Abstract
The identification of stromal cell–derived factor (SDF)-1 as a chemoattractant for human progenitor cells suggests that this chemokine and its receptor might represent critical determinants for the homing, retention, and exit of precursor cells from hematopoietic organs. In this study, we investigated the expression profile of CXCR4 receptor and the biological activity of SDF-1 during megakaryocytopoiesis. CD34+ cells from bone marrow and cord blood were purified and induced to differentiate toward the megakaryocyte lineage by a combination of stem-cell factor (SCF) and recombinant human pegylated megakaryocyte growth and development factor (PEG-rhuMGDF). After 6 days of culture, a time where mature and immature megakaryocytes were present, CD41+ cells were immunopurified and CXCR4mRNA expression was studied. High transcript levels were detected by a RNase protection assay in cultured megakaryocytes derived from cord blood CD34+ cells as well as in peripheral blood platelets. The transcript levels were about equivalent to that found in activated T cells. By flow cytometry, a large fraction (ranging from 30% to 100%) of CD41+cells showed high levels of CXCR4 antigen on their surface, its expression increasing in parallel with the CD41 antigen during megakaryocytic differentiation. CXCR4 protein was also detected on peripheral blood platelets. SDF-1 acts on megakaryocytes by inducing intracellular calcium mobilization and actin polymerization. In addition, in in vitro transmigration experiments, a significant proportion of megakaryocytes was observed to respond to this chemokine. This cell migration was inhibited by pertussis toxin, indicating coupling of this signal to heterotrimeric guanine nucleotide binding proteins. Although a close correlation between CD41a and CXCR4 expession was observed, cell surface markers as well as morphological criteria indicate a preferential attraction of immature megakaryocytes (low level of CD41a and CD42a), suggesting that SDF-1 is a potent attractant for immature megakaryocytic cells but is less active on fully mature megakaryocytes. This hypothesis was further supported by the observation that SDF-1 induced the migration of colony forming unit–megakaryocyte progenitors (CFU-MK) and the expression of activation-dependent P-selectin (CD62P) surface antigen on early megakaryocytes, although no effect was observed on mature megakaryocytes and platelets. These results indicate that CXCR4 is expressed by human megakaryocytes and platelets. Furthermore, based on the lower responses of mature megakaryocytes and platelets to SDF-1 as compared with early precursors, these data suggest a role for this chemokine in the maintenance and homing during early stages of megakaryocyte development. Moreover, because megakaryocytes are also reported to express CD4, it becomes important to reevaluate the role of direct infection of these cells by the human immunodeficiency virus (HIV)-1 in HIV-1–related thrombocytopenia.
MATURE BLOOD CELLS are derived from hematopoietic precursor cells that are present in specific (hematopoietic) tissues. Within this environment, hematopoietic cells lodge, proliferate, and differentiate. Upon maturation, cells must traverse specialized venus sinus walls to enter the circulation.1 Cellular interactions between precursor and stroma cells are thought to serve multiple functions, including retention of precursor cells within the hematopoietic tissue and regulation of the release of mature hematopoietic cells into the circulation, although the exact cellular and molecular mechanisms involved are not well understood.2 Like other blood leukocytes, platelets are continuously generated from precursors cells in the bone marrow.3,4 Megakaryocyte maturation proceeds by a series of steps characterized by the appearance of cell surface markers, including platelet glycoproteins.5
In the past few years, a new superfamily of chemotactic cytokines named chemokines has been described. These chemokines have been implicated in many aspects of leukocyte behavior, including regulation of leukocyte adhesion, locomotion, and chemotaxis.6 Depending on the number and spacing of conserved cysteines, the chemokine superfamily can be divided into four groups designed C, CC, CXC, and CX3C. The C and CX3C chemokines include only one known member, whereas the CC and CXC groups each have several members. CXC chemokines mainly target neutrophils but also show some action on T cells. CC chemokines exert their action on multiple leukocyte populations, including monocytes, eosinophils, basophils, T cells, natural killer (NK), and dendritic cells with variable selectivity, but in most cases they do not target neutrophils. Stromal cell–derived factor 1 (SDF-1), also called pre–B stimulating factor, is a CXC chemokine cloned from mouse bone marrow stromal cells.7 Besides its powerful chemoattactant effect on T cells,8,9 SDF-1 has been shown to be a chemoattractant for early hematopoietic cells including uncommitted and committed progenitor cells.10 It has also been shown that SDF-1 is a chemoattractant for early B-cell precursors and may partially replace the need for stromal cells for in vitro generation of B cells.8,10,11 Moreover, inactivation of the SDF-1 gene induces a marked defect in B-cell lymphopoiesis and bone marrow myelopoiesis, although normal numbers of myeloid progenitors were observed in fetal liver, suggesting that SDF-1 is the major chemoattractant of hematopoietic cells into the marrow.12
SDF-1 was found to be the ligand for a previously identified orphan receptor called HUMSTR or LESTR.8,9,13 Following the conventions established for the nomenclature of chemokine receptors, this receptor has been renamed CXC chemokine receptor 4 (CXCR4). This receptor, also called fusin, has been identified as one of the major coreceptors that in association with CD4 allows the entry into cells of lymphocytotropic HIV-1 strains.14-18
Knowledge of the expression and regulation of this receptor is of prime importance for understanding what drives the homing, retention, and exit of megakaryocytes and their precursors from hematopoietic organs and for understanding the mechanisms involved in HIV-1 related thrombocytopenia.
Until recently, expression of CXCR4 chemokine receptors on cells of the megakaryocyte/platelet lineage had not been studied. The goal of this study was to investigate whether CXCR4 was expressed on cells of the megakaryocyte/platelet lineage. We could demonstrate, as also recently shown,19 20 that megakaryocytes expressed very high level of CXCR4. CXCR4 expression increased with maturation and was also found in platelets. Our results also show that this receptor is only functional in a subset of megakaryocytes expressing both low levels of CD41a and CD42a, suggesting that SDF-1 action predominates during early stages of human megakaryocytic differentiation.
MATERIALS AND METHODS
Cord Blood, Bone Marrow Cells, and Platelets
Cord blood samples from normal full-term newborn infants were obtained from a cord blood bank (Dr Van Nifderick, Hopital St Vincent de Paul, Paris, France). After informed consent, bone fragments from adult patients undergoing hip surgery were collected in heparin-containing medium. Marrow cells were collected by vigorous shaking of bone fragments in α–minimum essential medium (MEM) supplemented with 100 μg/mL of deoxyribonuclease (Sigma, St Louis, MO; DNase type I). Low-density mononuclear cells (LDMC) were prepared by centrifugation on Lymphoprep (Nyegaard, Oslo, Norway) and were used for immunomagnetic bead separation.
Primary megakaryocytes were enriched from bone marrow using fractionation over a discontinuous Percoll (Pharmacia, les Ulis, France) density gradient as previously reported.21 Blood platelets were purified by gel filtration on Sepharose 2B in a buffer containing NaCl 129 mmol/L, Na3 citrate 13.6 mmol/L, glucose 11.1 mmol/L, KH2PO4 1.6 mmol/L, and NaH2PO4 8.6 mmol/L, pH 7.3.
Antibodies
Directly conjugated monoclonal antibodies (MoAb) R-phycoerythrin (PE)–HPCA2 (anti-CD34), PE–anti-CD62 (anti–P-selectin), and fluorescein isothiocyanate (FITC) anti-CD41a and CD42a were obtained from Becton Dickinson (Mountain View, CA). FITC-TAB (anti-CD41b) was provided by Dr R. McEver (Oklahoma Medical Research Foundation). PE-12G5 (anti-CXCR4) and a PE-CD41a MoAbs were obtained from Pharmingen (San Diego, CA) and FITC- and PE-conjugated IgG1 and IgG2a MoAb controls from Becton Dickinson. Unconjugated antibodies directed against CXCR4 (12G5) and an isotype-control MoAb were obtained from R&D Systems (Minneapolis, MN).
Human Cytokines
Recombinant human stem cell factor (rhuSCF) and PEG-rhuMGDF (gifts of Amgen Corp, Thousand Oaks, CA) were usually used at a final concentration of 50 ng/mL and 10 ng/mL, respectively. Recombinant human SDF-1α was obtained from R&D Systems.
Isolation of CD34+ Cells or Cultured Megakaryocytes
Mononuclear cells were separated using a magnetic cell sorting system (miniMACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) in accordance with the manufacturer’s recommendations. The purity of CD34+ cells recovered was determined by flow cytometry using PE-HPCA2 and was over 90%. Cultured megakaryocytes were purified by the same technique using an anti-CD41b MoAb. Purity after purification was over 95% as attested by labeling with a PE–anti-CD41a MoAb.
Cell Cultures
Liquid cultures.
CD34+ cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM) with penicillin/streptomycin/glutamine and 11.5 mmol/L α-thioglycerol (Sigma). Cultures were performed in serum-free conditions in which IMDM was supplemented with 1.5% bovine serum albumin (BSA; Cohn’s fraction V; Sigma), sonicated lipids, and iron-saturated human transferrin and stimulated by the combination of PEG-rhuMGDF and SCF (MK medium).22
Semisolid cultures.
Cultures were performed in serum-free clot in the presence of PEG-rhuMGDF and SCF. Ingredients for serum-free cultures were similar to those of liquid culture in which were added bovine plasma fibrinogen (1 mg/mL, Sigma), 0.01 mol/L ε amino caproic acid and horse thrombin (6 mU/mL; Stago, Asnières, France). Target cells were either CD34+ cells (1 × 103 cells/mL) or cultured cells before and after in vitro migration. Cultures were incubated at 37°C in a fully humidified atmosphere containing 5% CO2 in air and scored after 10 to 12 days. Colonies were quantitated by an indirect immuno-alkaline phosphatase labeling technique using an anti-GPIIIa MoAb (CD61, Y2-51) as previously described.23 Dishes were scanned in toto under an inverted microscope at 4× or 100× magnification.
Flow Cytometry Analysis
After washing, cells were stained with an appropiate dilution of the antibody. Double staining was performed using differently conjugated IgG1 as a control. Cells were suspended in phosphate buffer (PBS), kept at 4°C, and analyzed on a FACsort (Becton Dickinson) with the Cell Quest software package.
Chemotactic Assay
To analyze megakaryocyte migration, CD34+ cells were cultured for 6 to 12 days in MK medium. Unless otherwise specified, the cells were used directly for migration assays. In a limited number of experiments (n = 6), cultured cells were immunopurified on the basis of CD41a expression before the migration assay. The cells were then resuspended in serum-free medium to a final concentration of 2.5 × 106 cells/mL, and 100 μL of the cell suspension was placed into the upper chamber, whereas 600 μL of medium with or without SDF-1α was introduced in the lower chamber. The migration assay was performed using 5-μm or 8-μm pore filters (Transwell, 24-well cell clusters; Costar, Cambridge, MA). SDF-1α at different concentrations was diluted in serum-free medium and placed in the lower wells. Cells (2.5 × 105) were suspended in the same medium and added to upper wells. The chambers were incubated for 1 hour at 37°C in 5% CO2 and 95% air. The cells in the upper chamber were recovered. The upper chamber was then carefully recovered and cells in the bottom chambers were recovered in the same volume for counting. The different cell fractions were then labeled with a PE–anti-CD41a and FITC anti-CD42a MoAbs and analyzed by flow cytometry. For the experiment using pertussis toxin, cells were preincubated for 2 hours at 37°C with pertussis toxin (100 ng/mL; Sigma) before migration. All assays were done in triplicate. Data are presented as the chemotaxis index calculated by the following ratio: number of cells migrating to SDF-1α/number of cells migrating to medium. For morphological studies, the different cell fractions obtained were cytospun on slides and observed at light microscopy after May-Grunwald Giemsa staining. Cell diameter of cells in suspension was measured using a microscope equipped with an ocular micrometer. For each group, 300 cells were analyzed.
Actin Polymerization
To analyze actin polymerization in megakaryocytes, cultured cells were washed once in PBS and incubated at 37°C between 30 seconds and 2 minutes in the presence of either SDF-1α (300 ng/mL) or thrombin (1U/mL; Stago) and fixed for 10 minutes with an equal volume of 0.5% paraformaldehyde. After washing and permeabilization in 0.01% triton solution in PBS for 10 minutes, the cells were incubated with a PE–anti-CD41a MoAb and either 4.10−7 mol/L FITC-conjugated phalloidin (Sigma) or Oregon green–conjugated DNase1 (Molecular Probes, Eugene, OR). Data were analyzed by flow cytometry after gating the CD41+ cells.
Calcium Efflux Assay
To analyze the cellular calcium mobilization in megakaryocytes, cultured cells were washed twice in Hanks’ balanced salt solution (HBSS) and labeled with a PE–anti-CD41a MoAb. Cells were then suspended in HBSS and incubated at 37°C for 30 minutes in presence of 5 μg/mL Fluo-3/AM cell permeant (Molecular Probes). The cells were then washed twice with HBSS and resuspended at 1 × 106 cells/mL and stored in the dark at 22°C until analysis. A sample was analyzed on FACSort for basal level of fluorescence, then either SDF-1α (300 ng/mL) or thrombin (1 U/mL) was added. After stimulation, fluorescence changes were monitored during 10 minutes. Data were analyzed by gating the CD41+ cells and calculating the mean fluorescence intensity every 10 seconds.
Detection of the Activation-Dependent Antigen P-Selectin (CD62) on Megakaryocytes and Platelets
Cultured cells or platelets were stimulated at 37°C with either SDF-1α (300 ng/mL) or thrombin (1 U/mL). After 10 minutes of stimulation, samples were incubated for 15 minutes at 4°C with both R-PE–anti-CD62 (2 μg/mL) and FITC anti-CD41 MoAbs. Cells were then fixed for 1 hour with an equal volume of 0.5% paraformaldehyde. Control cells were fixed in the same manner without prior activation. Cells were subsequently resuspended in PBS.
Ribonuclease Protection Assay
The coding region of human CXCR4 was cloned by polymerase chain reaction (PCR) as previously described,16 as a 1.1-kbHindIII-Xho I fragment in pCDNA3(PCD-CXCR4). The BamHI-Dra I fragment, containing nucleotides 566 to 1009 of the cDNA of CXCR4, was transferred from pCD-CXCR4 into the pBluescript SK+ vector in theEcoRV polylinker site (pSK-CXCR4). A32P-UTP–labeled antisense probe was transcribed from the T7 promoter of the BamHI linearized pSK-CXCR4 plasmid. As an internal control for each reaction sample, a 32P-labeled antisense human actin probe was transcribed from the T7 promoter of theBamHI-digested pSK-actin plasmid. This plasmid was constructed by inserting a Nar I-Bsa I actin fragment, containing nucleotides 147 to 413, into the polylinker site of the pBluescript SK+ vector. Total RNA (20 μg) prepared from peripheral blood mononuclear cells, phytohemagglutinin (PHA)-activated mononuclear cells, CD41+ cord blood cells, and platelets was hybridized with radioactive probes at 50°C overnight. Nonhybridizing RNA was digested with RnaseA(10 μg/mL) and RnaseT1 (1,000 U/mL) for 1 hour at 37°C. To stop RNase action, sodium dodecyl sulfate (SDS; 0.6%) and proteinase K (145 μg/mL) were added for 15 minutes at 37°C. Protected fragments were extracted in the presence of 15 μg of carrier transfer RNA with phenol/chloroform/isoamyl alcohol and precipitated with absolute ethanol at −20°C. Fragments were resolved on a 4% polyacrylamide, 7 mol/L urea gel, and autoradiographed on hyperfilm MP (Amersham). The size of the protected fragments was determined using labeled Msp I-digested pBR322 (New England Biolabs, Beverly, MA).
Determination of Platelet Numbers Produced in Culture
SDF-1α at different concentrations was added after 6 days of culture of cord blood–derived CD34+ cells in serum-free conditions in presence of stem cell factor (SCF) and PEG-rhuMGDF. Six days after SDF-1α addition, platelet production was assessed by flow cytometry as previously described.24 After collection and rinsing with PBS/EDTA, cultured cells were centrifuged at 350g for 15 minutes, incubated with the R-PE–anti-CD41a MoAb for 30 minutes, and fixed with 0.5% paraformaldehyde (Serva, Heidelberg, Germany) for 20 minutes. Cells from each culture condition were diluted to the same volume (400 μL). For each sample, the acquisition rate was 1 mL/s for 100 seconds. Events were collected using an analytical gate based on scatter properties of normal blood platelets treated similarly using a log scale for forward light scatter (FSC) and side scatter (SSC). This gate excluded large contaminating cells (MKs) and small debris or microparticles. Samples were analyzed with a FACsort flow cytometer (Becton Dickinson).
Statistics
Results of experimental points obtained from multiple experiments were reported as the mean ± SD. Statistical analysis was performed using the two-tailed Student’s t-test for paired data.
RESULTS
Presence of CXCR4 Transcripts in Megakaryocytes and Platelets
CXCR4 expression was investigated using the RNAse protection assay. To obtain a large number of megakaryocytic cells, CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. After 6 days in culture at a time where a significant proportion (25%) of CD41+ cells were present, CD41+ cells were purified by the immunomagnetic bead technique with a purity exceeding 95% and total mRNA were extracted. This time point was chosen for these experiments because few megakaryocytes could be analyzed at earlier time points. Later in the cultures, megakaryocytes were more fragile and their immunoselection using immunomagnetic beads resulted in substantial cell clumping, which is associated with lower purity and viability.
Freshly isolated peripheral blood mononuclear cells and PHA-activated cells as well as platelets were also analyzed. As shown in Fig 1, a protected fragment of the predicted size, which comigrated with that found in PBMNC, was detected at high levels in purified megakaryocytes and platelets.
Detection of CXCR4 transcripts by RNase protection assay. mRNA was extracted from peripheral blood mononuclear cells, PHA-activated mononuclear cells, CD41+ cells, and platelets. tRNA served as a negative control. Samples of mRNA were hybridized with a specific CXCR4 riboprobe and a specific actin riboprobe, then digested with RNase T1. Protected fragments were analyzed on denaturing acrylamide gels. Sizes of the fragments were determined using labeled Msp I–digested pBR322.
Detection of CXCR4 transcripts by RNase protection assay. mRNA was extracted from peripheral blood mononuclear cells, PHA-activated mononuclear cells, CD41+ cells, and platelets. tRNA served as a negative control. Samples of mRNA were hybridized with a specific CXCR4 riboprobe and a specific actin riboprobe, then digested with RNase T1. Protected fragments were analyzed on denaturing acrylamide gels. Sizes of the fragments were determined using labeled Msp I–digested pBR322.
Flow Cytometric Expression of CXCR4 by Megakaryocytes and Platelets
We next investigated whether cell-surface expression of CXCR4 could be detected by multiparameter flow cytometric analysis. Cells derived from CD34+ cell liquid cultures at different days of culture were dually labeled with a FITC anti-CD41a MoAb and PE-12G5 MoAb to detect CXCR4 protein from day 6 to day 12 of culture.
In the representative experiment shown in Fig 2A and B, CXCR4 was expressed by the majority of CD41a+ cells at all days of culture. A positive correlation was found between CXCR4 and CD41a expression. CXCR4 was also detected but at a lower level on a minority of CD41a− cells present in the culture. A mean of 74 ± 25% (range 30% to 100%, n = 12) of cord blood–derived CD41a+ cells showed CXCR4 staining. Similar results were obtained with megakaryocytes derived from adult marrow CD34+ cells with a mean of 90 ± 5% (range 72% to 100%, n = 5).
CXCR4 expression on megakaryocytes and platelets. (A, B) Representative data of flow cytometric analysis of CD41a and CXCR4 on megakaryocytes grown in culture are shown. Cord blood CD34+ cells were cultured for 6 days in the presence of a combination of SCF and PEG-rhuMGDF. Cells were stained using an anti-CD41a MoAb in combination with either an isotype control antibody (A) or the anti-CXCR4 MoAb (B). (C) CXCR4 expression on freshly isolated megakaryocytes after percoll enrichment. (D) Platelets, depleted in leukocytes, were labeled using a similar procedure. Labeling with control MoAb is shown by the thin line. The thick line shows the staining with the anti-CXCR4 MoAb.
CXCR4 expression on megakaryocytes and platelets. (A, B) Representative data of flow cytometric analysis of CD41a and CXCR4 on megakaryocytes grown in culture are shown. Cord blood CD34+ cells were cultured for 6 days in the presence of a combination of SCF and PEG-rhuMGDF. Cells were stained using an anti-CD41a MoAb in combination with either an isotype control antibody (A) or the anti-CXCR4 MoAb (B). (C) CXCR4 expression on freshly isolated megakaryocytes after percoll enrichment. (D) Platelets, depleted in leukocytes, were labeled using a similar procedure. Labeling with control MoAb is shown by the thin line. The thick line shows the staining with the anti-CXCR4 MoAb.
We also studied the expression of CXCR4 during megakaryocyte differentiation in vivo. Bone marrow megakaryocytes were enriched using a percoll gradient and tested by two-color fluorescence. The expression pattern of CXCR4 on primary CD41a+ cells is shown in Fig2C. A mean of 88% (range 82% to 100%, n = 3) of primary CD41+ cells expressed CXCR4. Moreover, CXCR4 was expressed on the surface of freshly isolated platelets CXCR4 (Fig 2D). These experiments allowed us to establish that CXCR4 expression on megakaryocytes was not simply related to the culture conditions.
Short-Term Effects of SDF-1α on Megakaryocytes and Platelets
On the basis of expression data and because primary megakaryocytes were difficult to obtain, the following experiments were performed on cultured cells derived from cord blood CD34+ cells after 6 to 12 days of culture. Because similar results were obtained at day 6, day 9, and day 12 of culture, unless otherwise stated, the data shown were those obtained on day 6 of culture. At day 6 of culture, repeated experiments showed that the percentage of CD41a+ cells averaged 25% CD41a+ cells (n = 13), with a range of 12% to 33% including some mature megakaryocytes. Most of the cells present in day 6 cultures were CD34+ blast cells, whereas less than 2% expressed lineage markers (T cells, B cells, neutrophil-macrophage, and erythroid lineage). Later in the culture at day 12, the percentage of CD41a+ cells slightly increased with the appearance of CD15+ cells.
To address the functional role of CXCR4 on megakaryocytes, we first tested the ability of SDF-1α to induce calcium mobilization. As shown in Fig 3, we observed a transient calcium flux in all of the CD41a+ cells, but this effect of SDF-1α was much weaker than that elicited by thrombin (not shown). Additional experiments were designed to determine whether SDF-1α influences intracellular actin reorganization. This effect is thought to be a prerequisite for cell movement. After labeling by a PE–anti-CD41a MoAb, cells were treated with an optimal concentration of SDF-1α (500 ng/mL) or thrombin (2 U/mL) for 5 minutes at 37°C. After fixation, permeabilization, and labeling with fluorescent phalloidin or DNase I, cells were analyzed by flow cytometry. As shown in Fig 4, these two reagents augmented the expression of filamentous actin and decreased expression of actin G. This effect is similar to that reported in T cells.
Calcium flux response of megakaryocytes to SDF-1. After CD41a labeling, cells were loaded with Fluo-3 and exposed to SDF-1 (300 ng/mL). Changes in fluorescence were monitored over time by flow cytometry after SDF-1 addition. The results are derived from one representative experiment of a total of three separate experiments.
Calcium flux response of megakaryocytes to SDF-1. After CD41a labeling, cells were loaded with Fluo-3 and exposed to SDF-1 (300 ng/mL). Changes in fluorescence were monitored over time by flow cytometry after SDF-1 addition. The results are derived from one representative experiment of a total of three separate experiments.
Actin polymerization in megakaryocytes after SDF-1 addition. Cultured cells were incubated at 37°C for 30 seconds and 2 minutes in the presence of either SDF-1 (300 ng/mL) or thrombin (Stago, 1 U/mL). Intracellular F actin (phalloidin) or G actin (oregon green conjugated DNase 1) was determined by flow cytometry after gating the CD41a+ cells. Results of one representative experiment are shown. Two other experiments gave similar results.
Actin polymerization in megakaryocytes after SDF-1 addition. Cultured cells were incubated at 37°C for 30 seconds and 2 minutes in the presence of either SDF-1 (300 ng/mL) or thrombin (Stago, 1 U/mL). Intracellular F actin (phalloidin) or G actin (oregon green conjugated DNase 1) was determined by flow cytometry after gating the CD41a+ cells. Results of one representative experiment are shown. Two other experiments gave similar results.
In these experiments, we also sought to determine if SDF-1α induces actin polymerization directly or indirectly via a secondary effect on accessory cells. To address this question, day-6 CD41a+cells were purified using immunomagnetic beads. Purity of the CD41a+ cells in this population consistently exceeded 96%. Purified cells were then exposed to SDF-1α or thrombin, and actin polymerization was analyzed by flow cytometry. Results were highly reproductible with respect to unseparated cultures supporting the hypothesis that SDF-1α exerted a direct effect on megakaryocytes (data not shown).
Because thrombin and SDF-1α have common properties, we tested to see if SDF-1α induced activation of megakaryocytes and platelets by studying translocation of CD62 (P-Selectin) on the cell surface. As illustrated in Fig 5A, B, C, D, E, and F, SDF-1α increased cell surface expression of CD62 in CD41alow (Fig 5D) cells but not on CD41ahigh(Fig 5E) cells and platelets (Fig 5F).
Effects of SDF-1 on the expression of CD62 by megakaryocytes. (A,B,C) Examples of double-color staining with anti-CD62 in combination with anti-CD41a (A) or with control antibodies (B) and (C). Primary cultured cells were either left unstimulated (thick line) or stimulated for 10 minutes with either thrombin (1 U/mL, thin line) or SDF-1 (300 ng/mL, broken line). CD62 staining in combination with CD41a staining was then compared by FACS analysis as described in Materials and Methods. The analysis was performed in CD41alow (D) and CD41ahigh (E) gates as defined in (A) and in platelets (F). These data are representative of four experiments.
Effects of SDF-1 on the expression of CD62 by megakaryocytes. (A,B,C) Examples of double-color staining with anti-CD62 in combination with anti-CD41a (A) or with control antibodies (B) and (C). Primary cultured cells were either left unstimulated (thick line) or stimulated for 10 minutes with either thrombin (1 U/mL, thin line) or SDF-1 (300 ng/mL, broken line). CD62 staining in combination with CD41a staining was then compared by FACS analysis as described in Materials and Methods. The analysis was performed in CD41alow (D) and CD41ahigh (E) gates as defined in (A) and in platelets (F). These data are representative of four experiments.
Chemotactic Activity of SDF-1α on Megakaryocytes
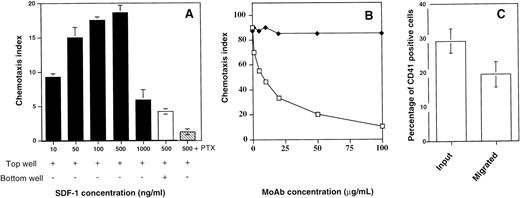
We subsequently investigated the ability of SDF-1α to induce migration of megakaryocytes in vitro. Previous studies have established that this method is sensitive and reliable in the evaluation of chemokine receptor function.10Figure 6A shows that SDF-1α induced a significant migration of megakaryocytes in vitro with a typical bi-modal dose-response curve and with a maximum effect at 500 ng/mL. Pretreatment of the cells with pertussis toxin completly inhibited the SDF-1α–induced migration.
Chemotaxis assay and cell surface expression of CD41a. (A) Chemotaxis responses of the CD41a+ cell population. CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. The cells were subjected to chemotaxis through 5-μm pores to various concentrations of SDF-1 and stained with an anti-CD41a MoAb (black bars). Checkerboard analysis was performed by adding SDF-1 (500 ng) both in the bottom and in the top well (white bar). This migration is inhibited by preincubating the cells with pertussis toxin (PTX; hatched bar). Data are expressed as chemotaxis index and represent the mean for one representative experiment done in triplicate. (B) Effect of a blocking MoAb against CXCR4 (12G5). The cells were preincubated either with increasing concentrations of 12G5 MoAb (white squares) or an isotype-control MoAb (black squares) before the migration assay. (C) Chemotaxis responses of megakaryocytes. After migation in response to SDF-1 (500 ng/mL), the cells were stained with an anti-CD41a MoAb. The percentage of CD41+cells was determined in starting and migrated cells. The results shown are the mean and SD of five experiments.
Chemotaxis assay and cell surface expression of CD41a. (A) Chemotaxis responses of the CD41a+ cell population. CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. The cells were subjected to chemotaxis through 5-μm pores to various concentrations of SDF-1 and stained with an anti-CD41a MoAb (black bars). Checkerboard analysis was performed by adding SDF-1 (500 ng) both in the bottom and in the top well (white bar). This migration is inhibited by preincubating the cells with pertussis toxin (PTX; hatched bar). Data are expressed as chemotaxis index and represent the mean for one representative experiment done in triplicate. (B) Effect of a blocking MoAb against CXCR4 (12G5). The cells were preincubated either with increasing concentrations of 12G5 MoAb (white squares) or an isotype-control MoAb (black squares) before the migration assay. (C) Chemotaxis responses of megakaryocytes. After migation in response to SDF-1 (500 ng/mL), the cells were stained with an anti-CD41a MoAb. The percentage of CD41+cells was determined in starting and migrated cells. The results shown are the mean and SD of five experiments.
To prove the specificity of the effects of SDF-1α, cells were preincubated either with increasing concentrations of a blocking MoAb against CXCR4 (12G5)25 or an isotype-control MoAb. Figure6B shows that the megakaryocyte chemotactic response to SDF-1α could be inhibited by 12G5 MoAb indicating that CXCR4 is the relevant receptor.
To distinguish between SDF-1α–induced chemokinesis (increased random migration) and chemotaxis (directed movement of cells along a chemotactic gradient), we performed checkerboard analysis. Cultured cells were resuspended in medium containing various concentrations of SDF-1α (10 ng/mL to 1,000 ng/mL) just before transferring the cells to the upper chamber. As shown in Table 1, a gradually increasing concentration gradient of SDF-1α between the lower and the upper compartment led to increased migration of CD41a+ cells toward the lower compartment. Hence, the megakaryocyte response to SDF-1α is a chemotactic response rather than the result of an increase in random migration.
Effects of SDF-1 on Migration of CD41+Cells by Checkerboard Assay
| SDF-1α Concentration (ng/mL) in Lower Chamber . | SDF-1α Concentration (ng/mL) in Upper Chamber . | |||
|---|---|---|---|---|
| 0 . | 10 . | 50 . | 1,000 . | |
| 0 | 2.5 | 1.5 | 2 | 1.6 |
| 10 | 16 | 2.5 | 2 | 2.3 |
| 50 | 28.8 | 10.7 | 3.5 | 2.5 |
| 500 | 24.2 | 18.1 | 9.3 | 3 |
| 1,000 | 13 | 8.8 | 5.9 | 2.6 |
| SDF-1α Concentration (ng/mL) in Lower Chamber . | SDF-1α Concentration (ng/mL) in Upper Chamber . | |||
|---|---|---|---|---|
| 0 . | 10 . | 50 . | 1,000 . | |
| 0 | 2.5 | 1.5 | 2 | 1.6 |
| 10 | 16 | 2.5 | 2 | 2.3 |
| 50 | 28.8 | 10.7 | 3.5 | 2.5 |
| 500 | 24.2 | 18.1 | 9.3 | 3 |
| 1,000 | 13 | 8.8 | 5.9 | 2.6 |
Cells were added to the upper chamber and SDF-1α was added to either the lower or upper chamber. Results shown are the percentages of cells that migrated into the lower chamber and are representative of two independent experiments performed in duplicate.
Characterization of SDF-1α Responsive Megakaryocyte Subsets
CD34+ cell–derived megakaryocytes grown in the presence of PEG-rhuMGDF and SCF represent an asynchronous population of cells at various stages of maturation with different ploidy and sizes.26 Analysis of the cells migrating to the bottom well showed that the relative proportion of CD41a+ cells did not change significantly as compared with their relative proportion in the starting population (Fig 6C), although these cells expressed the highest level of CXCR4 receptors among the cultured cells. Due to this lack of enrichment, we thought that it was possible that only a subpopulation of CD41a+ cells responded to SDF-1α. To examine this possiblity, we compared the starting population to the cells that migrated in response to SDF-1α and to the cells that migrated to medium alone for their FSC versus SSC properties as well for their morphology after May-Grunwald-Giemsa staining. As shown in Fig 7A,B,and C, the SSC and FSC properties of the migrated cells and the starting cells differ greatly. On average, migrated cells exhibited a low SSC properties as compared with the starting population or to the population that migrated spontaneously. It has been shown that megakaryocyte maturation is associated with marked increase of SSC.27 Furthermore, as shown in Fig 7E, no morphologically identifiable megakaryocytes as defined on the basis of their large size and a polylobulated nucleus were seen in the cell fraction that migrated in response to SDF-1α in contrast to the starting population (Fig 7D), suggesting either that mature megakaryocytes cannot fit through the pores of the transwell due to their large size or that the megakaryocyte migration in response to SDF-1α is downregulated during their maturation. However, the use of 8-μm pore size transwells did not modify these results (data not shown), suggesting that cell size was not the limiting parameter.
Morphological characteristics of cells migrating in response to SDF-1. After migration, cultured cells were analyzed for their morphological characteristics. Dot plot analysis (FSC versus SSC) of starting population (A), cells migrated in response to SDF-1 (B), and cells migrated in absence of SDF-1 (C) are shown. May-Grunwald-Giemsa staining of the starting cell fraction (D) and the cell fraction that migrated in response to SDF-1 (E) are shown.
Morphological characteristics of cells migrating in response to SDF-1. After migration, cultured cells were analyzed for their morphological characteristics. Dot plot analysis (FSC versus SSC) of starting population (A), cells migrated in response to SDF-1 (B), and cells migrated in absence of SDF-1 (C) are shown. May-Grunwald-Giemsa staining of the starting cell fraction (D) and the cell fraction that migrated in response to SDF-1 (E) are shown.
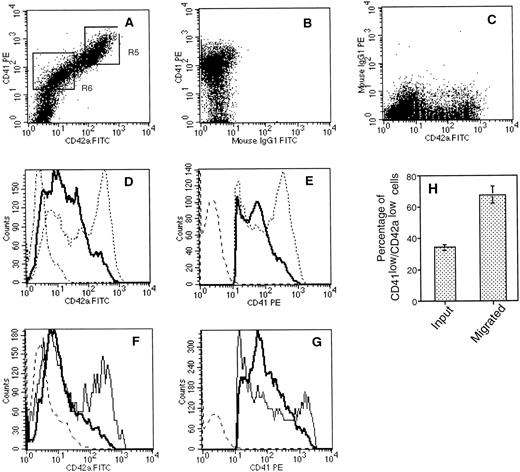
To further characterize the properties of the cells that respond to SDF-1α and to investigate whether megakaryocyte migration was regulated during their maturation, we compared the starting population with the cells that migrated in response to SDF-1α for the expression of CD41a and CD42a. The rationale for this experiment was based on previous studies showing that CD41a appears at an earlier stage of megakaryocyte-lineage development than CD42a. In these experiments, large-sized cells were specifically excluded by analyzing the cells with low SSC properties as defined in gate R1 (Fig 7). As illustrated in Fig 8A,B, and C, a direct relationship between CD41a and CD42a was observed. However, two populations of CD41a+ cells could be determined: CD41alow/CD42alow cells (region R6) and CD41ahigh/CD42ahigh cells (region R5). CD41a+ cells that chemotaxed to SDF-1α were both CD42alow and CD41alow (Fig 8D and E). Thus, when compared with the starting cell population, a marked enrichment in the proportion of the CD41alow/CD42alow was observed after migration: 34 ± 2% versus 67.6 ± 5.4%, respectively (Fig 8H). Moreover, the staining was compared between the cells that migrated in response to SDF-1α to the cells that migrated spontaneously and indicate a specific migration of CD42alowand CD41alow cells to SDF-1α (Fig 8F and G). To confirm this higher response of CD41alow/CD42alow to SDF-1α, we carried chemotaxis assays with isolated megakaryocyte subsets after 10 days of culture. To obtain sufficient number of cells, cultured cells were first immunopurified as described in Materials and Methods. The enriched fraction was then stained and separated using the gate R1 defined in Fig 7 and the gates R5 and R6 defined in Fig 8A. Purified CD41alow/CD42alow cells showed twofold to threefold higher migrating ability to SDF-1α (chemotaxis index 22.5 ± 8; mean ± SD of three experiments ) as compared with purified CD41ahigh/CD42ahigh (chemotaxis index 6 ± 2; mean ± SD of three experiments). To investigate whether these observations may relate to a different size between these two subsets in two experiments, we evaluated the mean size of megakaryocytes in the upper and the lower wells after migration using a micrometer. This analysis revealed that the mean size of the cells was 8.2 ± 0.6 μm and 9.6 ± 1.8 μm in the lower and upper well, respectively. This difference in size was weak but statistically significant (P > .0005).
Flow cytometric analysis of cell surface expression of CD41a and CD42a on starting and migrated cell populations. After migration, cultured cells were labeled with an R-PE–conjugated anti-CD41a MoAb and a FITC-conjugated anti-CD42a MoAb. (A, B, C) An example of double-color fluorescence beween CD41a and CD42a (A) and the respective controls (B, C). The cells within the lymphoid blast window (R1, Fig 7) were analyzed for the expression of CD42a (D) and CD41a (E). The thick line shows the staining of cells that migrated in response to an optimal concentration of SDF-1 (500 ng/mL). Broken lines represent the staining of the starting cell population, and dotted lines represent the staining with control antibodies. In two experiments, CD42a (F) and CD41a (G) staining was compared between the cells that migrated in response to SDF-1 (thick line) and to control media (thin line). (H) Migration of CD41alow/CD42alow cells. The mean and SD percentages of CD42alow/CD41alow were determined by gating on the entire population of CD41a+cells.
Flow cytometric analysis of cell surface expression of CD41a and CD42a on starting and migrated cell populations. After migration, cultured cells were labeled with an R-PE–conjugated anti-CD41a MoAb and a FITC-conjugated anti-CD42a MoAb. (A, B, C) An example of double-color fluorescence beween CD41a and CD42a (A) and the respective controls (B, C). The cells within the lymphoid blast window (R1, Fig 7) were analyzed for the expression of CD42a (D) and CD41a (E). The thick line shows the staining of cells that migrated in response to an optimal concentration of SDF-1 (500 ng/mL). Broken lines represent the staining of the starting cell population, and dotted lines represent the staining with control antibodies. In two experiments, CD42a (F) and CD41a (G) staining was compared between the cells that migrated in response to SDF-1 (thick line) and to control media (thin line). (H) Migration of CD41alow/CD42alow cells. The mean and SD percentages of CD42alow/CD41alow were determined by gating on the entire population of CD41a+cells.
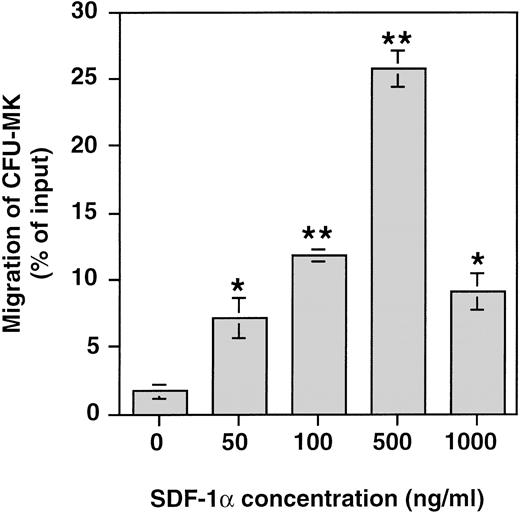
Finally, the presence of colony forming unit–megakaryocyte progenitors (CFU-MK) among the cultured cells that migrated in response to SDF-1α was investigated by performing CFU-MK assays on the cells before and after in vitro migration. Figure 9 shows that SDF-1α induced a significant migration of CFU-MK among cultured cells in vitro with a typical bimodal dose response curve and a maximum effect at 500 ng/mL. The proportion of CFU-MK migrating in response to SDF-1α ranged between 22% and 30% (n = 3). Similar results were obtained when the migration of CFU-GM and burst-forming unit–erythroid (BFU-E) was studied (data not shown). These data directly show that CFU-MK as other hematopoietic progenitors respond efficiently to SDF-1α.
Chemotaxis responses of CFU-MK in response to various concentrations of SDF-1. CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. After 6-day culture, the cells were subjected to chemotaxis through 5-μm pores and plated in CKU-MK assay. Results are shown of one representative experiment performed in triplicate. Two other experiments gave similar results.*P < .05, **P < .01 when compared with control.
Chemotaxis responses of CFU-MK in response to various concentrations of SDF-1. CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. After 6-day culture, the cells were subjected to chemotaxis through 5-μm pores and plated in CKU-MK assay. Results are shown of one representative experiment performed in triplicate. Two other experiments gave similar results.*P < .05, **P < .01 when compared with control.
Effects of SDF-1α on Megakaryopoiesis and Platelets Production
In a first set of experiments, SDF-1α (10 to 1,000 ng) was added at the onset of the culture in the CFU-MK assays stimulated by a combination of PEG-rhuMGDF and SCF at optimal concentrations. No effect of SDF-1α was observed on MK colony growth (Fig 10).
Effects of SDF-1 on CFU-MK growth. After CD34+ selection, 1 × 103cells were assayed in fibin-clot cultures in presence of increasing concentrations of SDF-1. Data are expressed as the mean and SD of two experiments performed in triplicate.
Effects of SDF-1 on CFU-MK growth. After CD34+ selection, 1 × 103cells were assayed in fibin-clot cultures in presence of increasing concentrations of SDF-1. Data are expressed as the mean and SD of two experiments performed in triplicate.
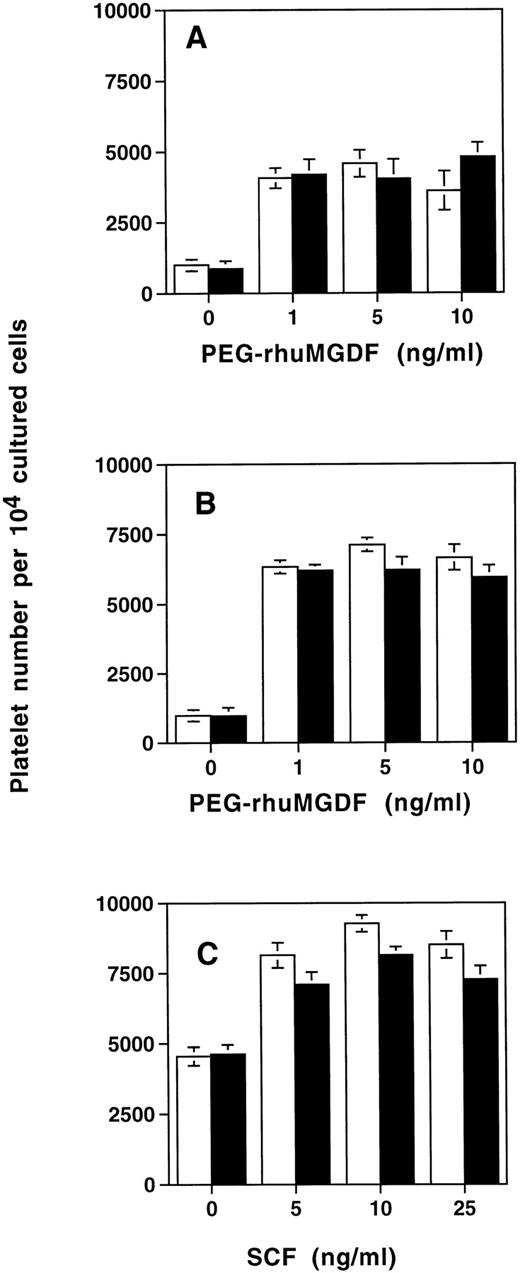
We then studied whether SDF-1α modified platelet production in vitro. For this purpose SDF-1α (500 ng/mL) was added after 6 days of culture of cord blood–derived CD34+ cells stimulated by SCF and PEG-rhuMGDF. Six days later (12 days of culture), platelet production was assessed by flow cytometry after CD41a labeling. SDF-1α did not modify platelet production in the cultures stimulated by PEG-rhuMGDF alone, whatever the concentration of PEG-rhuMGDF that was used (Fig 11A). Similarly, no effect of SDF-1α on platelet formation was observed when the cells were cultured in the presence of a combination of PEG-rhuMGDF at saturating concentration (10 ng/mL) and SCF at different doses (Fig 11B), or in the presence of SCF at saturating concentration (25 ng/mL) and PEG-rhuMGDF at different doses (Fig 11C).
Effects of SDF-1 on platelet production. CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. After two washes, SDF-1 was added at optimal concentration (500 ng/mL) in a second phase of the culture in the presence of increasing concentrations of PEG-rhuMGDF without any other factor (A) or in presence of an optimal concentration of SCF (25 ng/mL) (B). Conversely, platelet production was tested in the presence of an optimal concentration of PEG-rhuMGDF(10 ng/mL) and increasing concentrations of SCF (C). Six days after SDF-1 addition, platelet production was assessed by flow cytometry after anti-CD41a labeling. Cells from each culture condition without SDF-1 (white bar) and in presence of SDF-1 (black bar) were distributed in the same volume (400 μL), and for each sample the acquisition rate was 1 μL/s for 100 seconds. Data represent the mean for two experiments done in triplicate.
Effects of SDF-1 on platelet production. CD34+ cells were seeded in serum-free liquid culture containing SCF and PEG-rhuMGDF for up to 6 days. After two washes, SDF-1 was added at optimal concentration (500 ng/mL) in a second phase of the culture in the presence of increasing concentrations of PEG-rhuMGDF without any other factor (A) or in presence of an optimal concentration of SCF (25 ng/mL) (B). Conversely, platelet production was tested in the presence of an optimal concentration of PEG-rhuMGDF(10 ng/mL) and increasing concentrations of SCF (C). Six days after SDF-1 addition, platelet production was assessed by flow cytometry after anti-CD41a labeling. Cells from each culture condition without SDF-1 (white bar) and in presence of SDF-1 (black bar) were distributed in the same volume (400 μL), and for each sample the acquisition rate was 1 μL/s for 100 seconds. Data represent the mean for two experiments done in triplicate.
DISCUSSION
The aim of this study was to determine the expression and the function of CXCR4 chemokine receptors that would have important implications both in understanding what drives the homing, retention, and exit of precursor cells from hematopoietic organs and the mechanisms involved in HIV-1–related thrombocytopenia. CXCR4 is a member of the G-protein–coupled seven transmembrane domain receptor family.18 It is expressed in many cell tissues including brain, spleen, and lung.13,28 Among hematopoietic cells, CXCR4 is differentially expressed during T-cell differentiation29,30 and is present on mature T cells, B cells, and monocytes. Recently, it was shown by reverse-transcription (RT)-PCR that CXCR4 transcripts were detected in CD34+cells.31 It also seems very likely that CD34+cells express a functional CXCR4 receptor because its ligand SDF-1 is a chemoattractant for all CD34+ cell subsets.10
Using RNase protection assays, CXCR4 transcripts were readily detected in purified megakaryocytes derived from cord blood and adult bone marrow. Transcript levels were quite similar to that detected in PHA-activated T cells. This was not the consequence of transcript upregulation by culture conditions, because primary megakaryocytes and peripheral blood platelets also strongly expressed the CXCR4 transcript.
This expression study was greatly facilitated by the availability of an anti-CXCR4 MoAb.25 The expression pattern of the membrane protein correlated with the CD41a antigen. Among cultured myeloid cells, megakaryocytes were those that expressed the highest protein level. It is noteworthy that the protein could also be detected on the platelet surface. Similar results were recently obtained by two other groups.19,20 The only other chemokine receptors that have been previously found on platelets are the interleukin-8 (IL-8) receptor A and a CC chemokine receptor named K5.5.32
The role of chemokines in the regulation of megakaryocytopoiesis has been extensively studied. It was shown that several CXC or CC chemokines inhibited in vitro megakaryocytopoiesis.33Studies were only performed on the functions of PF4 and β-thromboglobulin that are produced by megakaryocytes.34-36 These chemokines may induce a specific inhibition of megakaryocytopoiesis, suggesting a negative autocrine loop of regulation.34-36 SDF-1α, the ligand of CXCR4, has not yet been involved in the regulation of cell proliferation and apoptosis. In contrast, it is a powerful chemoattractant for lymphocytes, monocytes, and hematopoietic progenitors.8,10,11 Results obtained with the SDF-1−/− mice as well as with in vitro migration assays on CD34+ cells suggest that SDF-1α is the physiological chemoattractant of hematopoietic cells into the marrow and regulates their migration into the blood.10 12
In the present work, we have shown that SDF-1α is also a potent chemoattractant for megakaryocytes. It is, as yet, the first chemokine that is involved in this function. As for lymphocytes and CD34+ cells, this migratory function is preceded by an intracellular calcium mobilization.8 It is associated with an increase in actin polymerization, which leads to the reorganization of intracellular actin necessary for cell movement.8 The CXCR4 expression by peripheral blood platelets was unexpected because platelets are devoid of chemotaxis. However, despite a high level of expression of CXCR4, only a fraction (30%) of megakaryocytes were induced to migrate by SDF-1α. The megakaryocytes that responded to SDF-1α were immature and included CFU-MK progenitors. In addition, a majority of CD41a+ blasts were present in cells that migrated, suggesting either that mature megakaryocytes cannot fit through the pores of the transwell due to their large size or that the megakaryocyte migration in response to SDF-1α is downregulated during their maturation. Nevertheless, phenotypic studies of a cell population with similar scatter properties (with about similar size) showed that CD41alow/CD42alow cells exhibited twofold to threefold higher migrating ability in response to SDF-1α than CD41ahigh/CD42ahigh cells, suggesting that megakaryocyte migration in response to SDF-1α is downregulated during maturation. This hypothesis is further supported by the fact that SDF-1α was able to induce P-selectin (CD62) translocation on CD41alow but not on CD41ahigh cells and on platelet surface membranes. Because early observations have shown that almost all CD41ahigh/CD42ahigh cells failed to undergo cell division as compared to CD41alow/CD42alow cells,5 this may imply that SDF-1α is a chemoattractant for immature proliferating megakaryocytes in the marrow. In addition, as SDF-1α induces P-selectin expression on immature megakaryocytes, it may be involved in modulation of their adhesion properties.
Similar loss of function of CXCR4 has been already reported in B-cell development, suggesting that SDF-1α may function in a stage-specific manner.11,37 Moreover, as in this study, a difference in CXCR4 expression and response patterns was observed in mature B cells that, while expressing significant levels of CXCR4, are not responsive to SDF-1α.11 37 These data suggest that the CXCR4 receptor can be functionally uncoupled or disengaged. Further work will be necessary to clarify the mechanisms involved in this uncoupling of expression and function.
The physiological role of SDF-1α in megakaryocyte/platelet differentiation remains totally speculative. Megakaryocytes are the only marrow cells that produce blood cells by cytoplasmic fragmentation. This production may be due to long extensions that pass through the fenestrated bone marrow endothelium barrier (proplatelet formation) and break into platelets under the forces of blood flow.38 Alternatively, the entire megakaryocyte may migrate into the sinusoid.39 Platelets are subsequently formed in the circulation, especially in the lung circulation, which may act as a filter for circulating megakaryocytes.40 Regulation of these late stages of platelet production is poorly understood. In the present study, we had no evidence that SDF-1α is involved in proplatelet formation because this process as well as in vitro platelet production were not modulated by addition of this chemokine. As in the present report, Wang et al19 reported no marked effect of SDF-1α on megakaryocytopoiesis in vitro. Thus, SDF-1α may be involved in the trafficking of megakaryocytes by controlling their exit from the marrow into blood and eventually their migration to the lung. In support of this hypothesis, it was recently shown that SDF-1α was able to induce a transendothelial migration of megakaryocytes and to enhance platelet formation by favoring the interaction between megakaryocytes and endothelial cells.20 However, extension of this finding to in vivo megakaryopoiesis must be considered with caution as SDF-1α is unlikely to be the only chemokine factor acting on megakaryocytes.
It has already been shown that a fraction of megakaryocytes expresses CD4.41-44 Our data imply that a fraction of megakaryocytes may be infected by lymphocytotropic strains of HIV-1. This possibility is supported by the observation that megakaryocytes from thrombocytopenic HIV patients express HIV transcripts or proteins.21,45 However, it has also been shown that HIV particles can be taken up by megakaryocyte and platelets without true internalization because viral particles are trapped in the demarcation membrane system.46 Knowledge of the expression of CXCR4 on megakaryocytes may facilitate the understanding of the mechanisms of HIV-related thrombocytopenia.
ACKNOWLEDGMENT
We are grateful to J.-L. Nichol (Amgen) for providing the SCF and PEG-rhuMGDF. We are grateful to surgeons for providing bone marrow samples and to Dr Van Nifderick from the St Vincent de Paul hospital for cord blood samples.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Institut Gustave Roussy, the Agence Nationale pour la Recherche sur le SIDA (ANRS), and SIDACTION. C.R. is a fellowship of the French Ministere de la recherche.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Fawzia Louache, MD, INSERM U 362, Institut Gustave Roussy, PR1, 39 Rue Camille Desmoulins, 94805 Villejuif, France.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal