Abstract
Interleukin-12 (IL-12) is a cytokine that plays a central role in the control of cell-mediated immunity. We have previously shown that transforming growth factor-β1 (TGF-β) inhibitory effects on human primary allogeneic cytotoxicity and proliferative responses interfere with IL-12 pathway. The present study was undertaken to further elucidate the biochemical basis of the functional interaction between these two cytokines and to define the site of TGF-β action on the signaling pathway activated by IL-12. Our data indicate that TGF-β induced an inhibition of interferon-γ (IFN-γ) production without affecting the IL-12Rβ1 and IL-12Rβ2 subunits mRNA expression by activated T cells. We further show that TGF-β has a significant inhibitory effect on the early signal transduction events following interaction of IL-12 with its receptor on activated T cells, resulting in the inhibition of both JAK2 and Tyk2 phosphorylation. In addition, TGF-β was found to significantly inhibit IL-12–induced phosphorylation of the STAT4 transcription factor. Electrophoretic mobility shift assay indicated that TGF-β induced a decrease in IL-12–induced STAT4 DNA binding activity in T lymphocytes. This study suggests that TGF-β influences IL-12 responsiveness at least in part by inhibiting early signaling events essential to gene induction in IL-12–activated T cells.
INTERLEUKIN-12 (IL-12), a potent proinflammatory cytokine, plays a central role in the initiation and control of cell-mediated immunity.1 It acts on T and natural killer (NK) cells as costimulator of proliferation, inducer of cytokine production,2-7 and by enhancing the generation as well as the cytolytic activity of both cell types.8-13IL-12 produced by accessory cells during early antigenic stimulation has been shown to be a powerful inducer of Th1 responses,14,15 and a defect in its production has been suggested to be a factor contributing to immune depression.16,17 The biologic activity of IL-12 is mediated by the binding of the IL-12 heterodimer to cell-surface receptors on activated T and NK cells.18,19 The existence of multiple forms of IL-12 receptor (IL-12R) has been reported.20,21One component of the IL-12R, originally called IL-12 receptor β chain (IL-12Rβ) and more recently designated IL-12Rβ1, is a gp130-like member of the hematopoietin receptor superfamily.21 When expressed in COS cells, IL-12Rβ1 binds IL-12 with low affinity. Although the IL-12Rβ1 chain per se is not sufficient for IL-12 signaling, the inability of IL-12Rβ1 knock-out mice to respond to IL-12 suggests that this chain is an essential component of the functional IL-12R.22 Recently, a second component of the human IL-12 receptor has been cloned.23This component, called IL-12Rβ2, belongs to the same receptor family as the IL-12Rβ1 subunit. Several studies suggest that this subunit acts as a high-affinity converter and is necessary for IL-12 signaling.23 To date, the JAK/STAT signal transduction pathway is the best characterized signal transduction pathway used by IL-12. IL-12 treatment of activated human T cells induces rapid tyrosine phosphorylation of two members of the JAK (Janus kinases) tyrosine kinases family, JAK2 and Tyk2, implicating these kinases in the immediate biochemical response to IL-12.24,25 A number of studies have characterized a family of transcription factors called STATs (signal transducers and activators of transcription), which are involved in the signal transduction cascades of many cytokines known to activate JAK kinases.26 Evidence has been provided that IL-12 induces STAT4 tyrosine phosphorylation and DNA binding in phytohemagglutinin (PHA)-activated human T cells.27 28 Given the role of IL-12 in determining the nature of immune responses, understanding the regulation of its production and its signaling is of major interest.
Transforming growth factor-β1 (TGF-β) is arguably the most potent immunosuppressive cytokine. It belongs to a family of pleiotropic polypeptide factors that regulate cell growth and differentiation.29 Among its immune properties, TGF-β modulates proliferation, differentiation, and functions of macrophages, T cells, B cells, and NK cells, thus regulating the innate, non–Ag-specific as well as the Ag-specific immunity. Several studies have indicated that TGF-β–induced functional inhibition involves modulation of cytokine production (ie, tumor necrosis factor-α [TNF-α], interferon-γ [IFN-γ], IL-1, IL-2) and cytokine-receptor surface expression cells.30-35 In this context, we have previously shown that TGF-β inhibits the development of cytotoxic and proliferative allogeneic responses by a mechanism involving downregulation of IL-12 production following allostimulation.36 Addition of exogenous IL-12 in the primary mixed lymphocyte reaction (1° MLR) cultures in the presence of TGF-β did not result in reversal of CTL generation and T-cell proliferation, suggesting that TGF-β may interfere with IL-12R expression or with the signal transduction pathway initiated through the IL-12R. The present study was undertaken to further examine the molecular and biochemical mechanism of TGF-β inhibitory effect on IL-12 pathway in human activated T cells. We show that TGF-β interferes with IL-12 responsiveness at least by a mechanism involving an alteration of IL-12–induced tyrosine phosphorylation of the kinases JAK2 and Tyk2 as well as the activation of the transcription factor STAT4.
MATERIALS AND METHODS
Cytokines, antibodies (Abs), and reagents.
Recombinant human IL-12 (1.7 × 107 U/mg) was kindly provided by S. Wolf (Genetics Institute, Cambridge, MA). Recombinant human TGF-β1 was purchased from R&D systems (Abingdon-Oxon, UK). Polyclonal rabbit antiserum against JAK2 and monoclonal antiphosphotyrosine Ab (clone 4G10) were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). Polyclonal rabbit Tyk2 antiserum and the T10-2 monoclonal mouse Ab anti-human Tyk2, used for immunoprecipitation and immunoblotting, respectively, have been previously described.37 Polyclonal rabbit antisera against human STAT4 (C-20, L-18) were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Goat anti-mouse and anti-rabbit Abs conjugated with horseradish peroxidase were respectively purchased from Amersham (Arlington Heights, IL) and Immunotech (Marseille, France).
Isolation and activation of T cells.
Normal human T cells (>90% CD3+) were isolated from the peripheral blood of healthy donors (Banque du Sang, Hôpital Saint-Louis, Paris) by Percoll gradient centrifugation as previously described.38 Mixed lymphocyte reaction (MLR): T cells (0.5 × 106 cells/mL) were alloactivated with irradiated (6,000 rads) stimulating B lymphoblastoid cells, E418 (0.125 × 106 cells/mL) at 37°C in 5% CO2 for 6 days in RPMI 1640 medium (Biochrom KG, Berlin, Germany) supplemented with 15% heat-inactivated human serum (Institut J. Boy, Reims, France), L-glutamine (2 mmol/L), penicillin (100 IU/mL), and streptomycin (100 μg/mL) (complete medium).
Before any stimulation with or without cytokines, 6-day alloactivated T cells were acid-treated (RPMI, pH 6.4) for 1 minute and washed with RPMI and resuspended in starvation medium (Dulbecco’s modified Eagle’s medium [DMEM; Biochrom KG, Berlin, Germany]) for 4 hours.
IFN-γ assay.
Six-day alloactivated T cells were resuspended (1 × 106/mL) in RPMI 1640 + 10% human serum in medium alone, IL-12 (2 U/106 cells), TGF-β (2.5 ng/106 cells), or IL-12 plus TGF-β. Supernatants were collected and tested for INF-γ production after 48 hours of culture (found to be optimal under our experimental conditions as determined by kinetics studies). IFN-γ secretion was measured by a specific enzyme-linked immunosorbent assay (ELISA; Genzyme; Cambridge, MA).
Ribonuclease protection assay for IL-12R mRNA expression.
For analysis of IL-12Rβ1 and IL-12Rβ2 chains mRNA expression, alloactivated T cells (day 6 of the MLR) were resuspended (1 × 106/mL) in RPMI 1640 + 10% human serum in the presence or the absence of TGF-β (2.5 ng/106 cells). After 24, 48, and 72 hours of incubation, T cells were obtained and total RNA was extracted using a standard guanidium thiocyanate method. Ribonuclease protection assays (RPA) were performed with 7 μg/lane total RNA using the Pharmingen probe kit hCR-3 (San Diego, CA) according to the company’s protocol. Products were resolved on 6% denaturing polyacrylamide gels and the protected fragments were visualized and quantitated using a PhosphorImager 445 SI (Molecular Dynamics, Sunnyvale, CA). Relative radioactivity values for IL-12Rβ1 and IL-12Rβ2 transcripts were determined by normalizing to the values obtained for L32, which was used as internal control for equal RNA loading.
Preparation of cytosolic cell extracts and immunoprecipitation.
Alloactivated T cells were resuspended (20 × 106cells/mL) in DMEM and incubated for 20 minutes with IL-12 (5 U/106 cells), TGF-β (1 ng/106 cells), or IL-12 plus TGF-β at 37°C. The reaction was terminated by addition of ice-cold phosphate-buffered saline (PBS) containing 100 μmol/L sodium orthovanadate (Na3VO4). After centrifugation, cells were washed rapidly with PBS/Na3VO4 and lysed in RIPA buffer containing 50 mmol/L Tris-HCl, pH 8, 150 mmol/L NaCl, 1 mmol/L EDTA, 0.5% sodium deoxycholate, 1% NP40, 0.05% sodium dodecyl sulfate (SDS), 1 mmol/L phenyl-methyl-sulfonyl fluoride (PMSF), 1 mmol/L O-Phenantroline, 2 mmol/L sodium orthovanadate, 1 mmol/L NaF, 10 μg/mL aprotinin, leupeptine, and 1 μg/mL pepstatin A. After 15 minutes of incubation on ice, detergent insoluble materials were removed by centrifugation at 4°C for 20 minutes at 13,000g. The protein concentration was determined using a BCA protein assay (Pierce Chemical Co, Rockford, IL).
For immunoprecipitations, cell lysates were precleared by incubation with 20 μL of protein A–coupled Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) for 1 hour under rotation at 4°C. After removal of the beads, the lysates (0.5 to 1 mg of protein) were incubated under rotation with an appropriate Ab (1 to 3 μg for each sample) for 1 hour at 4°C, followed by addition of 30 μL of protein A–Sepharose and incubation for 1 hour under the same conditions. The immunoprecipitates were then washed three times with cold lysis buffer and prepared in Laemmli-reducing buffer.
Gel electrophoresis and immunoblotting.
SDS-polyacrylamide gels were prepared according to the Laemmli protocol and used for immunoblotting. The concentration of polyacrylamide varied from 7% to 10% depending on the molecular weight range of the studied proteins. Equal amounts of protein were used in immunoblotting experiments. All samples were prepared in the Laemmli-reducing buffer and boiled for 5 minutes before application. Gels were blotted onto protean nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) for 1 hour at 100 V. Blots were developed by incubating in a blocking buffer containing 3% bovine serum albumin (BSA) and 0.3% Tween 20 in Tris-buffered saline (TBS) for 2 hours, followed by incubation in the primary Abs for 1 hour. After washing three times in TBS-Tween, blots were incubated for 1 hour with the secondary Ab conjugated with horseradish peroxidase. Detection was performed by the use of enhanced chemiluminescence (ECL; Amersham). When a membrane was reprobed, it was first stripped in acetic acid 0.1 mol/L at room temperature for 12 minutes.
Autoradiograms were scanned and intensity of each band was densitometrically quantitated using the MacBAS image-analysing software (v2.2, Fuji, France). Relative inhibition of the phosphotyrosine signal was determined by first normalizing each signal with the values obtained for the corresponding sample of immunoprecipitated protein.
Nuclear extracts and electrophoretic mobility shift assay (EMSA).
After cytokine stimulation as above, nuclear extracts were prepared from cytokine-stimulated T cells as previously described.39EMSAs were done using a 32P end-labeled double-stranded oligonucleotide (5′-GTATTTCCCAGAAAAAG-3′) corresponding to the IFN-γ response element (GRR) of the human Fcγ receptor I (FcγRI) gene.39 40 Briefly, 5 μg of nuclear extracts were incubated with end-labeled probe (50,000 cpm/sample) and 1 μg of poly(dI-dC) in binding buffer (20 mmol/L HEPES, pH 8, 0.2 mmol/L EDTA, 100 mmol/L NaCl, 100 mmol/L KCl, 10 mmol/L MgCl2, 8 mmol/L spermidine, 4 mmol/L DTT, 200 μg/mL BSA, 5% glycerol, 8% Ficoll 400) for 30 minutes at 4°C before electrophoresis on 6% polyacrylamide gels, drying, and autoradiography. For supershift experiments, nuclear extracts were preincubated with polyclonal rabbit anti-STAT4 (L18X, 3 to 4 μg/sample) for 60 minutes at 4°C before the start of the binding reaction. Autoradiograms were scanned and intensity of each band was densitometrically quantitated using the MacBAS image-analyzing software (v2.2).
RESULTS
TGF-β inhibits IL-12–induced IFN-γ production by activated T cells.
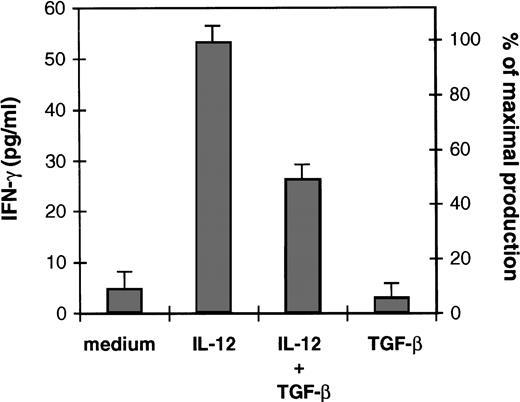
We have recently reported that IL-12 added exogenously at the initiation of the 1° MLR failed to restore CTL generation and T-cell proliferation in the presence of TGF-β.36 In the present study, we asked whether TGF-β intereferes with IL-12 responsiveness of activated T cells. Because IL-12 is a potent inducer of IFN-γ, we have examined the effect of TGF-β on the ability of IL-12 to induce IFN-γ production by alloactivated T cells. Figure 1 shows that IL-12 induced IFN-γ production by these cells upon 48 hours of incubation. In the presence of exogenous TGF-β (2.5 ng/106 cells), a 50% inhibition (obtained after substracting the amount of IFN-γ produced by the T cells in response to medium alone) of IL-12–induced IFN-γ production was observed, suggesting that TGF-β inhibits IL-12 T-cell responsiveness.
Effect of TGF-β on IL-12–induced IFN-γ production of alloactivated T cells. Alloactivated T cells were incubated at 1.106/mL for 48 hours in medium alone, IL-12 (2 U/106 cells), TGF-β (2.5 ng/106 cells), or IL-12 plus TGF-β. Culture supernatants were then collected and tested for IFN-γ production. Results are presented as mean ± SD of duplicate determinations. IFN-γ maximal production represents the IFN-γ production obtained with IL-12. Similar results were obtained with TGF-β ranging from 1 to 5 ng/106 cells. Equivalent results were obtained in three separate experiments.
Effect of TGF-β on IL-12–induced IFN-γ production of alloactivated T cells. Alloactivated T cells were incubated at 1.106/mL for 48 hours in medium alone, IL-12 (2 U/106 cells), TGF-β (2.5 ng/106 cells), or IL-12 plus TGF-β. Culture supernatants were then collected and tested for IFN-γ production. Results are presented as mean ± SD of duplicate determinations. IFN-γ maximal production represents the IFN-γ production obtained with IL-12. Similar results were obtained with TGF-β ranging from 1 to 5 ng/106 cells. Equivalent results were obtained in three separate experiments.
Effect of TGF-β on IL-12Rβ1 and IL-12Rβ2 subunits expression in alloactivated T cells.
To determine whether the TGF-β–induced alteration of IL-12 responsiveness was associated with modulation of IL-12R expression, we examined by the RPA IL-12Rβ1 and IL-12Rβ2 mRNA expression in alloactivated T cells incubated for 24, 48, and 72 hours in the presence or absence of TGF-β (2.5 ng/106 cells). As shown in Fig 2A, IL-12Rβ2 mRNA transcription was slightly affected by incubation with TGF-β for 48 and 72 hours. In a study with 4 different donors (Fig 2B), this modest inhibition (20% to 25%) was found to be not significant, as judged by a nonparametric Wilcoxon test, thus indicating that TGF-β had no significant effect on the regulation of the IL-12Rβ2 subunit gene expression in activated T lymphocytes. As seen in Fig 2, TGF-β had no effect on the mRNA expression of the IL-12Rβ1 subunit nor on its protein surface expression, observed by immunofluorescence analysis (data not shown). These data suggest that TGF-β–induced inhibition of IL-12 responsiveness is not associated with modulation of IL-12R expression. Thus, TGF-β may act downstream of the IL-12R.
Effect of TGF-β on IL-12R expression in alloactivated T cells. Six-day (d6) alloactivated T cells were incubated at 1.106/mL in the presence or absence of TGF-β (1 ng/106cells) at 37°C. Total RNA was extracted after 24, 48, and 72 hours of incubation, and transcripts encoding the IL-12Rβ1 and IL-12Rβ2 subunits, L32 and GAPDH, as loading control were quantitated by ribonuclease protection assays as described in Materials and Methods. (A) RPA bands of selected mRNA accumulation from a representative donor. Relative radioactivity values for IL-12Rβ1 and IL-12Rβ2 transcripts (upper row), after normalization against L32 (lower row) values, expressed in arbitrary units for lanes 1 through 7, respectively, were: 37143, 33773, 28275, 30560, 23923, 19729, and 16587. (B) Relative expression of IL-12Rβ1 and IL-12Rβ2 mRNA obtained from four donors. Data are given as mean (± SD) relative radioactivity values (expressed in arbitrary units) for IL-12Rβ1 and IL-12Rβ2 transcripts from four donors after normalization against the corresponding L32 values.
Effect of TGF-β on IL-12R expression in alloactivated T cells. Six-day (d6) alloactivated T cells were incubated at 1.106/mL in the presence or absence of TGF-β (1 ng/106cells) at 37°C. Total RNA was extracted after 24, 48, and 72 hours of incubation, and transcripts encoding the IL-12Rβ1 and IL-12Rβ2 subunits, L32 and GAPDH, as loading control were quantitated by ribonuclease protection assays as described in Materials and Methods. (A) RPA bands of selected mRNA accumulation from a representative donor. Relative radioactivity values for IL-12Rβ1 and IL-12Rβ2 transcripts (upper row), after normalization against L32 (lower row) values, expressed in arbitrary units for lanes 1 through 7, respectively, were: 37143, 33773, 28275, 30560, 23923, 19729, and 16587. (B) Relative expression of IL-12Rβ1 and IL-12Rβ2 mRNA obtained from four donors. Data are given as mean (± SD) relative radioactivity values (expressed in arbitrary units) for IL-12Rβ1 and IL-12Rβ2 transcripts from four donors after normalization against the corresponding L32 values.
TGF-β effect on IL-12–induced JAK2 and Tyk2 phosphorylation.
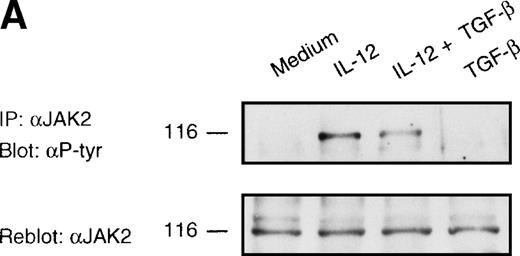
To determine the molecular and biochemical basis of TGF-β–induced attenuation of IL-12 responsiveness, we asked whether this was associated with the inhibition of IL-12–induced tyrosine phosphorylation of JAK2 and Tyk2. Western blot analysis of JAK2 and Tyk2 immunoprecipitates from alloactivated T cells stimulated with IL-12 (5 U/106 cells) for 20 minutes exhibited an induction of tyrosine phosphorylation of JAK2 and Tyk2 (Fig 3A and B). Treatment with TGF-β (1 ng/106 cells) led to a significant decrease in the IL-12–induced phosphorylation of Tyk2 kinase (75% of inhibition as evaluated by densitometric quantitation, Fig 3B), and to a lesser extent (34% of inhibition) of JAK2 kinase (Fig 3A). The TGF-β effect on IL-12–induced tyrosine phosphorylation of Tyk2 and JAK2 can be seen as early as 20 minutes after incubation with TGF-β, suggesting that this effect is independent from protein synthesis. When the blots were reprobed with anti-Tyk2 or anti-JAK2 Abs, no difference was observed in protein levels following treatment with TGF-β. Although the level of TGF-β–induced inhibition varied from donor to donor (mean ± SD on 5 different donors = 55 ± 20% for Tyk2 and = 38 ± 16% for JAK2), it was found to be very significant by a nonparametric Wilcoxon test (P = .0036 and .0069 for Tyk2 and JAK2, respectively, data not shown).
Inhibition of IL-12–induced tyrosine phosphorylation of JAK2 and Tyk2 kinases by TGF-β. Western blot analysis of 6-day alloactivated T cells incubated in medium alone, IL-12 (5 U/106 cells), TGF-β (1 ng/106 cells), or IL-12 + TGF-β at 37°C for 20 minutes. The JAK2 (A) and Tyk2 (B) immunoprecipitates were resolved on 7% SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-phosphotyrosine Ab 4G10 (upper panels). The blots were stripped and reprobed with anti-JAK2 or anti-Tyk2 Abs (lower panels). Similar results were obtained in five separate experiments.
Inhibition of IL-12–induced tyrosine phosphorylation of JAK2 and Tyk2 kinases by TGF-β. Western blot analysis of 6-day alloactivated T cells incubated in medium alone, IL-12 (5 U/106 cells), TGF-β (1 ng/106 cells), or IL-12 + TGF-β at 37°C for 20 minutes. The JAK2 (A) and Tyk2 (B) immunoprecipitates were resolved on 7% SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-phosphotyrosine Ab 4G10 (upper panels). The blots were stripped and reprobed with anti-JAK2 or anti-Tyk2 Abs (lower panels). Similar results were obtained in five separate experiments.
Inhibition of IL-12–induced STAT4 phosphorylation in alloactivated T cells by TGF-β.
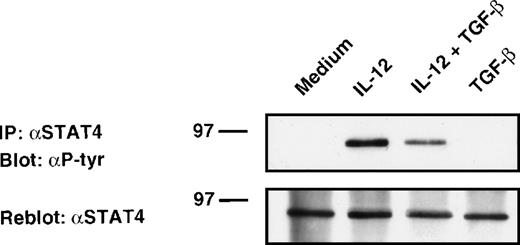
Activation of JAKs leads to tyrosine phosphorylation of STAT factors.26,41 In both human and murine T cells, the transcription factor STAT4 appears to be quite specifically activated by IL-12 and is essential for IL-12–mediated responses in lymphocytes.42 43 To determine whether TGF-β inhibits IL-12–induced STAT4 tyrosine phosphorylation, alloactivated T cells were stimulated with IL-12 in the presence or absence of TGF-β. Cell lysates were then immunoprecipitated with STAT4 Abs, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and analyzed by antiphosphotyrosine immunoblotting. As shown in Fig 4, stimulation of T cells with IL-12 induced tyrosine phosphorylation of STAT4. Treatment of these cells with TGF-β partially inhibited IL-12–induced tyrosine phosphorylation of STAT4 protein (45% inhibition as evaluated by densitometric quantitation). This inhibition was observed after 20 minutes of treatment with TGF-β, suggesting that this phenomenon was also independent of protein synthesis. As seen on reprobed blots with anti-STAT4 Ab, neither TGF-β nor IL-12 altered STAT4 expression level under our stimulating conditions. The TGF-β–induced inhibition (mean ± SD on 6 different donors = 50 ± 28%) was found to be significant by a nonparametric Wilcoxon test (P = .0081, data not shown).
Attenuation of IL-12–induced tyrosine phosphorylation of STAT4. Western blot analysis of 6-day alloactivated T cells incubated in medium alone, IL-12 (5 U/106 cells), TGF-β (1 ng/106 cells), or IL-12 + TGF-β at 37°C for 20 minutes. The STAT4 immunoprecipitates were resolved on 10% SDS-PAGE, transferred to nitrocellulose membrane, and blotted sequentially with anti-phosphotyrosine (upper panel) and anti-STAT4 (C-20) Abs (lower panel). Similar results were obtained in six separate experiments.
Attenuation of IL-12–induced tyrosine phosphorylation of STAT4. Western blot analysis of 6-day alloactivated T cells incubated in medium alone, IL-12 (5 U/106 cells), TGF-β (1 ng/106 cells), or IL-12 + TGF-β at 37°C for 20 minutes. The STAT4 immunoprecipitates were resolved on 10% SDS-PAGE, transferred to nitrocellulose membrane, and blotted sequentially with anti-phosphotyrosine (upper panel) and anti-STAT4 (C-20) Abs (lower panel). Similar results were obtained in six separate experiments.
Alteration of IL-12–induced STAT4 DNA binding in alloactivated T cells by TGF-β.
Because tyrosine phosphorylation of STAT proteins is associated with and is required for the binding of STATs transcription factors to conserved promoter elements,44 we asked whether the inhibition of STAT4 tyrosine phosphorylation by TGF-β observed in our model was associated with an inhibition of IL-12–induced DNA binding activity. Nuclear cell extracts from 6-day alloactivated T cells stimulated with IL-12 in the presence or absence of TGF-β were examined by EMSA for binding to an oligonucleotide probe corresponding to the GRR of the human Fcγ receptor I gene. This probe is known to contain a high-affinity binding site for various STAT factors, including STAT4. As shown in Fig 5, treatment of T cells with IL-12 induced a strong binding activity (IL-12–stimulated factor, IL-12 SF) at the FcγRI probe in 20 minutes (lane 3 compared with lane 1). In contrast to the constitutive complex migrating under the IL-12 SF, the IL-12 SF complex could be displaced with 100-fold excess of unlabeled FcγRI oligonucleotide indicating that this protein/DNA interaction was specific (data not shown). Supershift experiments confirmed that the IL-12 complex contained STAT4, because rabbit antiserum to STAT4 but not normal rabbit serum (data not shown) interfered with formation of the IL-12–induced protein/DNA complex (lane 4 compared with lane 2). As shown in lanes 5 and 6, IL-12 stimulation of T cells in the presence of TGF-β exhibited a lower induced DNA binding activity of STAT4 (36% of inhibition as evaluated by densitometric quantification), as compared with IL-12 alone (lanes 3 and 4), indicating that TGF-β downregulates IL-12–induced STAT4 activation.
TGF-β inhibits IL-12–induced DNA binding of STAT4-containing complexes. Six-day alloactivated T cells were incubated in medium alone (lanes 1 and 2), IL-12 (5 U/106cells, lanes 3 and 4), TGF-β (1 ng/106 cells, lanes 7 and 8), or IL-12 + TGF-β (lanes 5 and 6) at 37°C for 20 minutes. Nuclear cell extracts were prepared and an EMSA was done using the GRR oligonucleotide probe. Where indicated, antiserum against STAT4 (lanes 2, 4, 6, and 8) was incubated with the cell extracts for 60 minutes before addition of the probe. Similar results were obtained in three separate experiments.
TGF-β inhibits IL-12–induced DNA binding of STAT4-containing complexes. Six-day alloactivated T cells were incubated in medium alone (lanes 1 and 2), IL-12 (5 U/106cells, lanes 3 and 4), TGF-β (1 ng/106 cells, lanes 7 and 8), or IL-12 + TGF-β (lanes 5 and 6) at 37°C for 20 minutes. Nuclear cell extracts were prepared and an EMSA was done using the GRR oligonucleotide probe. Where indicated, antiserum against STAT4 (lanes 2, 4, 6, and 8) was incubated with the cell extracts for 60 minutes before addition of the probe. Similar results were obtained in three separate experiments.
DISCUSSION
Cytokines form a network of regulatory signals with considerable overlaps in their activities that lead to unexpected patterns of synergism or antagonism resulting in the modulation of the clonal expansion and differentiation of Ag-reactive cells. It is well established that IL-12 has a major role in the cytokine network and is an immunoregulatory cytokine important in the promotion and control of cell-mediated immunity.45 This cytokine represents an important link between innate immunity and specific immune responses.1 Therefore, the elucidation of its interaction with the immunosuppressive cytokine TGF-β may be of considerable importance in the understanding of T-cell activation and the development of T-cell immune response.
We showed in a recent study that addition of exogenous TGF-β at the sensitizing phase of the 1° MLR resulted in the inhibition of both allogeneic cytotoxic and proliferative human T-cell responses.36 This inhibitory effect of TGF-β involved an abrogation of IL-12/p70 production. Based on the failure of exogenous IL-12 to restore the allogeneic response, we postulated that TGF-β may exert its inhibitory effect by additional mechanisms distinct from inhibition of IL-12 production. When alloactivated T cells, expressing both IL-12Rβ1 and IL-12Rβ2 subunits, were examined for their ability to produce IFN-γ in response to exogenous IL-12 in the presence of TGF-β, a significant decrease in IFN-γ production was observed (Fig 1), suggesting that TGF-β treatment resulted in an alteration of the ability of T cells to respond to IL-12.
Because TGF-β has been reported to modulate the expression of surface receptors important in cell activation and growth,29 we investigated further whether the effect of TGF-β on IL-12 responsiveness was associated with regulation of IL-12R expression. Recent evidence indicates that the IL-12R subunits play a distinct functional role in the triggering of IL-12 responsiveness. The expression of a functional IL-12R (high affinity) requires the IL-12Rβ1 chain and the IL-12Rβ2 chain, which acts as a high-affinity converter crucial in IL-12 signaling.18,19,21,23 Several studies have clearly shown that the pattern of IL-12 responsiveness correlated with the expression of this IL-12Rβ2 subunit.46-49 Although it has been reported by Wu et al50 that TGF-β can inhibit the upregulation of the IL-12Rβ1 and IL-12Rβ2 expression during anti-CD3 activation of naive T cells, our data clearly show that TGF-β has no significant effect on the regulation of IL-12Rβ1 and IL-12Rβ2 subunits gene expression in alloactivated T lymphocytes (Fig2). However, when added at the initiation of the allostimulation, TGF-β selectively interferes with the upregulation of the IL-12Rβ2 mRNA expression (44% to 61% of inhibition at day 6 of the allostimulation) during alloactivation of naive T cells (data not shown). Thus, it appears that TGF-β inhibitory effect on IL-12Rβ2 mRNA expression depends on the experimental conditions, more precisely on the state of activation of T cells. Although TGF-β exerts an inhibitory effect on the primary induction of the IL-12Rβ2 expression, it is unable to downregulate IL-12Rβ2 expression on activated T cells. Under our experimental conditions, TGF-β did not significantly inhibit the expression of IL-12Rβ1 mRNA or its protein expression during alloactivation of naive T cells.36 This discrepency could be explained by the nature of the stimuli delivered to the T cells (anti-CD3 v B-cell line expressing costimulatory molecules). We show in the present study that TGF-β has no significant effect on the regulation of IL-12Rβ1 and IL-12Rβ2 subunits mRNA expression in alloactivated T lymphocytes, thus we can not exclude the possibility that TGF-β may regulate IL-12Rβ2 expression by a posttranscriptional mechanism. Based on the report of Rogge et al49 indicating the existence of a correlation between the expression of the transcript encoding the β2 component of the IL-12 receptor and the presence of high-affinity IL-12 binding sites on Th1 cells, it is reasonable to assume that the lack of TGF-β effect on IL-12Rβ2 mRNA expression observed in alloactivated T lymphocytes also reflects a lack of TGF-β effect on IL-12Rβ2 subunit protein surface expression. This suggests in our model that TGF-β may act presumably downstream the IL-12R to alter IL-12 responsiveness and prompted us to investigate the effect of TGF-β on IL-12 signaling. Our data clearly indicate that TGF-β inhibits the IL-12–induced tyrosine phosphorylation of Tyk2 and JAK2, without altering the expression of these kinases (Fig 3). It is well established that the transcriptional factor STAT4 is essential for mediating responses to IL-12 in lymphocytes and regulating the differentiation of both Th1 and Th2 cells.42,43 Our data show that TGF-β exerts an inhibitory effect on the IL-12–induced tyrosine phosphorylation and DNA binding activity of STAT4 (Figs 4 and5). Again, the TGF-β inhibitory effect is exerted rapidly and is not due to a lack of STAT4 protein. Our data are consistent with previous studies showing that a defect in IL-12 signaling via the JAK/STAT pathway can be asssociated with an absence of STAT4 activation.47,51 It has been shown that T and NK cells from STAT4 knock-out mice do not produce IFN-γ in response to IL-12, suggesting that STAT4 is directly involved in IFN-γ gene expression.22 Furthermore, in a recent study, Barbulescu et al52 identified a STAT4 binding site at the IFN-γ promoter and showed its functional importance for mediating IL-12 effects in primary human T cells.52 This fits with our data demonstrating a correlation between inhibition of STAT4 and IFN-γ production. It should be noted that TGF-β inhibited only partially IL-12 signaling, which resulted in a partial inhibition of IL-12 T-cell responsiveness (proliferation36 and IFN-γ production). Whether an additional transductional pathway activated by IL-12 that is not inhibited by TGF-β exists remains to be determined. Taken together, our observations clearly provide evidence for another site of TGF-β action on IL-12 responsiveness of T cells by its ability to interfere, at least in part, with IL-12 signaling and suggest the existence of a direct cross-talk between IL-12R and TGF-βR signal transduction pathways in T cells. The ability of TGF-β to interfere with other cytokine-induced signaling via the JAK/STAT pathway has been reported. Bright et al53 have recently shown that TGF-β inhibited IL-2–induced tyrosine phosphorylation and activation of JAK1 and STAT5 in murine T lymphocytes. In addition, Pazdrak et al54 have reported that in human eosinophils, TGF-β can interfere with IL-5 signaling by inhibiting tyrosine phosphorylation of JAK2 kinase and STAT1 nuclear factor. These observations suggest that the mechanisms that down-modulate signaling after ligand binding and JAK activation are critical to the regulation of cytokine action. Dephosphorylation events are likely to occur upon recruitement and activation of protein-tyrosine phosphatases, thus playing an important role in the regulatory control of JAK kinases in T cells.26In this regard, the activation of phosphatases by TGF-β has been already reported in keratinocytes.55 Furthermore, Bright et al53 have shown that the inhibition by TGF-β of IL-2–induced tyrosine phosphorylation and activation of JAK1 could be rapidly (within 10 minutes) restored following treatment with vanadate, a known tyrosine phosphatase inhibitor, suggesting the involvement of protein tyrosine phosphatase in the regulation of the JAK/STAT pathway by TGF-β in T cells. The activation of phosphatases may explain how TGF-β exerts its inhibitory effect on tyrosine kinases JAK2 and Tyk2 phosphorylation within 20 minutes in our model. It should also be noted that a TGF-β–inhibitory element consensus sequence GNNTTGGtGA has been found in a number of genes that are inhibited by TGF-β.56 Whether this sequence is found in IL-12–induced gene promoters would be of major interest.
It has become clear that cytokines play an important role in the conflict between tumor and immune system.57 Evidence has been provided that tumor cells produce immunosuppressive cytokines that may contribute to immune dysregulation. TGF-β is arguably the prototype of immunosuppressive cytokines reported to drive a shift toward Th2 type responses in tumor bearing mice.58Therefore, it is tempting to speculate that the presence of TGF-β in the T-cell microenvironment during Ag presentation and initiation of T-cell responses may alter IL-12 responsiveness of these cells, thus interfering with the development toward a Th1 phenotype and consequently with the induction of a specific cell-mediated immunity. Whether TGF-β facilitates the emergence of Th2 response in humans remains to be determined. Immunoregulatory cytokine-mediated immunotherapy aimed at manipulating the immune system still represents a potential strategy in immunologic intervention. Therefore, understanding the functional interaction between TGF-β and these cytokines may ultimately assist in the optimization of cytokine-based therapeutic intervention against human malignancies.
ACKNOWLEDGMENT
We are grateful to the volunteers (Banque du Sang, Hopital Saint Louis and Centre Transfusion Sanguine de Créteil) without whom this study would not have been possible. We wish to thank Genetics Institute for the generous gift of rhIL-12, and J. Chehimi for reading this manuscript and helpful comments. We specially wish to acknowledge Y. Lecluse for helpful technical advice concerning Western-blot.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, from the Association pour la Recherche sur le Cancer (ARC Contract no. C6227-C2036), from the Ligue Nationale contre le Cancer and from the Institut Gustave-Roussy. C.P. is supported by the Comité Val de Marne of the Ligue Nationale contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Salem Chouaib, PhD, Laboratoire Cytokines et Immunologie des Tumeurs Humaines, INSERM U487, Institut Gustave Roussy, 39 rue Camille Desmoulins, 94805 Villejuif Cedex, France; e-mail: chouaib@igr.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal