The AML1 gene encoding the DNA-binding -subunit in the Runt domain family of heterodimeric transcription factors has been noted for its frequent involvement in chromosomal translocations associated with leukemia. Using reverse transcriptase-polymerase chain reaction (RT-PCR) combined with nonisotopic RNase cleavage assay (NIRCA), we found point mutations of the AML1 gene in 8 of 160 leukemia patients: silent mutations, heterozygous missense mutations, and biallelic nonsense or frameshift mutations in 2, 4, and 2 cases, respectively. The mutations were all clustered within the Runt domain. Missense mutations identified in 3 patients showed neither DNA binding nor transactivation, although being active in heterodimerization. These defective missense mutants may be relevant to the predisposition or progression of leukemia. On the other hand, the biallelic nonsense mutants encoding truncated AML1 proteins lost almost all functions examined and may play a role in leukemogenesis leading to acute myeloblastic leukemia.
THE AML1 GENE IS KNOWN as the most frequent target of chromosomal translocations associated with leukemia.1 It belongs to the Runt domain gene family that encodes the major subunit, α, of heterodimeric transcription factor PEBP2/CBF.2 The Runt domain, named after theDrosophila segmentation gene, runt, is an evolutionarily conserved protein motif that consists of 128 amino acids and is responsible for both DNA binding and heterodimerization with the non-DNA binding regulatory subunit, β. Τhe gene encoding the β subunit was also shown to be the target of another leukemia-associated chromosomal rearrangement, inv(16).3 These observations have suggested that the two subunits play critical roles in hematopoiesis as close partners. In fact, AML1 is normally expressed in all lineages of hematopoietic cells and acts to regulate the expression of various genes specific to hematopoiesis. Furthermore, targeting mice lacking either Aml1 or Pebp2β/Cbfbshowed no fetal liver hematopoiesis.4-6 Thus, PEBP2/CBF has proved to be essential for definitive hematopoiesis of all lineages.
The translocations involving AML1 produce chimeric proteins: AML1-ETO(MTG8) in t(8;21); AML1-EVI1, AML1-MDS1, and AML1-EAP (out of frame fusion) in t(3;21); and TEL-AML1 in t(12;21). The leukemogenicity of these chimeric genes have been experimentally tested for a few cases. The antisense oligonucleotides complementary to the fusion transcript, AML1-ETO, inhibited the growth and induced differentiation of cell lines carrying the chimeric gene.7Furthermore, AML1-ETO and AML1-EVI1 were shown to block the differentiation of a murine myeloid precursor cell line, 32Dcl3, as induced by granulocyte colony-stimulating factor (G-CSF).8All of the above-noted chimeric proteins retained the entire Runt domain, but the transactivation region is largely deleted or replaced by foreign proteins. They were shown to interfere with transactivation by the normal AML1 in a transdominant manner. In addition, mice heterozygously knocked-in with AML1-ETO were defective in definitive hematopoiesis in the same way as wereAml1−/− mice.9 These observations led to the general notion that one role of the chimeric proteins in leukemogenesis could be to repress normal AML1 functions.1,2 However, AML1-ETO can also activate some selected promoters,10 11 raising the possibility that it might contribute to leukemogenesis through such again-of-function activity as well.
Despite the indisputable importance of chromosomal translocations in leukemogenesis, the versatile roles of AML1 in hematopoiesis led us to hypothesize that its micro lesions, overlooked in routine cytogenetic screening, might be involved in some cases of leukemia. Thus, we began to search for point mutations of AML1 among various types of leukemia patients. An initial probative survey covering 160 leukemic patients has indeed detected such mutations in 5 cases (3%) alongside 3 cases (2%) suspected of functionally neutral polymorphisms. This report describes in detail the molecular and functional characterization of these mutations. While this work was in progress, hereditary and sporadic mutations in the Runt domain of another α subunit gene, PEBP2αA/CBFA1, were identified for patients with cleidocranial dysplasia.12 13
MATERIALS AND METHODS
Patients and cell preparation.
Screening was performed for 160 leukemia patients with the following categories in indicated numbers: acute myeloblastic leukemia (AML), 109 [M0, 9; M1, 10; M2 with t(8;21), 8; M2 without t(8;21), 20; M3 with t(15;17), 13; M4 with inv(16), 11; M4 without inv(16), 25; M5, 12; M6, 1]; acute lymphoblastic leukemia (ALL), 37 including 8 with t(9;22); leukemic transformation from myelodysplastic syndrome (MDS), 6; and chronic myeloid leukemia (CML) blastic phase, 8. Diagnoses of leukemia and MDS were made by the morphological and immunophenotypic analyses according to French-American-British (FAB) criteria.14 All patients gave informed consent according to the guidelines set by the Institutional Committees for the Protection of Human Subjects. Mononuclear cells were isolated from peripheral blood or bone marrow samples of patients by Ficoll-Conray density gradient centrifugation and were immediately immunophenotyped. The remaining mononuclear cells were cryopreserved in liquid nitrogen for molecular analysis. More than 70% of the mononuclear cells from all patients were morphologically regarded as blasts on cytospin with May-Giemsa staining. Peripheral blood samples from 8 healthy volunteers were also tested as controls.
Reverse transcriptase-polymerase chain reaction (RT-PCR).
Total cellular RNA was extracted from cryopreserved mononuclear cells by ultracentrifugation in a guanidinium-isothiocyanate/CsCl2 gradient or TRIzol reagent (GIBCO BRL, Gaithersburg, MD). cDNA was synthesized using total RNA and oligo(dT)12-18 primer with SuperScript II reverse transcriptase (GIBCO BRL). To improve the resolution of the subsequent NIRCA analysis, the primary cDNA product was amplified by PCR in two blocks, a 5′ proximal region containing the Runt domain and a 3′ terminal remainder. These blocks collectively cover most of the protein coding sequence of AML1 (isoform b identified by Miyoshi et al15), except for some 20 amino acids on either end (Fig 1A). The PCR with Taqpolymerase (GIBCO BRL) was first performed using primer sets S21/AS21 for the 5′ region and S41/AS41 for the 3′ region. A 1/50 portion of the first PCR solution was used to seed the second round of PCR with new sets of primers tagged with T7 or SP6 promoter, S22T7/AS22SP6, and S42T7/AS42SP6. Because AML1 transcripts skipping exon 6 were supposed to occur in part,15 one of the primers in each set was designed to fall within exon 6 so that amplifications of those transcripts could be avoided (AS21, AS22SP6, S41, and S42T7). The sequences of the primers are as follows: S21, 5′-AGGCAAGATGAGCGAGGCGTTG-3′ (1644-1665); AS21, 5′-CTGAGGGTTAAAGGCAGTGGAGT-3′ (2283-2261); S22T7, 5′-TGTAATACGACTCACTATAGGGCAAGATGAGCGAGGCGTT-3′ (1645-1664); AS22SP6, 5′-AGATTTAGGTGACACTATAGGAGCTGCTCCAGTTCACTGA-3′ (2189-2171); AS23, 5′-CATTGCCAGCCATCACAGTGAC-3′ (1906-1885); S41, 5′-CCGGGAGCTTGTCCTTTTCC-3′ (2144-2163); AS41, 5′-TCGCTCTGGTTCGGGAGGCT-3′ (2849-2829); S42T7, 5′-TAATACGACTCACTATAGGGAGCTTGTCCTTTTC-3′ (2146-2162); and AS42SP6, 5′-ATTTAGGTGACACTATAGGAGGCTGGGGTTGAGCA-3′ (2836-2819), where the numbers in the parentheses indicate nucleotide positions in the AML1 cDNA according to the GenBank entry, D43968, and the primers with suffixes T7 and SP6 contain the sequences derived from the respective promoters.
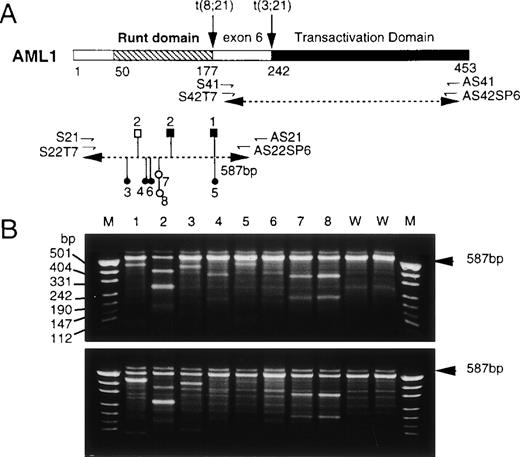
Screening of AML1 mutations by a nonisotopic RNase cleavage assay (NIRCA). (A) Structure of the AML1 protein and strategy of RT-PCR. The primers for PCR are shown by small directed arrows below the diagrammatic structure of AML1. The dotted lines with double arrowheads indicate the segments amplified by RT-PCR. Mutations found in 8 patients are mapped onto the segment containing the Runt domain: (○) silent; (•) missense; (□) frameshift; (▪) nonsense. (B) Gel electrophoretic patterns of RNAs generated by NIRCA. The sense strand from each test sample was hybridized with the antisense strand from the wild-type in the upper panel and vice versa in the lower panel. Lanes 1 through 8, patients numbered respectively; lanes 9 and 10, wild-type controls. M denotes Hpa II-digested pUC19 DNA as size markers. The arrowheads on the right indicate the position of original RNA duplexes. For patients no. 4 through 6, cleavage products were recognizable only in the upper panel.
Screening of AML1 mutations by a nonisotopic RNase cleavage assay (NIRCA). (A) Structure of the AML1 protein and strategy of RT-PCR. The primers for PCR are shown by small directed arrows below the diagrammatic structure of AML1. The dotted lines with double arrowheads indicate the segments amplified by RT-PCR. Mutations found in 8 patients are mapped onto the segment containing the Runt domain: (○) silent; (•) missense; (□) frameshift; (▪) nonsense. (B) Gel electrophoretic patterns of RNAs generated by NIRCA. The sense strand from each test sample was hybridized with the antisense strand from the wild-type in the upper panel and vice versa in the lower panel. Lanes 1 through 8, patients numbered respectively; lanes 9 and 10, wild-type controls. M denotes Hpa II-digested pUC19 DNA as size markers. The arrowheads on the right indicate the position of original RNA duplexes. For patients no. 4 through 6, cleavage products were recognizable only in the upper panel.
To assess the quality of RNA, the β2-microglobulin gene was also amplified as an internal control. All the samples examined produced clear signals on RT-PCR for both AML1 and the β2-microglobulin, thus warranting their further analyses.
Nonisotopic RNase cleavage assay (NIRCA).
NIRCA was performed using the Mismatch Detect II kit (Ambion, Austin, TX) as described.16 The second PCR products were converted to RNA by transcription with T7 and SP6 polymerase for the sense and antisense strands, respectively. Each RNA strand was hybridized with the corresponding complementary transcript from the wild-type cDNA, cleaved with optimized RNase mixtures, and electrophoresed in 2.0% agarose gels containing ethidium bromide. Samples from 8 normal individuals, including 7 Japanese and 1 Egyptian, as wild-type controls showed no cleavage either on their own or in cross-examinations with each other. For those patients found positive, the reproducibility of NIRCA was confirmed by duplicate or triplicate experiments using different lots of cryopreserved cells, which ascertained that the observed mutations were not PCR-derived artefacts.
DNA sequencing.
The first PCR products were subcloned into plasmid pCRII and subjected to cycle sequencing (Applied Biosystems, Foster City, CA). The sequence of each identified mutation was reconfirmed with at least three independent plasmid clones.
Restriction fragment length polymorphism (RFLP) analysis and Southern blotting.
For those mutations accompanied by any change in restriction endonuclease sites, RFLP analysis with RT-PCR products or genomic DNA was performed to confirm their sequence data as well as to determine their zygosity. Southern blotting was performed using standard procedures. Probe M2G3 was a 1.7-kb EcoRI genomic fragment from the AML1 intron 5. Probe AP2 was the first PCR product amplified with S21/AS21 primers from the wild-type AML1 cDNA. The probe for the Ig heavy chain J region gene (JH) was kindly provided by Dr T.H. Rabbitt (MRC Laboratory of Molecular Biology, Cambridge, UK). The signal intensity was quantitated with a densitometer (Fuji BAS2000; Fuji Photo Film, Tokyo, Japan).
Electrophoretic mobility shift assay (EMSA).
An N-terminal proximal part of AML1 containing the Runt domain (amino acids 24-189) was expressed in Escherichia coli as a fusion N-terminally tagged with hexahistidines, purified in a nickel nitrilotriacetic resin (Ni-NTA) column (Qiagen, Hilden, Germany), and subjected to EMSA with a probe carrying a polyomavirus enhancer-derived PEBP2 site, essentially as described previously.17 The expression plasmid was constructed by reinserting the targeted region of AML1, as cloned in pCRII, betweenBamHI and Pst I sites of pQE9 (Qiagen). For S114ter and C72ins, an alternative vector, pQE13, was used instead so as to express them as fusions with a more bulky N-terminal appendage containing hexahistidines and dihydrofolate reductase (DHFR). The structures of these constructs were confirmed by sequencing.
Affinity column assay of AML1-PEBP2β association.
The heterodimerization activity for AML1 mutants impaired in DNA binding was assayed as described previously.17 Briefly, the hexahistidine-tagged AML1 fragment (1 μg) was incubated with tag-less PEBP2β2 (0.5 μg) and loaded onto an Ni-NTA column. This column was successively washed with buffers containing 8 mmol/L and 250 mmol/L imidazole. Proteins in each fraction were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie brilliant blue.
Subcellular localization.
For in vivo functional studies of mutated AML1 proteins, theBan I-HindIII fragments from pQE9-AML1(24-189) orSma I fragments from pCRII were substituted into the compatible site(s) of pEF-AML1(1-453), a mammalian expression plasmid driven by the powerful EF-1α promoter.18 The resultant plasmids were transfected into rat fibroblast cells, REF52, by electroporation. Immunofluorescence labeling of AML1 was performed as described previously19 20 using rabbit anti-αB1 and fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG. The cells were visualized and photographed with a fluorescent microscope.
Transcriptional assay.
The luciferase reporter plasmid pM-CSF-R-luc21 and the effector plasmid pEF-AML1(1-453) containing a mutation in question were transfected at a fixed ratio (8 and 12 μg per assay, respectively) into human myelomonoblastic leukemia cells, U937, by electroporation. After 18 hours of transfection, cell extracts were prepared and assayed for the luciferase activity using a luciferase assay system (Picagene; Toyo Inc, Tokyo, Japan) in a luminometer. In experiments to study functional competitions between the normal and mutant AML1 proteins, the cotransfection of plasmids was performed using a nonliposomal transfection reagent, FuGENE6 (Boehringer Mannheim, Mannheim, Germany), instead of electroporation. The efficiency of transfection was greatly increased with this reagent, so that the inputs of plasmids per assay were reduced to 0.5 μg for pM-CSF-R-luc and 0.2-0.7 μg for pEF-constructs expressing AML1 with or without mutations, AML1-ETO,20 or PEBP2β-MYH11.19 The total input of DNA was kept constant by supplementing appropriate amounts of the backbone pEF plasmid so as to avoid potential artefacts due to uneven overall DNA dosages.
RESULTS
Frequent occurrence of mutations clustered within the Runt domain of the AML1 gene.
Of 160 patients thus far examined for point mutations by NIRCA, 8 patients showed positive results as indicated in Fig 1B. The sample from patient no. 2, in particular, gave 4 cleaved fragments, including two comigrating ones (lane 2, the thickest band at 290 bp), suggesting the occurrence of two independent mutations (lane 2). All of these mutations, summing 9 in total, were further identified by sequencing at positions consistent with their respective patterns on NIRCA (Table 1 and Fig 1A). Interestingly, the mutations were all localized within the Runt domain. They contained three major groups with distinct translational effects: (1) silent mutations: Ileu87 changed to an identical synonymous codon in both patients no. 7 and 8 (I87syn); (2) missense mutations: His58 to Asn (H58N), Lys83 to Asn (K83N), Arg177 to Gln (R177Q), and Arg80 to Cys (R80C) in patients no. 3 through 6, respectively; (3) nonsense or frame shift mutations: Arg177 to the TGA termination codon (R177ter) in patient no. 1 and two mutations, Ser114 to the TAG stop (S114ter) and a four-base insertion (AGAC) after Cys72 (C72ins), in patient no. 2. C72ins resulted in a frame shift with an eventual termination at the position corresponding to codon 111. Clinical and cytogenetical findings of the patients carrying these mutations are summarized in Table 1. None of these patients showed any recognizable abnormality in chromosome 21. A PML/RARA fusion transcript in patient no. 4 and a BCR/ABL fusion transcript in patient no. 6 were detected by RT-PCR.
Clinical and Cytogenetical Features of the Patients With AML1 Mutations
| Patient No. . | Age/ Sex . | Diagnosis (FAB) . | Karyotype . | Mutant/ WT* . | First Allele (nucleotide sequence change) . | Second Allele . |
|---|---|---|---|---|---|---|
| 1 | 65/M | AML M0 | Complex† | 8 | R177ter (CGA → TGA) | R177ter |
| 2 | 69/M | AML M0 | 46,XY,20q-[20] | 3 + 5‡/1 | S114ter (TCG → TAG) | C72ins‡ |
| 3 | 60/F | AML M0 | 46,XX,20q-[20] | 6/3 | H58N (CAC → AAC) | WT |
| 4 | 40/M | AML M3 relapse | NA | 3/6 | K83N (AAG → AAC) | WT |
| 5 | 75/F | AML M5a | NA | 4/8 | R177Q (CGA → CAA) | WT |
| 6 | 61/M | CML BP | NA | 4/2 | R80C (CGC → TGC) | WT |
| 7 | 30/M | AML M4 | 46,XY[20] | 5/4 | I87syn (ATC → ATA) | WT |
| 8 | 67/M | ALL L2 | 46,XY[20] | 4/2 | I87syn (ATC → ATA) | WT |
| Patient No. . | Age/ Sex . | Diagnosis (FAB) . | Karyotype . | Mutant/ WT* . | First Allele (nucleotide sequence change) . | Second Allele . |
|---|---|---|---|---|---|---|
| 1 | 65/M | AML M0 | Complex† | 8 | R177ter (CGA → TGA) | R177ter |
| 2 | 69/M | AML M0 | 46,XY,20q-[20] | 3 + 5‡/1 | S114ter (TCG → TAG) | C72ins‡ |
| 3 | 60/F | AML M0 | 46,XX,20q-[20] | 6/3 | H58N (CAC → AAC) | WT |
| 4 | 40/M | AML M3 relapse | NA | 3/6 | K83N (AAG → AAC) | WT |
| 5 | 75/F | AML M5a | NA | 4/8 | R177Q (CGA → CAA) | WT |
| 6 | 61/M | CML BP | NA | 4/2 | R80C (CGC → TGC) | WT |
| 7 | 30/M | AML M4 | 46,XY[20] | 5/4 | I87syn (ATC → ATA) | WT |
| 8 | 67/M | ALL L2 | 46,XY[20] | 4/2 | I87syn (ATC → ATA) | WT |
Abbreviations: WT, wild-type; NA, not available; CML BP, chronic myeloid leukemia blastic phase.
Ratio of the mutant and wild-type clones in sequencing.
47,XY,+19[10] 48,XY,+8,+9[4] 48,XY,+13,+19[4].
AGAC insertion after Cys72 resulting in a frame shift and a termination at codon 111.
Mutations were biallelic in patients carrying nonsense mutations and monoallelic in the remainder.
In patients no. 3 through 8, which carried silent or missense mutations, both wild-type and mutated sequences were detected at comparable frequencies (Table 1, column Mutant/WT), indicating that their mutations were heterozygous. This conclusion was further confirmed by RFLP analysis of cDNA and genomic DNA for R177Q in patient no. 5 (1 Taq I site lost; see Fig2A for the result with genomic DNA) and for I87syn in patients no. 7 and 8 (1 Alu I site gained; data not shown).
RFLP analysis of genomic DNA. (A) Genomic DNAs digested with Taq I were hybridized with probe M2G3. The restriction map generated from the sequence, dbj0˙00057, is shown on the top: B,BamHI; E, EcoRI; T, Taq I. The Taq I site eliminated by mutations at codon 177 is boxed. Lanes 1, 4, and 5, wild-type; lane 2, patient no. 1 (R177ter); lane 3, patient no. 5 (R177Q). (B) The gene dosage analysis by Southern blotting of theAML1 gene with the Ig heavy chain J region (JH) gene as control. Genomic DNA was digested withBamHI and hybridized with probes M2G3 and JH. Lane 1, patient no. 1; lane 2, HL60. (C) Genomic DNAs digested withPst I were hybridized with cDNA probe AP2 that covers exons 3 through 6. The restriction map, based on the same sequence as in (A), is shown on the top. P stands for Pst I sites, among which the one created by mutation C72ins is boxed. Lanes 1 through 3, wild-type; lane 4, patient no. 2. Note that two fragments were newly produced in lane 4 with a reciprocal partial attenuation of the 1.9-kb fragment from which they were derived.
RFLP analysis of genomic DNA. (A) Genomic DNAs digested with Taq I were hybridized with probe M2G3. The restriction map generated from the sequence, dbj0˙00057, is shown on the top: B,BamHI; E, EcoRI; T, Taq I. The Taq I site eliminated by mutations at codon 177 is boxed. Lanes 1, 4, and 5, wild-type; lane 2, patient no. 1 (R177ter); lane 3, patient no. 5 (R177Q). (B) The gene dosage analysis by Southern blotting of theAML1 gene with the Ig heavy chain J region (JH) gene as control. Genomic DNA was digested withBamHI and hybridized with probes M2G3 and JH. Lane 1, patient no. 1; lane 2, HL60. (C) Genomic DNAs digested withPst I were hybridized with cDNA probe AP2 that covers exons 3 through 6. The restriction map, based on the same sequence as in (A), is shown on the top. P stands for Pst I sites, among which the one created by mutation C72ins is boxed. Lanes 1 through 3, wild-type; lane 4, patient no. 2. Note that two fragments were newly produced in lane 4 with a reciprocal partial attenuation of the 1.9-kb fragment from which they were derived.
By contrast, the wild-type cDNA clone was absent in patient no. 1 (none of 8). In patient no. 2 as well, there was only one wild-type clone against 8 mutant clones, among which S114ter and C72ins were found in comparable frequencies, ie, 3 and 5, respectively. Besides, cytogenetical analysis indicated that leukemia cells from this patient were monoclonal and contained two complements of chromosome 21 with no apparent abnormality as judged from their characteristic karyotype (46, XY, 20q-[20]; Table 1). Taken together, these observations showed that the AML1 gene in patient no. 2 was altered to contain C72ins on one allele and S114ter on the other. Additional evidence consistent with this contention was obtained from RFLP analysis of cDNA with Bfa I (data not shown) and genomic DNA with Pst I (Fig 2C), for which the new sites were generated by S114ter and C72ins, respectively.
That leukemia cells in patient no. 1 also lacked the wild-typeAML1 allele was confirmed by RFLP analysis of cDNA products and genomic DNA using Taq I, for which one site was abolished by the R177ter mutation, just as in the case of R177Q noted above (Fig 2A, only the result for genomic DNA is shown). In patient no. 1 (lane 2), the normal restriction fragment (1.5 kb) cleaved at that site became barely detectable and its fused product (4.9 kb) was reciprocally intensified, whereas the corresponding bands were observed in more balanced proportions with a heterozygous control (patient no. 5; lane 3). To examine whether the R177ter mutation is monoallelic or biallelic, we measured the relative dosages of the AML1 andJH genes in leukemia cells from patient no. 1 by Southern blotting of genomic DNA using HL-60 cells, which retains chromosome 14 and chromosome 21 intact, as a control.22 If one AML1 allele was deleted, the signal ratio,AML1/JH, should be reduced by about one half compared with the control. However, as shown in Fig 2B, the actual value was virtually equivalent between the samples from patient no. 1 and HL-60 cells (1.6 v 1.4). Thus, we conclude that leukemia cells in patient no. 1 retained two AML1 alleles, both of which carried the same R177ter mutation.
It is notable that, in addition, the wild-type AML1 sequence detected at low levels in patient no. 1 (Fig 2A, the faint 1.5-kb band) and patient no. 2 (Table 1, the single wild-type cDNA clone found) were likely due to contaminating nonleukemic cells. This implies that their biallelic mutations were generated in somatic cells for at least one allele. We were unable to examine whether both alleles had been intact in the germline, because no authentic samples of nonleukemic cells were available.
Mutational effects on the DNA binding and heterodimerization activities of the Runt domain.
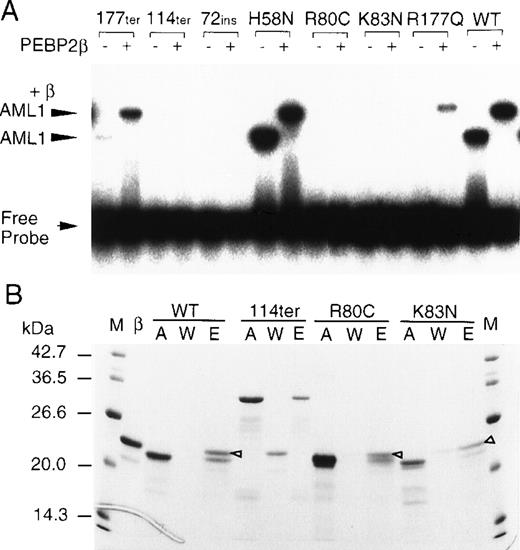
To examine how each mutation affected the Runt domain’s function, we overproduced partial AML1 proteins (amino acids 24-189 for the wild-type and missense mutants) in E coli and subjected them to EMSA (Fig 3A). In this assay, the DNA binding and heterodimerization activities can readily be detected by the shifting and supershifting of the DNA band in the absence and presence of the β subunit, respectively. H58N was virtually normal in both activities. This is coincidental with the fact that residue 58 is located just outside of the minimum essential region (residues 59-178) for DNA binding and heterodimerization as determined by the previous deletion analysis.17 R177ter and R177Q showed barely detectable DNA binding by themselves. However, in the presence of the β subunit, they produced supershifted bands with markedly increased intensities, indicating that they were still active in the heterodimerization activity. This implies that the Runt domain having its R177 residue lost or altered to a nonconservative substitute retains a cryptic potential for DNA binding, which can be unmasked by its conformational change upon heterodimerization with the β subunit. The remaining 4 mutants (R80C, K83N, C72ins, and S114ter) showed no DNA binding regardless of the presence or absence of the β subunit. These mutants were further tested for the heterodimerization ability by the affinity column assay (Fig 3B). This activity was clearly detected in the missense mutants, R80C and K83N, but not at all in the grossly truncated products from C72ins and S114ter. Interesting to note, both R80 and K83 are closely flanking either side of the C81 residue, which has been implicated in the redox regulation of DNA binding by the Runt domain.23 24 Thus, these basic amino acids were supposed to play critical roles in not only conferring redox-susceptibility on the cysteine residue, but also making direct interactions with DNA for themselves.
Mutational alterations in the DNA binding and heterodimerization activities of the Runt domain. (A) The partial AML1 proteins indicated above the panel were produced in E coli and subjected to EMSA. Symbols + and − signify the presence and absence of the β2 subunit, respectively. (B) The indicated partial AML1 proteins were subjected to an affinity column assay. M, molecular weight marker; β, PEBP2β2; A, input AML1 protein; W, unbound proteins in washed fractions; E, bound proteins eluted at 250 mmol/L imidazole. The bands marked with open triangles indicate the β subunits associated with AML1 proteins.
Mutational alterations in the DNA binding and heterodimerization activities of the Runt domain. (A) The partial AML1 proteins indicated above the panel were produced in E coli and subjected to EMSA. Symbols + and − signify the presence and absence of the β2 subunit, respectively. (B) The indicated partial AML1 proteins were subjected to an affinity column assay. M, molecular weight marker; β, PEBP2β2; A, input AML1 protein; W, unbound proteins in washed fractions; E, bound proteins eluted at 250 mmol/L imidazole. The bands marked with open triangles indicate the β subunits associated with AML1 proteins.
Subcellular localization of the AML1 mutants.
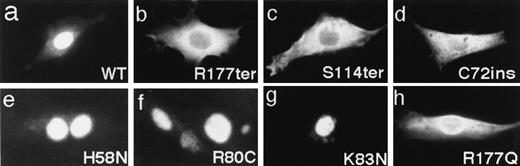
Previous studies have indicated that the Runt domain is also important for the nuclear localization of the AML1 protein.19,20Thus, we investigated the subcellular localization of the mutated AML1 proteins by transfecting their full-length cDNAs into REF52 fibroblasts and then immunostaining the expressed products.19,20 Three missense mutants carrying amino acid changes inside of the Runt domain, H58N, R80C, and K83N (Fig 4e through g) were entirely localized to the nucleus, just as was the wild-type (Fig4a). Another missense mutant, R177Q, showed a weakened nuclear localization concomitant with increased staining of the cytoplasm (Fig4h). By contrast, the nonsense and frameshift mutants, R177ter, S114ter, and C72ins, were almost exclusively localized in the cytoplasm (Fig 4b through d). These results are consistent with the report that the nuclear localization of the AML1 product critically depends on the integrity of the Runt domain with a specific requirement for arginines clustered around the C-terminal boundary of this domain.20
Subcellular localization of the indicated AML1 proteins was detected by immunofluorescence staining with anti-B1.
Subcellular localization of the indicated AML1 proteins was detected by immunofluorescence staining with anti-B1.
Transcription activation abilities of the AML1 mutants.
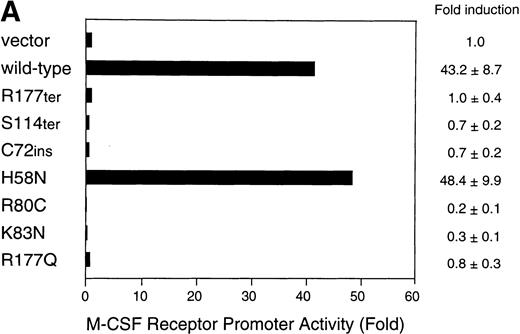
Finally, we measured the transactivation potential of the mutant AML1 proteins using a reporter construct based on the macrophage colony-stimulating factor (M-CSF) receptor promoter, which has been well-characterized as a myeloid-specific AML1-target.20,21 This promoter is also notable for its potential implications in leukemogenesis due to AML1-ETO and PEBP2β/CBFβ-MYH11, because its activity undergoes positive and negative regulations by these fusion proteins, respectively.10 25 With U937 cells as host, the transfection of the wild-type AML1 at an optimal dose effected a strong transactivation up to 40-fold over the mock-transfected control (Fig 5A). H58N again showed an apparently normal activity in this assay as well. In contrast, the remaining nonsilent mutants were unable to elicit any such stimulation at all, showing a good parallelism with their impaired DNA binding or their deleted C-terminal transactivation domain.
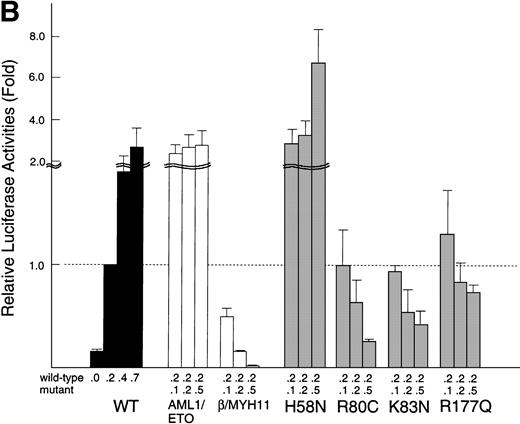
Transactivation of the M-CSF receptor promoter by exogenously expressed AML1 proteins in U937 cells. (A) Cells were transfected with a reporter plasmid (8 μg) and indicated AML1 expression constructs (12 μg) by electroporation. Luciferase activities were measured and presented as the fold increase relative to the control transfected with the backbone expression vector. (B) The wild-type AML1 and missense AML1 mutants were coexpressed in varying doses as indicated. Transfection of plasmids was performed with the aid of a nonliposomal transfection reagent, FuGENE6. Luciferase activities are expressed as fold changes relative to the activity observed at the standard dose (0.2 μg) of the wild-type AML1 alone. In both (A) and (B), each value represents the mean of three separate experiments. Standard deviations of the measurements are given either numerically or by thin vertical bars.
Transactivation of the M-CSF receptor promoter by exogenously expressed AML1 proteins in U937 cells. (A) Cells were transfected with a reporter plasmid (8 μg) and indicated AML1 expression constructs (12 μg) by electroporation. Luciferase activities were measured and presented as the fold increase relative to the control transfected with the backbone expression vector. (B) The wild-type AML1 and missense AML1 mutants were coexpressed in varying doses as indicated. Transfection of plasmids was performed with the aid of a nonliposomal transfection reagent, FuGENE6. Luciferase activities are expressed as fold changes relative to the activity observed at the standard dose (0.2 μg) of the wild-type AML1 alone. In both (A) and (B), each value represents the mean of three separate experiments. Standard deviations of the measurements are given either numerically or by thin vertical bars.
In the above-noted analysis, the promoter activity in the presence of R80C and K83N were persistently lower by severalfold than the mock-transfected control, suggesting that these mutants could dominantly interfere with the action of the endogenous wild-type AML1. To further examine this possibility, we conducted competition experiments in which mutant and wild-type AML1 constructs were cotransfected in varying ratios (Fig 5B). The wild-type AML1 was held at a constant, nonsaturating dose, such that any regulatory perturbation to the AML1-mediated transactivation could be sensitively monitored (see the left-most group of bars marked WT) . In control experiments using AML1-ETO and PEBP2β-MYH11 instead of AML1 point mutants, these fusion proteins indeed elicited prominent stimulations and repressions of the M-CSF receptor promoter, respectively, as previously reported.10 25 In confirmation of our initial inference, R80C and K83N caused substantial dose-dependent inhibitions, although not so strongly as did PEBP2β-MYH11, when their ratio to the wild-type AML1 was increased to unity or more. Rather unexpectedly, R177Q also showed similar progressive inhibitions, although considerably weaker than those observed with the above-noted two mutants. Of interest, in addition, H58N caused marked stimulations by far exceeding those attained with the wild-type AML1 at corresponding supplementary doses. Possible regulatory implications of these findings will be considered in the Discussion below.
DISCUSSION
This study has provided the first demonstration of nontranslocation generated mutations in AML1 among patients with various types of leukemias. The 8 distinct mutations identified were all clustered within the Runt domain and the majority of them, except I87syn and H58N, resulted in production of functionally defective AML1 proteins. These findings have not only deepened our insights into the molecular determinants of the Runt domain functions, but also showed the special importance of this domain as a frequent target of leukemogenic mutations other than and in addition to the known variety of translocations involving AML1. Among the 8 mutations, four major categories are recognizable in terms of their translational context, zygosity, functional influence, and putative leukemogenic significance (Table 1).
The first category consists of biallelic premature-terminating mutations with no functional AML1 allele left: R177ter in patient no. 1 and C72ins plus S114ter in patient no. 2. Coincidentally, the 2 patients were both assigned to the same diagnostic subtype, minimally differentiated acute myeloid leukemia (AML-M0), which is characterized by blast cells that are positive for myeloid antigens (CD13 and CD33) but negative for a cytochemical myeloid marker, myeloperoxidase (MPO).14 These features appear to be consistent with the reported involvements of AML1 in the regulation of various myelopoiesis-related genes, including MPO.26Moreover, the nominal incidence of these mutations among the AML-M0 patients (2 of 9) was considerably high, albeit the number of patients examined was small. Thus, it is tempting to speculate that a biallelic loss of AML1 activity may be one important, relatively frequent route leading to AML-M0. In light of the previous gene targeting studies with mice,4 5 the complete absence of AML1 would inevitably result in a blocked differentiation of stem cells or early committed progenitors for definitive hematopoiesis. AML-M0 cells might well correspond to such differentiation-blocked precursor cells that underwent clonal expansion either as such or in consequence of an additional growth-promoting mutation(s). To test this hypothesis, it would be instrumental to construct animal models in which Aml1can be conditionally disrupted at a postnatal stage. Obviously, it is also required to confirm the putative relation between premature-terminating mutations and AML-M0 in the more large populations.
The second category contains the three hemizygous missense mutations of loss-of-function type: R80C, K83N and R177Q in patients no. 6, 4, and 5, respectively. These mutants were all defective in DNA binding and hence also in transactivation. They were further suggested to act as transdominant inhibitors of the normal AML1, presumably because they could compete with the latter for interactions with the β subunit or other cooperating transcription factors.2 An alternative attractive target for this competition may be the nuclear matrix, to which AML1 has been shown to bind in a manner tightly linked to its transactivation potential as well as its ability to enhance the DNA replication of polyomavirus DNA.27 Whichever be the case, these inhibitory interactions are supposed to occur mainly in the nucleus. On this ground, the weakened but detectable inhibition observed with R177Q may be ascribed to its remaining partial ability for nuclear translocation. In their negative transdominant effects, these mutants are reminiscent of AML1ΔN, a novel isoform of AML1N-terminally truncated to the midst of the Runt domain as previously identified by Zhang et al.28 AML1ΔN was shown to interfere with AML1-dependent transactivation and granulocytic differentiation. By analogy, R80C and K83N, and perhaps also R177Q, may well block the myeloid differentiation to certain aspects and degrees, whereby to contribute to the generation or progression of leukemia. However, the leukemic phenotypes of the 3 patients were different from each other. In this regard, it should be noted that patients no. 4 and 6 additionally harbored translocation-generated alterations,PML/RARΑ and BCR/ABL, respectively, and that they had undergone either a relapse from once controlled AML-M3 (patient no. 4) or a blastic phase of CML (patient no. 6). These situations remarkably resemble those observed with the therapy-related AML or leukemia progressions accompanied by t(3;21).1 Therefore, hemizygous missense mutations in AML1 may play a role in aggravating leukemia in concert with other mutational alterations, if they were not leukemogenic on their own.
The third category with a sole entry of H58N was functionally proficient in all assays tested. Thus, H58N may most simply be taken to represent a polymorphism. However, this mutant was actually demonstrated to cause a hypernormal activation of the M-CSF receptor promoter. This suggests that it might be either intrinsically more potent or metabolically more stable than the wild-type AML1. In any event, there appears to exist an alternative possibility that H58N could contribute to the generation or progression of leukemia through an aberrant upregulation of some AML1-targets. Worthy of note in this connection is the fact that the H58 residue is perfectly conserved in all the three mammalian Runt domain homologs (αA, αB, and αC) as well as the αB homologs in chicken29 andXenopus,30 which implies its functional indispensability.
The fourth, most enigmatic group is the I87syn, which was found in 2 unrelated patients. If this mutation was literally silent, how could it be clonally maintained within leukemic cell populations? We thus infer either that the mutation might exist as a relatively common polymorphism in humans, at least in the local Japanese population studied, or else it might affect the expression of AML1 at a step(s) other than and before translation. These possibilities remain to be explored in the future studies.
The preceding mutational classification raises an intriguing question as to why biallelic alterations were observed recurrently and exclusively with premature-terminating mutations, even though the probability of occurrence of such dual-mutational hits would be supposedly very small. As a simplest conceivable explanation, one mutation might have come from the germline. This possibility was left unresolved, because we were unable to analyze nonleukemic cell samples from patients no. 1 and 2. Alternatively, a somatic mutation that occurred first might confer a proliferative advantage on the mutant clone over the wild-type, so that it could stably persist or expand until another mutation would hit the second allele. In support of this possibility, Aml1+/− ES cells in chimeric mice were shown to contribute to bone marrow and peripheral blood cells more proficiently than the wild-type cells, despite no apparent development of leukemia.4 In either case, a single premature-terminating mutation in AML1 would play a role in generating a preleukemic state, on the basis of which an overt leukemia due to biallelic mutations could develop more frequently than predicted from a simple statistic calculation. For this leukemogenic scenario, the second mutation may have to be of nonsense or frameshift type, because a missense AML1 mutation with negative transdominant effects could be detrimental, rather than beneficial, to cell growth in the absence of any counteraction from the wild-type AML1 product.
With reservation of a few cases, the results of the present study point to a new unifying view that a mutation of AML1 with any qualitative or quantitative anomaly could lead to leukemia in one way or another. The AML1 coding sequence, especially within the Runt domain, contains many scores of nucleotide positions that could give rise to either premature-terminating mutations or deleterious missense mutations31 similar to those identified herein. Thus, we predict that increasing numbers and varieties of point mutations inAML1 will be found upon extended screening with larger populations of leukemia patients, possibly at an overall frequency close to or higher than 3%, as presently observed with the three putative polymorphic cases excluded. Analogous mutations of another Runt domain family gene, PEBP2αA/CBFA1, identified in patients with cleidocranial dysplasia contained broader types of mutations, including deletions and insertions of various sizes as well as point mutations.14 15 In closing, further careful and systematic screening for these kinds of AML1 mutations among leukemia patients, with extended technical approaches, will be awaited to fully delineate their biological, pathogenic, and clinical impacts.
ACKNOWLEDGMENT
The authors thank Drs Masao Matsuoka, Takumi Era, Yu-Wen Zhang, Woo-Young Kim, Tetsuya Ohno, and Kazuhiko Umesono for advice and Drs Shintaro Nishimura and Fumio Kawano for samplings.
Supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture, Grants-in-Aid for Cancer Research from Japanese Ministry of Health and Welfare, and a grant to N.A. from the Okukubo Memorial Fund for Medical Research in Kumamoto University School of Medicine.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Norio Asou, MD, Second Department of Internal Medicine, Kumamoto University School of Medicine, 1-1-1 Honjo, Kumamoto 860-8556, Japan; e-mail:ktcnasou@kaiju.medic.kumamoto-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal