factor-dependent cells with the TPO-receptor gene containing a point mutation in the dimer interface homology domain of the TPO receptor (also known as c-mpl) induces constitutive receptor activation and factor-independent cell proliferation.1,4 By engineering expression of the EPO receptor with a single amino acid change, Watowich et al2 observed that mutants resulting in disulfide-linked receptor homodimerization cause constitutive activation of the EPO receptor and induction of factor-independent cell proliferation. Kitayama et al3 reported a point mutation of the c-Kit receptor tyrosine kinase that confers factor-independent growth and tumorigenicity of factor-dependent hematopoietic cells. Theoretically, mutation of any cytokine receptors that signal through JAK2 and STAT5 or downstream signal transduction pathways could cause the constitutive activation of JAK2/STAT5 in Meg-01 and Dami/HEL cells (Dami/HEL cells also have constitutive phosphorylation of Raf-1). Further study is needed to examine the possibility of whether aberrant receptors or other signaling molecules are involved in the constitutive activation of JAK2/STAT5, as well as the growth factor-independent survival and proliferation of Dami/HEL cells.
We provide substantial evidence to show that the constitutive activation of the JAK2/STAT5 signaling pathway is not likely to be via autocrine stimulatory cytokines. This conclusion is supported by the following observations. (1) We and others showed that Dami/HEL and Meg-01 cells grow very well, even in serum-free medium, in the absence of exogenous cytokines; and addition of cytokines such as TPO, GM-CSF, IL-3, IL-6, and TNF-α does not result in any stimulatory effect on cell growth. (2) The addition of anti–IL-3, anti–GM-CSF, and anti-TPO neutralizing antibodies does not have any effect on proliferation of Dami/HEL or Meg-01 cells. (3) Using freshly prepared Mo7e or TF-1 cells, which are often used as bioassay systems to detect stimulatory cytokines, neither Dami/HEL nor Meg-01 cell-conditioned medium support Mo7e or TF-1 survival or proliferation. (4) If growth factor-independent cells produce secreted or unsecreted cytokines that cause autocrine stimulation of the cells, one should see not only the constitutively activated JAK2/STAT5, but also increased MEK activity and MAPK phosphorylation, as we and others have shown that IL-3, GM-CSF, TPO, and EPO all activate both JAK2/STAT5 and MAPK signaling pathways in erythroleukemic (TF-1 and UT-7) and megakaryocytic (Mo7e) cell lines.11,12 (5) Other cytokines, such as G-CSF and those which signal through gp130 (IL-6, IL-11, etc), induce JAK and STAT family members other than JAK2 and STAT5. (6) We and others8 10 showed that even persistent cytokine-treatment only induces the transient activation of JAK2/STAT5 (Fig 3). Thus, an autocrine stimulatory factor effect seems not to be a reasonable explanation for the constitutive activation of JAK2/STAT5 in Dami/HEL or Meg-01 cells.
We found constitutive activation of a STAT-like DNA-binding factor in the growth factor-independent Dami/HEL and Meg-01 cells and cytokine-induced activation of a STAT-like DNA-binding factor in Mo7e cells, which raised the question of whether STAT-like proteins in factor-independent cells are the same as those induced in factor-dependent cells. Because other STATs also have the capacity to bind to the GAS promoter, albeit to a lesser degree, it was necessary to identify the specific STAT(s) that is/are activated constitutively in Dami/HEL and Meg-01 cells. Our results show clearly that the constitutively activated STAT-like DNA-binding factor in Dami/HEL and Meg-01 cells and the cytokine-induced STAT-like protein in Mo7e cells is STAT5. This conclusion is based on the following findings: (1) both the cytokine-dependent and the cytokine-independent DNA-binding factors have the capacity to bind to the IRF-1 GAS oligonucleotide probe; (2) the formation of DNA-protein complexes is abolished by competing oligonucleotides containing the STAT5-binding sequence (MGFe), but not by ISRE, SIE, and FIRE, which do not have a consensus STAT5 binding site; (3) anti-STAT5 antibody supershifts both the constitutively activated (from Dami/HEL and Meg-01 cells) and cytokine-induced (from Mo7e cells) DNA-protein complexes; and (4) antibodies to STAT1, STAT2, STAT3, STAT4, and STAT6 do not supershift the DNA-protein complexes in nuclear extracts from either unstimulated Dami/HEL and Meg-01 cells or TPO-stimulated Mo7e cells. Our observation that STAT5 or a STAT5-like factor, but not STAT3, is activated shortly after exposure of Mo7e cells to cytokines is consistent with prior reports8 10 in which only the STAT5 activation, but no STAT3 activation, was reported in hematopoietic cell lines exposed to these cytokines.
Our data suggest that the activation of JAK2/STAT5 is triggered by different mechanisms in Dami/HEL, Meg-01, and Mo7e cells. The constitutive activation of JAK2/STAT5 correlates well with the factor-independence of Dami/HEL and Meg-01 cell lines. Future detailed investigation will be important to determine the underlying reason(s) for the constitutive activation of the JAK2/STAT5 pathway in Dami/HEL and Meg-01 cell lines, the relationship of the constitutively activated Raf-1 and JAK2/STAT5 in Dami/HEL cells and the relationship between p185BCR/ABL and JAK2/STAT5 activation in Meg-01 cells. A better understanding of these processes will provide insight into signal transduction events leading to uncontrolled cell survival and proliferation in leukemias and ultimately may lead to new diagnostic and therapeutic strategies.
Supported by Grants No. CA56072, CA55652, and P30 CA76292 from the National Institutes of Health/National Cancer Institute, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard Y. Liu, PhD, H. Lee Moffitt Cancer Center, MRC 3007, 12902 Magnolia Dr, Tampa, FL 33612; e-mail:liur@moffitt.usf.edu.

![Fig. 1. Effects of TPO on DNA synthesis in human leukemic cell lines. Dami/HEL, Meg-01, and Mo7e cells were incubated with the indicated concentrations of TPO for 72 hours and labeled with 2 μCi/mL [3H]-TdR for 4 hours. The cpm of [3H]-TdR incorporation into newly synthesized DNA was determined by counting the radioactivity from triplicate samples and expressed as the mean cpm. This figure is representative of the data from three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718001x.jpeg?Expires=1769138318&Signature=3ISwPu4WjOe0Hw4YgcG3vPRy6AG9WLRrOkSoYUIizxouiBROSPnX2GX1UQn-h9eAqZo5wnqmZtashVBb4vGOva~YFtYQ~RgDjz9-c6bPmIfCbG-ZlXunJSZIUi2JlBcDSCFq725cyRouSPqD5G0Re~DPr6BKDcF3xm2NkHH2LpsgIcEmzwY1JReM346g9-UVK2j61EAGyydWm12R-QEE8XQjqB3DjbKsGUsztQy~pQvPGp0H2KgmmZ82kNUuGatpwzhYxKnj-97JBCd87yR0seJXLxogY6SIhl5NJJIRCE4wkyC~N2t3UAqeO8xm9vSkLKDAge9iH2JEmziU6jvVzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Constitutive activation of a STAT-like DNA-binding factor in Dami/HEL and Meg-01 cells. Nuclear extracts from each cell line cultured in the absence of cytokine (CTL) or in the presence of as much as 400 ng/mL TPO or 40 ng/mL IL-3, were incubated with [32P]-labeled, double-stranded IRF-1 GAS oligonucleotide. The DNA-binding complex and unbound probes were separated electrophoretically on 5% nondenaturing polyacrylamide gels. The autoradiograph shows the STAT-like DNA-binding factor (DBF) and the nonspecific bands (NS).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718002w.jpeg?Expires=1769138318&Signature=AY8YcAVxoSnCh9GqyqSTCzKVp6KEVurv~hzvmapK0u3XiMvXEtYyXYB-dRchAbsnHvltlc-3-pp3pi1qikCc1Rg9A0AF7V5Y1bYe-yXynVKUTH57ssDeK89VKnBj960C7ALZQkTjzXajiA8VbFa0Fnj9-ShmXC6Mc2kmXpa70hS8uPsXqAkSvlMsEk-uyJ2tfyOFYVvYPTqVCDmRpB1QnEJz3Nd6hNOP~ijg1BH9FbsOmnB-kajhUDI8O91UkxSogwUrTD7L2MLlcVXsSxXhF9hRDNIwRf0lVom8qBscIdyxU~Vknc7iARTfI5GCxvbsw~QPk7JCukrTQNH588qxqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effects of cytokines on activation of a STAT-like DNA-binding factor in Mo7e cells. Nuclear extracts from Mo7e cells grown in the absence of cytokine (CTL), or in the presence of 40 ng/mL EPO, 400 ng/mL TPO, 40 ng/mL GM-CSF or 40 ng/mL IL-3, were incubated with [32P]-labeled IRF-1 GAS oligonucleotide. The STAT-like DNA-binding complex and unbound probe were separated on 5% native polyacrylamide gel. The autoradiograph shows the DBF, NS, and free probes (P).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718003w.jpeg?Expires=1769138318&Signature=vb~vX~a6roS6N2XhgM0FSFz8wCcMi7jfJ0JA7R4UoZEjXqGzsSkwpg9Pz-d5IHBHVao3Uf5wEG3i-F1vXeDknOJmEtJcW2jSP0tnBBViMrBhSXIEGEUUOqF~5vkmyRc4a7PIc9hLwWX9CSdOvpaADB4ooGSIG7nJtxk61k7ipAyBTkN8IyYnxCvmXjmYEk3GaP43wh7AA4lcf5KcTrmWNq7awVVFXIeuLiGFWre96dwpf-eEhPUZnpKDmDuEIBR8~efVeK0UqFKhPMl1vzoB7rViHI2eBpRqTBsoq-nEOIoDsbAmpRYbxxy9XmD7KDmBUQ-JS0SkOIEqck-ax77RTQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Specificity of binding of STAT-like DNA-binding factors in Dami/HEL and Mo7e cells to DNA containing the IRF-1 GAS sequence. (A) Dami/HEL cell nuclear extracts were incubated with [32P]-labeled IRF-I GAS probe (lane 1) or with [32P]-labeled probe plus a 50-fold excess of unlabeled oligonucleotide FIRE, ISRE, SIE, or MGFe, respectively (lanes 2 to 5). The DNA-binding complexes were separated in a 5% nondenaturing polyacrylamide gel. (B) Mo7e cell nuclear extracts from untreated control cells (lane 1) or cells treated with 400 ng/mL TPO (lane 2) were incubated with [32P]-labeled IRF-1 GAS probe or with [32P]-labeled probe plus a 50-fold excess of unlabeled oligonucleotide FIRE, ISRE, SIE, or MGFe, respectively (lanes 3 to 6). The DNA-binding complexes were separated in a 5% nondenaturing polyacrylamide gel. The autoradiograph shows the STAT-like DBF and NS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718004w.jpeg?Expires=1769138318&Signature=tYAaShRUoFzhxPr0~~qoOhwrOpnnBbGFgGPb0dAzt0fSqKvqw5W85qdXI82~rDB7ECbTBYVnyyA5zBR33K4pEe4h5dAFeQcZ8Jyk8BEx3EWCN5zWElPcN6S7UvXkAgaY2YzEXIwtbUNmNGrJvT0GQN3kkN9Go~ACkZsOBJ~JLT4osalnv55gfJ5PJ50dn3QJbnfNyGUhjnzjCSmauvY65xZVVc3~voaK5698cQDswrbpnBDOhskyh8o1JbFBfVjdRY-i-k~-wRs8nNwuOoIlEsUpxyDS36bJdVgXTLZxFh-rNY3SjDqIVIy-nlStsYCAgeEu~cmse7nxGXO-ezbrEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Identification of the constitutively activated DNA-binding factor in Dami/HEL and Meg-01 cells and the cytokine-induced DNA-binding factor in Mo7e cells by gel electrophoretic mobility supershift assays. Nuclear extracts prepared from Dami/HEL cells without cytokine exposure were incubated with [32P]-labeled IRF-1 GAS plus anti-STAT3 or anti-STAT5 antibodies (lanes 1 and 2). Nuclear extracts from Meg-01 cells without cytokine exposure were incubated with the [32P]-labeled probe plus anti-STAT3 or anti-STAT5 antibodies (lanes 3 and 4). Nuclear extracts from Mo7e cells without cytokine exposure (lane 5) or treated with 400 ng/mL TPO (lanes 6 to 8), were incubated with [32P]-labeled IRF-1 GAS probe (lanes 5 and 6), or with [32P]-labeled probe plus anti-STAT3 or anti-STAT5 antibodies (lanes 7 and 8). Nuclear extracts, from SK-BR–3 cells incubated without cytokine exposure (lane 9) or with 20 ng/mL EGF (lanes 10 to 14), were incubated with [32P]-labeled IRF-1 GAS probe alone (lanes 9, 10, and 13), or with [32P]-labeled probe plus anti-STAT1, anti-STAT3, or anti-STAT5 antibodies (lanes 11, 12, and 14). The EGF-treated SK-BR–3 cells, which were reported by our group to have activated STAT1, STAT3, and STAT5,36 were used to show the antibody-induced supershift of STAT1, STAT3, and STAT5. The autoradiograph shows the STAT-like DBF, NS, and free IRF-1 GAS probe (P). The arrowheads indicate the supershifted complexes. These results are representative of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718005w.jpeg?Expires=1769138318&Signature=vI7nOvGsgFw0qqhjLMb9w1AyT4L-L~AHAYVDCjMyE0XErYRnL~vXy~KczJO4bvh0AD3ot0S2sp8phRD6EhysM68TtPH2wMm3dlSgtMTLCNwQJDQWY8LN3C3DLVHPuXXpyzBwmYc55jgBPCX-XATrBXO5dU-qAyIMSWeyBl17ssC023WHaHGxBAgpL7VHLoNxEQndKN8ZOzcMQIjLtnuKnqTn5~FXl5ruap~O~UHoC7gswXzWq9FD2bxYU3Wb6rG86b8KHvesBYzhsx1FAl7d0p4Go6qvjKHIpQ6Gej7fS0bx~gL1LTbX69LyclM~~PVYnxt4jhdKG90s~MUe3XAIRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Phosphorylation of proto-oncogene Raf-1 and activation of MEK in Mo7e, Meg-01, and Dami/HEL cells. (A) Phosphorylation of Raf-1 was examined by band shift on Western blot. Equal amounts of cellular proteins (40 μg) from Mo7e, Dami/HEL, Meg-01, and U937 cells incubated with no cytokine (lanes marked as C) or 40 ng/mL GM-CSF (lanes, GM) were subjected directly to 10.5% SDS-PAGE. The separated proteins were transferred to a PVDF membrane and probed with anti–Raf-1 antibody. The arrow indicates the location of unphosphorylated Raf-1 and the arrowhead indicates the band shift of phosphorylated Raf-1. (B) Activation of MEK was detected by the kinase-induced phosphorylation of a kinase-defective p42mapk (K52R) labeled with [32P]-ATP. Equal amounts of cellular proteins (30 μg) from Mo7e, Meg-01, and Dami/HEL cells incubated with no cytokine (lanes marked as C) or 40 ng/mL GM-CSF (lanes, GM) were used to detect MEK activity. Phosphorylation of p42 is used as the readout to detect MEK activity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718008w.jpeg?Expires=1769138318&Signature=SQpqGPleXwCUBBkH7-34VVVfNblaxltoohbv3fZ0I7x4fbhNVArTa2ec5rfVmuKXEHQK8deieTCpX2Y2TWD-BfkVVSK8yiSofE9IVQr8iLQS13C5YRYmrkG3tMsdGDwX8pm3P-9jqXUHIuA1kQ7YwktZm8ZYTf8qj7Skl3ZOOONlgX4YoAOK7daKOdZD-vL9oqRjQtwIM0taDzn1WcQb1UJgAeOyNHvMLSU7C1G94ZEuh8clLjR5UAUkC-zeF4DJXitWjPlVIzsRW4UqAzIKkpq8hFP4F7NVZTr5wldfaWE5K-4bbyXjRGA-R16zXu62D-VYpWhuhck4V6ZzIJtZ2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 10. Bioassays of possible stimulatory factors in Dami/HEL and Meg-01 conditioned medium. Mo7e cells, pretreated for 18 hours in medium without cytokines, were incubated for 72 hours with control or conditioned medium from cultures of Dami/HEL cells (A) or Meg-01 cells (B), using either (1) culture medium not exposed to Dami/HEL or Meg-01 cells (CTL), (2) conditioned medium collected from the 3-day culture of Dami/HEL or Meg-01 cells (CM), or (3) conditioned medium plus 2 ng/mL GM-CSF (CM + GM-CSF). The cells then were labeled with [3H]-TdR for an additional 4 hours. CPM of [3H]-TdR incorporation was determined from triplicate samples and expressed as the mean. These results are representative of three separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2369/5/m_blod40718010x.jpeg?Expires=1769138318&Signature=XxgODH2qeeuIQGdsaKnUlUxJrnAPWO6r3S8c0EP2Tlz1eJA~FRCyopYP45Btp-8-P9iggZU-v92oTTqPZI6KIePZU7CId5RFRYj7AE6SMpi9rPvJu3W3qQyV~8Xhn-p-fCwYZJg23jChJkYjxTEn5I5RY5djncVqp2hrF29vampgr~u1X4xsOTPmvb1SQUyKcrmQdUl8On2j7OBgl5ntWMnpDaiKiadVZOSFC-DlNcDZSkw-cufAnxGwl0LGFYtAwaT876BeO~rfnoWEIEpeN1Li2L2cLmn21yBjFmrtmz2xAXcVWK9WcolLdcfdmtNnE2wRlwo1jNouD7THL-QdEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal