Vitronectin (VN) binds to plasminogen activator inhibitor-1 (PAI-1) and integrins and may play an important role in the vascular response to injury by regulating fibrinolysis and cell migration. However, the role of VN in the earliest response to vascular injury, thrombosis, is not well characterized. The purpose of this study was to test the hypothesis that variation in vitronectin expression alters the thrombotic response to arterial injury in mice. Ferric chloride (FeCl3) injury was used to induce platelet-rich thrombi in mouse carotid arteries. Wild-type (VN +/+, n = 14) and VN-deficient (VN −/−, n = 15) mice, matched for age and gender, were studied. Time to occlusion after FeCl3 injury was determined by application of a Doppler flowprobe to the carotid artery. Occlusion times of VN −/− mice were significantly shorter than those of VN +/+ mice (6.0 ± 1.2 minutesv 17.8 ± 2.3 minutes, respectively, P < .001). Histologic analysis of injured arterial segments showed that thrombi from VN +/+ and VN −/− mice consisted of dense platelet aggregates. In vitro studies of murine VN +/+ andVN −/− platelets showed no significant differences in ADP-induced aggregation, but a trend towards increased thrombin-induced aggregation in VN −/− platelets. Purified, denatured VN inhibited thrombin-induced platelet aggregation, whereas native VN did not. Thrombin times of plasma from VN −/− mice (20.5 ± 2.1 seconds, n = 4) were significantly shorter than those ofVN +/+ mice (34.2 ± 6.7 seconds, n = 4, P < .01), and the addition of purified VN to VN −/− plasma prolonged the thrombin time into the normal range, suggesting that VN inhibits thrombin-fibrinogen interactions. PAI-1-deficient mice (n = 6) did not demonstrate significantly enhanced arterial thrombosis compared with wild-type mice (n = 6), excluding a potential indirect antithrombin function of VN mediated by interactions with PAI-1 as an explanation for the accelerated thrombosis observed in VN−/− mice. These results suggest that vitronectin plays a previously unappreciated antithrombotic role at sites of arterial injury and that this activity may be mediated, at least in part, by inhibiting platelet-platelet interactions and/or thrombin procoagulant activity.
VITRONECTIN (VN) IS A major plasma protein that is also found in platelets and the extracellular matrix of many tissues.1,2 VN binds multiple ligands, including integrins,3 plasminogen activator inhibitor-1 (PAI-1),4 the urokinase receptor (uPAR),5collagen,6 complement C5b-7,7 and heparin.8 These interactions suggest that VN plays an important role in regulating several biologic processes, such as cell adhesion and migration, hemostasis, and immune defense.9 VN is a single-chain 78-kD glycoprotein that consists of an N-terminal somatomedin B domain and two hemopexin-type domains. The somatomedin B domain contains an Arg-Gly-Asp (RGD) sequence that binds integrins and serves as a cell attachment site.3 Plasma VN exists in a native conformation that does not bind integrins.10 Binding to certain ligands, such as PAI-1 or thrombin-antithrombin III, induces conformational changes in VN that expose binding sites for integrins, heparin, and other molecules.11,12 In addition, VN exists in both monomeric and multimeric forms that may serve distinct biologic functions.13
VN appears to play an important role in the response of the blood vessel to injury. VN may control the clearance of vascular thrombi by binding and stabilizing PAI-1, a key regulator of fibrinolysis.4 VN may regulate neointima formation after injury through interactions with αVβ3 and uPAR, receptors expressed on the surface of migrating vascular smooth muscle cells.14,15 However, the role of VN in thrombosis, the earliest response of the blood vessel to injury, is not well defined. VN binds to platelet glycoproteins IIb/IIIa (αIIββ3) and αVβ3 and may mediate platelet adhesion and aggregation at sites of vascular injury.16 In vitro studies have yielded conflicting results regarding the role of VN in platelet function. Asch and Podack17 showed that anti-VN antibodies inhibit platelet aggregation in vitro, suggesting that VN contributes to platelet accumulation at sites of vascular injury. However, Mohri and Ohkubo18 demonstrated that VN inhibited platelet aggregation and competed with fibrinogen and von Willebrand factor for binding to glycoprotein IIb/IIIa, suggesting that VN may prevent platelet-dependent thrombosis. In addition to its platelet interactions, VN may control the thrombotic response to vascular injury by regulating thrombin function. The capacity of PAI-1 to inhibit thrombin is accelerated greater than 200-fold by VN.19,20In addition, thrombin-antithrombin III complexes bind VN, suggesting that VN controls clearance of thrombin from the circulation.21 Recently, VN-deficient mice were generated by a gene-targeting strategy.22 Mice lacking VN develop normally and do not exhibit any discernible phenotypic abnormalities. In this study, we have subjected wild-type (VN +/+) mice and VN-deficient (VN −/−) mice to carotid artery injury to test the hypothesis that VN is an important determinant of the acute thrombotic response to vascular injury. Our results suggest that VN plays an important antithrombotic role in vivo.
MATERIALS AND METHODS
Mice.
C57BL/6J mice were purchased from Jackson Labs (Bar Harbor, ME). The generation of VN-deficient mice by homologous recombination in embryonic stem cells has been reported previously.22PAI-1–deficient (PAI-1 −/−) mice were a gift from Dr P. Carmeliet (University of Leuven, Leuven, Belgium).23To eliminate potential effects of genetic differences among mouse strains on experimental results, consecutive generations of mice carrying the null VN allele were backcrossed to C57BL/6J mice. Only mice that were the product of ≥8 backcrosses were used in experiments comparing VN−/− mice with VN +/+ mice. PAI-1−/− mice used in experiments were the product of greater than 10 backcrosses to the C57BL/6J genetic background. Genotyping of mice was performed by polymerase chain reaction (PCR) analysis of tail DNA as described previously.22 24 All animal care and experimental procedures complied with the Guide for Care and Use of Laboratory Animals (Department of Health, Education, and Welfare Publication No. NIH 78-23) and were approved by the University of Michigan Committee on Use and Care of Animals.
Reagents.
Native human VN was purchased from Molecular Innovations Inc (Royal Oak, MI). Human α-thrombin was from CalBiochem (La Jolla, CA). Thrombin substrate (Spectrozyme TH) was from American Diagnostica (Greenwich, CT). Ferric chloride (FeCl3) was from Mallinckrodt Chemical (Paris, KY). Human VN purified by heparin-affinity chromatography, reptilase (Atroxin), and human fibrinogen (plasminogen-free) were from Sigma (St Louis, MO).
Thrombosis protocol.
A previously described carotid artery thrombosis protocol was used.25 26 Adult mice (6 to 8 weeks old; weight, ∼25 g) were anesthetized by intraperitoneal injection of pentobarbital (120 mg/kg). The left common carotid artery was surgically exposed and a miniature Doppler flowprobe (Model 0.5VB; Transonic Systems, Ithaca, NY) was placed on the surface of the artery. Sodium chloride solution (0.9%) was placed in the surgical wound to allow Doppler monitoring, and baseline blood flow was recorded using a Transonic Model T106 flowmeter. Thereafter, sodium chloride solution was removed from the wound and filter paper (0.5 × 1.0 mm) saturated with 10% FeCl3 was applied to the adventitial surface of the carotid artery, immediately proximal to the flow probe. After 3.0 minutes, the filter paper was removed, saline solution was again placed in the wound, and carotid blood flow was monitored (ie, it was not possible to monitor carotid artery blood flow during the application of FeCl3). Time to thrombotic occlusion after initiation of arterial injury was defined as the time required for blood flow to decline to 0 mL/min. If the carotid artery was observed to be thrombosed at the earliest time point that flow could be monitored after initiation of injury (ie, 3.0 minutes), time to occlusion was recorded as ≤3.0 minutes. The operator was blinded to mouse genotype while performing all experiments.
Histologic analyses.
For some animals, the arterial vasculature was perfusion fixed immediately after completing the thrombosis protocol, as described previously.27 Injured arterial segments were excised, embedded in paraffin, sectioned, and subjected to hematoxylin and eosin staining.
Bleeding assay.
Mice (6 to 8 weeks old) were anesthetized by intraperitoneal injection of phenobarbital (100 mg/kg) and placed in a restraining chamber from which the tail protruded. The distal 1 mm of the tail was amputated and the tail was immersed for 10 minutes in 1 mL of 0.9% NaCl warmed to 37°C. Blood loss was determined by measuring the absorbance of saline at 560 nm and comparing the result to a standard curve constructed from known volumes of mouse blood.
In vitro platelet aggregation.
Platelet aggregation was studied using a previously described microtiter plate assay.28 Blood was collected into citrate anticoagulant from anesthetized mice by inferior vena cava puncture with a 25-gauge needle. Platelet-rich plasma (PRP) was prepared by centrifuging blood (120 g for 6 minutes) in 0.5 mL polypropylene tubes at room temperature in a swing-out rotor. After adjustment to a platelet count of 2.5 × 108/mL by the addition of citrated platelet-poor plasma, 95 μL of count-adjusted PRP was placed in 96-sample microtiter plate wells and incubated at 37°C in a SpectraMax 340 microtiter plate reader (Molecular Devices, Sunnyvale, CA). ADP (12.5 μmol/L) was added, and the absorbance of wells at 595 nm was monitored at 20- to 30-second intervals. Plates were shaken automatically for 15 seconds between each reading. The percentage of aggregation was calculated as described.28 Gel-filtered platelets suspended in Tyrode’s buffer,29 prepared by Sepharose 2B chromatography (Sigma), were used to study thrombin-induced aggregation as described above.
Coagulation and hematologic assays.
Platelet-poor plasma was prepared by centrifuging citrated mouse blood for 8 minutes at 16,000g. Thrombin times, activated partial thromboplastin times (APTTs), and reptilase times were performed using a KC4A Micro apparatus (Amelung GmbH, Lemgo, Germany) according to the manufacturer’s instructions. Thrombin amidolytic activity was measured by incubating thrombin and Spectrozyme TH at room temperature in 0.01 mol/L Tris-HCl, 0.14 mol/L NaCl, pH 7.5, and monitoring the absorbance of reaction mixtures at 405 nm in a microtiter plate reader. Platelet counts and hematocrits of citrated whole blood were measured using a Model H-10 blood cell counter (Texas Instruments Laboratories, Houston, TX). Fibrinogen/fibrin degradation products (FDP) were measured with a Staphylococcal clumping factor assay (Catalog 850-ST; Sigma) according to the manufacturer’s instructions. Plasma fibrinogen concentrations were determined by the fibrin clot opacity method, as described.30 31 In this assay, the limit optical density of dilute plasma during prolonged incubation with thrombin is directly proportional to plasma fibrinogen concentration. Briefly, 20 μL of citrated plasma were added to a spectrophotometer cuvette containing 400 μL of 0.05 mol/L Tris-HCl (pH 7.5), 0.15 mol/L NaCl. Thrombin/calcium solution (20 μL) was added to yield final concentrations of 0.45 U/mL and 0.9 mmol/L, respectively. After 10 minutes of incubation at room temperature, absorbance at 340 nm was measured. Fibrinogen concentration in pooled plasma prepared from 4VN +/+ mice was defined as 100%. Fibrinogen levels in pooled (n = 4 mice) VN −/− plasma were determined by comparison to a standard curve constructed from dilutions of pooledVN +/+ plasma. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting of diluted human plasma was performed with the PhastSystem (Pharmacia, Uppsala, Sweden). Primary antibody was goat antimouse fibrinogen (Accurate Inc, Westbury, NY). Secondary antibody was peroxidase-conjugated rabbit antigoat IgG (Zymed Labs, South San Francisco, CA). Blots were developed by the chemiluminescence method (ECL reagent kit; Amersham, Little Chalfont, UK).
Statistical analyses.
Data are presented as mean ± 1 standard error of the mean (SEM), unless otherwise indicated. The two-sample t-test or Mann-Whitney Rank Sum test were used to determine if significant differences existed between experimental groups.
RESULTS
Arterial thrombosis studies.
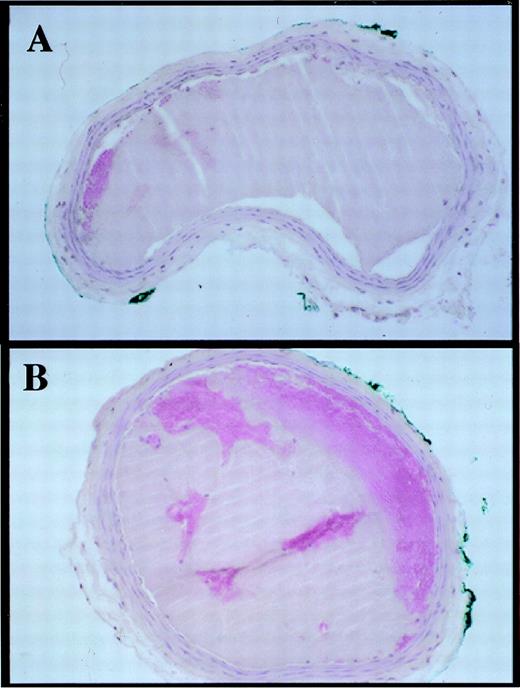
Experimental groups consisted of 14 VN +/+ mice (5 male and 9 female) and 15 VN −/− mice (6 male and 9 female). Representative carotid artery blood flow tracings before and after vascular injury are shown in Fig 1. Thrombotic occlusion occurred ≤3.0 minutes after initiation of vascular injury in 8 of 15 VN −/− mice, but only in 1 of 14 VN +/+ mice (Table 1). Median occlusion times were 16.4 minutes for VN +/+ mice and ≤3.0 minutes for VN −/− mice. Mean occlusion times (calculated by using values of 3.0 minutes as occlusion times for mice whose arteries were already occluded when flow monitoring was resumed after vascular injury) were significantly shorter in VN−/− mice (6.0 ± 1.2 minutes) than in VN +/+ mice (17.8 ± 2.3 minutes, P < .001). Blood platelet counts and hematocrits did not differ significantly between VN+/+ and VN −/− mice (Table 2). APTTs, reptilase times, and bleeding in response to tail tip amputation were similar between groups. Plasma fibrinogen measured by the fibrin clot opacity method did not differ between groups (100% ± 10.8% and 93.8% ± 7.2% in VN +/+ mice and VN −/− mice, respectively; P > .7). Similarly, Western blot analysis of diluted plasma samples showed no apparent differences in plasma fibrinogen between VN +/+ mice and VN −/− mice (data not shown). Fibrinogen/fibrin degradation products were not detectable in pooled serum obtained from VN +/+ mice (n = 4) orVN −/− mice (n = 4). Control experiments showed that the FDP assay readily detected murine fibrin degradation products (data not shown). Mean carotid artery blood flow prior to injury did not differ between VN +/+ mice and VN −/− mice (0.78 ± 0.2 mL/min and 0.85 ± 0.2 mL/min, respectively,P > .2). No significant differences in gross or microscopic appearance of uninjured arteries were noted between VN +/+ andVN −/− mice (n = 3 each group, data not shown). Histologic analysis of thrombi (n = 4) recovered immediately after carotid injury showed that they consisted predominantly of platelets, with no discernible differences between genotypes (Fig2).
Carotid artery blood flow tracings obtained from a wild-type mouse and a VN-deficient mouse. Period of ferric chloride injury is indicated by hatched bar. Artifactual reduction in flow during injury is due to removal of saline from the surgical site to allow application of FeCl3. Thrombotic occlusion occurs 18.7 minutes and ≤3.0 minutes after initiation of injury in theVN +/+ mouse and theVN −/− mouse, respectively.
Carotid artery blood flow tracings obtained from a wild-type mouse and a VN-deficient mouse. Period of ferric chloride injury is indicated by hatched bar. Artifactual reduction in flow during injury is due to removal of saline from the surgical site to allow application of FeCl3. Thrombotic occlusion occurs 18.7 minutes and ≤3.0 minutes after initiation of injury in theVN +/+ mouse and theVN −/− mouse, respectively.
Occlusion Times (in Minutes) of VN +/+ Mice and VN −/− Mice
| . | VN +/+ (n = 14) . | VN −/− (n = 15) . |
|---|---|---|
| 13.0 | ≤3.0 | |
| 28.5 | ≤3.0 | |
| 14.8 | 5.5 | |
| 16.8 | ≤3.0 | |
| 3.8 | 4.5 | |
| 16.0 | 4.2 | |
| 19.7 | ≤3.0 | |
| 33.8 | ≤3.0 | |
| 22.2 | 12.0 | |
| 28.7 | ≤3.0 | |
| ≤3.0 | 12.3 | |
| 20.3 | ≤3.0 | |
| 14.7 | 11.6 | |
| 13.5 | 15.8 | |
| ≤3.0 | ||
| Median | 16.4 | ≤3.0 |
| Mean ± SEM | 17.8 ± 2.3 | 6.0 ± 1.2 |
| . | VN +/+ (n = 14) . | VN −/− (n = 15) . |
|---|---|---|
| 13.0 | ≤3.0 | |
| 28.5 | ≤3.0 | |
| 14.8 | 5.5 | |
| 16.8 | ≤3.0 | |
| 3.8 | 4.5 | |
| 16.0 | 4.2 | |
| 19.7 | ≤3.0 | |
| 33.8 | ≤3.0 | |
| 22.2 | 12.0 | |
| 28.7 | ≤3.0 | |
| ≤3.0 | 12.3 | |
| 20.3 | ≤3.0 | |
| 14.7 | 11.6 | |
| 13.5 | 15.8 | |
| ≤3.0 | ||
| Median | 16.4 | ≤3.0 |
| Mean ± SEM | 17.8 ± 2.3 | 6.0 ± 1.2 |
For calculation of means, occlusion times of ≤3.0 minutes were assigned the value of 3.0 minutes.
Hemostatic Parameters of VN +/+ Mice and VN −/− Mice
| Parameter . | VN +/+ . | VN −/− . | PValue . |
|---|---|---|---|
| Platelet count (×109mL) | 1.18 | 1.21 | |
| Hematocrit (%) | 38.9 | 37.8 | |
| APTT (seconds) | 31.3 ± 0.5 (4) | 30.5 ± 0.4 (4) | >5 |
| Reptilase time (seconds) | 40.6 ± 5.8 (4) | 38.4 ± 1.0 (4) | >.5 |
| Tail bleeding assay (mL blood loss) | 0.52 ± .05 (5) | 0.43 ± 0.04 (4) | >.3 |
| Plasma fibrinogen (% normal pooled plasma) | 100 ± 10.8 | 93.8 ± 7.2 | >.7 |
| FDP | Not detctable | Not detectable |
| Parameter . | VN +/+ . | VN −/− . | PValue . |
|---|---|---|---|
| Platelet count (×109mL) | 1.18 | 1.21 | |
| Hematocrit (%) | 38.9 | 37.8 | |
| APTT (seconds) | 31.3 ± 0.5 (4) | 30.5 ± 0.4 (4) | >5 |
| Reptilase time (seconds) | 40.6 ± 5.8 (4) | 38.4 ± 1.0 (4) | >.5 |
| Tail bleeding assay (mL blood loss) | 0.52 ± .05 (5) | 0.43 ± 0.04 (4) | >.3 |
| Plasma fibrinogen (% normal pooled plasma) | 100 ± 10.8 | 93.8 ± 7.2 | >.7 |
| FDP | Not detctable | Not detectable |
Platelet counts and hematocrits were measured with pooled, heparinized blood obtained from 3 mice. Fibrinogen was measured in triplicate with pooled, citrated plasma obtained from 4 mice. Fibrinogen/fibrin degradation products (FDP) were measured with pooled serum obtained from 5 mice. Other parameters were performed on individual samples obtained from the number of mice indicated in parentheses.
Transverse sections of carotid arteries excised immediately after ferric chloride-induced thrombosis. (A) VN+/+ mouse. (B) VN −/− mouse. Thrombi consist of dense platelet aggregates (hematoxylin and eosin staining, original magnification × 200). The diameter of the mouse carotid artery is approximately 0.5 mm.
Transverse sections of carotid arteries excised immediately after ferric chloride-induced thrombosis. (A) VN+/+ mouse. (B) VN −/− mouse. Thrombi consist of dense platelet aggregates (hematoxylin and eosin staining, original magnification × 200). The diameter of the mouse carotid artery is approximately 0.5 mm.
Characterization of in vitro platelet function.
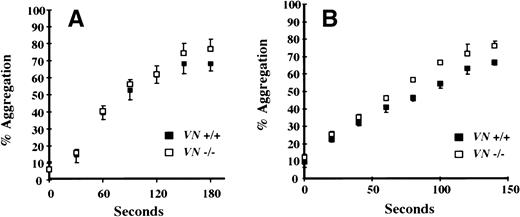
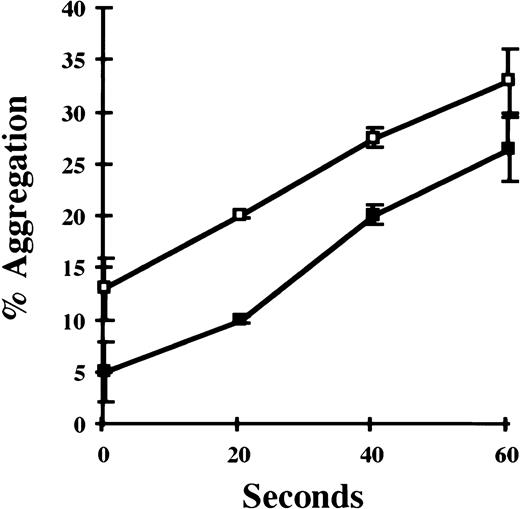
Given the platelet-rich composition of carotid artery thrombi generated in this model, platelet aggregation studies were performed to compare platelet function of VN +/+ and VN −/− mice. Pooled samples of platelet-rich plasma were prepared and in vitro aggregation was induced with ADP (12.5 μmol/L). As shown in Fig 3A, no significant differences were observed between VN +/+ and VN −/− mice. There was a trend towards enhanced thrombin-induced aggregation of washed VN −/− platelets compared with VN+/+ platelets (Fig 3B). Consistent with this observation, addition of heparin-affinity-purified (ie, denatured-renatured) human VN (200 μg/mL) to washed VN −/− platelets significantly inhibited thrombin-induced aggregation (P < .005; Fig 4). However, native human VN (350 μg/mL) had no detectable effect on thrombin-induced aggregation (data not shown).
In vitro function of mouse platelets. (A) ADP-induced platelet aggregation. Citrated PRP was prepared from VN +/+ mice (n = 2) and VN −/− mice (n = 2). After adjusting platelet counts to 2.5 × 108/mL, ADP (12.5 μmol/L)-induced platelet aggregation was studied in 96-well microtiter plates that were warmed to 37°C and automatically shaken. (B) Thrombin-induced platelet aggregation. Washed platelets were prepared from VN +/+ mice (n = 3) and VN−/− mice (n = 3) and suspended in Tyrode’s buffer at a concentration of 2.5 × 108/mL. Thrombin (1 U/mL)-induced platelet aggregation was studied as described for ADP. Data points represent the mean of triplicate experiments ± 1 SD.
In vitro function of mouse platelets. (A) ADP-induced platelet aggregation. Citrated PRP was prepared from VN +/+ mice (n = 2) and VN −/− mice (n = 2). After adjusting platelet counts to 2.5 × 108/mL, ADP (12.5 μmol/L)-induced platelet aggregation was studied in 96-well microtiter plates that were warmed to 37°C and automatically shaken. (B) Thrombin-induced platelet aggregation. Washed platelets were prepared from VN +/+ mice (n = 3) and VN−/− mice (n = 3) and suspended in Tyrode’s buffer at a concentration of 2.5 × 108/mL. Thrombin (1 U/mL)-induced platelet aggregation was studied as described for ADP. Data points represent the mean of triplicate experiments ± 1 SD.
Effect of purified VN on in vitro platelet aggregation. Gel-filtered VN −/− platelets (2.0 × 108/mL) were incubated with thrombin (1 U/mL) in the absence (□) or presence (▪) of denatured human VN (200 μg/mL), and platelet aggregation was studied as described in Fig 3. Data points represent the mean of triplicate experiments ± 1 SEM.
Effect of purified VN on in vitro platelet aggregation. Gel-filtered VN −/− platelets (2.0 × 108/mL) were incubated with thrombin (1 U/mL) in the absence (□) or presence (▪) of denatured human VN (200 μg/mL), and platelet aggregation was studied as described in Fig 3. Data points represent the mean of triplicate experiments ± 1 SEM.
Effects of VN on thrombin function.
Because thrombin is an important determinant of platelet-dependent arterial thrombosis,32 we performed experiments to test the hypothesis that VN produces its anticoagulant effect by inhibiting thrombin procoagulant activity. Thrombin times were performed by adding human α-thrombin (4.4 U/mL) to pooled samples (n = 4) of citrated plasma. Thrombin times of plasma from VN −/− mice (20.5 ± 2.1 seconds) were significantly shorter than those ofVN +/+ mice (34.2 ± 6.7 seconds, P < .01). Furthermore, addition of native human VN (350 μg/mL) to VN−/− mouse plasma prolonged the thrombin time into the normal range, whereas the addition of an equal volume of buffer containing bovine serum albumin (BSA; 350 μg/mL) had no effect (Table 3). VN also inhibited clotting of purified human fibrinogen (thrombin times, 32.9 ± 4.9 seconds and 42.9 ± 2.4 seconds in the absence and presence of VN [350 μg/mL], respectively; P < .05), but had no effect on thrombin amidolytic activity, measured by thrombin (25 nmol/L) hydrolysis of low molecular weight substrate (Spectrozyme TH; 150 μmol/L; data not shown).
Effect of VN on Thrombin Time
| Pooled Plasma . | Thrombin Time (seconds) . | P Value . |
|---|---|---|
| VN +/+ (n = 4) | 34.2 ± 6.7 | |
| VN −/− (n = 4) | 20.5 ± 2.1 | <.01 v VN +/+ |
| VN−/− plus purified VN (n = 3) | 41.2 ± 5.5 | <.001 v VN −/− |
| Pooled Plasma . | Thrombin Time (seconds) . | P Value . |
|---|---|---|
| VN +/+ (n = 4) | 34.2 ± 6.7 | |
| VN −/− (n = 4) | 20.5 ± 2.1 | <.01 v VN +/+ |
| VN−/− plus purified VN (n = 3) | 41.2 ± 5.5 | <.001 v VN −/− |
Thrombin times were performed by mixing 100 μL of citrated pooled plasma, 55.5 μL of BSA or VN (1.3 mg/mL in PBS), and 50 μL of thrombin (18 U/mL) and measuring time to clot formation at 37°C.
Effects of VN on PAI-1 function.
VN accelerates the capacity of PAI-1 to inhibit thrombin by greater than 200-fold.19 20 If the antithrombotic effect of VN observed in our model were mediated by enhancement of the antithrombin function of PAI-1, then PAI-1 deficiency would be expected to mimic VN deficiency, resulting in accelerated thrombus formation. To test this hypothesis, we measured the time to occlusive thrombus formation after FeCl3 carotid artery injury in PAI-1 +/+ mice (n = 6) and PAI-1 −/− mice (n = 6). However, mean occlusion times did not differ significantly between groups (15.5 ± 1.9 minutes and 13.8 ± 2.4 minutes forPAI-1 +/+ mice and PAI-1 −/− mice, respectively; P > .5).
DISCUSSION
In this study, we observed a significantly enhanced rate of thrombus formation after arterial injury in VN −/− mice compared with VN +/+ mice. We used ferric chloride injury to trigger thrombosis in our experiments. This method has been used in a variety of species and vascular sites to trigger platelet-dependent thrombosis.26,33,34 Iron ions enhance conversion of O2− and H2O2 to oxidizing species, such as hydroxyl radical, that injure endothelial cells and markedly increase tissue factor expression in vitro and in vivo.35-38 The markedly enhanced rate of thrombosis inVN −/− mice compared with VN +/+ mice suggests that VN plays a previously unsuspected role in protecting the injured arterial wall against thrombus formation.
Our in vitro studies suggest that the antithrombotic effect of VN may be mediated by inhibition of platelet-platelet and thrombin-substrate interactions. VN binds platelet glycoprotein IIb/IIIa,16,18providing a mechanism by which VN may modulate platelet function. Because VN contains a single RGD and mutagenesis of this sequence blocks platelet binding,3 monomeric VN would not be expected to support platelet aggregation. In fact, prior in vitro studies demonstrated that VN inhibits platelet aggregation and competes with fibrinogen and von Willebrand factor for binding to platelets, leading to the hypothesis that VN inhibits platelet-dependent thrombosis.18 Our experiments provide the first in vivo data to support this hypothesis. We observed that VN purified under denaturing conditions, which expose its cell attachment site,10 inhibited thrombin-induced platelet aggregation. However, native VN, which does not bind integrins,10 did not. A hypothesis that is consistent with our in vivo and in vitro data is that plasma VN undergoes a conformational change at sites of vascular injury, thereby exposing its integrin-binding site and inhibiting platelet-platelet interactions by competing with fibrinogen, von Willebrand factor, or other factors for binding to glycoprotein IIb/IIIa on activated platelets. Such a negative feedback mechanism could serve to prevent excessive platelet accumulation and vascular occlusion after injury to the blood vessel wall. The proportion of VN capable of binding heparin is less than 2% in plasma, but increases over threefold upon formation of serum,39 suggesting that activation of the coagulation cascade triggers conversion of native VN to a conformationally altered form capable of binding platelets. Several factors generated or released at sites of arterial injury bind VN and expose its RGD site, including thrombin-antithrombin complex, PAI-1, and C5b-7.7,11,12 This functional activation of VN by ligand binding also may explain the inhibition of platelet aggregation by anti-VN antibodies in experiments by Asch and Podack.17 It is possible that the antibodies used in these experiments induced a conformational change in VN that exposed its RGD site, thereby inhibiting platelet aggregation by enabling VN to compete with fibrinogen for binding to platelet glycoprotein IIb/IIIa. In addition to the plasma, VN is present in platelets and the blood vessel wall.40,41 Although platelet VN and blood vessel wall VN exist to a significant extent as multimeric forms capable of supporting platelet aggregation,13 we observed accelerated platelet thrombus formation in VN −/−mice. These results suggest that, in this model, the platelet or vessel wall pools of VN are not required for platelet accumulation at sites of arterial injury and that the antithrombotic properties of VN are dominant over its potential procoagulant function.
An additional mechanism by which VN may inhibit thrombosis is by downregulating thrombin function. We demonstrated that thrombin induces clot formation more rapidly in VN-deficient plasma than in normal plasma and that addition of purified VN to VN-deficient plasma prolongs the thrombin time. Similar VN effects were observed on thrombin clotting of purified fibrinogen. VN deficiency had no effect on reptilase clotting times, suggesting that VN does not affect polymerization of fibrin monomer. VN did not inhibit thrombin amidolytic activity. Together, these results suggest that VN inhibits thrombin-fibrinogen interactions by binding to thrombin at a site distinct from its active-site, as suggested previously by Naski et al.20 A direct antithrombin effect of VN could contribute to the enhanced thrombotic response observed in VN−/− mice. In addition to its direct effects, it has been proposed that VN may inhibit thrombin function indirectly by accelerating PAI-1–mediated thrombin inhibition greater than 200-fold.19 20 However, we did not observe a significantly accelerated thrombotic response in PAI-1 −/− mice, which appears to exclude VN-dependent thrombin inhibition by PAI-1 as a mechanism responsible for the longer thrombosis times observed inVN +/+ mice.
In summary, we have shown that VN-deficient mice form occlusive arterial thrombi at an accelerated rate compared with wild-type mice. We hypothesize that the antithrombotic properties of VN are mediated by its interaction with platelet glycoprotein IIb/IIIa and by its capacity to inhibit thrombin-fibrinogen interactions, although we cannot exclude that other mechanisms may be operative as well. These findings represent the first phenotypic abnormality observed in VN−/− mice and suggest an important role for VN in inhibiting platelet-dependent thrombosis at sites of arterial injury, a previously unrecognized function of this adhesive glycoprotein.
ACKNOWLEDGMENT
The authors thank Randal Westrick for assistance with mouse breeding and genotyping, Mary Ellen Wechter for assistance with phlebotomy, and Drs Alvin Schmaier and Benedict Lucchesi for sharing laboratory equipment.
Supported by National Institutes of Health Grants No. HL-57346 and HL-49184. D.G. is a Howard Hughes Medical Institute investigator.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to William P. Fay, MD, University of Michigan Medical Center, 7301 MSRB III, 1150 W Medical Center Dr, Ann Arbor, MI 48109-0644; e-mail: wfay@umich.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal