Abstract
Clearance of apoptotic neutrophils (polymorphonuclear leukocyte [PMN]) by macrophages is thought to play a crucial role in resolution of acute inflammation. There is increasing evidence that ingestion of apoptotic cells modulates macrophage behavior. We therefore performed experiments to determine whether ingestion of apoptotic PMN modulated the uptake process itself. Rat bone marrow-derived macrophages (BMDM) ingested apoptotic PMN by a process that was enhanced by tumor necrosis factor (TNF) and attenuated by interferon (IFN)-γ, interleukin (IL)-4, and IL-10. It was inhibitable by the tetrapeptide arg-gly-gln-ser (RGDS), therefore implicating the vβ3/CD36/thrombospondin pathway. Interaction of apoptotic PMN with BMDM for 30 minutes, 48 hours before rechallenge reduced uptake of apoptotic PMN by 50% compared with previously unchallenged BMDM. Blocking initial uptake with RGDS abrogated the effect of preexposure. Comparable and sustained attenuation of uptake was obtained by ligating vβ3 with the monoclonal antibody (MoAb), F11, after a delay of more than 90 minutes, whereas MoAbs to CD25 and CD45 had no effect. Ligation of 6β1 and 1β2, integrins not previously implicated in the engulfment of apoptotic cells also decreased uptake with similar kinetics to F11. Therefore, apoptotic PMN regulate their own uptake through an integrin-dependent process, which can be reproduced by ligation of other integrins expressed by macrophages.
MACROPHAGES INFLUENCE almost all aspects of immunological and inflammatory responses and play an essential role in linking the innate and acquired immunity.1 Macrophages not only induce injury, but also control key events in the resolution of inflammation and the repair processes that follow it. One of the critical functions in this process is phagocytosis of apoptotic cells via specific recognition mechanisms. To date, a number of recognition mechanisms for apoptotic cells have been described: (1) an uncharacterized lectin-dependent interaction2; (2) a complicated charge sensitive process involving the CD36/vitronectin receptor (αvβ3) complex on the macrophage surface interacting with unknown moieties on the apoptotic polymorphonuclear leukocyte (PMN) surface via a thrombospondin bridge3,4; (3) a stereo-specific recognition of phosphatidylserine that is expressed on the surface of the apoptotic cell after loss of membrane asymmetry5,6; (4) macrophage scavenger receptors7; (5) the lipopolysaccharide (LPS) receptor CD148-10 and macrosialin or CD68.11 12
The specific removal of apoptotic thymocytes,13eosinophils,14 and neutrophils15 by macrophages has been well described. Extensive tissue damage and inflammation both precede and follow neutrophil death by necrosis. The cellular debris is phagocytosed by macrophages, which are activated by the process. In contrast, apoptosis of neutrophils is associated with the swift recognition of intact cells by macrophages followed by their ingestion and degradation. Local inflammation and tissue injury are avoided not only because neutrophils are prevented from releasing their toxic contents, but also because the macrophages usual proinflammatory secretory response to phagocytosis is not activated16 and may be biased towards release of the anti-inflammatory cytokine transforming growth factor (TGF)-β.17
These results suggest that uptake of apoptotic neutrophils by macrophages does not merely fail to induce synthesis of proinflammatory cytokines, but actively modulates macrophage function and biases the profile of cytokines they release. This raises the question whether uptake of apoptotic cells “imprints” a pattern of behavior on macrophages analogous to the effect of exposure to some cytokines.18 The specific purpose of the experiments described here was to ascertain whether uptake of apoptotic neutrophils modulates the ability of macrophages to ingest a second challenge with apoptotic PMNs. The results show a substantial reduction in the proportion of macrophages that ingest a second challenge of apoptotic PMNs, but that uptake can still be modulated appropriately by cytokines. The bone marrow-derived macrophages (BMDM) uptake of apoptotic PMN is RGDS-dependent and presumptively occurs by the αvβ3/CD36/thrombospondin pathway. After a delay of at least 90 minutes, ligation of αvβ3 also downmodulates uptake of apoptotic cells specifically, and ligation of two other integrins, α6β1 and α1β2, have the same effect. This shows that uptake of apoptotic cells is regulated by events that induce signalling through integrin receptors irrespective of whether or not they are directly associated with uptake. This raises the question whether uptake of apoptotic cells via αvβ3 reciprocally influences functions of integrins responsible for cell adhesion and facilitate the emigration of macrophages from an inflamed focus as described by Bellingan et al.19
MATERIALS AND METHODS
Reagents.
Recombinant human tumor necrosis factor (rhTNF)-α, rhTGF-β, and recombinant rat interferon (IFN)-γ were obtained from Boehringer (Ingelheim, Germany), Sigma Chemical Co (Dorset, UK), and Bradsure Biologicals Ltd (Loughborough, UK), respectively. Recombinant rat interleukin (IL)-4 was produced in-house as described previously20 using a Chinese hamster ovary (CHO) cell line generously donated by Dr Neil Barclay (MRC Cellular Immunology Unit, Oxford, UK). The rat monoclonal antibody (MoAb), F11, against the integrin β3 chain21 was a gift from Prof Michael Horton (Bone and Mineral Centre, University College London Medical School, London, UK). The mouse antirat integrin antibody α6β1, CD18, CD116, anti-CD45, anti-CD25, anti-ED3, and mouse antihuman CD21 were obtained from Serotec (Oxford, UK). The rabbit antihuman erythrocyte membrane antibody was obtained from DAKO (Glostrup, Denmark). The tetrapeptides arg-gly-asp-ser (RGDS), arg-gly-glu-ser (RGES), and phospho-L-serine were obtained from Sigma Chemical Co.
Isolation and culture of BMDM.
Rat BMDM were obtained using a technique previously described in detail.22 Briefly, bone marrow cells were flushed aseptically from the dissected femurs of male Spraque Dawley rats with a jet of complete medium directed through a 25-gauge needle to form a single cell suspension. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 2 mmol/L glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin, 10% heat-inactivated fetal calf serum, and 10% L929 conditioned medium as a source of macrophage-colony stimulating factor (M-CSF). After 7 days in culture, BMDM were dispensed into 24-well culture plates (Corning, Corning, NY) at a concentration of 5 × 105 cells/well and rested in medium without added M-CSF for 24 hours before use in experiments.
Inhibition of uptake of apoptotic neutrophils.
BMDM were incubated with a series of inhibitors at concentrations of 1 mmol/L for 15 minutes at 4°C and were washed immediately before interaction with apoptotic neutrophils. Phospho-L-serine was used as a stereo-specific inhibitor of the macrophage phosphatidylserine receptor, using conditions described by Fadok et al.5 The tetrapeptide arg-gly-asp-ser (RGDS) was used as described,4and the noninhibitory peptide arg-gly-glu-ser (RGES) added as a control.
Assay for uptake of apoptotic neutrophils.
BMDM were transferred to 24-well plates at a density of 5 × 105 cells/well and rested for 24 hours before the medium was changed and the cells incubated with various cytokines. Uptake of apoptotic neutrophils was assessed after 48 hours using a microscopically quantified phagocytic assay, which has previously been described and illustrated in detail.4,23 Apoptotic neutrophils were prepared from PMN isolated from fresh heparinized normal human blood by dextran sedimentation and Percoll centrifugation. They were aged in teflon bags for approximately 24 hours in RPMI 1640 supplemented with antibiotics and 10% fetal calf serum. More than 98% of these cells excluded trypan blue while apoptosis was verified by oil immersion light microscopy of May-Giemsa–stained cytospin preparations as previously described.23 The apoptotic cells were washed once and resuspended in RPMI at a concentration of 2.5 × 106/mL. A total of 1 mL of cells was added to each well and allowed to interact with the macrophages for 30 minutes at 37°C in a 5% CO2 atmosphere. The wells were washed in saline at 4°C to remove noningested PMN, fixed with 2% gluteraldehyde in 0.9% saline, and stained for myeloperoxidase to identify ingested PMN. The proportion of macrophages that had ingested neutrophils was then counted by inverted light microscopy.
To determine the effect of previous ingestion of apoptotic neutrophils on macrophages, rat BMDM were transferred to 24-well plates at a density of 5 × 105 cells/well and rested for 24 hours before the medium was changed and cells were incubated with either medium alone, RGDS peptide followed by apoptotic neutrophils, or apoptotic neutrophils alone. After 30 minutes incubation, the wells were washed in saline at 4°C to remove noningested PMN, and the macrophages were rested for 48 hours in control medium or medium containing various cytokines. They were then reincubated for 30 minutes with a second challenge of apoptotic neutrophils and the proportion of macrophages that took up PMN assessed. The assay for uptake of opsonized erythrocytes was performed exactly as previously described.24
Ligation of the integrin receptors.
MoAbs to αvβ3, α6β1, α1β2, CD25, and CD45 were used to assess the effect of ligation of macrophage cell surface receptors on uptake of apoptotic neutrophils. Macrophages were incubated at various MoAb concentrations ranging from 0.01 to 10 μg/mL, for 30 minutes, at 4°C in saline, or were incubated with various concentrations of mouse antihuman CD21 as an irrelevant isotype-matched control. The cells were then washed before incubation in medium for various times before the start of the standard interaction assay with apoptotic PMN.
Quantitation of nitric oxide (NO) generation.
Generation of NO was measured by assaying culture supernatants for nitrite, a stable reaction product of NO. Aliquots of 200 μL of each cell-free culture supernatant were incubated with 50 μL of Griess reagent (0.5% sulphanilamide, 0.05% N-(1-naphtyl) ethylendiamine dihydrochloride in 2.5% phosphoric acid) in 96-flat–bottomed tissue culture plates for 10 minutes at room temperature. The optical densities of the assay samples were then measured at 540 nm using a solution of phenol red free DMEM. In most experiments, nitrite was measured 48 hours after exposure to cytokines.
RESULTS
Cytokines regulate uptake of apoptotic neutrophils by BMDM.
The initial experiment was designed to confirm our previous observations that pro and antiinflammatory cytokines influence uptake of apoptotic human neutrophils by uncommitted rat BMDM.20TNF caused a 36% increase in the proportion of BMDM that took up apoptotic neutrophils compared with controls, whereas IL-4, IL-10, and IFN-γ inhibited uptake by 56%, 22%, and 42%, respectively, and TGF-β had no effect (Table 1). Incubation with cytokines modulated not only the number of macrophages taking up apoptotic cells, but also the average number of neutrophils per macrophage, ie, IL-4 caused a 56% decrease in the number of macrophages taking up apoptotic neutrophils and a 40% reduction in the number of neutrophils per macrophage. These findings differ from those reported for human monocyte-derived macrophages. In these cells, incubation with proinflammatory cytokines (IFN and TNF) increased their ability to ingest neutrophils, whereas antiinflammatory cytokines (IL-4, IL-6, and IL-10) had no effect.25 These differences could reflect the source and species of the macrophages used or the conditions in which they were matured. Recently, Bonder et al26 have shown that human 7-day–cultured monocytes did not express the functionally active IL-2 receptor γ-chain, a component of the IL-4 receptor, whereas macrophages did, which may explain the different effect of IL-4 on uptake of apoptotic neutrophils by monocyte-derived macrophages and BMDM.
Effect of Cytokines on the Number of Macrophages That Take up Apoptotic Neutrophils
| Cytokine (Concentration) . | Uptake of Apoptotic PMNs (%) . |
|---|---|
| Control | 31 ± 2.8 |
| IFN + TNF (20 U/mL, 10 ng/mL) | 18 ± 1.9* |
| IFN (20 U/mL) | 21.6 ± 2† |
| TNF (10 ng/mL) | 42.6 ± 2.8* |
| TGF-β (7.5 ng/mL) | 28.2 ± 2.5 |
| IL-4 (5 μL/mL) | 13 ± 2.5* |
| IL-10 (100 ng/mL) | 22.8 ± 2.7 |
| Cytokine (Concentration) . | Uptake of Apoptotic PMNs (%) . |
|---|---|
| Control | 31 ± 2.8 |
| IFN + TNF (20 U/mL, 10 ng/mL) | 18 ± 1.9* |
| IFN (20 U/mL) | 21.6 ± 2† |
| TNF (10 ng/mL) | 42.6 ± 2.8* |
| TGF-β (7.5 ng/mL) | 28.2 ± 2.5 |
| IL-4 (5 μL/mL) | 13 ± 2.5* |
| IL-10 (100 ng/mL) | 22.8 ± 2.7 |
N = 10.
P < .01 relative to unstimulated controls.
P < .05 relative to unstimulated controls.
BMDM use an integrin-dependent mechanism to recognize apoptotic PMN.
Human monocyte-like cell lines and murine peritoneal macrophages use the phosphatidylserine receptor (PSR) for recognition of apoptotic cells.27 Human monocyte-derived macrophages and murine BMDM have been reported to use the αvβ3/CD36/thrombospondin pathway.3 In our studies, 1 mmol/L RDGS specifically inhibited uptake of apoptotic PMN by unstimulated rat BMDM and by macrophages incubated for 48 hours with IFN-γ, TNF, IL-4, or TGF-β. Neither the control peptide RGES, nor phospho-L-serine, which inhibits PS-mediated recognition of apoptotic cells,5 had any effect on uptake by cytokine-stimulated or unstimulated macrophages (Table 2). Thus, both uncommitted or cytokine-stimulated rat BMDM use an integrin-dependent recognition mechanism, presumptively the CD36/αvβ3/thrombospondin system rather than a PSR-dependent mechanism. This conclusion is strengthened by the demonstration of αvβ3 on the surface of BMDM by immunofluorescence using the MoAb, F11, directed against the β3 subunit of the receptor (data not shown). To verify the recognition mechanism, it would be necessary to block either CD36 or αvβ3 on the macrophage surface. To our knowledge, the one antirat antibody available for this purpose is the mouse MoAb F11 against the β3 subunit of the vitronectin receptor, which is a poor blocking antibody under our experimental conditions.
Effect of Inhibitors on the Number of Macrophages Taking up Apoptotic PMN (%)
| . | Control . | RGDS (1 mmol/L) . | RGES (1 mmol/L) . | Phospho-L Serine (1 mmol/L) . |
|---|---|---|---|---|
| Control | 32 ± 2.4 | 9.2* ± 1 | 30.6 ± 2 | 28.2 ± 2.2 |
| IFN-TNF (20 U/mL, 10 ng/mL) | 18.0 ± 2.3 | 7.9* ± 1.5 | 17.9 ± 4.8 | 16.3 ± 3.2 |
| TNF (10 ng/mL) | 42.6 ± 2.4 | 15* ± 1.8 | 39.7 ± 3.1 | 43 ± 3 |
| TGF (7.5 ng/mL) | 28 ± 4.2 | 14.1† ± 2.1 | 27.5 ± 3.3 | 24.8 ± 3 |
| IL-4 (5 μL/mL) | 12.6 ± 2.2 | 5.7† ± 1.9 | 13.5 ± 1 | 12.1 ± 1.3 |
| . | Control . | RGDS (1 mmol/L) . | RGES (1 mmol/L) . | Phospho-L Serine (1 mmol/L) . |
|---|---|---|---|---|
| Control | 32 ± 2.4 | 9.2* ± 1 | 30.6 ± 2 | 28.2 ± 2.2 |
| IFN-TNF (20 U/mL, 10 ng/mL) | 18.0 ± 2.3 | 7.9* ± 1.5 | 17.9 ± 4.8 | 16.3 ± 3.2 |
| TNF (10 ng/mL) | 42.6 ± 2.4 | 15* ± 1.8 | 39.7 ± 3.1 | 43 ± 3 |
| TGF (7.5 ng/mL) | 28 ± 4.2 | 14.1† ± 2.1 | 27.5 ± 3.3 | 24.8 ± 3 |
| IL-4 (5 μL/mL) | 12.6 ± 2.2 | 5.7† ± 1.9 | 13.5 ± 1 | 12.1 ± 1.3 |
This table shows the effect of inhibitors on recognition of apoptotic PMNs by control and cytokine-stimulated BMDM.
P < .01 relative to controls.
P < .05 relative to controls.
BMDM incubated with F11 for 45 minutes and then seeded in vitronectin-coated plates adhered as efficiently as control macrophages. There was no difference in the number of nonadherent cells (less than 1% of the seeded cells in both groups) when aliquots of the supernatants of control and F11-treated macrophages were examined 2, 4, 12, and 24 hours after seeding (data not shown).
Previous uptake of apoptotic PMNs reduces the ability of BMDM to ingest apoptotic PMN.
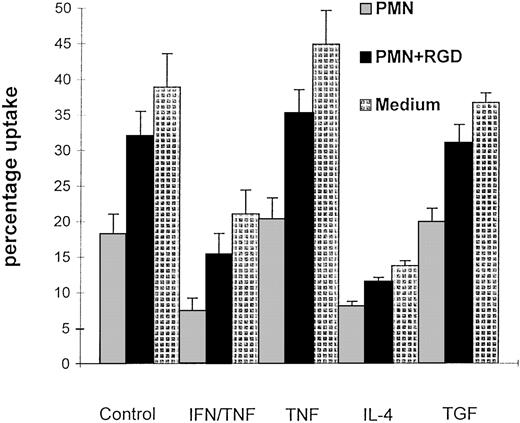
To determine the effect of uptake of apoptotic neutrophils on macrophage function, uncommitted rat BMDM were challenged for 30 minutes with apoptotic neutrophils in medium alone or in the presence of RGD peptide to prevent uptake. They were then rested for 48 hours in medium before being reexposed to freshly prepared apoptotic neutrophils. Macrophages that had previously ingested apoptotic PMN had a markedly reduced ability to engulf apoptotic neutrophils compared with control macrophages (Fig 1), whereas their ability to take up opsonized erythrocytes was unchanged (data not shown). The difference cannot be attributed to a nonspecific effect of the neutrophils because macrophages challenged with PMN in the presence of RGDS-peptide retain their subsequent ability to take up apoptotic neutrophils (Fig 1).
Figure 1 shows the percentage uptake of apoptotic neutrophils by BMDM. The macrophages were incubated 48 hours before the interaction assay with apoptotic neutrophils, RGDS followed by apoptotic neutrophils or medium. They were then washed and cultured in medium containing cytokines or medium alone before washing and a 30-minute interaction with apoptotic PMN; mean ± standard error (SE), n = 10; * P < .01.
Figure 1 shows the percentage uptake of apoptotic neutrophils by BMDM. The macrophages were incubated 48 hours before the interaction assay with apoptotic neutrophils, RGDS followed by apoptotic neutrophils or medium. They were then washed and cultured in medium containing cytokines or medium alone before washing and a 30-minute interaction with apoptotic PMN; mean ± standard error (SE), n = 10; * P < .01.
The degree of inhibition was comparable to that observed when BMDM are exposed to IFN-γ, IL-4, or IL-10, which we have previously shown cannot be reversed by treatment with TNF.28 By contrast, uptake of apoptotic cells by BMDM did not abrogate the modulatory effects of TNF or other cytokines on uptake of apoptotic cells when added to the medium after the initial challenge. However, in each, their capacity to take up apoptotic PMNs was reduced by 50%. Furthermore, prior uptake of PMNs did not affect the ability of IFN-γ to prime macrophages for generation of NO (Table 3). In this set of experiments, there was no significant difference in IFN/TNF-induced NO generation between uncommitted BMDM, macrophages that had ingested apoptotic neutrophils, and macrophages that have been incubated with RGDS peptide followed by apoptotic neutrophils. Thus, uptake of apoptotic cells specifically inhibits BMDM ability to engulf apoptotic cells without interfering with their ability to respond to a range of pro and antiinflammatory cytokines.
Effect of Uptake of Apoptotic PMN or Ligation of Integrins on IFN/TNF-Induced NO Generation (Arbitrary Units)
| . | PMN . | RGDS (1 mmol/L) +PMN . | Medium . | F11 (1 μg/mL) . | CD18 (1 μg/mL) . |
|---|---|---|---|---|---|
| IFN-TNF (20 U/mL, 10 ng/mL) | 21.2 ± 1.2 | 23.4 ± 0.9 | 22.7 ± 1.1 | 19.4 ± 3.4 | 22.5 ± 1.9 |
| Control | 2.1 ± 1.2 | 2.9 ± 0.5 | 2.5 ± 0.7 | 2.09 ± 0.9 | 1.8 ± 1.1 |
| . | PMN . | RGDS (1 mmol/L) +PMN . | Medium . | F11 (1 μg/mL) . | CD18 (1 μg/mL) . |
|---|---|---|---|---|---|
| IFN-TNF (20 U/mL, 10 ng/mL) | 21.2 ± 1.2 | 23.4 ± 0.9 | 22.7 ± 1.1 | 19.4 ± 3.4 | 22.5 ± 1.9 |
| Control | 2.1 ± 1.2 | 2.9 ± 0.5 | 2.5 ± 0.7 | 2.09 ± 0.9 | 1.8 ± 1.1 |
Ligation of the αvβ3 receptor and other integrins reduce uptake of apoptotic PMNs.
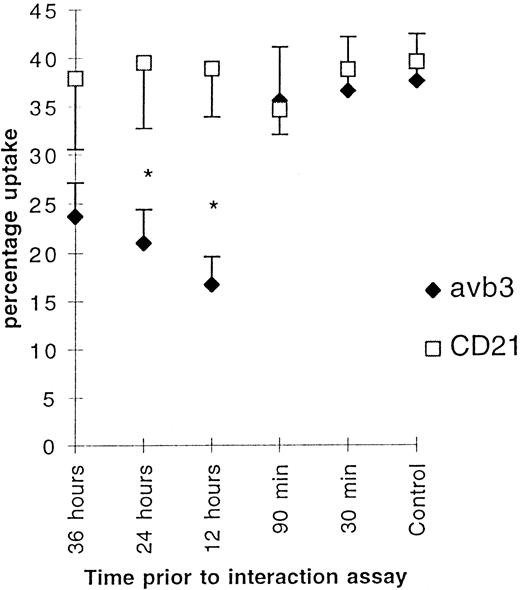
Overloading the macrophage phagocytic capacity provides the most obvious explanation as to why uptake of apoptotic cells prevented further uptake. However, this seems unlikely for three reasons. First, uptake of opsonized erythrocytes did not downmodulate the ability of macrophages to ingest apoptotic neutrophils 48 hours later (Table 4). Second, incubation of BMDM with neutrophils from different donors known to induce high or low uptake showed that irrespective of whether 20% or 40% of macrophages took up apoptotic cells and regardless of substantial differences in the number of PMN ingested per macrophage, the degree of downmodulation was about 50% (data not shown). Finally, the 48 hours between the PMN challenges should be sufficient to allow the macrophages to recover. The fact that inhibition was prevented by incubation in the presence of RGDS suggested that the mechanism might involve the αvβ3/CD36/thrombospondin pathway.4 This was addressed by incubation of BMDM for 30 minutes with various concentrations of the MoAb, F11, against the β3 subunit of the αvβ3 receptor.21 F11 blocks the calcium response after peptide binding to the vitronectin receptor in rat osteoclast.29 It binds to αvβ3 on the surface of macrophages, but did not alter their adhesion to vitronectin, nor did it block uptake of apoptotic PMN by BMDM. Despite this, ligation of αvβ3 with F11 12 to 36 hours before the interaction assay caused substantial reduction of PMN uptake (Fig 2), comparable to that induced by apoptotic PMN themselves.
Effect of Uptake of Opsonized Erythrocytes on the Number of Macrophages That Take up Apoptotic Neutrophils 48 Hours Later
| Initial Challenge . | Uptake of Apoptotic PMNs (%) . |
|---|---|
| Control | 25.8 ± 7.3 |
| Opsonized erythrocytes | 27.2 ± 4.8 |
| Initial Challenge . | Uptake of Apoptotic PMNs (%) . |
|---|---|
| Control | 25.8 ± 7.3 |
| Opsonized erythrocytes | 27.2 ± 4.8 |
N = 5.
Figure 2 shows the effect of ligation of vβ3 (1 μg/mL) and an isotype-matched control CD21 (10 μg/mL) on uptake of apoptotic neutrophils by BMDM. The macrophages were incubated for 30 minutes at various times before the start of the interaction assay. Mean (percentage of uptake) ± SE, n = 8. * P < .01.
Figure 2 shows the effect of ligation of vβ3 (1 μg/mL) and an isotype-matched control CD21 (10 μg/mL) on uptake of apoptotic neutrophils by BMDM. The macrophages were incubated for 30 minutes at various times before the start of the interaction assay. Mean (percentage of uptake) ± SE, n = 8. * P < .01.
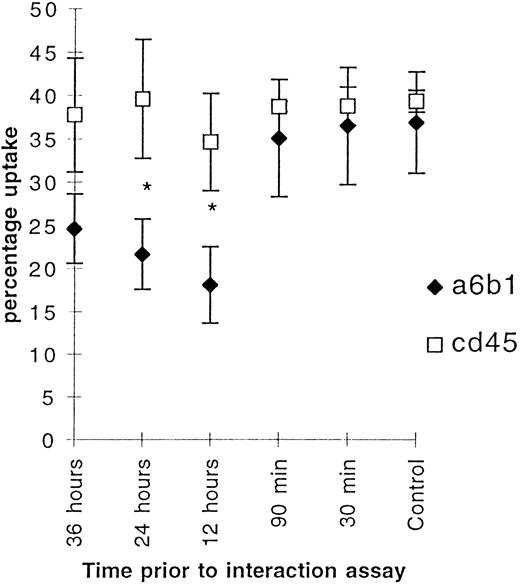
These data indicate that ligation of αvβ3 integrin decreases uptake of apoptotic neutrophils after a delay of at least 90 minutes, whereas an isotype-matched control mouse antihuman CD21 MoAb, which recognized neither PMNs nor macrophages, had no effect. To examine the specificity of the effects of F11, the experiments were repeated, first using MoAb against α6β1, another integrin receptor expressed by macrophages, but not known to be involved in recognition of apoptotic neutrophils, and secondly using a MoAb against CD45, another molecule on the macrophage plasma membrane. Strikingly the MoAb against α6β1 also downregulated uptake of PMNs with identical kinetics to antibodies against αvβ3, whereas the MoAb against CD45 had no effect (Fig 3). Thus, ligation of integrins, but not of other receptors on the surface of macrophages, specifically downregulates uptake of apoptotic cells after a delay of more than 90 minutes, but did not affect the uptake of other particles such as opsonized red blood cells (data not shown).
Figure 3 shows the effect of ligation of 6β1 and CD45 (10 μg/mL) on uptake of apoptotic neutrophils by BMDM. The macrophages were incubated for 30 minutes at various times before the start of the interaction assay. Mean (percentage of uptake) ± SE, n = 8. * P < .01.
Figure 3 shows the effect of ligation of 6β1 and CD45 (10 μg/mL) on uptake of apoptotic neutrophils by BMDM. The macrophages were incubated for 30 minutes at various times before the start of the interaction assay. Mean (percentage of uptake) ± SE, n = 8. * P < .01.
To confirm these observations, we performed another set of experiments examining the role of receptor ligation on uptake of apoptotic PMNs by macrophages after ligation of the receptors for 45 minutes 12 hours before the interaction assay. Ligation of the integrin receptors CD11b (percentage uptake 14.4 ± 2.1, P < .01) and CD18 (13.4 ± 3.4, P < .01) significantly decreased uptake of apoptotic cells by macrophages, whereas incubation with MoAbs against the IL-2 receptor CD25 (28.7 ± 3.5) present on the macrophage surface, or ED3 (29 ± 4), only expressed by activated or tissue macrophages, were not different from controls (29.8 ± 3.4). Thus, ligation of three different integrin receptors specifically downmodulated macrophage ingestion of apoptotic neutrophils, whereas ligation of two other receptors on the macrophage surface did not. It seems likely that at least some of the modulating effects of apoptotic PMNs themselves can be attributed to this mechanism. In addition, there was no significant difference in IFN/TNF-induced NO generation between uncommitted BMDM and macrophages, which have been incubated with antibodies that ligate their integrin receptors (Table 3). Thus, similar to uptake of apoptotic cells, ligation of integrin receptors specifically inhibits BMDM ability to engulf apoptotic cells without interfering with their ability to respond to a range of pro and antiinflammatory cytokines.
DISCUSSION
The specific uptake of apoptotic neutrophils by macrophages is one of the critical steps in the resolution of inflammation.30 It provides a way to remove neutrophils before granulocyte lysis and release of the neutrophils’ cytotoxic contents16 and does not activate the macrophages usual proinflammatory response to phagocytosis. Indeed, Fadok et al17 have recently provided evidence that uptake of apoptotic cells induces macrophages to synthesize the antiinflammatory cytokine, TGF-β. The importance of the process is illustrated by the observation that insufficient or impaired capacity for phagocytic clearance leads to disintegration of the cells undergoing apoptosis and worsening of tissue damage.31 We hypothesized that alterations in the process responsible for removal of apoptotic neutrophils might contribute to these observations. In some situations, induction of a single episode of acute inflammation resolves quickly, whereas a second episode results in progressive tissue damage. One explanation for this might be that the difference was caused by a reduced capacity to remove apoptotic neutrophils.32 This prompted us to analyze the effect of ingestion of apoptotic cells on the ability of macrophages to take up a second pulse of apoptotic cells 48 hours later. The results show that uptake of the second pulse 48 hours after the first is consistently reduced by 50%, which could have a substantial effect on tissue repair.
The characteristics of the mechanisms responsible for impaired uptake demonstrate that it involves specific interaction between the neutrophils and macrophages: (1) neutrophil uptake by BMDM was inhibited by RGDS, which interrupts integrin-dependent recognition; (2) it was not influenced by uptake of opsonized erythrocytes and was independent of the magnitude of the first “neutrophil meal” and thus unlikely to be due simply to macrophage “indigestion”; (3) the effect was sustained for at least 48 hours; (4) it did not interfere with cytokine-induced modulation of uptake of apoptotic cells; and (5) uptake of apoptotic neutrophils does not influence IFN-γ/TNF-induced generation of NO by macrophages. Taken together, these characteristics suggest that modulation is caused by the specific interactions between macrophage receptors and ligands on the PMN.
A number of different macrophage receptor-mediated pathways have been described to be involved in uptake of apoptotic neutrophils: (1) an uncharacterized lectin-dependent interaction2; (2) a complicated charge sensitive process involving the CD36/vitronectin receptor (αvβ3) complex on the macrophage surface interacting with unknown moieties on the apoptotic PMN surface via a thrombospondin bridge3,4; (3) a stereo-specific recognition of phosphatidylserine that is expressed on the surface of the apoptotic cell after loss of membrane asymmetry5,6; (4) macrophage scavenger receptors7; (5) the LPS receptor, CD148-10 and macrosialin or CD68.11,12Inhibition by RGDS, but not PS, suggests that uptake by rat BMDM in our experiments is mediated by the αvβ3/CD36/thrombospondin recognition pathway, which has been extensively characterized by Savill et al.3 4 The importance of αvβ3 was identified in blocking experiments using MoAbs. Our experiments were conducted using the MoAb, F11, an antibody to the β3 chain, which blocks some αvβ3-dependent functions, but not the ability of BMDM to bind to vitronectin under our experimental conditions. Despite this, it caused a sustained downmodulation of the macrophages’ ability to take up apoptotic cells after a delay of more than 90 minutes, comparable in degree to that seen after ingestion of apoptotic cells. This effect was not observed with isotype-matched control antibodies or with antibodies to CD25 or CD45 on the macrophage surface. Strikingly, however, antibodies to three other integrin receptors, α6β1, CD11b, and CD18, not known to be associated with uptake of apoptotic neutrophils, had the same effect. Thus, ligation of β1, β2, and β3 integrins all cause sustained specific downmodulation of uptake of apoptotic cells, but no effect on uptake of opsonized erythrocytes, and presumptively β1 and β2 integrins downmodulate the function of αvβ3 in neutrophil uptake.
There are precedents for cross-inhibition between integrin receptors, including interactions involving αvβ3. Blystone et al33 have previously shown that ligation of αvβ3 blocks high-affinity phagocytic function, but not adhesive function of the fibronectin receptor α5β1, possibly by influencing serine/threonine kinase activity of the cytoplasmic portion β1 chain. Similarly α4β1 ligation inhibits α5β1-dependent expression of metalloproteinases.34 Diaz-Gonzalez et al35 and Fenczik et al36 have analyzed the cross-talk between different integrins in detail and introduced the term transdominant inhibition to describe this phenomenon. They showed that ligation of αllbβ3 suppresses adhesive properties of α5β1 and α2β1.35,36 They demonstrated that the phenomenon was dependent on the assumption of the high-affinity state of αllbβ3 and was attributable to conformational changes in the cytoplasmatic portion of the β1 chain.37It is not yet clear at what level changes in integrins might modulate uptake of apoptotic cells, partly because of uncertainties about the signalling pathways involved.
The intracellular signalling pathways that control uptake of apoptotic cells have not been studied systematically. However, Rossi et al38 have recently reported that activation of cyclic adenosine monophosphate (cAMP) signalling pathways by inflammatory mediators downmodulates macrophage ingestion of apoptotic cells and that alteration in cAMP concentrations might be responsible for the observation that ligation of CD44 specifically enhances phagocytosis of apoptotic neutrophils.39 Our results suggest that uptake is also regulated by cross-talk between integrins and emphasize the multiple levels of control for macrophage removal of apoptotic cells. The ability of macrophages to ingest apoptotic cells can be dynamically regulated, providing a potential target for modulation of inflammation.
Furthermore, our observations also have the important implication that ligation of αvβ3 caused by uptake of apoptotic cells might downmodulate integrin-dependent macrophage adhesion and facilitate migration of macrophages from an inflamed site to the local lymph nodes, as has been described by Bellingan et al.19 We are currently conducting experiments to test this possibility.
ACKNOWLEDGMENT
The authors are indebted to A. Woodger for preparing this manuscript.
Supported by Grant No. ER 254/1-1 from the Deutsche Forschungsgemeimschaft (to L.-P.E.), Grant No. 044988/2/95/2 from the Wellcome Trust (to G.M.W.), and the National Kidney Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Lars-Peter Erwig, MD, University of Aberdeen, Department of Medicine and Therapeutics, Institute of Medical Sciences, Foresterhill, Aberdeen AB25 2ZD, UK; e-mail: L.P.Erwig@abdn.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal