THE GENERATION of hematopoietic cells requires the coordinated response to a plethora of stimulatory and inhibitory signals that cells receive from their extracellular environment. The “positive” regulators are relatively well defined: the molecules that stimulate the proliferation, differentiation, and survival of hematopoietic cells have been extensively studied. These include the colony-stimulating factors (CSFs) and the majority of the interleukins (ILs). Several of these molecules are available for clinical use, and much is known about their structure, the consequences of their over-production, and the biological effects that result from their complete absence. The multi-protein receptor complexes that are used by these molecules at the cell surface have been characterized to reveal a complicated interplay of shared receptor components and unique receptor elements. More recently the intracellular pathways that are triggered by growth factor/receptor interactions have begun to be dissected, revealing an interdigitating network of signaling molecules that is striking both in terms of its complexity, and in terms of the recurring themes that are revealed in otherwise apparently divergent experimental systems.1 By comparison, the “negative” regulators of hematopoiesis have been relatively neglected. However, it has been clear for a number of years that molecules like transforming growth factor-β (TGF-β) are potent negative regulators of hematopoiesis and function as important molecules in determining hematopoietic responses.
The identification of the JAK/STAT signaling pathway provided an understanding of one mechanism by which stimulatory signals received at the cell surface are rapidly transmitted to the nucleus. The JAKs (or Janus kinases) are receptor-associated molecules that are phosphorylated on tyrosine in response to cytokine-receptor interactions. As a consequence, the STAT (for signal transducers and activators of transcription) molecules are recruited to the receptor and phosphorylated.2,3 This leads to their dimerization and translocation to the nucleus where they bind and activate transcription of target genes. The recruitment of particular JAK/STAT family members overlaps but differs for different cytokines. Thus, for example, IL-6 type cytokines use JAK1, JAK2, and the related molecule TYK2, and STAT1 and STAT3,4,5 although the critical molecules appear to be JAK1 and STAT3.6,7 In comparison, the IL-12 signaling pathway results in phosphorylation of JAK2 and TYK28,9 and requires STAT4 for IL-12–generated responses.10 11
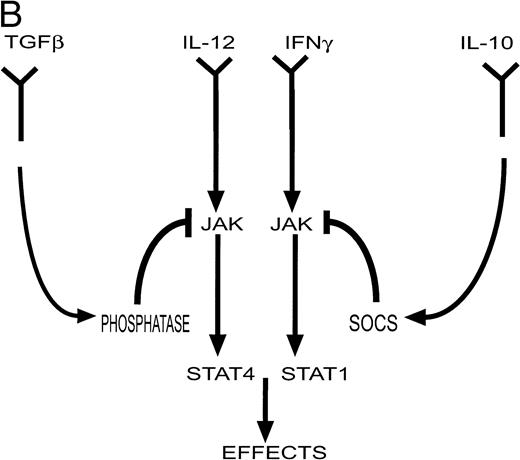
The mechanism by which “negative” regulators exert their inhibitory action is less clear. One possible mechanism involves the recruitment of protein tyrosine phosphatases that can then serve to inactivate JAK proteins. One such is SHP-1, the defective function of which results in the hyperproliferation and accumulation of several hematopoietic cell lineages and in the development of autoimmune disease as evidenced by the motheaten mouse.12Specific targeting of this molecule to the erythropoietin receptor can inhibit ligand-stimulated tyrosine phosphorylation and result in dephosphorylation of JAK2.13 The negative regulator TGF-β has been reported to activate protein tyrosine phosphatases14 and this may be one mechanism by which it exerts its inhibitory effect on the JAK/STAT pathway.15
Another family of signaling molecules that are potentially important mediators of inhibitory signals have recently been described. There appear to be at least 8 of these “SOCS” proteins (for suppressors of cytokine signaling) including CIS, an early response gene, that encode SH2-domain containing proteins16-19 and 12 others that also share a C-terminal domain called the SOCS box.17These proteins were identified simultaneously using three different approaches. One involved a functional screen for cDNAs encoding proteins that blocked IL-6 function.20 A second approach involved a yeast two-hybrid strategy for proteins interacting with the kinase domain of JAK2.21 The third searched for proteins with antigen similarity to the SH2 domain of the STAT molecules.22 Cytokines induce the expression of the SOCS genes, and the SOCS proteins then serve to downregulate the JAK/STAT pathways and therefore curtail the biological response. Thus, for example, SOCS1 (also known as JAB and SSI-1) is induced within 20 minutes after stimulation by IL-6 and suppresses the cellular response to IL-6 via its interaction with activated JAK proteins through its SH2 domain.23 Levels of SOCS1 expression return to baseline within about 4 hours. The transcriptional activation of SOCS genes is mediated, at least in part by the STAT proteins; CIS expression is modulated by STAT5 and STAT3 is important for SOCS1 expression.24 SOCS1 also attenuates the biological response to a number of other cytokines including leukemia inhibitory factor, oncostatin-M, interferon-γ (IFN-γ), thrombopoietin, and growth hormone.20,25 Moreover, as well as acting to reduce phosphorylation of JAK kinases and STAT proteins, SOCS proteins may function as more general inhibitory regulators.26 27Therefore, these proteins are induced by cytokines, and act to suppress cytokine signaling, acting in a classical negative feedback loop.
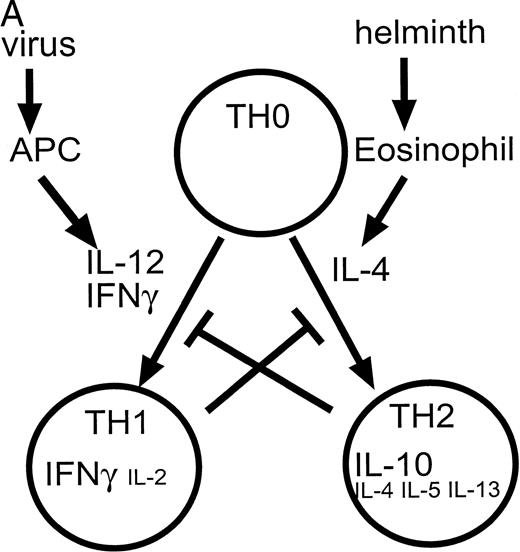
One situation in which a complex array of competing “positive” and “negative” regulators is clearly evident, is in the generation of cells important in antigen-specific or cell-mediated immune responses. Studies of T-lymphocyte clones have identified two major subpopulations of CD4+ cells, T-helper lymphocyte (TH) 1 induced by IL-12 and IFN-γ and TH2 cells induced by IL-4.28-30 The development of the TH1 versus TH2 phenotype is dependent on cell-intrinsic as well as extrinsic stimuli.31 These cells differ in the cytokines that they produce after activation. Thus, a cellular immune response dominated by a TH1 response is characterized by cells that produce IFN-γ, IL-2, and TNF-β. Conversely, a TH2 response is typified by the production of IL-4, IL-5, IL-6, IL-13, and IL-10 (Fig 1). Both TH1 and TH2 cells produce granulocyte-macrophage CSF (GM-CSF), tumor necrosis factor-α (TNF-α), and IL-3; however, the relative levels may vary. These patterns of cytokine expression can be used to categorize most murine CD4+ T-lymphocyte clones, and as such, allow classification in terms of the principal immune response induced in experimental systems.32 This may also be relevant to some human disease states.33 As expected from the differing cytokine expression patterns, these subsets of cells also differ in their functional properties, with TH1 cells being predominant in assisting microbiocidal macrophage responses (via IFN-γ and TNF-β) while TH2 cytokines augment B-cell responses. This divergence in function is particularly evident during infection with the protozoa, Leishmania, where macrophage activation is required for protection from this intracellular parasite.34 In this system TH1-responses are protective while TH2 responses fail to offer protection and lead to a nonhealing of disease. In BALB/c mice, a bias toward a TH2 response, with production of IL-4 and the requirement for STAT6-mediated signaling,35 results in the genetic susceptibility toLeishmania infection.
(A) Cytokines controlling the development of cell-mediated (TH1) and humoral (TH2) responses. Certain infections such as viruses induce the production of IL-12 and IFN-γ by antigen presenting cells (APC). These factors promote the differentiation of TH0 cells to the TH1 phenotype. Other infections such as helminths induce the production of IL-4 (by eosinophils) which induces differentiation to the TH2 phenotype. TH1 cytokines such as IFN-γ inhibit the production of IL-4 and IL-10 while TH2 cytokines such as IL-10 inhibit the production and action of IL-12. (Part B) Factors such as IL-12 and IFN-γ activate a cascade of intracellular signaling molecules including molecules in the JAK/STAT pathway. In contrast, inhibitory molecules may produce their inhibitory effects in part via the activation of molecules such as phosphatases in the case of TGF-β, and SOCS genes in the case of IL-10. These pathways would serve to attenuate a biological response.
(A) Cytokines controlling the development of cell-mediated (TH1) and humoral (TH2) responses. Certain infections such as viruses induce the production of IL-12 and IFN-γ by antigen presenting cells (APC). These factors promote the differentiation of TH0 cells to the TH1 phenotype. Other infections such as helminths induce the production of IL-4 (by eosinophils) which induces differentiation to the TH2 phenotype. TH1 cytokines such as IFN-γ inhibit the production of IL-4 and IL-10 while TH2 cytokines such as IL-10 inhibit the production and action of IL-12. (Part B) Factors such as IL-12 and IFN-γ activate a cascade of intracellular signaling molecules including molecules in the JAK/STAT pathway. In contrast, inhibitory molecules may produce their inhibitory effects in part via the activation of molecules such as phosphatases in the case of TGF-β, and SOCS genes in the case of IL-10. These pathways would serve to attenuate a biological response.
In this context, IL-12 and IFN-γ are important, proinflammatory cytokines that act as a powerful inducers of a TH1 response.36,37 IL-12 is also active on natural killer (NK) cells. IL-12 is produced by antigen-presenting cells (APC) as a result of an interaction, via CD40 and its ligand, between the APC and activated T lymphocytes.38,39 In this incestuous relationship, the production of IL-12 by APC is further stimulated by the TH1 cells and their product IFN-γ and vice versa.40,41 Conversely, a defect in the production of IL-12 and IFN-γ may be important in impaired TH1-mediated immune responses,42,43 with evidence that IL-12 and IFN-γ production, and the IL-12 responsiveness of TH1 cells, can be antagonized by TH2 cells and the cytokines that they produce (IL-10, IL-4, IL-13).44 45
The target cell for the action of IL-12, the activated T cell, is also subject to additional opposing, inhibitory cytokine influences. TGF-β is an immunosuppressive molecule that, in addition to its inhibitory action on lymphoid cells, inhibits proliferation, differentiation, and functional activity of myeloid cells and NK cells. TGF-β can interfere with IL-2–mediated tyrosine phosphorylation and activation of JAK1 and STAT515 and with multiple components of the IL-5 signaling pathway including an inhibitory effect on JAK2 and STAT1.46 In the report in this issue by Pardoux et al,47 the mechanism by which the inhibitory effect of TGF-β is able to oppose the stimulatory action of IL-12 on activated T cells is addressed: how does an activated T-cell resolve these conflicting signals?
As outlined above, IL-10 (along with IL-4 and IL-13) is an anti-inflammatory cytokine produced by TH2 cells.48,49 It serves to maintain a TH2-type immune response in part by preventing the development of TH1 cells, thereby preventing production of the macrophage activating molecules such as IFN-γ. IL-10 is also produced by monocytes in response to lipopolysaccharides. In addition to its action on lymphoid cells, IL-10 acts as a direct inhibitor of macrophages, inhibiting gene transcription and inhibiting production of inflammatory cytokines.50-52 Thus, as well as being a product of TH2 cells, IL-10 has an auto-regulatory role in monocytes53 and its production by monocytes is inhibited by the proinflammatory molecule IFN-γ.54 What are the mechanisms by which the opposing influences of IL-10 and IFN-γ are reconciled within the monocyte? In the accompanying report by Ito et al55 these authors examine the mechanisms by which the action of IL-10 and IFNs are balanced.
Pardoux et al have taken advantage of their recent demonstration that TGF-β inhibited the development of cytotoxic and proliferative allogeneic (TH1) responses by a mechanism that involved decreased production of IL-12.56 They further observed that TGF-β–mediated inhibition of alloreactive T cells was not overcome by the addition of exogenous IL-12. This was confirmed in their report in this issue by the demonstration that addition of IL-12 was not able to completely overcome the inhibitory effect of TGF-β on IFN-γ production by these cells. They reasoned that this failure implied that TGF-β also interfered either with IL-12 receptor expression on the activated T cell, or with the signal transduction pathway initiated by IL-12 receptor. They showed that the former was not the case; there was no consistent difference in IL-12 receptor expression in response to TGF-β. The addition of TGF-β did not alter the levels of JAK2 and Tyk2, two intracellular signaling molecules normally phosphorylated in response to IL-12; however, there was a significant decrease in the tyrosine phosphorylation of both molecules within 20 minutes of incubation with TGF-β. A consequence of activation of JAKs is the phosphorylation of STAT molecules, with STAT4 being activated in response to IL-12 signaling. Again there was no decrease in the level of STAT4 protein but tyrosine phosphorylation and DNA binding activity of STAT4 were decreased in response to TGF-β within 20 minutes. Thus, these results suggest that TGF-β directly interferes with intracellular signaling by IL-12 and the authors suggest that this may be mediated by TGF-β–induced tyrosine phosphatases (Fig 1).
The results presented by Ito et al55 are remarkably similar, but they provided additional evidence that may further explain the mechanism by which interference at the level of intracellular signaling occurs. The authors showed that IL-10 inhibited the transcription of some genes normally induced in human monocytes in response to stimulation with IFN-γ and IFN-α. Consistent with the studies by Pardoux et al, there was no change in number or affinity of IFN-γ receptors in response to treatment with IL-10. Similarly, IL-10 did not alter the levels of STAT1, the principal target for IFN-γ signaling in these cells, but decreased the phosphorylation of STAT1 and decreased DNA binding activity. These inhibitory effects of IL-10 were more evident when lower concentrations of IFN were examined. There was no evidence that this effect of IL-10 was due to induction of phosphatase activity. However, this study presents a result that potentially could explain the observations reported in both papers. These authors showed that treatment of monocytes with IL-10 induced the expression of the SOCS3 gene within 30 minutes and maintained its expression for at least 120 minutes. These kinetics are consistent with the observed action of IL-10 to inhibit IFN-induced STAT1 activation and IFN-stimulated gene expression. Although studies have shown that in myeloid and fibroblast cell lines SOCS1 but not SOCS3 was an inhibitor of IFN-γ signaling,57,58 more recent results have also implicated SOCS3 as an inhibitor of IFN signaling.59 It will be important to determine whether other SOCS proteins are induced by IL-10.
Together, these results suggest that one mechanism by which inhibitory cytokines exert their effect is via the direct activation of inhibitory signaling pathways like phosphatases and SOCS proteins. The SOCS pathway is a potential candidate for mediating this type of response because SOCS proteins are normally activated in response to positive cytokine signals, thus ensuring that a biological response is transient. Direct access to these same signaling molecules by inhibitory cytokines provides an attractive and efficient mechanism whereby competing signals are resolved to redirect a cellular response. It seems possible that this might prove a common mechanism for cells faced with alternate choices and competing extracellular demands.
REFERENCES
Author notes
Address reprint requests to C.G. Begley, MD, The Walter and Eliza Hall Institute of Medical Research, PO Royal Melbourne Hospital, Parkville, 3050 Victoria, Australia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal