Abstract
Human immunodeficiency virus (HIV)-specific cytotoxic T lymphocytes (CTL) probably play the major role in controlling HIV replication. However, the value of adoptive transfer of HIV-specific CTL expanded in vitro to HIV+ patients has been limited: this contrasts with the success of CTL therapy in treating or preventing Epstein-Barr virus and cytomegalovirus disease after bone marrow transplantation (BMT). We investigated the fate of expanded HIV-specific CTL clones in vivo following adoptive transfer to a patient with acquired immunodeficiency syndrome (AIDS). Two autologous CTL clones specific for HIV Gag and Pol were expanded to large numbers (>109) in vitro and infused into an HIV-infected patient whose viral load was rising despite antiretroviral therapy. The fate of one clone was monitored by staining peripheral blood mononuclear cells (PBMCs) with T-cell receptor–specific tetrameric major histocompatibility complex (MHC)-peptide complexes. Although the CTL transfer was well tolerated, there were no significant changes in CD4 and CD8 lymphocyte counts and virus load. By tracking an infused clone using soluble MHC-peptide complexes, we show that cells bearing the Gag-specific T-cell receptors were rapidly eliminated within hours of infusion through apoptosis. Thus, the failure of adoptively transferred HIV-specific CTL to reduce virus load in AIDS may be due to rapid apoptosis of the infused cells, triggered by a number of potential mechanisms. Further trials of adoptive transfer of CTL should take into account the susceptibility of infused cells to in vivo apoptosis.
SEVERAL INDEPENDENT observations suggest that cytotoxic T lymphocytes (CTL) are critical for the control of human immunodeficiency virus (HIV) infection. Virus-specific CTL can be detected early in HIV infection and are temporally associated with the rapid decreases in plasma virus load after primary infection.1,2 These early vigorous responses have been shown to drive the selection of viruses with mutations in the targeted epitopes, providing convincing evidence for CTL-directed pressure.3,4 CTL responses have also been detected in infants and sex workers with HIV exposure who remain uninfected, suggesting that CTL could be associated with clearance or prevention of infection in some patients.5,6 In a closely related animal model, the inhibition of CTL by infusion of CD8-blocking antibodies resulted in marked increases of simian immunodeficiency virus (SIV) in the plasma and lymph nodes of infected macaques.7 More recently, a strong inverse correlation between plasma viral load and the numbers of circulating HIV-specific CTL, measured by the novel technique of soluble peptide-HLA tetramers which represent the target of CTL recognition, has been shown.8 Taken together, these and other reports are consistent with the view that CTL responses are an integral part of virus control.9
These observations raised the possibility that treating HIV-infected patients by the adoptive transfer of CTL could be beneficial, particularly because such therapy has proved to be safe and effective for the prevention and treatment of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation in bone marrow transplant (BMT) recipients.10-12 However, the transfer of HIV-specific CTL lines or clones in HIV-infected patients, although also safe, has so far produced only modest results.13-15
We have transferred two autologous CTL clones specific for epitopes derived from Gag and Pol to a patient with a high virus load and clinical disease. To trace the fate of the infused cells in vivo, we have used soluble major histocompatibility complex (MHC)-peptide molecules (tetramers) specific for the T-cell receptor (TCR) of one of the infused clones.
PATIENT AND METHODS
Patient 868 is a white homosexual male (HLA-A*0201, A*2401,B*2705,B*3501) who had been HIV+ for over 6 years and whose clinical course included episodes of Pneumocystis carinii pneumonia and recurrent Herpes simplex infections. At transfer, the patient’s CD4 count was 430/μL and he experienced symptoms such as fatigue and sweats, which were attributed to a high viral load only partially controlled by antiretroviral drugs. His medications included zidovudine, didanosine, and prophylactic trimethoprim-sulfamethoxazole. Ethical approval was obtained from Central Oxford Regional Ethics Committee and informed consent from the patient.
HIV-specific CTL oligoclonal lines were produced by incubating fresh peripheral blood mononuclear cells (PBMC) obtained from the patient with irradiated, autologous EBV-transformed lymphoblastoid cell lines (LCL) coated with peptides derived from Gag and Pol. Individual CTL clones were established by limiting dilution. Large-scale in vitro expansion of CTL was based on published methods.14 Cells were grown in tissue culture flasks, each containing the clone, irradiated allogeneic PBMC, irradiated allogeneic LCL, and anti-CD3 (OKT3, a gift from Orthoclone, Raritan, NJ). The culture medium was RPMI-1640 supplemented with 10% human AB serum, glutamine, penicillin, and streptomycin. Aliquots of cells and medium were sent for microbiological testing to ensure sterility. Chromium-51 (Cr-51) release assays were performed according to standard methods.
Anchored polymerase chain reaction (PCR) was performed as previously described to determine the TCR of each clone and establish clonality.16 17
Synthesis of MHC-peptide tetrameric complexes has been described.18 Purified HLA-A2 heavy chain and β2 microglobulin were synthesized using a bacterial expression system (pET; R&D Systems, Abingdon, Oxon, UK). The heavy chain was modified by the deletion of the transmembrane/cytosolic tail and the addition of a C-terminal sequence containing the BirA enzymatic biotinylation site. Heavy-chain, β2 microglobulin and peptide were refolded by dilution. The 45-kD refolded product was isolated by fast protein liquid chromatography (FPLC), and biotinylated by BirA. Streptavidin-phycoerythrin conjugate (Leinco Technologies, St. Louis, MO) was added and the tetrameric product was concentrated to 1 mg/mL.
The CTL clones were phenotyped by dual-staining with phycoerythrin (PE)-conjugated anti-CD8 monoclonal antibody (MoAb) (Dako, Cambridgeshire, UK) and fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (Dako), anti-CD28 (Immunotech, Westbrook, ME), or anti-Fas MoAb, and analyzed on a Becton Dickinson FACScan using Cellquest software (Becton Dickinson, San Jose, CA). Negative isotype control MoAbs were FITC- and PE-conjugated MoAbs of irrelevant specificity (Dako). CD4 and CD8 cell counts were done in a clinical immunology laboratory by standard methods. The plasma HIV-RNA concentrations were determined using a commercial PCR assay (Roche Amplicor, Branchburg, NJ).
To analyze apoptosis in vivo, PBMC were stained with 3 μL of PE-conjugated A2 Gag-specific tetramer (1 mg/mL) and 2 μL of Tricolour-conjugated anti-CD8 (Caltag, Burlingame, CA) at 4°C for 30 minutes. After two washes the cells were stained with 100 μL Annexin-V-Fluos (Boehringer Mannheim, Mannheim, Germany) for 15 minutes and analyzed by flow cytometry as described above. In this study, the gate was set on live cells, which removes more than 90% of the necrotic cell population (data not shown).
RESULTS
HIV-specific clones.
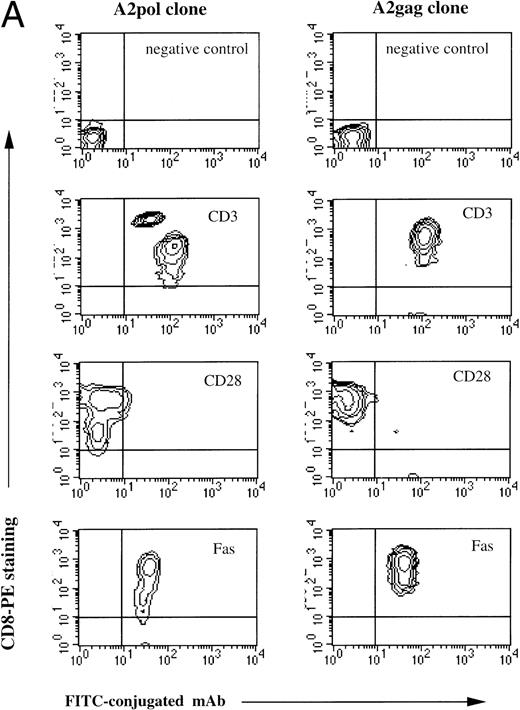
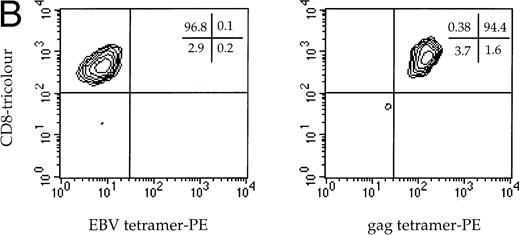
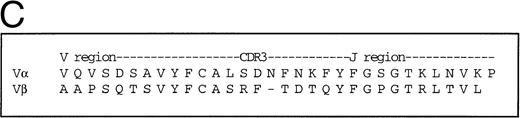
Two HLA A*0201-restricted CTL clones derived from patient 868 were selected for adoptive transfer. The first recognized the immunodominant HLA-A*0201-restricted epitope from Gag p17-8, SLYNTVATL,19and the other recognized a conserved but infrequently recognized epitope from Pol, VIYQYMDDL.20 Both the A2 Gag clone and the A2 Pol clone expressed CD3, CD8, and Fas, but not CD28 (Fig1A). Dual staining of the A2 Gag clone with soluble MHC-Gag tetramer and anti-CD8 confirmed its specificity (Fig1B), but attempts to stain clone 10 failed because of the instability of the Pol-tetramer complex, thought to be a consequence of the low affinity of this epitope for HLA A*0201 (data not shown). Thus, the Vα and Vβ usage and complementarity determining region 3 (CDR3) of the clone was determined by sequencing using anchored PCR. Of 18 transcripts sequenced, all contained the same CDR3 nucleotide segment corresponding to Vα22/Jα9.11 and Vβ13.3/Jβ2.3/Cβ2 (Fig 1C), thereby providing evidence of clonality.
(A) Phenotype of the clones. The A2 Gag and the A2 Pol clones were analyzed by flow cytometry. The y-axes indicate staining with CD8-PE and the x-axes represent staining with control antibody, CD3-FITC, CD28-FITC, and Fas-FITC. (B) Specificity of the A2 Gag clone. The panel shows the A2 Gag clone stained with anti–CD8-tricolor and either an irrelevant A2 tetramer- (complexed with an EBV peptide) or A2 Gag tetramer-PE. (C) The sequences of the CDR3 regions of the A2 pol clone T-cell receptor. The V and Vβ usage and CDR3 sequences of the TCR of the pol-specific CTL clone were the same in 18 transcripts, providing evidence of clonality.
(A) Phenotype of the clones. The A2 Gag and the A2 Pol clones were analyzed by flow cytometry. The y-axes indicate staining with CD8-PE and the x-axes represent staining with control antibody, CD3-FITC, CD28-FITC, and Fas-FITC. (B) Specificity of the A2 Gag clone. The panel shows the A2 Gag clone stained with anti–CD8-tricolor and either an irrelevant A2 tetramer- (complexed with an EBV peptide) or A2 Gag tetramer-PE. (C) The sequences of the CDR3 regions of the A2 pol clone T-cell receptor. The V and Vβ usage and CDR3 sequences of the TCR of the pol-specific CTL clone were the same in 18 transcripts, providing evidence of clonality.
Adoptive transfer.
The A2 Gag and Pol clones were grown to 1.1 × 109 and 1.7 × 109 cells, respectively, for adoptive transfer and the expanded clones were shown to have maintained both HIV-specific cytotoxic activity and an ability to suppress HIV replication in vitro (not shown). The cells were infused into a peripheral vein over a period of 30 minutes. The transfer was well tolerated except for a single episode of chills and rigors within 4 to 6 hours of infusion which rapidly subsided: the patient was subsequently well.
CD4/CD8 and virus loads postinfusion.
Table 1 shows the peripheral blood CD4 and CD8 lymphocyte counts and the virus load before and after adoptive transfer. Despite the substantial number of specific CTL infused, the number of CD8+ cells had not increased at 1 hour or 20 hours after infusion, and there were no significant changes in the virus load following therapy.
Changes in the CD4/CD8 Counts and Viral Load Before and After CTL Infusion
| Time . | . | +1 h . | +20 h . | +48 h . | +7 d . | +14 d . | +21 d . |
|---|---|---|---|---|---|---|---|
| CD4 (cells/μL) | 430 | 440 | 390 | 490 | 480 | 510 | 400 |
| CD8 (cells/μL) | 1,720 | 1,760 | 1,600 | 2,030 | 1,910 | 2,100 | 1,810 |
| Virus load (RNA copies/mL) | 31,000 | 26,600 | 49,300 | 45,970 | 63,500 | 56,300 | ND |
| Time . | . | +1 h . | +20 h . | +48 h . | +7 d . | +14 d . | +21 d . |
|---|---|---|---|---|---|---|---|
| CD4 (cells/μL) | 430 | 440 | 390 | 490 | 480 | 510 | 400 |
| CD8 (cells/μL) | 1,720 | 1,760 | 1,600 | 2,030 | 1,910 | 2,100 | 1,810 |
| Virus load (RNA copies/mL) | 31,000 | 26,600 | 49,300 | 45,970 | 63,500 | 56,300 | ND |
CD4 and CD8 counts were measured by flow cytometry and plasma viral load was measured using the Roche Amplicor assay.
Abbreviation: ND, not determined.
Tracking clone 19 in vivo.
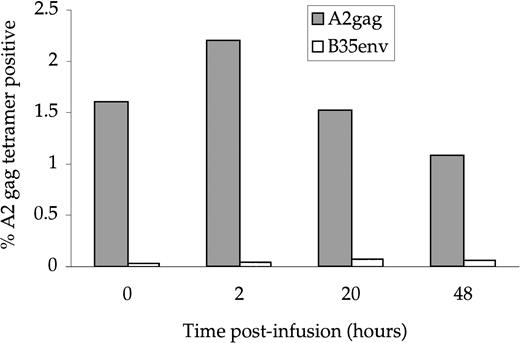
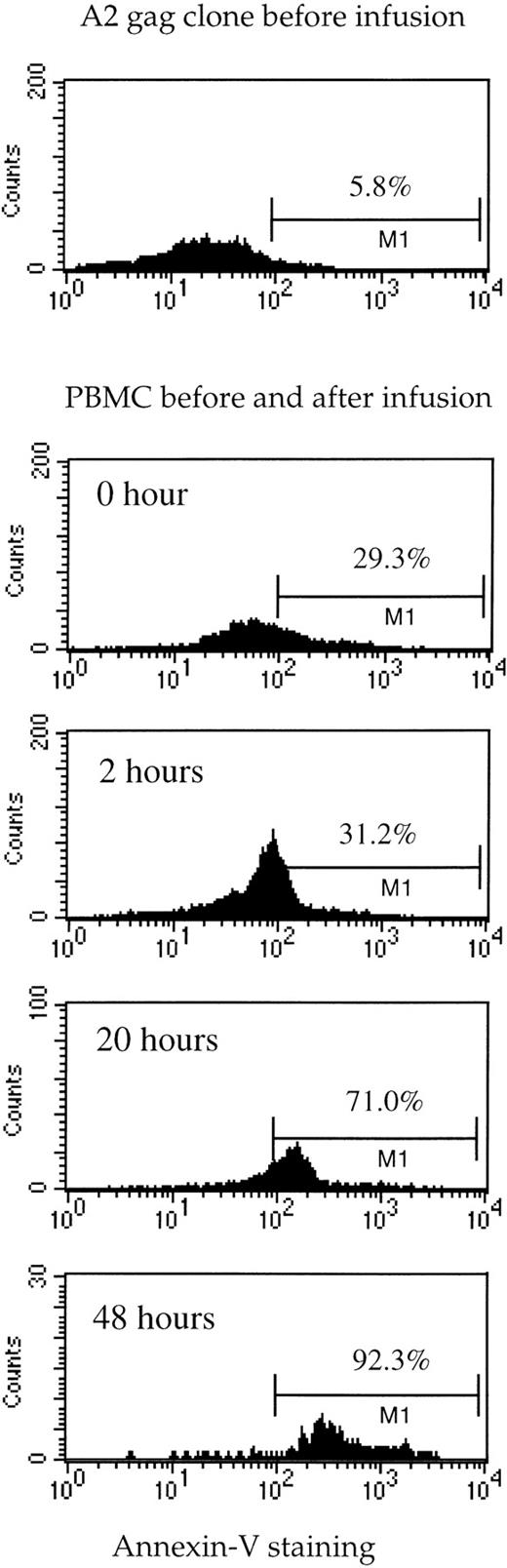
PBMC obtained from the patient before and after adoptive transfer were stained with the TCR-specific tetramer (which recognizes the TCR on the A2 Gag clone) and CD8-tricolor (Fig2). Parallel studies were done using a tetramer of HLA-B35 complexed with an HIV Env peptide, for which CTL were not infused. Tetramer-positive cells accounted for 1.6% of CD8 cells in the patient before the infusion, and this increased to 2.2% after infusion. Because 2.5 × 105 staining events were measured at each time-point with the same reagents under the same conditions, this represents a real and substantial increase in tetramer-positive cells of 37%. This is close to what would be expected assuming total body CD8+ T cells were 2 × 1011 preinfusion, so that an additional 1.1 × 109 cells were added to 3.2 × 109 preexisting tetramer-positive cells. To determine the percentage of Gag-specific CTL undergoing cell death, tetramer-positive cells from the patient were costained with CD8-tricolor and a marker of apoptosis, Annexin V-FITC (Fig 3). The proportion of dead tetramer-positive cells showed a massive increase from 29% before infusion to over 90% of cells 48 hours after infusion.
Tetramer-specific A2 Gag cells before and after infusion. PBMC taken from the patient at the indicated timepoints were stained with anti–CD8-tricolor, A2 Gag-specific tetramer-PE and a control, B35 Env-specific tetramer-PE. The percentages shown represent CD8+/tetramer-positive cells.
Tetramer-specific A2 Gag cells before and after infusion. PBMC taken from the patient at the indicated timepoints were stained with anti–CD8-tricolor, A2 Gag-specific tetramer-PE and a control, B35 Env-specific tetramer-PE. The percentages shown represent CD8+/tetramer-positive cells.
Death of tetramer-specific A2 Gag cells in vivo before and after infusion. The A2 Gag clone (before infusion) and PBMC obtained from the patient at the time-points indicated were stained with anti–CD8-tricolor, Gag-specific tetramer-PE, and Annexin-V-FITC. The histograms represent Annexin V staining of the CD8+/tetramer-positive cells.
Death of tetramer-specific A2 Gag cells in vivo before and after infusion. The A2 Gag clone (before infusion) and PBMC obtained from the patient at the time-points indicated were stained with anti–CD8-tricolor, Gag-specific tetramer-PE, and Annexin-V-FITC. The histograms represent Annexin V staining of the CD8+/tetramer-positive cells.
DISCUSSION
The utility of adoptive transfer for the treatment and prevention of EBV and CMV infections in patients immunosuppressed after BMT11,12,21 suggested that a similar strategy might be useful in HIV: early reports of infusion of nonspecific, autologous CD8+ cells to HIV-infected patients showed the method to be generally safe, although it had little impact on viral replication.22,23 However, subsequent studies using HIV-specific CTL lines and clones produced only modest or even detrimental outcomes.13-15
We have taken advantage of the recently developed peptide-HLA tetramer technology to follow the fate of a transferred CTL clone and understand its apparent lack of in vivo efficacy. The number of cells we infused represented close to 1% of the patient’s total CD8+cells, which is a high frequency even for HIV+ subjects and should reasonably be expected to have a significant impact on viral replication: however, virus load remained unchanged. We then used fluorescently labeled, tetrameric HLA-A*0201-Gag molecules, which by binding to the TCR of the infused clone permit specific staining of these CTL from patient PBMC samples, to examine the survival of the clone after infusion. There was only a short-lived increase, from a total of 1.6% to 2.2% in the tetramer-stained CD8+ cells after infusion. This near-40% increase at the earliest time-point postinfusion, although substantial, is less than the predicted increase of 62%, suggesting that some cells were eliminated or sequestered very early. By costaining patient PBMC with anti-CD8, tetramer, and Annexin V, we determined that the CTL were being rapidly eliminated through apoptosis, with over 90% of tetramer-positive cells dying within 48 hours.
In mouse models, adoptive CTL transfer is effective in controlling infection with influenza and lymphocytic choriomeningitis virus (LCMV), and the infused cells appear both to survive and expand24-26: this is facilitated by prior depletion of lymphocytes by irradiation, which may provide a stimulus for repopulation of lymphoid tissue.27 Successful adoptive transfer in humans has been limited so far to BMT recipients, whose own immune system has been ablated and in whom cell transfer is performed in a setting of an actively regenerating immune system: this is in contrast to acquired immunodeficiency syndrome (AIDS) patients or recipients of solid-organ transplants. In addition, it is often resting spleen cells that are transferred in murine experiments, whereas we used antigen-stimulated, cultured CTL, which may possess different characteristics because of the procedures necessary for in vitro expansion. The development of the mature, stimulated phenotype seen in our clones, characterized by upregulation of Fas and absence of CD28 expression, may be a result of overstimulation of HIV-specific lymphocytes both in vivo (before culture) and in vitro, and may render them particularly susceptible to premature apoptosis, particularly in the absence of sufficient interleukin-2 (IL-2). Hamann et al28 have previously described two populations of primed, memory CTL and, interestingly, the CD28− subset is dependent on exogenous cytokines such as IL-2 and IL-15 for proliferation. While this may affect the long-term survival of infused clones, it does not address the rapid death of the cells. The rapid turnover of HIV-specific CTL in vivo was confirmed by our observation that before transfer, 29% of the Gag-specific cells were undergoing cell death. The infusion of nearly 40% more CTL did not alter the antiviral response; instead, a higher percentage of tetramer-positive cells underwent apoptosis after infusion, implying that a cascade of antigen-specific cell death was triggered. Follow-up of the patient 40 days after cell transfer indicated that the number of Gag-specific CTL had not returned to pretreatment levels (data not shown), and suggests that the lost CTL were not replenished by expansion of naive cells.
Interestingly, a previous trial of adoptive transfer in which a single Nef-specific clone was infused in large numbers to a patient with AIDS showed that the clone was apparently driving the selection of viruses containing deletions in the targeted Nef epitope.13 The selection of escape mutants in this setting implies that the infused CTL survived in vivo and exerted pressure on HIV-infected cells. However, some of the cell infusions in that trial were accompanied by very large doses of IL-2, raising the possibility that the survival of CTL in vivo is contingent on CD4+ T-cell help, mediated predominantly through IL-2: this is likely to be absent or dysfunctional in HIV-infected patients. The suggestion that CTL require proper CD4+ cell function for optimal activity is supported by data in mice and humans. In mice with a null mutation of CD4, infection by LCMV leads to rapid disappearance of antiviral CTL and poor control of viral replication.29Similarly, in immunosuppressed patients whose anti-CMV cellular immunity was restored by adoptive transfer, CTL activity correlated with the level of CMV-specific CD4+ T cells.12,30 This hypothesis is also supported by recent data which show that HIV-1–specific CD4+ proliferative responses to p24 are inversely related to viral load.31
The control of lymphocyte life span is thought to be dependent on the regulated expression of the tumor necrosis factor (TNF)-receptor family molecule, Fas, which induces a signal cascade leading to cellular apoptosis upon activation by Fas ligand.32 We and others have shown that SIV and HIV-infected cells upregulate FasL, enabling them to reverse-kill antigen-specific CTL in a Fas-dependent manner.33 We also confirmed that both infused clones showed in vitro susceptibility to apoptosis induced by a Fas-ligand–expressing cell line (data not shown). This may represent another mechanism by which CTL are eliminated that probably merits further investigation, particularly for trials of adoptive therapy for malignancies in which Fas ligand is frequently upregulated.
Because trials of adoptive cell transfer in HIV are laborious and infrequent, the generality of these finding will need to be confirmed by different groups. Subsequent trials should take into account the vulnerability of cells cultured in vitro to apoptosis and use means for quantifying their survival in vivo. These data also suggest coinfusion of IL-2 may increase the survival of CTL. Engineering apoptosis-resistant antigen-specific CTL may circumvent the obstacle, but the success of this strategy depends on better elucidating the mechanisms of cell death in vivo.
ACKNOWLEDGMENT
We thank the patient for his generous cooperation, the Wycombe GUM Clinic for provision of blood samples, Dr Graham Bird for CD4/CD8 flow cytometry, and Dr S. Riddell and K. Watanabe for their very helpful technical advice.
R.T. and X.X. contributed equally to this work.
Supported by the UK Medical Research Council. R.T. was a Samuel McLaughlin Fellow of Canada, and S.R.-J. is an MRC Senior Fellow.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sarah Rowland-Jones, MD, Molecular Immunology Group, Institute of Molecular Medicine, John Radcliffe Hospital, Oxford OX3 9DS, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal