We previously found that the adapter protein Gab1 (110 kD) is tyrosine-phosphorylated and forms a complex with SHP-2 and PI-3 kinase upon stimulation through either the interleukin-3 receptor (IL-3R) or gp130, the common receptor subunit of IL-6–family cytokines. In this report, we identified another adapter molecule (100 kD) interacting with SHP-2 and PI-3 kinase in response to various stimuli. The molecule displays striking homology to Gab1 at the amino acid level; thus, we named it Gab2. It contains a PH domain, proline-rich sequences, and tyrosine residues that bind to SH2 domains when they are phosphorylated. Gab1 is phosphorylated on tyrosine upon stimulation through the thrombopoietin receptor (TPOR), stem cell factor receptor (SCFR), and T-cell and B-cell antigen receptors (TCR and BCR, respectively), in addition to IL-3R and gp130. Tyrosine phosphorylation of Gab2 was induced by stimulation through gp130, IL-2R, IL-3R, TPOR, SCFR, and TCR. Gab1 and Gab2 were shown to be substrates for SHP-2 in vitro. Overexpression of Gab2 enhanced the gp130 or Src-related kinases–mediated ERK2 activation as that of Gab1 did. These data indicate that Gab-family molecules act as adapters for transmitting various signals.
Src HOMOLOGY 2-containing tyrosine phosphatase 2 (SHP-2) is a tyrosine phosphatase bearing two SH2 domains in the amino-terminal region.1-3 In comparison to SHP-1, which is a negative regulator for signaling cytokine receptors4-8 and the B-cell antigen receptor (BCR),9 SHP-2 is a positive regulator of signaling from receptor tyrosine kinases and cytokine receptors.
SHP-2 is phosphorylated on tyrosine in response to growth factors and cytokines such as platelet-derived growth factor (PDGF),10,11 prolactin,12 interferon-α/β (INF-α/β),13 granulocyte-macrophage colony-stimulating factor (GM-CSF)14 and to stimulation through gp130,15 the common receptor subunit of inteleukin-6 (IL-6)–family cytokines.16 SHP-2 contains YXNX motifs, which is the consensus sequence for Grb2 binding, in its carboxy-terminal region.2,3 Upon stimulation, SHP-2 associates with Grb2, which forms a link to the Ras pathway through Sos.10,11,15 These data suggest that SHP-2 may act as an adapter molecule for activating Ras. In addition to the adapter function of SHP-2, its catalytic activity is necessary for transmitting signals to the ERK MAP kinases. Catalytically inactive mutants of SHP-2 inhibit the activation of ERK MAP kinases in response to insulin,17 EGF,18 and FGF,19providing evidence for the existence of substrates for SHP-2 and suggesting a role they might play in signal transduction. Candidates for SHP-2 substrates include several molecules reported to associate with SHP-2. These include the SIRP/SHPS family of transmembrane proteins,20,21 IRS1,17 IRS2,22Gab1,23 and as yet unidentified 97- to 100-kD molecules.24-28 Especially, 97- to 100-kD tyrosine-phosphorylated proteins (pp97 and pp100) were shown to associate with SHP-2 in response to IL-2,24IL-3,25 macrophage colony-stimulating factor (M-CSF),26 the stimulation of the T-cell receptor (TCR),27 and transformation bybcr-abl.28 pp97 and pp100 interact with the SH2 domains of the p85 PI-3 kinase and CrkL and the SH3 domain of Grb2.24 These data suggested that pp97 and pp100 are adapter molecules that contain various tyrosine motifs for binding to the SH2 domains and prolinerich sequences for binding to the SH3 domain, although their identities were not yet known. However, these characteristic features of pp97 and pp100 quite resembled those of Gab1.
Gab1 was originally isolated as a binding protein for Grb2. Gab1 is tyrosine-phosphorylated and interacts with SHP-2 and PI-3 kinase in response to insulin, EGF,23 HGF,29NGF,30 lysophosphatidic acid (LPA),31 IL-3, and gp130 stimulation.32 It contains a PH domain, tyrosine-based motifs, and proline-rich sequences including MBD (c-Met binding domain). Furthermore, the Drosophila Gab1 homologue Daughter of Sevenless (DOS) is a substrate for the Drosophila SHP-2 homologue Corkscrew (CSW). DOS was shown to act downstream of the receptor tyrosine kinase Sevenless and upstream of or in parallel to the Ras pathway.33 34 Therefore, Gab1 or Gab1-related molecules are good candidates for substrates or signal transducers for SHP-2.
We previously demonstrated that Gab1 acts downstream of gp130 in transmitting signals to ERK MAP kinase.32 However, in BAF-B03 cells, ERK2 was activated upon the stimulation of gp130 without the expression of Gab1. Instead, we observed a tyrosine-phosphorylated 100-kD molecule to associate with SHP-2 and PI-3 kinase upon stimulation of gp13015 and the IL-3 receptor. In this report, we identified pp100 as a member of the Gab1 family of adapter molecules and named it Gab2. We show that both Gab1 and Gab2 act downstream of cytokine and growth factor receptors as well as the antigen receptors on T and B cells.
MATERIALS AND METHODS
cDNA cloning and plasmid construction.
The amino-terminal fragment of human Gab2 cDNA was obtained by 5′ rapid amplification of 5′-cDNA ends (5′-RACE) from a cDNA library of the human myeloma cell line U266. The U266 cDNA library was constructed by using a Marathon cDNA Amplification Kit (Clontech, La Jolla, CA). The fragment was amplified by polymerase chain reaction (PCR) with the adapter primers and primers CCGGCTGAGGAAACATTTCTCAGG and AGCCTGATTGAAGCCACAGATCTGGC. Their design was based on the nucleotide sequence of KIAA0571 (accession no.AB011143) according to the manufacturer’s protocol. The PCR fragment and KIAA0571 were ligated at the Pst I site and subcloned into the expression vector pcDNA3 (Invitrogen, San Diego, CA). The Flag-tagged expression vectors for Gab2 were constructed as previously described32 and subcloned into pcDNA3. The partial fragments of mouse Gab2 were obtained by screening a λZipLox BAF-B03 cDNA library with the KIAA0571 fragment (kindly provided by Kazusa DNA Research Institute, Chiba, Japan) as a hybridization probe. The BAF-B03 cDNA λ phage library was constructed using the Superscript λ system for cDNA synthesis (GIBCO-BRL, Grand Island, NY) from granulocyte colony-stimulating factor (G-CSF)–stimulated BAF-B03 G133 transfectants.15 A cDNA fragment containing the entire coding sequence of mouse Gab2 was obtained by the ligation of a product of 5′RACE PCR that was obtained using the adapter primers and primers CTGTGCTCTCTTCAGCCTGATTGAAG and AGCCTGATTGAAGCCGCAGATCTGGC and a fragment obtained by PCR using the primers CATGAATAAGTGGGTCCAGAGCATC and GGTCAACAACTTTCAACACAAACACATTC. A BAF-B03 cDNA library was used as a template in the RACE and PCR reactions. It was constructed from G-CSF–stimulated BAF-B03 G133 transfectants. The cDNAs were sequenced by an automated ALF sequencer (Amersham Pharmacia, Arlington Heights, IL) and an ABI377 sequencer (ABI, Foster City, CA). The bacterial expression vectors for GST-SHP-2 CAT and GST-SHP-2 C/S CAT were constructed by inserting the catalytic domain (amino acid 263-594) of SHP-2 and SHP-2 C/S into the NcoI and BamHI sites of pGEX-KG. The bacterial expression vector for GST-Gab2 was constructed by inserting the BglII-Sca I fragment of human Gab2 cDNA (amino acids 380-563) into the BamHI and Sma I sites of pGEX-KG. The expression vector for Gab2 was constructed by inserting theHindIII-Pvu II fragment of human Gab2 into pcDNA3. The expression vectors for Flag-tagged ERK2, Gab1, JAK1, STAT3, and v-Src were described previously.32 Expression vectors for v-Src, Btk, and Tec were gifts from Drs M. Karin, S. Tsukada, and H. Mano and were described previously.35
Antibodies.
Anti-Gab2 antibody was raised by immunizing rabbits with GST-Gab2. For immunization, female rabbits were first injected with 1.0 mg of GST-Gab2 fusion protein in complete Freund’s adjuvant and then boosted every 2 weeks with 0.5 mg of the antigen in incomplete Freund’s adjuvant. Anti-Gab1 antibody was described previously.32The anti-HA (12CA5) and anti-Flag (M2) antibodies were purchased from Boehringer Manheim (Indianapolis, IN) and Eastman Kodak (Rochester, NY), respectively. Anti-SHP-2 (sc280) and anti-Grb2 (sc255) antibodies were purchased from Santa Cruz Biotechnology Co (Santa Cruz, CA). Anti-p85 PI-3 kinase (06-195) and antiphosphotyrosine (4G10) antibodies were purchased from UBI Corp (Lake Placid, NY).
Cell lines and stimulation.
HepG2, 293T, 293T transfectants, BAF-B03, BAF-B03-G277, and BAF-B03-G68 cells were maintained as described previously.15,32 KT-3 cells were maintained in RPMI1640 supplemented with 10% fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 μg), and 5 ng/mL of recombinant human IL-6 (Ajinomoto, Tokyo, Japan). NIH3T3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS and penicillin (100 U/mL). MO7E cells were maintained in RPMI1640, 10% FCS, antibiotics, and 10 ng/mL of recombinant human IL-3. Jurkat and Ramos cells were maintained in RPMI1640, 10% FCS, and antibiotics. For the stimulation of cell lines, KT-3 cells were starved of IL-6 for 12 hours and stimulated with 20 ng/mL of human recombinant IL-2 for 10 minutes. HepG2 cells were starved of serum for 24 hours and stimulated with 100 ng/mL of IL-6 for 10 minutes. NIH3T3 cells were starved of serum for 24 hours and stimulated with 50 ng/mL of human recombinant PDGF for 10 minutes. MO7E cells were starved of IL-3 for 12 hours and stimulated with 30 ng/mL of human recombinant SCF for 10 minutes. TF-1 cells were starved of IL-3 for 12 hours and stimulated with 30 ng/mL of human recombinant thrombopoietin (TPO) for 10 minutes.36Jurkat cells were incubated with RPMI1640 and 0.5% FCS for 4 hours and stimulated with 10 μg/mL of anti-CD3 antibody (UCHT-1) for 10 minutes. Ramos cells were stimulated with 10 μg/mL of F(ab′)2 fragments of goat antihuman IgM for 5 minutes. TF-1 and MO7E cells were kindly provided by Drs Y. Kanakura and I. Matsumura.
Immunoprecipitation and immunoblotting.
The methods of immunoprecipitation and immunoblotting were essentially as described previously.32 After stimulation, cell lysates were prepared in lysis buffer (20 mmol/L Tris HCl, pH 7.4, 150 mmol/L NaCl, 1% NP40, 500 μmol/L sodium vanadate, 1 mmol/L dithiotheritol, 5 μg/mL aprotinin, 5 μg/mL leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride) and incubated with 1 μL of anti-Gab1, Gab2 serum, 4 μL of anti-SHP-2, or 0.5 μL of anti-p85 antibodies and 10 μL of protein A-sepharose for 12 hours at 4°C. Immunoprecipitates were washed three times with 1 mL of lysis buffer without protease inhibitors. Proteins were eluted with 20 μL of 3× Laemmli’s sodium dodecyl sulfate (SDS) loading buffer, separated on a 4% to 20% polyacrylamide gel (Dai-ichi Kagaku, Tokyo, Japan), and electrotransferred to a polyvinyliden difluoride membrane (Immobilon-P; Millipore, Bedford, MA). The membranes were blocked with TBST (20 mmol/L Tris HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Tween 20) containing 1% gelatin and incubated with the primary antibodies (1 μg/mL of monoclonal antibodies, 5,000×-diluted anti-Gab1, Gab2, and SHP-2 antibodies, 10,000×-diluted anti-p85 antibody) for 1 hour at room temperature. The membranes were washed with TBST for 10 minutes three times and incubated with horseradish peroxidase (HRP)-conjugated goat antimouse (for monoclonal antibodies) diluted 1:5,000 or HRP-conjugated goat antirabbit (for polyclonal antibodies) diluted 1:10,000 Ig antibodies (Zymed, South San Francisco, CA). The membranes were washed with TBST three times. The immune complexes were visualized using a chemiluminescence system (Renaissance; Dupont NEN Products, Boston, MA).
Phosphatase assay.
Tyrosine-phosphorylated Gab1 and STAT3 were immunoprecipitated from 5 × 106 IL-6–stimulated HepG2 cells. Tyrosine-phosphorylated Gab2 was immunoprecipitated from 5 × 106 IL-3–stimulated BAF-B03 cells. The precipitates were washed three times with 1 mL of lysis buffer without sodium vanadate or protease inhibitors and once with phosphatase buffer (100 mmol/L MES, pH 6.8, 150 mmol/L NaCl, 5 mmol/L dithiothreitol [DTT], and 2 mmol/L EDTA). The dephosphorylation reaction was performed by incubating with 50 μL of phosphatase buffer containing 3 μg of GST-SHP-2 CAT or GST-SHP-2 C/S CAT for 30 minutes at 30°C. The immunoprecipitates were washed with 1 mL of lysis buffer containing sodium vanadate. Proteins were eluted with SDS loading buffer. Tyrosine phosphorylation of Gab1, Gab2, and STAT3 was analyzed by immunoblotting with anti-phosphotyrosine antibody.
Other assays.
RESULTS
Identification of Gab2 and its expression.
We previously observed that, when G-CSF15 or IL-332 were used to stimulate BAF-B03 transfectants expressing a chimeric receptor that contained the extracellular domains of the G-CSF receptor and the transmembrane and cytoplasmic domains of gp130, a tyrosine-phosphorylated 100-kD molecule (pp100) that was associated with SHP-2 appeared (Fukada et al15 and see Fig3B). SHP-2 and pp100 were also coimmunoprecipitated with anti-p85 PI-3 kinase antibody immunoprecipitates (see Fig 3B). pp100 bound GST-Grb2 in vitro (see Fig 3D). These data indicate that the biochemical characteristics of pp100 are similar to those of Gab1, which was shown to interact with SHP-2, PI-3 kinase, and Grb2, prompting us to search for homologues of Gab1.
We found that a human cDNA from an entire cDNA sequencing project (KIAA0571 accession no. AB011143; Kazusa DNA Research Institute) displayed strong homology to Gab1. Although Gab1 contains a PH domain, the KIAA0571 clone lacked part of the PH domain in the amino-terminal region. We isolated the PH domain by 5′RACE PCR from a cDNA library made from the human myeloma cell line U266. The mouse cDNA was also isolated using a combination of hybridization and PCR. The entire coding region of the human and mouse clones exhibited 37% and 35% identity to human Gab1 at the amino acid level (Fig 1), indicating that these clones encode a novel member of human and mouse Gab1-family adapter proteins. Given the similarities in sequence and function (described below), we have named this molecule Gab2. In addition to the PH domain, Gab2 has many functional domains that are well conserved between it and Gab1. Like Gab1, Gab2 contains various tyrosine-based motifs for SH2-domain binding. In particular, the binding motifs for Grb2, Crk, PI-3 kinase, and SHP-2 are well conserved (Fig 1). Gab2 also has a region similar to the c-Met binding domain (MBD) of Gab1 (37% and 36% identity in human and mouse Gab2 versus that in human Gab1) and it possesses proline-rich sequences (amino acids 351-358 in human Gab2) that are also conserved.
Comparison of the amino acid sequences of Gab1 and Gab2. The sequences of human and mouse Gab2 cDNA were deposited in the DNA Data Bank of Japan (DDBJ). Their accession numbers are AB018413 andAB018414, respectively. The PH domain, MBD, and tyrosine-based motifs for Grb2, Crk, p85 PI-3 kinase, and SHP-2 are underlined.
Comparison of the amino acid sequences of Gab1 and Gab2. The sequences of human and mouse Gab2 cDNA were deposited in the DNA Data Bank of Japan (DDBJ). Their accession numbers are AB018413 andAB018414, respectively. The PH domain, MBD, and tyrosine-based motifs for Grb2, Crk, p85 PI-3 kinase, and SHP-2 are underlined.
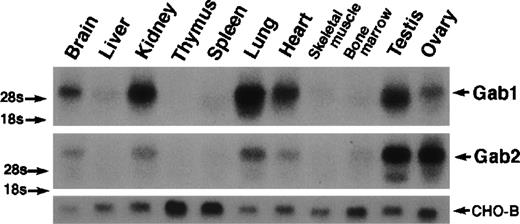
Both Gab1 and Gab2 were expressed ubiquitously, but they were most highly expressed in the brain, kidney, lung, heart, testis, and ovary (Fig 2).
Ubiquitous expression of Gab1 and Gab2. Total RNA was isolated from mouse tissues. RNA (20 μg) was analyzed by Northern blotting with mouse Gab1 (upper panel) and Gab2 cDNAs (lower panel) as probes. Note that RNA for both Gab1 and Gab2 was detected in all of the tissues after a long exposure. CHO-B cDNA was used as a loading control.
Ubiquitous expression of Gab1 and Gab2. Total RNA was isolated from mouse tissues. RNA (20 μg) was analyzed by Northern blotting with mouse Gab1 (upper panel) and Gab2 cDNAs (lower panel) as probes. Note that RNA for both Gab1 and Gab2 was detected in all of the tissues after a long exposure. CHO-B cDNA was used as a loading control.
Gab2 is pp100, which interacts with SHP-2, PI-3 kinase, and Grb2. The Box1 and Box2 region of gp130 was sufficient for its tyrosine phosphorylation.
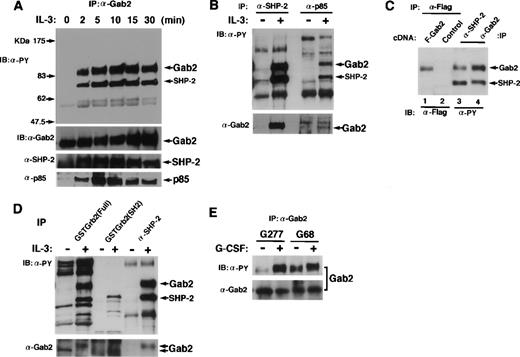
To demonstrate that Gab2 is pp100, we raised the polyclonal antibody against a carboxy-terminal region (amino acids 380-563) of Gab2 and used it for immunoprecipitation and immunoblotting. BAF-B03 cells, which did not express Gab1, were stimulated with IL-3, and immunoprecipitated Gab2 was tyrosine-phosphorylated after 2 minutes of stimulation (Fig 3A). Concomitantly, tyrosine-phosphorylated SHP-2 was coimmunoprecipitated with Gab2. The p85 PI-3 kinase was not tyrosine-phosphorylated (data not shown) but was also coimmunoprecipitated with Gab2 (Fig 3A). Gab2 was the major tyrosine-phosphorylated molecule interacting with SHP-2 and PI-3 kinase in response to IL-3 stimulation in BAF-B03 cells (Fig 3B). Furthermore, when we transfected 293T cells with an expression vector for Flag-tagged Gab2, the Gab2 immunoprecipitated with anti-Flag antibodies was 100 kD (Fig 3C). It comigrated on a SDS polyacrylamide gel with the 100-kD protein that was immunoprecipitated with either anti–SHP-2 or anti-Gab2 antibodies from IL-3–stimulated BAF-B03 cells (Fig 3C). Gab2 was not detected in the anti-Grb2 immunoprecipitates from either nonstimulated or stimulated cells (data not shown). However, Grb2 bound both nonphosphorylated and tyrosine-phosphorylated Gab2 in vitro (Fig3D). These data indicate that Gab2 is the pp100 interacting with SHP-2 and PI-3 kinase. Gab2 may also interact with Grb2 in cells.
Gab2 associates with SHP-2 and PI-3 kinase. (A) BAF-B03 cells were stimulated with IL-3 for the indicated periods of time. Proteins were immunoprecipitated with an anti-Gab2 antibody, separated on an SDS-polyacrylamide gel, and subjected to immunoblotting with anti-phosphotyrosine, anti-Gab2, anti-SHP-2, and anti-p85 PI-3 kinase antibodies. The locations of Gab2, SHP-2, and p85 are indicated by arrows. (B) BAF-B03 cells were stimulated with IL-3 (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti–SHP-2 or anti-p85 PI-3 kinase antibodies and subjected to immunoblotting with anti-phosphotyrosine (upper panel) and anti-Gab2 (lower panel) antibodies. (C) 293T cells were transfected with an expression vector for Flag-tagged Gab2 (lane 1) or a control vector (lane 2), and cell lysates were immunoprecipitated with anti-Flag antibodies. Cell lysates from IL-3–stimulated BAF-B03 cells were immunoprecipitated with anti-SHP-2 (lane 3) or anti-Gab2 (lane 4) antibodies. The immunoprecipitates were separated on the same SDS-polyacrylamide gel. The anti-Flag, anti-SHP-2, and anti-Gab2 immunoprecipitates were analyzed by immunoblotting with anti-Flag and anti-phosphotyrosine antibodies, respectively. (D) Cell lysates from BAF-B03 cells that were stimulated or unstimulated with IL-3 were mixed with GST fusion proteins containing the entire coding fragment (Full) or the SH2 domain of Grb2. GST fusion protein-bound fractions were isolated by glutathione sepharose and subjected to immunoblotting with anti-phosphotyrosine (upper panel) and anti-Gab2 (lower panel) antibodies. Anti-SHP-2 immunoprecipitates were also analyzed in the same membrane. Note that the slower migrating form of Gab2 is the tyrosine-phosphorylated form. (E) BAF-B03 cells expressing the chimeric receptor G277 (containing the entire cytoplasmic domain) or G68 (containing 68 amino acids from the membrane region) were stimulated with G-CSF (+) or left unstimulated (−), and immunoprecipitated Gab2 proteins were subjected to immunoblotting with anti-phosphotyrosine (upper panel) and anti-Gab2 (lower panel) antibodies.
Gab2 associates with SHP-2 and PI-3 kinase. (A) BAF-B03 cells were stimulated with IL-3 for the indicated periods of time. Proteins were immunoprecipitated with an anti-Gab2 antibody, separated on an SDS-polyacrylamide gel, and subjected to immunoblotting with anti-phosphotyrosine, anti-Gab2, anti-SHP-2, and anti-p85 PI-3 kinase antibodies. The locations of Gab2, SHP-2, and p85 are indicated by arrows. (B) BAF-B03 cells were stimulated with IL-3 (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti–SHP-2 or anti-p85 PI-3 kinase antibodies and subjected to immunoblotting with anti-phosphotyrosine (upper panel) and anti-Gab2 (lower panel) antibodies. (C) 293T cells were transfected with an expression vector for Flag-tagged Gab2 (lane 1) or a control vector (lane 2), and cell lysates were immunoprecipitated with anti-Flag antibodies. Cell lysates from IL-3–stimulated BAF-B03 cells were immunoprecipitated with anti-SHP-2 (lane 3) or anti-Gab2 (lane 4) antibodies. The immunoprecipitates were separated on the same SDS-polyacrylamide gel. The anti-Flag, anti-SHP-2, and anti-Gab2 immunoprecipitates were analyzed by immunoblotting with anti-Flag and anti-phosphotyrosine antibodies, respectively. (D) Cell lysates from BAF-B03 cells that were stimulated or unstimulated with IL-3 were mixed with GST fusion proteins containing the entire coding fragment (Full) or the SH2 domain of Grb2. GST fusion protein-bound fractions were isolated by glutathione sepharose and subjected to immunoblotting with anti-phosphotyrosine (upper panel) and anti-Gab2 (lower panel) antibodies. Anti-SHP-2 immunoprecipitates were also analyzed in the same membrane. Note that the slower migrating form of Gab2 is the tyrosine-phosphorylated form. (E) BAF-B03 cells expressing the chimeric receptor G277 (containing the entire cytoplasmic domain) or G68 (containing 68 amino acids from the membrane region) were stimulated with G-CSF (+) or left unstimulated (−), and immunoprecipitated Gab2 proteins were subjected to immunoblotting with anti-phosphotyrosine (upper panel) and anti-Gab2 (lower panel) antibodies.
We previously showed that neither tyrosine residues nor the carboxy-terminal region (amino acid 710) of gp130 was necessary for the tyrosine-phosphorylation of Gab1. We also determined which part of gp130 is responsible for the tyrosine-phosphorylation of Gab2 (Fig 3E). When BAF-B03 cells expressing the G68 chimeric receptors (containing only the box1 and box2 region of gp130) were stimulated with G-CSF, Gab2 was tyrosine-phosphorylated, as observed in G-CSF–stimulated BAF-B03 cells expressing the G277 chimeric receptor (containing the entire cytoplasmic domain of gp130; Fig 3E) or IL-3–stimulated BAF-B03 cells (data not shown). These data indicate that, as for Gab1, the box1 and box2 region of gp130 was sufficient and that the tyrosine residues of gp130 were not necessary for the tyrosine phosphorylation of Gab2.
Gab1 and Gab2 act as adapter proteins for various signaling pathways.
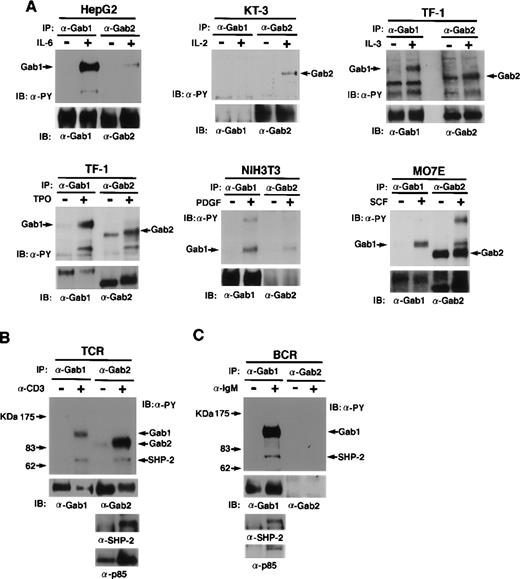
We next examined the roles of Gab1 and Gab2 in various signal transduction pathways (Fig 4A through C). When HepG2 cells, which express both Gab1 and Gab2, were stimulated with IL-6, Gab1 was phosphorylated on tyrosine but Gab2 was not strongly phosphorylated. When KT-3 cells, which express Gab2 but not Gab1, were stimulated with IL-2, Gab2 was phosphorylated on tyrosine. In TF-1 cells, both Gab1 and Gab2 were phosphorylated on tyrosine in response to both thrompoietin and IL-3. In MO7E cells, both Gab1 and Gab2 were phosphorylated on tyrosine in response to SCF. In NIH3T3 cells, which express Gab1 but not Gab2, Gab1 was phosphorylated on tyrosine in response to PDGF. These data indicate that both Gab1 and Gab2 act downstream of cytokine receptors and receptor tyrosine kinases (SCF and PDGF).
Gab1 and Gab2 are phosphorylated on tyrosine in response to various stimuli. (A) Cytokines and growth factor stimuli. Various cell lines were stimulated by the indicated cytokines or growth factors. Anti-Gab1 and anti-Gab2 immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine, anti-Gab1, and anti-Gab2 antibodies. Note that Gab2 was tyrosine-phosphorylated in unstimulated MO7E cells, but the band of Gab2 was shifted upon SCF stimulation, indicating that Gab2 was hyper-phosphorylated in the stimulated cells. HepG2, KT-3, TF-1, NIH3T3, and MO7E were human hepatoblastoma, human (Lennert’s) T lymphoma, human erythroleukemia, mouse fibroblast, and human megakaryocytic leukemia cell lines, respectively. HepG2, TF-1, and MO7E cells express both Gab1 and Gab2. But KT-3 and NIH3T3 cells do not express Gab1 or Gab2, respectively (confirmed by RT-PCR, data not shown). Proteins of 115 to 120 kD detected in the Gab2 immunoprecipitates from HepG2, NIH3T3, and MO7E cells are likely Gab1 proteins cross-reacted with anti-Gab2 antibodies. (B) TCR and (C) BCR stimuli. Jurkat cells were stimulated with anti-CD3 antibody or left unstimulated. Ramos cells were stimulated with anti-IgM antibody or left unstimulated. Cell lysates were immunoprecipitated with anti-Gab1 or anti-Gab2 antibodies and subjected to immunoblotting with anti-phosphotyrosine, anti-Gab1, anti-Gab2, anti-SHP2, and anti-p85 antibodies. Expression of Gab2 in Ramos cells was not detected by immunoblotting and RT-PCR (data not shown).
Gab1 and Gab2 are phosphorylated on tyrosine in response to various stimuli. (A) Cytokines and growth factor stimuli. Various cell lines were stimulated by the indicated cytokines or growth factors. Anti-Gab1 and anti-Gab2 immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine, anti-Gab1, and anti-Gab2 antibodies. Note that Gab2 was tyrosine-phosphorylated in unstimulated MO7E cells, but the band of Gab2 was shifted upon SCF stimulation, indicating that Gab2 was hyper-phosphorylated in the stimulated cells. HepG2, KT-3, TF-1, NIH3T3, and MO7E were human hepatoblastoma, human (Lennert’s) T lymphoma, human erythroleukemia, mouse fibroblast, and human megakaryocytic leukemia cell lines, respectively. HepG2, TF-1, and MO7E cells express both Gab1 and Gab2. But KT-3 and NIH3T3 cells do not express Gab1 or Gab2, respectively (confirmed by RT-PCR, data not shown). Proteins of 115 to 120 kD detected in the Gab2 immunoprecipitates from HepG2, NIH3T3, and MO7E cells are likely Gab1 proteins cross-reacted with anti-Gab2 antibodies. (B) TCR and (C) BCR stimuli. Jurkat cells were stimulated with anti-CD3 antibody or left unstimulated. Ramos cells were stimulated with anti-IgM antibody or left unstimulated. Cell lysates were immunoprecipitated with anti-Gab1 or anti-Gab2 antibodies and subjected to immunoblotting with anti-phosphotyrosine, anti-Gab1, anti-Gab2, anti-SHP2, and anti-p85 antibodies. Expression of Gab2 in Ramos cells was not detected by immunoblotting and RT-PCR (data not shown).
It was previously reported that SHP-2 interacts with 97- and 120-kD molecules upon stimulation of TCR27 and BCR37in lymphocytes. We determined whether Gab1 and Gab2 act downstream of antigen receptors. When TCR were stimulated with an anti-CD3 antibody on human Jurkat T cells, Gab2 was strongly tyrosine-phosphorylated, but the phosphorylation of Gab1 was relatively low (Fig 4B). Furthermore, SHP-2 and the p85 PI-3 kinase were detected in the Gab2 immunoprecipitates (Fig 4B), confirming that Gab2 is the previously reported pp97. In the human B-cell line Ramos, which expresses Gab1 but not Gab2, Gab1 was tyrosine phosphorylated when the cells were stimulated with anti-IgM antibodies (Fig 4C). These data showed that Gab family adapter molecules act downstream of not only cytokine receptors and receptor tyrosine kinases, but also of antigen receptors in lymphocytes.
Gab1 and Gab2 are substrates for SHP-2.
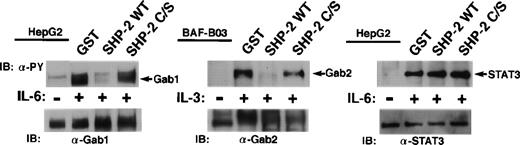
We next examined whether Gab1 and Gab2 are substrates for SHP-2. Tyrosine-phosphorylated Gab1, Gab2, and STAT3 were isolated from IL-6–stimulated HepG2 and IL-3–stimulated BAF-B03 cells by immunoprecipitation and subjected to an in vitro phosphatase assay using GST fusion proteins containing the catalytic domain of SHP-2 or its inactive mutant (C/S). Gab1 and Gab2 but not STAT3 were dephosphorylated by the catalytic domain of SHP-2. The dephosphorylation did not occur in the presence of the inactive catalytic domain (Fig 5). These data indicate that Gab1 and Gab2 could be substrates for SHP-2 in vivo.
Gab1 and Gab2 are substrates for SHP-2 in vitro. Tyrosine-phosphorylated Gab1 and STAT3 were isolated by immunoprecipitation from IL-6–stimulated HepG2 cells. Tyrosine-phosphorylated Gab2 was isolated from IL-3–stimulated BAF-B03 cells. These proteins were incubated with a GST fusion protein containing the catalytic domain of SHP-2 (GST-SHP-2 WT) or the catalytic inactive mutant (GST-SHP-2 C/S). Dephosphorylation of these proteins was determined by immunoblotting with anti-phosphotyrosine antibody (upper panel). The amounts of these proteins were analyzed by immunoblotting with anti-Gab1, anti-Gab2, and anti-STAT3 antibodies (lower panel), respectively.
Gab1 and Gab2 are substrates for SHP-2 in vitro. Tyrosine-phosphorylated Gab1 and STAT3 were isolated by immunoprecipitation from IL-6–stimulated HepG2 cells. Tyrosine-phosphorylated Gab2 was isolated from IL-3–stimulated BAF-B03 cells. These proteins were incubated with a GST fusion protein containing the catalytic domain of SHP-2 (GST-SHP-2 WT) or the catalytic inactive mutant (GST-SHP-2 C/S). Dephosphorylation of these proteins was determined by immunoblotting with anti-phosphotyrosine antibody (upper panel). The amounts of these proteins were analyzed by immunoblotting with anti-Gab1, anti-Gab2, and anti-STAT3 antibodies (lower panel), respectively.
Gab2 acts upstream of ERK MAP kinases.
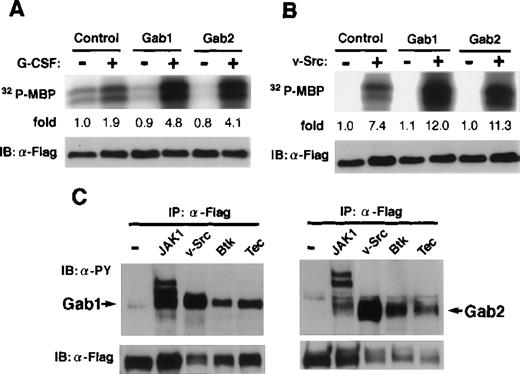
It was reported that the overexpression of Gab1 enhanced the c-Met- or gp130-mediated ERK MAP kinase activation.29 32 We analyzed the role of Gab2 in MAP kinase activation. When an expression vector for Flag-tagged ERK2 was transfected with and without vectors for Gab2 into 293T cells expressing the G-CSF-R/gp130 chimeric receptor, the gp130-mediated ERK2 activities were enhanced by the expression of Gab2 (Fig 6A), suggesting that Gab2 acts upstream of ERK MAP kinases, as Gab1 does. The ERK activation by v-Src, Tec, and Btk was also enhanced by the overexpression of both Gab1 and Gab2 (Fig 6B and data not shown). Moreover, Gab1 and Gab2 were phosphorylated on tyrosine in cells expressing v-Src, Tec, and Btk (Fig6C). These data suggest that Gab1 and Gab2 are substrates for these tyrosine kinases and that, after phosphorylation, they serve as adapter molecules for transmitting signals to ERK MAP kinases.
Gab2 is involved in the activation of ERK MAP kinase. (A) 293T cells expressing the chimeric receptor G277 were transfected with expression vectors for Flag-tagged ERK2, together with either Gab1, Gab2, or a control vector. Cells were stimulated with G-CSF for 30 minutes (+) or left stimulated (−) and the ERK2 activities were determined by an immunoprecipitation kinase assay using myelin basic protein (MBP) as a substrate. The amount of MBP-incorporated32P was quantified by an image analyzer and indicated as the ratios against that from control unstimulated cells. (B) 293T cells were transfected with vectors for Flag-tagged ERK2, together with either Gab1, Gab2, or a control vector. They were also transfected with the expression vectors for v-Src (+) or a control vector (−), as indicated. The ERK2 activities were determined as described above. (C) 293T cells were transfected with vectors for Flag-tagged-Gab1 or Gab2, together with either JAK1, v-Src, Btk, Tec, or a control vector. Gab1 and Gab2 were immunoprecipitated with anti-Flag antibodies and analyzed using anti-phosphotyrosine (upper panel) and anti-Flag antibodies (lower panel).
Gab2 is involved in the activation of ERK MAP kinase. (A) 293T cells expressing the chimeric receptor G277 were transfected with expression vectors for Flag-tagged ERK2, together with either Gab1, Gab2, or a control vector. Cells were stimulated with G-CSF for 30 minutes (+) or left stimulated (−) and the ERK2 activities were determined by an immunoprecipitation kinase assay using myelin basic protein (MBP) as a substrate. The amount of MBP-incorporated32P was quantified by an image analyzer and indicated as the ratios against that from control unstimulated cells. (B) 293T cells were transfected with vectors for Flag-tagged ERK2, together with either Gab1, Gab2, or a control vector. They were also transfected with the expression vectors for v-Src (+) or a control vector (−), as indicated. The ERK2 activities were determined as described above. (C) 293T cells were transfected with vectors for Flag-tagged-Gab1 or Gab2, together with either JAK1, v-Src, Btk, Tec, or a control vector. Gab1 and Gab2 were immunoprecipitated with anti-Flag antibodies and analyzed using anti-phosphotyrosine (upper panel) and anti-Flag antibodies (lower panel).
DISCUSSION
Although SHP-2 has been implicated in cytokine and growth factor receptor signaling, the molecular mechanism(s) by which SHP-2 controls downstream signaling has been largely unknown. Drosophila genetics showed that CSW and its substrate DOS are required for Sev-dependent eye development.33,34 In addition to the CSW-DOS pathway, Sos and an adapter molecule Drk (Drosphila homologue of Grb2) are also required for Sev signaling.38 This situation is very similar to that for cytokine receptors and receptor tyrosine kinases in mammals. Many of those receptors recruit Grb2 either directly or indirectly through Shc or SHP-2. In addition, the catalytic activity of SHP-2 was shown to be required for signaling to ERK MAP kinases. Therefore, it is quite important to find substrates for SHP-2. It was reported that 97-to 100-kD molecules are tyrosine-phosphorylated and associated with SHP-2 and PI-3 kinase in hematopoietic cells upon IL-2,24 IL-3,25 and TCR27stimulation and upon transformation by bcr-abl.28Gab2, identified here, is the pp97 and pp100 previously reported: it was phosphorylated on tyrosine in response to IL-2, IL-3, and TCR and in response to other cytokines (Fig 4A and B). Upon stimulation, Gab2 associated with SHP-2 and PI-3 kinase (Fig 3A and B). Grb2 interacted with Gab2 in vitro (Fig 3D). Tyrosine-phosphorylated Gab2 was dephosphorylated by the catalytic subunit of SHP-2 (Fig 5). These data indicate that Gab2 is an adapter molecule for transmitting signals from cytokines, growth factors, and antigen receptors in lymphocytes.
Although both Gab1 and Gab2 are phosphorylated on tyrosine in response to a wide variety of stimuli, there seems to be certain specificity. In HepG2 cells, stimulation of gp130 resulted in the tyrosine phosphorylation of, mainly, Gab1. In BAF-B03 cells, which express only Gab2, gp130-stimulation resulted in the tyrosine phosphorylation of Gab2 (Fig 3E), suggesting that Gab2 can replace the function of Gab1. Quantitative analyses will be required to show if the preferential use of Gab1 and Gab2 through various signaling pathways reflects any functional specificity. In this regard, targeted disruption of these genes will show the roles of Gab1 and Gab2 in these signal-transduction pathways.
Overexpression of Gab1 and Gab2 enhanced gp130-mediated ERK MAP kinase activation. It also enhanced the ERK activation induced by the expression of Src and its related tyrosine kinases (Fig 6A and B). These data suggest that both Gab1 and Gab2 are substrates for various tyrosine kinases and act as signal transducers for them. In T or B cells, various Src family kinases, such as Lck, Fyn, and Lyn, and Src-related kinases Tec and Btk, were shown to be involved in antigen receptor signaling.39-41 Furthermore, in TCR signaling, the phosphatase activity of SHP-2 was shown to be required for ERK activation.42 Therefore, Gab1 and Gab2 are good candidates for signal transducers for ERK activation in TCR and BCR signaling.
It is not yet clear how Gab1 and Gab2 are regulated by SHP-2 or how they transmit signals downstream. Our previous data on Gab132 and other reports on pp97 and pp100 show that SHP-2 and PI-3 kinase simultaneously interact with Gab1 or Gab2 upon stimulation.24,25 We also found that, in cells expressing a gp130 mutant that lacks the SHP-2 binding motif, SHP-2 was neither phosphorylated on tyrosine nor associated with Gab1; coincidentally, the interaction of PI-3 kinase with Gab1 was attenuated, although Gab1 was phosphorylated on tyrosine.32 These data suggest that the interaction of PI-3 kinase with the Gab proteins somehow depends on the interaction between SHP-2 and the Gab proteins. The involvement of the PI-3 kinase was also supported by the observation that a dominant-negative p85 PI-3 kinase or treatment with wortmanin, a PI-3 kinase inhibitor, blocked the Gab1-enhanced ERK activation.32 Thus, Gab family proteins may link SHP-2 to PI-3 kinase for transmitting signals. In support of this hypothesis, Gab1, Gab2, and the distantly related IRS1 and IRS2 all contain two SHP-2 binding motifs in their carboxy-terminal region. A cluster of PI-3 kinase-binding motifs (>3) are located 30 to 181 amino acids distant from the cluster of SHP-2 binding motifs in all these proteins. Either dephosphorylation of these adapter molecules by SHP-2 or the binding to them of SHP-2 may facilitate conformational changes that render them able to recruit or activate PI-3 kinase. Mutational analyses of Gab1, Gab2, and SHP-2 will be required to clarify these points. In any case, Gab1 and Gab2 play important roles in the signal transduction of cytokines, growth factors, antigen receptors, and possibly others.
ACKNOWLEDGMENT
The authors thank R. Masuda and T. Kimura for their excellent secretarial assistance. We thank Drs I. Matsumura, Y. Kanakura, S. Tsukada, H. Mano, M. Kasuga, T. Matozaki, and K. Matsuoka for various reagents. We thank Kazusa DNA Research Institute for providing us the KIAA0571 cDNA. We also thank Dr T. Kurosaki for his suggestion on the analysis of B-cell antigen receptor signaling.
Supported by grants and a Grant-Aid for COE Research from the Ministry of Education, Science, Sports, and Culture in Japan, the Searle Scientific Research Fellowship, and the Osaka Foundation for Promotion of Clinical Immunology.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
After acceptance of this manuscript, Gu et al (Mol Cell 2:729, 1998) reported the molecular cloning of p97/Gab2.
Author notes
Address reprint requests to Toshio Hirano, MD, PhD, Division of Molecular Oncology (C-7), Osaka University Medical School, 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail:hirano@molonc.med.osaka-u.ac.jp;http://www.med.osaka-u.ac.jp/pub/molonc/www/index.html.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal