Abstract
Chemokines regulate hematopoiesis in part by influencing the proliferative status of myeloid progenitor cells (MPC). Human MCP-1/murine JE, a myelosuppressive chemokine, specifically binds C-C chemokine receptor 2 (CCR2). Transgenic mice containing a targeted disruption in CCR2 that prevents expression of CCR2 mRNA and protein and have MPC that are insensitive to inhibition by MCP-1 and JE in vitro were assessed for potential abnormalities in growth of bone marrow (BM) and spleen MPC. MPC in both unseparated and c-kit+lin− populations of BM from CCR2-deficient (−/−) mice were in a greatly increased proliferation state compared with CCR2 littermate control (+/+) mice, an effect not apparent with progenitors from spleens of CCR2 (−/−) mice. Increased cycling status of CCR2 (−/−) BM MPC did not result in increased numbers of nucleated cells or MPC in BM or spleens of CCR2 (−/−) mice. Possible reasons for this apparent discrepancy were highlighted by flow cytometric analysis of c-kit+lin− BM cells and colony formation by MPC subjected to delayed addition of growth factors. The c-kit+lin− population of BM cells from CCR2 (−/−) mice had a significantly higher percentage of apoptotic cells than those from CCR2 (+/+) BM. However, elevated apoptosis was not associated with decreased numbers of c-kit+lin− cells. The increased percentage of apoptotic c-kit+lin− cells was due to elevated apoptosis within the c-kitdimlin−, but not the c-kitbrightlin−, subpopulations of cells. Consistent with enhanced apoptosis of phenotypically defined cells, MPC from CCR2 (−/−) BM and purified c-kit+lin− cells demonstrated decreased cell survival in vitro upon delayed addition of growth factors. The data suggest that signals received by CCR2 limit proliferation of progenitor cells in the BM, but also enhance survival of these cells.
IN THE ADULT, THE MAJOR source of blood cells is the bone marrow (BM). In the murine system, the spleen can serve as an extramedullary site for development of blood cells.1,2 All blood cells are produced from common primitive cells known as stem and progenitor cells. Hematopoiesis must be tightly regulated, because uncontrolled expansion of blood cells can be deleterious to the host and has the potential to result in leukemia or a myeloproliferative syndrome. The provision of blood cells and maintenance of hematopoiesis requires cellular proliferation with expansion of stem and progenitor cells, but suppression of proliferation and quiescence of these cells when they are not needed. Survival instructions must be received by quiescent stem and progenitor cells to insure their continued existence and also by more mature, differentiated cells whose functional activity is required. Primitive or mature cells that are not required can be eliminated through apoptotic programs (reviewed in McKenna and Cotter3).
Chemokines are a family of cytokines originally named because they are chemoattractant cytokines for mature blood cells.4,5Chemokines have been implicated in the control of myelopoiesis, especially as negative regulators.4,6-9 Attempts to understand the physiological relevance of chemokines on hematopoiesis have included assessment of the effects of in vivo administration of chemokines to mice10-17 and humans.18 These studies have substantiated in vivo the myelosuppressive effects of certain chemokines previously noted in vitro or ex vivo.15-17,19-21 Recently, a few chemokines have been implicated in migration of myeloid progenitor cells (MPC).22-25
Chemokines and chemokine receptors have been categorized into families based on sequence similarities (reviewed in Rollins4 and Baggiolini et al5). There are at least four families of chemokines (C, CC, CXC, and CX3C) that are distinguished by differences in the spacing of critical cysteine residues. Chemokine receptors are categorized based on the type of chemokine(s) that they bind. However, studies of the roles of chemokines and chemokine receptors are complicated by the existence of redundancy within the chemokine/chemokine receptor network.4,5 9 A single chemokine can often bind more than one receptor and a given chemokine receptor may bind a number of chemokines.
To assess whether certain chemokine receptors play dominant roles in the regulation of hematopoiesis, studies have been performed on mice in which specific chemokine receptor genes have been knocked out. Such studies have implicated the CXCR2 receptor (which binds human interleukin-8 [IL-8] and murine macrophage inflammatory protein-2 [MIP-2], among other CXC chemokines) in negative regulation of MPC proliferation26 and the CCR1 receptor in trafficking and mobilization of MPC.24,27 Most recently, we have noted that MPC in the BM of mice in which the CCR2 receptor has been functionally deleted (−/−) are not responsive in vitro to the suppressive effects of human monocyte chemoattractant protein-1 (MCP-1) or murine JE, chemokines known to bind CCR2.28These results were consistent with the in vitro16,17,21,28and in vivo16,17 myelosuppressive effects of MCP-1. However, we had also noted, as data not shown, that CCR2 (−/−) mice had the same absolute numbers of MPC per femur as CCR2 littermate control (+/+) mice.28 This was not what we would have expected if CCR2 was a dominant receptor for MCP-1/JE–mediated inhibition, as suggested by the in vitro studies with the CCR2 (−/−) and (+/+) BM cells, unless compensatory effects were involved.28 This contrasts with the observation of CXCR2-deficient mice, which have greatly enhanced numbers of MPC in BM, spleen, and blood compared with CXCR2 (+/+) mice,26 consistent with the in vitro15-17,21,26and in vivo15-17 suppressive activities of human IL-8 and murine MIP-2.26 We therefore decided to evaluate functional characteristics of MPC in the BM and spleen of CCR2 (−/−) and CCR2 (+/+) mice. The results presented here demonstrate that MPC in BM, but not spleen, of CCR2 (−/−) mice manifest a significantly enhanced endogenous proliferative state, but that they also undergo enhanced apoptosis.
MATERIALS AND METHODS
CCR2 (−/−) and CCR2 (+/+) mice.
CCR2-deficient (−/−) mice were produced using homologous recombination in embryonic stem cells to disrupt expression of the CC chemokine receptor CCR2 as described elsewhere.28 CCR2 (+/+) mice were littermate controls.
Analysis of myeloid progenitor cells in vitro.
The in vitro colony assays for granulocyte-macrophage (colony-forming unit–granulocyte-macrophage [CFU-GM]), erythroid (burst-forming unit-erythroid [BFU-E]), and multipotential (colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte [CFU-GEMM]) MPC were performed as previously described29 in the presence of fetal bovine serum (FBS; Hyclone, Logan, UT). CFU-GM colony formation (>50 cells/group) was stimulated in 0.3% agar culture medium with 10% FBS by using 10% pokeweed mitogen mouse spleen cell-conditioned medium (PWMSCM). BFU-E and CFU-GEMM, and sometimes CFU-GM, colony formation was stimulated in 0.9% methyl cellulose culture medium with 30% FBS by 1 U/mL recombinant human erythropoietin (Epo; Amgen, Thousand Oaks, CA), 0.1 mmol/L hemin (Eastman Kodak, Rochester, NY) and 5% PWMSCM with or without 50 ng/mL recombinant murine steel factor (SLF; Immunex Corp, Seattle, WA). Unseparated BM and spleen cells were plated at respective concentrations of 5.0 × 104 and 5.0 × 105 cells/mL. For the delayed growth factor addition studies, unseparated BM cells or purified c-kit+lin− cells were plated in methylcellulose cultures containing 30% FBS with or without 0.1 mmol/L hemin. The combination of 1 U/mL Epo, 5% PWMSCM, and 50 ng/mL SLF was added to plates at either time 0 or at 2, 6, 12, or 24 hours after the start of culture. In the cycling studies described below, c-kit+lin− BM cells were also assessed for colony formation. Cells were incubated in a humidified environment at 5% CO2 and lowered (5%) oxygen tension. Colonies were scored after 7 days of incubation as described.29
Cycling status of hematopoietic progenitor cells.
The proportion of progenitors in DNA synthesis (S-phase of the cell cycle) was estimated as reported previously.10,14,18 29 The high specific activity (20 Ci/mmol) tritiated thymidine (50 μCi/mL; New England Nuclear, Boston, MA) kill technique was used and is based on calculation of the reduction in the number of colonies formed after pulse exposure of cells for 20 minutes to radioactive tritiated thymidine as compared with a control (McCoy’s medium or a comparable amount of nonradioactive thymidine).
Statistical analysis.
Three plates were scored for each sample, and each mouse was evaluated separately. Results are expressed as a mean ± 1 SEM, and these are derived from the averages of each of the individual mice within a group. The probability of significant differences between groups was determined with use of the Student’s t-test.
Flow cytometry.
Femoral BM was dispersed into single-cell suspensions and red blood cells were lysed by incubation with red cell lysis buffer (0.155 mol/L NH4Cl, 0.01 mol/L KHCO3, and 0.1 mmol/L EDTA; pH 7.3) at room temperature for 5 minutes. Cells (1 × 106) were washed in cold phosphate-buffered saline (PBS) and stained with MC540 dye (Molecular Probes, Eugene, OR) as described elsewhere.30 Briefly, the cells were resuspended in 100 μL PBS to which 4 μL of a 10 μg/mL MC540 stock was added. The cells were incubated with the MC540 dye at room temperature, in the dark, for 20 minutes. At the end of the incubation period, the Fc receptors on the cell surface were blocked with anti-CD32/CD16 antibodies (1 μg/106 cells) before the addition of the following antibodies for a final concentration of 1 μg/106 cells: anti–c-kit conjugated to Cy-Chrome and fluorescein isothiocyanate (FITC)-conjugated anti-B220, anti-CD3, and anti-CD11b (all antibodies were obtained from Pharmingen, San Diego, CA). The cell/antibody solution was incubated on ice for 30 minutes in the dark. The cells were washed twice with cold PBS and then analyzed immediately with a FACScan flow cytometer and CellQuest software (Becton Dickinson, Co, Mountain View, CA). Selection of c-kit+ and lin+ cells and setting of quadrant markers was based on the background staining with irrelevant, isotype-matched, fluorescently conjugated antibody controls (Pharmingen).
RESULTS
BM-derived myeloid progenitor cells are cycling at a higher rate in CCR2 (−/−) compared with CCR2 (+/+) mice.
The cycling status (percentage of cells in S-phase) of BM and spleen CFU-GM, BFU-E, and CFU-GEMM were evaluated in a total of 11 CCR2 (−/−) and 8 CCR2 (+/+) mice (Table 1). A dramatic increase was found in the cycling status of the MPC derived from the BM of CCR2 (−/−) mice as compared with wild-type control mice. In contrast, CFU-GM, CFU-GEMM, and BFU-E in the spleens of CCR2 (−/−) and CCR2 (+/+) mice did not show a statistically significant difference in their cycling status (Table 1). These initial results suggested that signaling by CCR2 has an effect on the cycling status of MPC that reside in the BM, but not in the spleen.
Cycling Status of BM and Splenic Progenitors From CCR2 (−/−) and CCR2 (+/+) Mice
| . | Percentage of Progenitors in S-Phase . | ||
|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-GEMM . | |
| Femoral marrow | |||
| +/+ | 3.4 ± 1.8 | 8.1 ± 2.3 | 7.3 ± 3.3 |
| −/− | 62.9 ± 2.3 | 52.7 ± 3.5 | 70.1 ± 1.6 |
| P values | (<.001) | (<.001) | (<.001) |
| Spleen | |||
| +/+ | 3.7 ± 1.5 | 2.4 ± 1.9 | 4.0 ± 3.1 |
| −/− | 4.9 ± 2.0 | 7.9 ± 4.2 | 14.6 ± 5.7 |
| P values | (NS) | (NS) | (NS) |
| . | Percentage of Progenitors in S-Phase . | ||
|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-GEMM . | |
| Femoral marrow | |||
| +/+ | 3.4 ± 1.8 | 8.1 ± 2.3 | 7.3 ± 3.3 |
| −/− | 62.9 ± 2.3 | 52.7 ± 3.5 | 70.1 ± 1.6 |
| P values | (<.001) | (<.001) | (<.001) |
| Spleen | |||
| +/+ | 3.7 ± 1.5 | 2.4 ± 1.9 | 4.0 ± 3.1 |
| −/− | 4.9 ± 2.0 | 7.9 ± 4.2 | 14.6 ± 5.7 |
| P values | (NS) | (NS) | (NS) |
Results shown are the mean ± 1 SEM for a total of 11 CCR2 (−/−) and 8 CCR2 (+/+) mice from two experiments in which each mouse was assessed individually. CFU-GM was stimulated in agar culture by PWMSCM. BFU-E and CFU-GEMM were stimulated in methylcellulose cultures in the presence of hemin by Epo and PWMSCM.
Abbreviation: NS, P > .05.
Increased cycling rates of BM-derived progenitors from CCR2 (−/−) mice do not result in increased total cellularity or an increase in the absolute number of progenitor cells.
The increased cycling rates in BM-derived MPC from CCR2 (−/−) mice, if unchecked, would be expected to lead to a concomitant increase in total cellularity or absolute numbers of MPC in the BM or spleen, as previously noted for CXCR2 (−/−) mice.26 However, as shown in Table 2, the total nucleated cellularity in both the BM and spleen was not significantly different between CCR2 (−/−) and CCR2 (+/+) mice. Additionally, there were no significant differences in the absolute numbers of CFU-GM, BFU-E, or CFU-GEMM in BM or spleens of CCR2 (−/−) and CCR2 (+/+) mice (Table 2). These results suggested that MPC produced from increased proliferation in CCR2 (−/−) mice might be ablated before leaving the BM, because they were not found in increased numbers in the spleen, an organ with an environment conducive to receiving MPC that migrate from BM in response to stress-induced stimuli.1,2 8To test this hypothesis, we used flow cytometry to identify immature blood cells in the BM and to determine if there were differences in apoptosis between the CCR2 (−/−) and CCR2 (+/+) mice.
Total Nucleated Cellularity and Absolute Number of Progenitors From the BM and Spleen of CCR2 (−/−) and CCR2 (+/+) Mice
| . | Nucleated Cells ×10−6per Organ . | Absolute Numbers of Progenitors ×10−3 per Organ . | ||
|---|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-GEMM . | ||
| Femoral marrow | ||||
| +/+ | 22.8 ± 1.6 | 40.7 ± 6.1 | 13.4 ± 1.7 | 3.2 ± 0.8 |
| −/− | 21.1 ± 1.4 | 49.1 ± 6.7 | 10.9 ± 1.5 | 4.0 ± 0.8 |
| P values | (NS) | (NS) | (NS) | (NS) |
| Spleen | ||||
| +/+ | 103.1 ± 7.7 | 5.1 ± 0.8 | 6.3 ± 1.0 | 0.6 ± 0.3 |
| −/− | 100.9 ± 11.2 | 5.8 ± 1.5 | 7.3 ± 1.7 | 0.8 ± 0.3 |
| P values | (NS) | (NS) | (NS) | (NS) |
| . | Nucleated Cells ×10−6per Organ . | Absolute Numbers of Progenitors ×10−3 per Organ . | ||
|---|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-GEMM . | ||
| Femoral marrow | ||||
| +/+ | 22.8 ± 1.6 | 40.7 ± 6.1 | 13.4 ± 1.7 | 3.2 ± 0.8 |
| −/− | 21.1 ± 1.4 | 49.1 ± 6.7 | 10.9 ± 1.5 | 4.0 ± 0.8 |
| P values | (NS) | (NS) | (NS) | (NS) |
| Spleen | ||||
| +/+ | 103.1 ± 7.7 | 5.1 ± 0.8 | 6.3 ± 1.0 | 0.6 ± 0.3 |
| −/− | 100.9 ± 11.2 | 5.8 ± 1.5 | 7.3 ± 1.7 | 0.8 ± 0.3 |
| P values | (NS) | (NS) | (NS) | (NS) |
Results shown are the mean ± 1 SEM for a total of 11 CCR2 (−/−) and 8 CCR2 (+/+) mice from two experiments in which each mouse was assessed individually. MPC were stimulated in vitro as denoted in the legend to Table 1.
Abbreviation: NS, P > .05.
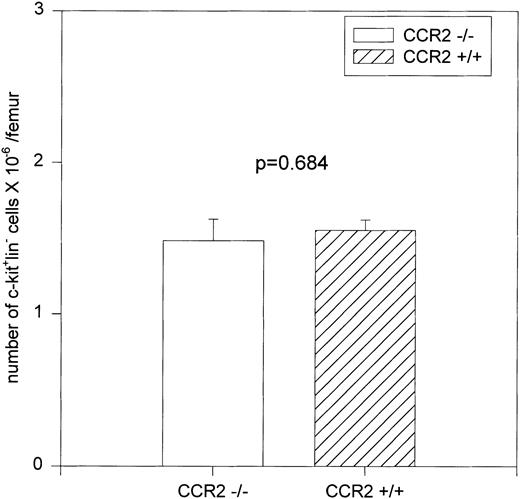
Flow cytometric analysis of c-kit+lin− primitive cell populations from the BM of CCR2 (−/−) and CCR2 (+/+) mice showed no differences in the numbers of these cells, but there was a greater percentage of apoptotic c-kit+lin− cells from CCR2 (−/−) mice.
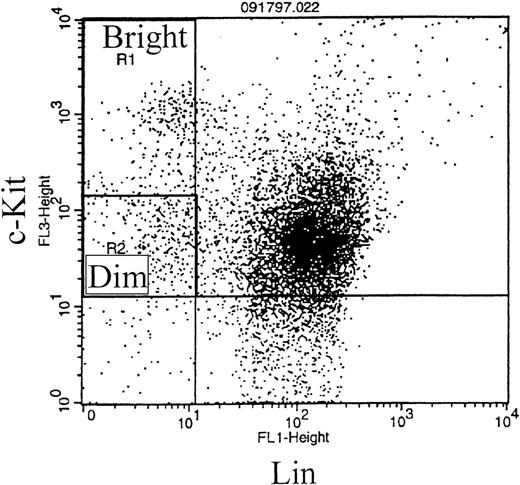
BM removed from killed mice was depleted of red blood cells and stained with fluorescently conjugated antibodies specific for c-kit and several lineage markers: B220 (expressed on B lymphocytes), CD3 (expressed on T lymphocytes), and CD11b (expressed on macrophages, monocytes, and neutrophils). c-kit is expressed on stem, progenitor, and immature cells in the BM.31-34 The anti-B220, anti-CD3, and anti-CD11b antibodies bind to and identify more mature cells that are committed to specific differentiation pathways. Therefore, staining with a combination of antibodies to c-kit and the lineage markers described above allows selection of the more immature cells within the BM (c-kit+lin− population). A representative dot plot of c-kit/lin selected regions is shown in Fig 1A. Percentages of cells of the total population for each region were determined separately for a total of 10 CCR2 (−/−) and 9 CCR2 (+/+) mice from two separate experiments, and the results were expressed as the absolute number per femur. As shown in Fig 2, there was no significant difference in the c-kit+lin−primitive cell population between the CCR2 (−/−) and CCR2 (+/+) mice, results consistent with no significant differences in MPC of the BM of these mice (Table 2).
Representative dot plot of murine BM cells stained with fluorescently conjugated antibodies that recognize c-kit (y axis) and the following lineage markers: B220, CD3, or CD11b (x axis) (A). The locations for the quadrant markers were set based on background, nonspecific staining using an irrelevant fluorescently conjugated antibody. (B) Representative dot plot of MC540 staining of c-kit+lin− gated cells (upper left-hand quadrant of dot plot in [A]). The fluorescence intensity of MC540 staining is plotted on the y axis against side scatter on the x axis. The MC540 gate to distinguish MC540bright, apoptotic cells from MC540dim, viable cells was determined by identification of viable and apoptotic cells from a forward versus side scatter analysis.
Representative dot plot of murine BM cells stained with fluorescently conjugated antibodies that recognize c-kit (y axis) and the following lineage markers: B220, CD3, or CD11b (x axis) (A). The locations for the quadrant markers were set based on background, nonspecific staining using an irrelevant fluorescently conjugated antibody. (B) Representative dot plot of MC540 staining of c-kit+lin− gated cells (upper left-hand quadrant of dot plot in [A]). The fluorescence intensity of MC540 staining is plotted on the y axis against side scatter on the x axis. The MC540 gate to distinguish MC540bright, apoptotic cells from MC540dim, viable cells was determined by identification of viable and apoptotic cells from a forward versus side scatter analysis.
Numbers of c-kit+lin− cells in total BM. c-kit+lin− cells (from the upper left-hand quadrant of Fig 1A) were gated and analyzed for their percentage in the total population of nucleated BM cells and the results are expressed as the number of cells per femur.
Numbers of c-kit+lin− cells in total BM. c-kit+lin− cells (from the upper left-hand quadrant of Fig 1A) were gated and analyzed for their percentage in the total population of nucleated BM cells and the results are expressed as the number of cells per femur.
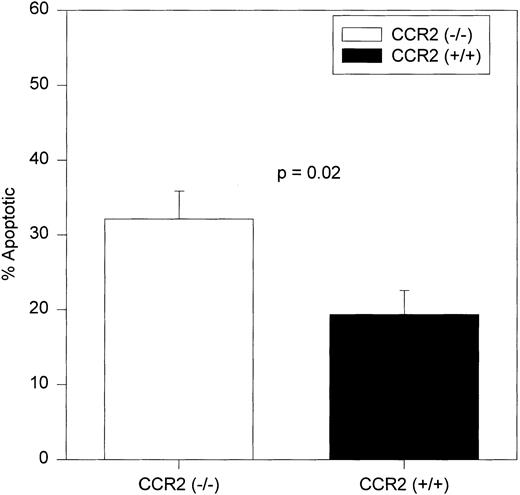
To evaluate apoptosis, BM cells from CCR2 (−/−) and (+/+) mice were stained with merocyanine 540 (MC540) dye in addition to staining with antibodies specific for c-kit and lineage markers. The MC540 dye detects apoptotic cells by associating with altered lipid moieties in the outer leaflet of the plasma membrane.30,35,36 Changes in the lipid composition of the outer leaflet of the plasma membrane represents one of the earliest stages of apoptotic cell death.37 Therefore, binding of MC540 dye detects cells that have just initiated an apoptotic program. Alternative methods that detect apoptotic cells by detecting chromosomal DNA fragmentation, an event that occurs in the later stages of apoptosis, may not be applicable to analysis of cells freshly isolated from a killed animal, because the late apoptotic cells may have already been cleared by resident macrophages.38-40
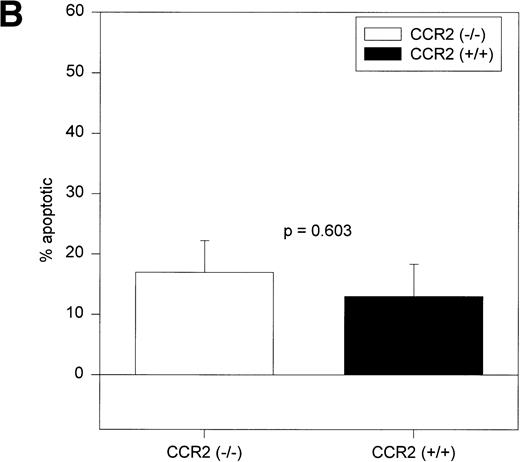
The c-kit+lin− cells shown in the upper left-hand region of Fig 1A were analyzed for MC540 staining. A representative dot plot of MC540-stained c-kit+lin− cells is shown in Fig 1B. As shown in Fig 3, the percentage of c-kit+lin− apoptotic cells (MC540 positive) found in the BM of CCR2 (−/−) mice was significantly greater than the percentage found in this same population from the CCR2 (+/+) mice.
Average percentages of apoptotic c-kit+/lin− cells in BM. c-kit+lin− cells were gated separately from the remainder of the BM population and analyzed for MC540 fluorescence intensity (MC540 bright cells represent the apoptotic fraction of the gated population). The same mice used to obtain results in Fig 1 were assessed independently in two separate experiments. The bars represent the mean ± 1 SEM.
Average percentages of apoptotic c-kit+/lin− cells in BM. c-kit+lin− cells were gated separately from the remainder of the BM population and analyzed for MC540 fluorescence intensity (MC540 bright cells represent the apoptotic fraction of the gated population). The same mice used to obtain results in Fig 1 were assessed independently in two separate experiments. The bars represent the mean ± 1 SEM.
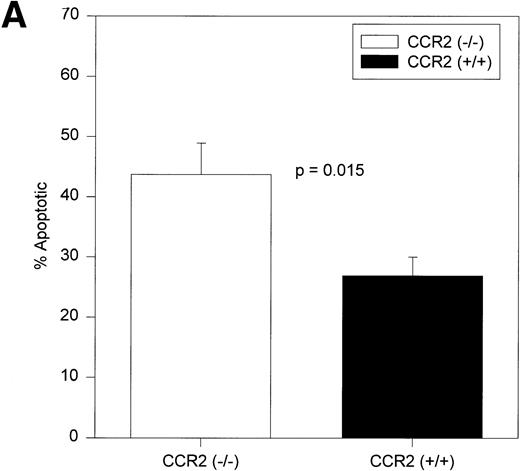
Increased apoptosis is seen only in the c-kitdimlin− primitive population of cells.
Analysis of the c-kit+lin+ and c-kit−lin+ gated regions shown in Fig 1A (upper right and lower right panels) showed no significant differences in the percentage of the total population or in the percentage of apoptotic cells between CCR2 (−/−) and CCR2 (+/+) mice (Table 3). The c-kit+lin− population shown in the dot plot of Fig 1A can be divided into two subpopulations of cells: c-kitbrightlin− and c-kitdimlin−(Fig 4). Both of these subpopulations contain progenitor cells, but studies are controversial as to which subpopulation contains the more immature progenitors.41-45As shown in Fig 5A, the CCR2 (−/−) mice had a significantly greater percentage of apoptotic cells within the c-kitdimlin−population. No significant difference in apoptotic cells was noted between CCR2 (−/−) and CCR2 (+/+) cells within the c-kitbrightlin− population (Fig 5B). Therefore, the increased apoptosis seen in the c-kit+lin− cells is due to increased apoptosis of the c-kitdimlin−subpopulation of cells.
Comparative Analysis of the Percentage of Cells and Apoptosis of c-kit+lin+ and c-kit−lin+ Cells From CCR2 (−/−) and CCR2 (+/+) Mice
| Cell Population . | Percentage of BM . | Percentage Apoptotic . | ||
|---|---|---|---|---|
| CCR2 (−/−) . | CCR2 (+/+) . | CCR2 (−/−) . | CCR2 (+/+) . | |
| c-kit+lin+ | 41.2 ± 6.8 | 47.1 ± 1.5 | 67.3 ± 3.9 | 62.2 ± 4.2 |
| c-kit−lin+ | 53.1 ± 6.6 | 47.0 ± 1.7 | 56.8 ± 6.7 | 56.8 ± 6.1 |
| Cell Population . | Percentage of BM . | Percentage Apoptotic . | ||
|---|---|---|---|---|
| CCR2 (−/−) . | CCR2 (+/+) . | CCR2 (−/−) . | CCR2 (+/+) . | |
| c-kit+lin+ | 41.2 ± 6.8 | 47.1 ± 1.5 | 67.3 ± 3.9 | 62.2 ± 4.2 |
| c-kit−lin+ | 53.1 ± 6.6 | 47.0 ± 1.7 | 56.8 ± 6.7 | 56.8 ± 6.1 |
Results shown are the mean ± 1 SEM. None of the comparisons within each cell type is significantly different (P > .05) between CCR2 (−/−) and (+/+) cells.
Representative dot plot that distinguishes c-kitbrightlin− from c-kitdimlin− cells. The upper left-hand quadrant (c-kit+lin−) can be divided into two separate subpopulations as shown: c-kitbrightlin− and c-kitdimlin−.
Representative dot plot that distinguishes c-kitbrightlin− from c-kitdimlin− cells. The upper left-hand quadrant (c-kit+lin−) can be divided into two separate subpopulations as shown: c-kitbrightlin− and c-kitdimlin−.
Percentages of c-kitdimlin− (A) and c-kitbrightlin− (B) apoptotic cells in BM of CCR2 (−/−) and (+/+) mice. The cells were gated separately from the remainder of the BM population and analyzed for apoptosis (MC540 binding). Ten CCR2 (−/−) and 9 CCR2 (+/+) mice were analyzed individually from two separate experiments. The bars represent the mean ± 1 SEM.
Percentages of c-kitdimlin− (A) and c-kitbrightlin− (B) apoptotic cells in BM of CCR2 (−/−) and (+/+) mice. The cells were gated separately from the remainder of the BM population and analyzed for apoptosis (MC540 binding). Ten CCR2 (−/−) and 9 CCR2 (+/+) mice were analyzed individually from two separate experiments. The bars represent the mean ± 1 SEM.
As seen in Fig 6A, there were no significant differences in absolute numbers of c-kitdimlin− cells between CCR2 (−/−) and CCR2 (+/+) mice. However, there was a very small but significant decrease in the number of c-kitbrightlin− cells in CCR2 (−/−) mice as compared with CCR2 (+/+) controls (Fig 6B).
Absolute numbers of c-kitdimlin− (A) and c-kitbrightlin− (B) cells per femur as assessed in 10 CCR2 (−/−) and 9 CCR2 (+/+) individual mice from two experiments. The bars represent the mean ± 1 SEM.
Absolute numbers of c-kitdimlin− (A) and c-kitbrightlin− (B) cells per femur as assessed in 10 CCR2 (−/−) and 9 CCR2 (+/+) individual mice from two experiments. The bars represent the mean ± 1 SEM.
Increased cycling rates of progenitors in the c-kit+lin− population of BM cells from CCR2 (−/−) mice.
Because it was the c-kit+lin− population of BM cells that demonstrated significant differences in apoptosis between CCR2 (−/−) and CCR2 (+/+) mice, we evaluated the cycling rates of MPC in this phenotypically defined population of early cells. Figure 7 presents data demonstrating that CFU-GM, BFU-E, and CFU-GEMM in c-kit+lin− BM cells from CCR2 (−/−) mice are proliferating at a higher rate than these cells in CCR2 (+/+) mice, consistent with the data shown in Table 1 for MPC in unseparated BM. The total cloning efficiencies for MPC in the c-kit+lin− population of the BM from the two CCR2 (−/−) and two CCR2 (+/+) mice were as follows: 8.2%, 8.1%, 8.8%, and 7.5% based on the number of colonies formed per 1,000 c-kit+lin− cells plated.
Cycling rates of myeloid progenitors in c-kit+lin− BM. The cycling rates of progenitors were determined by tritiated thymidine kill assay of sorted c-kit+lin− BM cells. Results represent the mean ± range of the assessment of separated cells from two individual mice each from the CCR2 (−/−) and (+/+) groups in which 1,000 cells were plated in methylcellulose in the presence of Epo, PWMSCM, and SLF.
Cycling rates of myeloid progenitors in c-kit+lin− BM. The cycling rates of progenitors were determined by tritiated thymidine kill assay of sorted c-kit+lin− BM cells. Results represent the mean ± range of the assessment of separated cells from two individual mice each from the CCR2 (−/−) and (+/+) groups in which 1,000 cells were plated in methylcellulose in the presence of Epo, PWMSCM, and SLF.
Decreased survival of progenitors from CCR2 (−/−) BM upon delayed growth factor addition.
The percentages of apoptotic cells noted above were for phenotypically defined populations of cells. It is known that MPC undergo apoptosis upon growth factor withdrawal.8 To better compare apoptosis in a functional assay, MPC from CCR2 (−/−) and CCR2 (+/+) mice were evaluated for survival as assessed by colony formation of CFU-GM, BFU-E, and CFU-GEMM with delayed additions of Epo, PWMSCM, and SLF. A comparative analysis of plates containing hemin at the initiation of the assay or added with the growth factors at the time points indicated was also performed. The results in Fig 8 demonstrate a decreased survival of CFU-GEMM, BFU-E, and CFU-GM from BM cells of CCR2 (−/−) compared with CCR2 (+/+) mice, effects apparent throughout the 24-hour span of delayed growth factor addition. Because the results were similar for each analysis, the plotted data represent a combination of delayed growth factor addition analysis for all assay variations, including unseparated BM cells, purified c-kit+lin− cells, and comparison of the addition of hemin at time 0 to all plates or in combination with the other growth factors at the time points shown. These colony results are consistent with the apoptotic differences noted between CCR2 (−/−) and (+/+) for phenotypically characterized cells.
Effects of delayed addition of growth factors on survival of CFU-GEMM, CFU-GM, and BFU-E. Results shown are a combined analysis for 6 mice each for CCR2 (−/−) and (+/+) mice using unseparated BM cells and purified c-kit+lin− cells as described in Materials and Methods, in which hemin was either in all plates before addition of growth factors or in which hemin was left out of the plates, but present in the cocktail of growth factors added at time 0 or during addition at later time points. Survival of MPC is based on time 0 control colony counts. Each point represents the mean of the combined data ±1 SEM. P values were less than .01 for each graph at each time point, except for the 24-hour point in the BFU-E graph, in which the P value is .04.
Effects of delayed addition of growth factors on survival of CFU-GEMM, CFU-GM, and BFU-E. Results shown are a combined analysis for 6 mice each for CCR2 (−/−) and (+/+) mice using unseparated BM cells and purified c-kit+lin− cells as described in Materials and Methods, in which hemin was either in all plates before addition of growth factors or in which hemin was left out of the plates, but present in the cocktail of growth factors added at time 0 or during addition at later time points. Survival of MPC is based on time 0 control colony counts. Each point represents the mean of the combined data ±1 SEM. P values were less than .01 for each graph at each time point, except for the 24-hour point in the BFU-E graph, in which the P value is .04.
DISCUSSION
The large redundancy in chemokine/chemokine receptor interactions offers the possibility of compensatory effects that could mask actions in mice in which a chemokine or chemokine receptor gene is knocked out. Thus, gene-knockout mice may not pick up all the functions of the gene that has been knocked out. However, it should pick up effects if that gene plays a dominant role in certain events within the animal. The problem of potential redundancy is especially apparent with chemokines in that a single chemokine receptor can bind more than one chemokine, as is the case for CCR2, which can bind human MCP-1 and its murine counterpart JE, as well as human MCP-2, MCP-3, and MCP-4 and murine MCP-5.4,9 Also, a number of chemokines can bind more than one chemokine receptor, and not all the receptors that bind the particular chemokine may signal in response to that chemokine. For example, MIP-1α binds at least CCR1, CCR5, and CCR9.4,9However, CCR9, a promiscuous receptor that binds MIP-1α as well as MCP-1, MCP-2, MCP-3, MCP-4, and MCP-5, among other chemokines, does not appear to signal in response to these chemokines.46 Added to this complexity is the fact that new chemokines continue to be discovered,9 and in some cases it is not known which receptors they interact with.
However, studies in gene-targeted mice demonstrate that certain cytokines or receptors play an important role in hematopoiesis.8 Dominant effects have been noted in interactions involving blood cell regulation in mice in which the expression of the chemokine receptors CXCR226 or CCR124,27 have been deleted. In this context, deletion of CCR2 results in the inability of MPC from BM to respond to the myelosuppressive effects of human MCP-1 and murine JE in vitro.28 The CCR2 (−/−) MPC were not compromised in their capacity to respond to the negative regulatory effects of other chemokines that use other chemokine receptors.28 We also noted, as data not shown, that the absolute number of progenitors in BM and spleen and the nucleated blood cell counts of CCR2 (−/−) mice were within the same ranges as CCR2 (+/+) mice.28 The tentative conclusion proposed was that CCR2 may not be required for normal steady-state myelopoiesis.28 This was in contrast to the results seen in CXCR2 knock-out mice in which hyperproliferation of MPC in the marrow and spleen was noted under normal environmental conditions.26 This hyperproliferative state was consistent with the inability of MPC from the BM of CXCR2 (−/−), but not of CXCR2 (+/+) mice, to respond to inhibition by human IL-8 or murine MIP-2.26 Based on this, CXCR2 was suggested to be involved in negative regulation of myelopoiesis. Knockout of CCR1 gene expression did not result in an increased number of myeloid progenitors per organ,24 a result similar to that of the CCR2 knockout. However, in contrast to the CCR2 (−/−) mice, the MPC from BM of the CCR1 (−/−) mice were just as sensitive to inhibition by the ligand MIP-1α as MPC from BM of CCR1 (+/+) mice and did not have a significantly increased cycling status.27Thus, the results in the CCR2 knockout were intriguing and suggested compensatory, CCR2-dependent mechanisms that would maintain homeostasis in the BM.
Evaluation of the cycling status of MPC is sometimes a more sensitive indicator of the proliferative responses of MPC than are changes in the absolute number of these progenitors per organ in mice.47-49 As noted in the present study, MPC in BM from the CCR2 (−/−) mice were cycling to a much greater extent than in BM from the CCR2 (+/+) mice. The cycling data in Table 1 and Fig 6 would indicate that as much as 70% of the early multipotential (CFU-GEMM) MPC from CCR2 (−/−) mice were in S phase at the time of pulse with high-dose 3H thymidine, which is at least a 10-fold increase over CCR2 (+/+) mice. This exceptionally high value is the result of enhanced proliferation of MPC in the BM of CCR2 (−/−) mice and is indicative of rapid cell cycle progression with a very short G1 phase.50-52 Work published by others showed that the percentage of cells in S phase as measured by3H thymidine kill assay can be as high as 70% within particular subsets of progenitor cells in mice receiving myeloablative treatment.53 Additionally, this work also estimated that the average cell cycle time for rapidly cycling progenitors can be as low as 6 to 9 hours. A significant amount of this time is required to duplicate chromosomal DNA during the S phase. Therefore, the increased, high percentage of cycling MPC in the BM of CCR2 (+/+) mice compared with CCR2 (−/−) mice indicates the extent of direct and/or indirect suppressive effects of CCR2 on the cell cycle progression of early MPC.
Unlike the cycling status of MPC in the BM, enhanced cycling of splenic MPC in CCR2 (−/−) mice was not observed. This may relate to the microenvironment of the BM compared with that of the spleen. In the adult, under steady-state conditions, the BM is considered to be the major source of production of stem cells and MPC. Although the spleen can be a hematopoietically active organ in the mouse, this is usually most noticeable after stress-induced stimuli.1,2,8 24 The lack of enhanced cycling of CCR2 (−/−) splenic MPC most likely reflects the lack of the appropriate stimuli in the spleen of these unperturbed mice.
Still, a major question was why the enhanced proliferative state of MPC in the BM of CCR2 (−/−) mice was not translating into increased numbers of MPC and nucleated cells in these mice. One possibility for this apparent discrepancy was that the MPC in the BM of CCR2 (−/−) mice were not only undergoing enhanced proliferation, but that they were also undergoing enhanced apoptosis. This hypothesis was verified at the level of c-kit+lin− BM cells from CCR2 (−/−) mice using the MC540 dye to asses apoptosis. Of interest is that the enhanced apoptosis of the c-kit+lin− cells was apparent for the c-kitdimlin− cells. Published reports are contradictory as to whether the c-kitdimlin− or c-kitbrightlin− subpopulation contains the more immature progenitor and stem cells.41-45 However, the growth factor requirements for colony formation differ between c-kitbright and c-kitdim cells and suggests that the more immature progenitors reside in the c-kitdimpopulation.44 The more immature progenitors are triggered to proliferate by a combination of growth factors such as those used in the present study: Epo, SLF, and the many stimulatory cytokines present in PWMSCM.8,9 29 The absence of CCR2 has apparent effects occurring at earlier stages of maturity on phenotypically defined cells and is consistent with the functional colony data. Enhanced apoptosis in the BM of CCR2 (−/−) mice as demonstrated by the delayed growth factor addition assay suggests a dominant role for CCR2 in the survival of MPC.
It is believed that apoptosis plays a critical role in maintaining homeostasis during the process of hematopoiesis. However, the extent of apoptosis within each stage of cell development in the BM has not been clearly defined. Recently published work suggests that the highest apoptotic rates (up to 99%) occur among the earliest progenitor cells but decreases to almost 0% as the cells differentiate and mature.54 Based on these observations, the high degree of apoptosis seen in the c-kit+lin−population of BM, particularly the c-kitdimlin− subpopulation of both CCR2 (−/−) and CCR2 (+/+) mice, suggests that these populations contain very immature cells in the BM compartment. Therefore, the apoptotic values of these phenotypically defined populations would be greater than observations of overall apoptosis noted in total BM. Additionally, the MC540 dye detects cells undergoing the earliest stages of apoptosis, whereas other methods used to evaluate apoptosis through the detection of DNA fragmentation, a late apoptotic event, would exclude apoptotic cells that have been engulfed by resident macrophages. Therefore, the MC540 dye may give a more accurate accounting of the extent of apoptosis within progenitor subsets in the BM and these values may be higher than apoptotic values found using alternative methods.
Several chemokines that bind chemokine receptors other than CCR2 have been shown to exhibit suppressive effects on the proliferation of MPC from murine BM (reviewed in Broxmeyer and Kim9). Therefore, it is interesting that other myelosuppressive chemokines, such as MIP-1α, do not have compensatory suppressive effects on the proliferation of BM progenitors in CCR2 (−/−) mice. This again indicates the apparent dominant effects of CCR2 signals on the cycling of MPC. MIP-1α was the first chemokine to be characterized as having in vitro suppressive effects on the cycling of early progenitor cells while enhancing proliferation of more mature progenitors.19 However, mice deficient in MIP-1α expression did not have an expanded progenitor pool.55 This may be due to compensatory actions of other chemokines, such as those that bind CCR2, or MIP-1α effects under steady-state conditions that are not relevant in vivo. The cycling and apoptotic status of progenitors in the MIP-1α knockout mice were not examined, and it is possible that the absence of this chemokine does allow for enhanced proliferation with compensatory increases in apoptosis, as seen in this study of CCR2 (−/−) mice. The apparent lack of compensatory effects by other suppressive chemokines in the CCR2 (−/−) mice may be directly or indirectly due to the absence of the CCR2 receptor. The lack of this receptor may skew expression of myelosuppressive chemokines and/or chemokine receptors such that compensatory negative regulation by additional members of the chemokine network is no longer possible. Studies published by Boring et al28 showed that CCR2 (−/−) mice have altered cytokine production in their lymphatics and in specific lymphocyte subsets. Perhaps chemokine expression profiles are altered as well. Increased levels of chemokines that are not suppressive themselves but are capable of inhibiting the suppressive effects of other chemokines may be yet another consequence of the absence of CCR2. The lack of compensatory negative regulation is intriguing but at this point open to speculation until further studies can be performed.
There are additional, interesting phenotypic aspects of the progenitor cells in the BM of CCR2 (−/−) mice. Assessment by colony assay of MPC in CCR2 (−/−) mice indicated that these populations and perhaps the stem cell pool had not been exhausted due to their increased cycling status. Substantial evidence has demonstrated the potential for exhaustion of progenitor and stem cell pools when sustained, intense, proliferative demands are placed on these cells.56-63 There is also contradictory evidence suggesting that the stem cell compartment does not become exhausted under similar circumstances.64 65 Additionally, these mice had not succumbed to leukemogenesis. The mice were typically only 6 to 8 weeks old at the time of analysis and may not have exhausted progenitor and stem cell pools or allowed expansion of leukemogenic cells. As the data indicates, effective apoptotic mechanisms remain intact in the CCR2 (−/−) mice, preventing overexpansion of MPC and thus keeping a potentially myeloproliferative disorder under control.
Which chemokines specifically mediate the negative proliferative and positive survival effects of CCR2 on MPC remains to be determined, and the same chemokines may not necessarily mediate both effects. MCP-1 and MCP-4, but not MCP-2 and MCP-3, have been shown to suppress the proliferation of MPC in vitro,16,17,21 and MCP-1 has been tested in vivo in mice and shown to be myelosuppressive.16 17 Thus, MCP-1 and MCP-4 are reasonable candidates for chemokines that might act in a negative sense to limit murine/human myelopoiesis through CCR2. Murine MCP-5 has not yet been tested in this setting.
Supported by Public Health Service Grants No. R01 HL 56416, R01 DK53674, and R01 HL 5407 (to H.E.B.) and R01 HL 5277 (to I.F.C.) from the National Institutes of Health (NIH). A.R. is supported by NIH Training Program T32 DK 07519 (to H.E.B.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hal E. Broxmeyer, PhD, Walther Oncology Center, Indiana University School of Medicine, 1044 W Walnut St, Room 302, Indianapolis, IN 46202-5254; e-mail: hbroxmey@iupui.edu.

![Fig. 1. Representative dot plot of murine BM cells stained with fluorescently conjugated antibodies that recognize c-kit (y axis) and the following lineage markers: B220, CD3, or CD11b (x axis) (A). The locations for the quadrant markers were set based on background, nonspecific staining using an irrelevant fluorescently conjugated antibody. (B) Representative dot plot of MC540 staining of c-kit+lin− gated cells (upper left-hand quadrant of dot plot in [A]). The fluorescence intensity of MC540 staining is plotted on the y axis against side scatter on the x axis. The MC540 gate to distinguish MC540bright, apoptotic cells from MC540dim, viable cells was determined by identification of viable and apoptotic cells from a forward versus side scatter analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1524/4/m_blod40507001x.jpeg?Expires=1767766714&Signature=2K1~P5uChwhcQaoh7b-Iu~UxG8won1TeR4ZluquSOeoDfiTM0q-7YDAFTCMhB~EFPDZY26mmXePSKS2wy7q7EneEShtX1mrMjGp-jlHVtFZ4t2BmJeDP5nZqKT4IWTU-s7LuWb9~fKlWpFayg1YP1M01jaB254QnxGySrOhf0nHD1cWWFWEuJgGkdPVlPURoPc89EwIspeYKScU6vdTDNRkMUMLPPqjhwCHVn4rTCMu7PpHys5YsxUY2E4NdeV12AJB-bwpgaq7Fffy4yTCpbK~5datShPe3AjC-ILGBVQ1oLsmj6P6hcWD6OBxZpLnjB1wg9ABR8w8~m4HprZKRnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal