Abstract
Because arachidonate metabolites are potent mediators of inflammation, we have studied the effects of leukotriene B4(LTB4) and the cysteinyl leukotrienes C4 and D4 (LTC4 and LTD4) on the release of nitric oxide (NO), in vitro, by human polymorphonuclear granulocytes (PMN). Two independent and highly sensitive real-time methods were used for these studies, ie, the NO-dependent oxidation of oxyhemoglobin (HbO2) to methemoglobin and a NO-sensitive microelectrode. When activated with LTB4, LTC4, or LTD4, but not with other lipoxygenase products such as 5S-HETE, 5-oxo-ETE or 5S,12S-diHETE, PMN produced NO in a stimulus- and concentration-dependent manner. The rank order of potency was LTB4 = LTC4 > LTD4, corresponding to 232 ± 50 pmol of NO/106 PMN for 100 nmol/L LTB4 after 30 minutes. The kinetic properties of the responses were similar for all three leukotrienes with a maximum response at 13 ± 3 minutes. Cysteinyl leukotriene and LTB4 antagonists inhibited the agonist-induced NO production by 70%, and treatment with Bordetella pertussis toxin, or chelation of cytosolic Ca2+, [Ca2+]i, also efficiently inhibited this response. In contrast, treatment of PMN with cytochalasin B (5 μg/mL) enhanced the LTB4-induced NO formation by 86%. Thus, this is the first demonstration that the cysteinyl leukotrienes LTC4 and LTD4, as well as LTB4, activate NO release from human PMN by surface receptor, G-protein and [Ca2+]i-dependent mechanisms. This effect differs from activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, for which only LTB4is an activator.
NITRIC OXIDE (NO) IS AN important effector and mediator of a multitude of biological functions.1 It is enzymatically formed from L-arginine by the nitric oxide synthase (NOS) family of enzymes, either constitutive (cNOS), or inducible (iNOS).2,3 The cNOS requires calcium and calmodulin for activation and produces only small amounts of NO, whereas iNOS is independent of added Ca2+ and produces large amounts of NO.1-3 Several lines of evidence suggest that NO plays an important role in the inflammatory process, such as being cytoprotective as well as cytotoxic, eg, in rheumatoid arthritis and vasculitides.4,5 Furthermore, increased NO production in the airways has been demonstrated in allergic inflammatory conditions in humans,6 in disorders with increased plasma leakage,7 and for functions in the control of the proinflammatory cascade systems in allergy.8
A cNOS has been purified from human polymorphonuclear granulocytes (PMN).9 In patients with urinary tract infections, PMN have been shown to express both iNOS and cNOS mRNA, and iNOS protein and activity was demonstrated.10 Still, the mechanisms for activation of NO production in human PMN as well as its biological significance are largely unknown. We have previously shown that human PMN produce NO rapidly on activation with bacterial oligopeptides or with a phorbol ester.11-13
Thus, we asked whether proinflammatory leukotrienes (LT) affect NO generation in PMN. Leukotriene B4 (LTB4) is a potent activator of PMN migration and chemotaxis,14,15 but it also stimulates the secretion of granule enzymes16 and superoxide ions in PMN.17-19 Furthermore, others have shown that LTB4 may activate nitrite production, conceivably dependent on NO formation, in human PMN.20 Contrary to LTB4, the cysteinyl leukotrienes (LTC4 and LTD4) do not elicit adhesive or secretory responses in PMN, but are powerful mediators in allergy and inflammation by induction of plasma leakage and reduction of myocardial contractility,21effects also reported for NO.7,22 Human PMN have been reported to possess receptors for LTC4,23,24and LTD4 may induce elevation of intracellular Ca2+ in PMN.25 However, whether LTC4 and LTD4 affect NO production from PMN is still unknown.
Based on these considerations, we have studied the effects of LTB4, LTC4, and LTD4 on NO release from human PMN. Because interaction between NO and the products of the oxidative metabolism in PMN, primarily the release of superoxide anions, may be of significance,26 we have also compared effects on NO production with the effects on superoxide release. For detection of these products, we have used highly sensitive, real-time methods.11-13 27-30
MATERIALS AND METHODS
Chemicals.
Percoll and Sephadex G25 were from Pharmacia Fine Chemicals (Uppsala, Sweden). Polymonoprep was from Nycomed Pharma AS (Oslo, Norway). Endotoxin-free, deionized water and Hanks’ balanced salt solutions (HBSS), with or without Ca2+, were from GIBCO (Paisley, Scotland). Tetrakis (3-methoxy-4-hydroxyphenyl) nickel(II)porphyrin was from Interchim (Montluçon, France), Nafion and pure NO gas from Aldrich (Milwaukee, WI). L-arginine, catalase, cytochrome C (horse heart type III), 1,2-bis(2-aminophenoxy)-ethane-N,N,N’,N’-tetraacetic acid (BAPTA-AM), luminol, N-formyl-methionyl-leucyl-phenylalanine (fMLP), sulfanilic acid, N-(1-naphtyl)ethylenediamine dihydrochloride, cytochalasin B, Bordetella pertussis toxin, and bovine hemoglobin were from Sigma Chemical Co (St Louis, MO). 5S-HETE, 5-oxo-ETE, and leukotrienes B4, C4, and D4 were from Cascade Biochemical (Berkshire, UK). 5S,12S-diHETE was from Biomol (Plymouth Meeting, PA). Superoxide dismutase (SOD) was from Boehringer Mannheim (Mannheim, Germany). NG-monomethyl-L-arginine (L-NMMA) was from Calbiochem (La Jolla, CA). SK&F 104 353 was a kind gift from Prof S.-E. Dahlén, Department of Physiology, the Karolinska Institute (Stockholm, Sweden), and CP-105,696 was a kind gift from Central Research Division, Pfizer Inc (Groton, CT).
Preparation of PMN.
Preparation of PMN was performed by Percoll density centrifugation, essentially as described previously.27 Only freshly prepared, nominally endotoxin-free solutions were used, and all preparations were performed under aseptic conditions. The cells were resuspended in HBSS with Ca2+ to 3.5 × 106 PMN/mL and kept at 4°C before analysis. The cells were then pelleted, washed three times in HBSS with or without Ca2+, and finally suspended to desired final concentration. Supernatants from the final wash were always assessed spectrophotometrically for residual hemoglobin, and the PMN used only if no such residues were found. The PMN preparation contained 98% to 99% granulocytes; the contaminating cells (1% to 2%) were principally monocytes and 0.1% to 0.7% eosinophils. To assess if this small monocyte contamination contributed to the NO produced, these cells were purified to 98% purity on Polymonoprep as described previously.31 In three separate experiments, performed in duplicate, no NO production was recorded (using the HbO2method; vide infra) from monocytes (105 cells per mL) either unstimulated or activated with fMLP, LTB4, or LTC4 (all at 100 nmol/L). Thus, we conclude that the contaminating monocytes do not add to the NO measured from PMN.
Preparation of oxyhemoglobin (HbO2).
The assay was performed as described previously.11-13Briefly, a solution of bovine hemoglobin was made in doubly distilled, deionized water at a concentration of 1 mmol/L and oxygenated by bubbling with O2 for 5 minutes. Subsequently, the hemoglobin was reduced with 1.5 mmol/L deoxygenated sodium dithionite in distilled water, and reoxygenated for 20 minutes. The sodium dithionite was then removed on a Sephadex G25 column. Oxyhemoglobin was prepared freshly for each experimental day and quality tested by scanning at 400 to 600 nm. The viability of the cells under study, assessed with trypan blue exclusion, was not affected by the used concentrations of, or by incubation times with, HbO2 (5 μmol/L). Neither did used concentrations of L-arginine, L-NMMA, nor incubation in Ca2+-free HBSS, with or without BAPTA-AM (5 μmol/L), decrease viability.
Measurement of NO release with oxyhemoglobin.
PMN (1.75 × 106/mL in HBSS with or without Ca2+, as indicated), or monocytes (105 cells per mL) in control experiments, were treated with either 300 μmol/L L-arginine or 1 mmol/L L-NMMA for 45 minutes at 37°C in a shaking water bath. Before incubation, SOD (150 U/mL), and catalase (300 U/mL), and in certain experiments, Bordetella pertussis toxin (PT; 750 ng/mL), were added. In experiments with the Ca2+-chelator BAPTA-AM (5 μmol/L), this was added 15 minutes before stimulation. HbO2 and stimuli, or corresponding volumes of HBSS, were added immediately before analysis.11 Cytochalasin B (5 μg/mL), when used, was added 3 minutes before stimulation. The metHb generation was followed for 30 minutes at 37°C, as indicated, by spectroscopy at 401 versus 411 nm in a Perkin-Elmer Lamda 7 spectrophotometer, essentially as described previously.11All samples were assessed as the difference between samples with L-arginine and identical samples with L-NMMA instead of L-arginine. Thus, only the L-NMMA inhibitable response was assessed and taken to represent the net amount of NO released from PMN. An ε = 19.7 mmol/L-1 cm-1 was used for calculating produced amount of NO.32
Electrochemical detection of NO release.
This was performed with a three-electrode potentiostatic Biopulse system (Tacussel, Lyon, France). The working electrode was a carbon fiber (8 μm diameter, approximately 1 mm length), coated with tetrakis(3-methoxy-4-hydroxyphenyl)nickel (II)porphyrin and Nafion films29,30 and the measurement of NO production was performed as described previously.29,30 The suspension of PMN (1.75 × 106/mL in HBSS with Ca2+) was incubated for 20 minutes at 37°C with L-arginine or L-NMMA (both at 1 mmol/L) and SOD (150 U/mL). Leukotriene B4 and C4 were added when a stable current baseline was reached, and the NO production was followed for 5 minutes. There were no changes in baseline current when L-NMMA, L-arginine, or SOD were added to unstimulated PMN, neither for different concentrations of H2O2 nor on preincubation with catalase (data not shown). The electrode was calibrated with standard NO solutions as described previously,29 30 and a standard curve with PMN present was made for each experiment and at the end of each measurement. All samples were assessed as the difference between samples with L-arginine and identical samples with L-NMMA instead of L-arginine. Thus, only the L-NMMA inhibitable response was assessed and taken to represent the net amount of NO released from PMN.
Measurements of superoxide anion release with cytochrome C-reduction.
This was performed as previously described.27 Briefly, PMN at 1.75 × 106/mL in HBSS, with 100 μmol/L cytochrome C, were activated and the reduction of cytochrome C was followed continuously at 550 nm in a Perkin Elmer Lamda 7 spectrophotometer with a thermostated multicuvette holder. Blanks were identical to samples, but with SOD present (150 U/mL). Thus, only the SOD-inhibitable reduction of cytochrome-C was measured in all instances. In experiments with L-arginine or L-NMMA, PMN were treated for 45 minutes at 37°C in a shaking water bath with arginines before O2--measurement. When used, as indicated, BAPTA-AM (5 μmol/L) was added 15 min before stimulation. An ε = 21.1 mmol/L-1 cm-1 was used to calculate the amount of O2--production.27 28
Chemiluminescence (CL).
CL was performed essentially as described previously27 28in a luminoaggregometer (Chronolog Havertown, PA). The CL response was measured from the magnitude of the peak response, given in mV, corrected for magnitude of the background (unstimulated) chemiluminescence. The reading was continuous during the luminescent reaction. All recordings were made from 0.5 or 1.0 mL samples to which reagents (5 to 20 μL) were added. Samples containing neutrophils (1.25 × 106 cells/mL) were adjusted to 37°C for 3 minutes before addition of stimulus.
Statistical assessment.
Statistical assessment was performed with Student’s t-test.
RESULTS
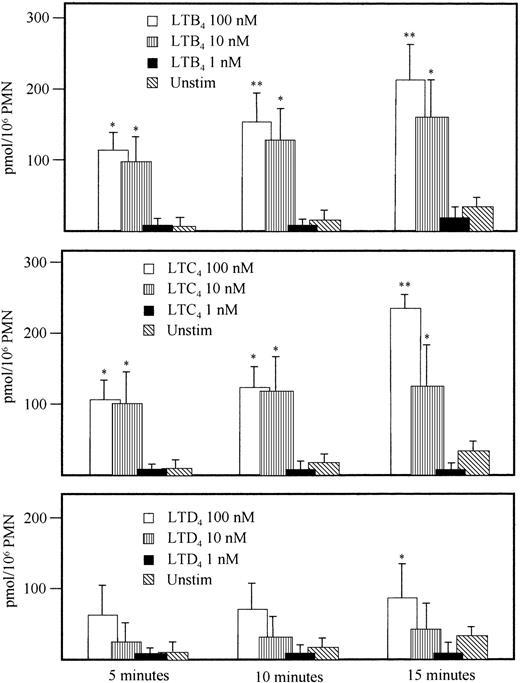
When PMN were activated with LTB4 (100 nmol/L), LTC4 (100 nmol/L) or LTD4 (100 nmol/L), they responded with a methemoglobin production in a stimulus-dependent manner, but less pronounced compared with a structurally unrelated activator of chemotaxis and oxidative metabolism, the oligopeptide fMLP (100 nmol/L) (Fig 1). The release of NO was dose-dependent for the three tested leukotrienes (Fig 2) and the rank order of potency in inducing NO release was LTB4 = LTC4 > LTD4 (Fig 2). The LTB4 response was completed after approximately 15 minutes, whereas the fMLP-stimulated PMN still showed NO production at this time.
Comparison between NO production in unstimulated PMN and LTC4, LTD4, LTB4, or fMLP (all agonists were used at 100 nmol/L) stimulated PMN, assessed with the HbO2 method as described in Materials and Methods. * .01 < P < .05; ** .005 < P < .01; *** P < .005 versus unstimulated PMN; mean ± SEM; n = 4.
Comparison between NO production in unstimulated PMN and LTC4, LTD4, LTB4, or fMLP (all agonists were used at 100 nmol/L) stimulated PMN, assessed with the HbO2 method as described in Materials and Methods. * .01 < P < .05; ** .005 < P < .01; *** P < .005 versus unstimulated PMN; mean ± SEM; n = 4.
Effect on NO production by various concentrations (1, 10, or 100 nmol/L) of indicated stimuli, performed with the HbO2 method as detailed in Materials and Methods. * 0.01 < P < .05; ** .005 < P < .01 versus unstimulated PMN; mean ± SEM; n = 4.
Effect on NO production by various concentrations (1, 10, or 100 nmol/L) of indicated stimuli, performed with the HbO2 method as detailed in Materials and Methods. * 0.01 < P < .05; ** .005 < P < .01 versus unstimulated PMN; mean ± SEM; n = 4.
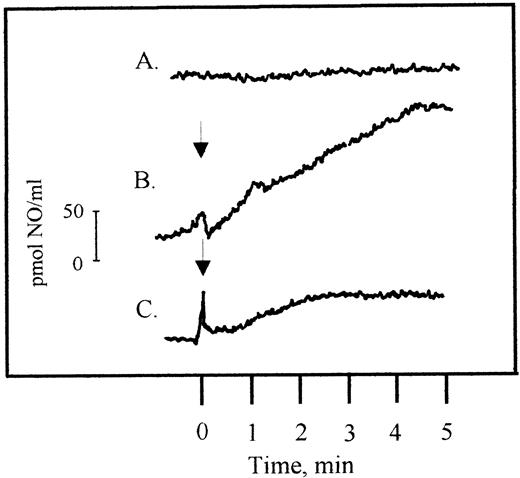
Next, we assessed if the detection of NO-release from PMN with the HbO2-method was as specific for NO when PMN were activated with leukotrienes, as previously shown when a formylpeptide or phorbolester were used as stimuli.11-13 To this end, a highly sensitive and specific porphyrinic microsensor was used.29 30 With LTB4 (100 nmol/L), a prompt and continuous release of NO from PMN was recorded (Fig 3). Production of NO 5 minutes after addition of LTB4 was 18.7 ± 4.5 pmol NO/106PMN (n = 4). This was in good agreement with the results obtained with the HbO2 method (12.2 ± 4.5 pmol NO/106 PMN and minutes; n = 8). Leukotrienes C4 or D4 (at 100 nmol/L) also conferred NO generation, measurable with the electrode, in a similar concentration as obtained with the HbO2 method (13.5 ± 5.5 and 5.65 ± 1.5 pmol NO/106 PMN, respectively; n = 2). Time to response was similar for all three leukotrienes with a lag period of 30 seconds. Because the HbO2 method permits kinetic studies also for longer time periods than the NO electrode, this method was selected for further studies.
NO production in LTB4-stimulated human PMN (3.5 × 106/mL), measured by the NO-specific electrode. The upper line (A) shows unstimulated PMN, line (B) stimulated PMN, and the line at the bottom (C) stimulated PMN preincubated with L-NMMA (1 mmol/L), performed as detailed in Materials and Methods. The arrow indicates when LTB4 (100 nmol/L) was added.
NO production in LTB4-stimulated human PMN (3.5 × 106/mL), measured by the NO-specific electrode. The upper line (A) shows unstimulated PMN, line (B) stimulated PMN, and the line at the bottom (C) stimulated PMN preincubated with L-NMMA (1 mmol/L), performed as detailed in Materials and Methods. The arrow indicates when LTB4 (100 nmol/L) was added.
Next, we assessed if the LTB4-induced NO formation was the result of a stereospecific, ie, receptor-mediated process, or an unspecified effect on PMN. To this end, we applied related eicosanoids, 5-hydroxy eicosatetraenoic acid (5S-HETE; 100 nmol/L), or its metabolite 5-oxo-ETE (100 nmol/L), or 5S,12S-diHETE (10 and 100 nmol/L),33,34which have been shown not to activate the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or chemotaxis in PMN. However, none of these eicosanoids showed any statistically significant release of NO from PMN (28 ± 10, 29 ± 11, 24 ± 13 and 0 pmol NO/106 PMN, respectively, 30 minutes after stimulation; mean ± standard error of mean [SEM]; n = 3). As reported previously, lipoxin A4 did not elicit NO production from human PMN, measured with a similar HbO2-technique as used here.5
To further study the receptor-dependence of the leukotriene-induced responses, we applied specific receptor antagonists. Treatment of PMN with the cysteinyl leukotriene receptor-antagonist SK&F 104 35323 significantly reduced the NO-release induced by LTC4 or LTD4(Table 1). Substituting the cysteinyl leukotrienes with LTB4 as stimulus assessed the specificity of this antagonist. However, no inhibition of LTB4-induced NO formation or CL by SK&F 104 353-treated PMN was found. Conversely, treatment of PMN with the LTB4-receptor antagonist CP-105,69635 significantly reduced NO production in LTB4-stimulated PMN (Table 1), whereas the responses to LTC4 or LTD4 were unaffected. Also, the effect of CP-105,696 on oxidative metabolism was assessed with CL. Because LTC4 and LTD4 did not induce a CL response from PMN, we used fMLP-activation of PMN as positive control. The CL response from CP-105,696-treated PMN was totally abolished on LTB4-activation (49.6 ± 9.9 mV without CP-105,696 compared with 0 mV with CP-105,696, mean ± SEM; n = 4), but no reduction of the response to fMLP was found (635 ± 19.6 and 650 ± 53.5 mV, with and without CP-105,696, respectively; mean ± SEM; n = 6). We have previously shown that SK&F 104 353 inhibits the rise of cytosolic calcium concentration in LTC4- and LTD4-stimulated PMN.24
Effect of Leukotriene Receptor Antagonists on NO Formation in PMN
| Percent Reduction of NO Production Stimulus (at 100 nmol/L) . | |||
|---|---|---|---|
| Inhibitor | LTD4 | LTC4 | LTB4 |
| SK&F 104 353 | 86.6 ± 18.5 | 70.8 ± 19.9 | 0 |
| CP-105,696 | 0 | 0 | 79.0 ± 12.2 |
| Percent Reduction of NO Production Stimulus (at 100 nmol/L) . | |||
|---|---|---|---|
| Inhibitor | LTD4 | LTC4 | LTB4 |
| SK&F 104 353 | 86.6 ± 18.5 | 70.8 ± 19.9 | 0 |
| CP-105,696 | 0 | 0 | 79.0 ± 12.2 |
Inhibition of the PMN-derived NO release activated by LTB4, LTD4, or LTC4 after treatment with SK&F 104 353 or CP-105,696 (both at 10 μmol/L), as percent of similarly treated PMN, but without the specific receptor antagonist. The release of NO from activated PMN, without receptor inhibitors, was for LTB4 232 ± 50, for LTC4240 ± 99, and for LTD4 145 ± 67 pmol/106PMN. The NO release was measured at 30 minutes after activation and performed as detailed in Materials and Methods section. Mean ± SEM; n = 4.
To further establish a role for serpentine receptor transduction mechanisms, ie, PT-sensitive G-proteins, PT (750 ng/mL) was added during incubation. We found a marked blunting of NO production in PT-treated PMN for all three stimuli, LTB4, LTC4, and LTD4 (at 100 nmol/L), the reduction being 91.2% ± 4.4%, 96.7% ± 10.4%, and 98.0% ± 5.1%, respectively (mean ± SEM; n = 4; P < .05) 15 minutes after stimulation compared with simultaneously run untreated controls. Likewise, PT treatment reduced fMLP- and LTB4-stimulated CL with 85% ± 4.5% (mean ± SEM; n = 3; P < .05). Consequently, these results support the concept that NO production in PMN, activated by these leukotrienes, is a cell surface receptor-dependent event involving receptors with specificity for LTB4 or the cysteinyl leukotrienes, respectively.
We next asked if NO release was dependent on cytosolic Ca2+-transients distal to G-protein activation, as previously described for LTB4 activation of other PMN functions.16,24 To this end, PMN were treated with the high-affinity cytosolic Ca2+-chelator BAPTA-AM (5 μmol/L), and subsequently suspended in nominally calcium-free HBSS. These conditions markedly reduced the NO release induced by LTB4 and LTD4, and to a significant, but slightly less extent, by LTC4(Fig 4). The same experimental conditions were used to assess NADPH oxidase activity induced by LTB4or fMLP, by means of CL or cytochrome-C reduction. We found that treatment with BAPTA-AM reduced the responses significantly in both assays. Addition of BAPTA-AM reduced LTB4 induced CL from 49.6 ± 9.9 to 7.5 ± 1.7 mV and for fMLP from 650 ± 53.0 to 15 ± 2.5 mV (mean ± SEM; n = 3 to 4; P< .05), in accordance with previous reports.18 25 The reduction of O2 production assessed with cytochrome-C reduction was in the same range; in fMLP-stimulated PMN, the production was reduced from 10.45 ± 0.9 to 1.7 ± 0.4 nmol O2-/106 PMN and for LTB4 from 2.0 ± 0.4 to 0 nmol O2-/106 PMN, 10 minutes after stimulation (mean ± SEM; n = 8). Neither LTC4 nor LTD4 induced a CL response or cytochrome-C reduction from PMN under normocalcemic conditions. To assess the possibility that this might be due to concomitant NO production and scavenging of O2-, we also assessed BAPTA-AM-treated PMN by CL after stimulation with LTC4 or LTD4. However, similar to nontreated PMN, the cysteinyl leukotrienes did not evoke a CL response from PMN treated with BAPTA-AM, irrespective of the Ca2+ content in the medium. Neither did the addition of BAPTA-AM during incubation reduce the PMA-induced CL response in human PMN (1505 ± 53 and 1415 ± 48 mV, respectively; mean ± SEM; n = 2).
Effect of [Ca2+]i on the release of NO from PMN, with Ca2+ present, assessed with the HbO2 method as detailed in Materials and Methods, activated with LTC4, LTD4, and LTB4(all at 100 nmol/L) compared with PMN treated with the Ca2+ chelator BAPTA-AM (5 μmol/L) in nominally Ca2+-free HBSS or with unstimulated PMN in HBSS with Ca2+. * .01 < P < .05; ** P< .01; mean ± SEM; n = 4.
Effect of [Ca2+]i on the release of NO from PMN, with Ca2+ present, assessed with the HbO2 method as detailed in Materials and Methods, activated with LTC4, LTD4, and LTB4(all at 100 nmol/L) compared with PMN treated with the Ca2+ chelator BAPTA-AM (5 μmol/L) in nominally Ca2+-free HBSS or with unstimulated PMN in HBSS with Ca2+. * .01 < P < .05; ** P< .01; mean ± SEM; n = 4.
Finally, we studied the dependence of the cytoskeleton and degranulation for the NO-response in LTB4-stimulated PMN treated with cytochalasin B, previously reported to enhance the chemiluminescence responses in LTB4-stimulated PMN and PMN aggregation.18 Both the initial and the sustained NO release was significantly increased in samples preincubated with cytochalasin B (Fig 5), and the mean increase in NO production during the observation period was 86% ± 32% (mean ± SEM; n = 3; P < .05 compared with identical samples without cytochalasin B).
Effect of cytochalasin B (5 μmol/L) incubation on the release of NO from human PMN, assessed with the HbO2 method as detailed in Materials and Methods, activated with LTB4(100 nmol/L) and measured 1, 5, 10, and 30 minutes after stimulation. Mean ± SEM; n = 3. * .01 < P < .05; **P < .01 compared with identical samples with cytochalasin B present.
Effect of cytochalasin B (5 μmol/L) incubation on the release of NO from human PMN, assessed with the HbO2 method as detailed in Materials and Methods, activated with LTB4(100 nmol/L) and measured 1, 5, 10, and 30 minutes after stimulation. Mean ± SEM; n = 3. * .01 < P < .05; **P < .01 compared with identical samples with cytochalasin B present.
DISCUSSION
The production of NO by human PMN has previously been demonstrated by us (using the same methods),11-13 as well as by others (using various methods for detection of NOS activity).10,20,36 Because NO is highly reactive and has a very short half-life in biological systems,32 its detection in phagocytes is technically demanding. We have previously reported the characteristics of the two methods.11-13,28,29 Thus, both methods are assessing NO directly and with considerably higher sensitivity than, for example, colorimetric analysis of nitrite. Moreover, one might speculate that superoxide anions could attenuate the NO assessments. However, previous studies in PMN from patients with chronic granulomatous disease show that these PMN, incapable of superoxide anion production, oxidized HbO2 to approximately the same extent as normal PMN.11 37
In previous studies, we17,18 and others25,38have demonstrated that functional responses of PMN, eg, activation of NADPH oxidase, homotypic aggregation, and [Ca2+]i transients elicited by LTB4 are more rapid in onset, as well as terminated earlier than those evoked by fMLP and, particularly, the phorbol ester PMA. The reason remains unclear, but was attributed to kinetic differences in stimulus-response coupling systems. Here, we show that a similar difference in activation kinetics for NOS exists, where leukotriene responses reached a plateau phase earlier than the fMLP responses (and were more rapid in onset than NO generation evoked by PMA).18,28 These findings point to consistent and stimulus-specific activation pathways that apply also for the NOS studied here. Furthermore, LTC4 or LTD4activate the production of NO in sharp contrast to their lack of activity in inducing NADPH-oxidase activation,17 25 and with kinetic properties similar to LTB4. There was also a difference between the NADPH oxidase and NOS in activation kinetics, in that all NO responses were slower in onset. Because our detection systems give instant recordings of NO production, we believe that this NOS requires more time to produce NO than the NADPH oxidase to assemble and generate superoxide ions.
The concept that the leukotriene-induced NO release was mediated by cell surface receptors was supported by the results of cysteinyl-leukotrienes and LTB4-receptor antagonists. These results are similar to findings for a variety of other responses to leukotrienes in PMN and other cells. Moreover, the inhibitory effect of PT, indicating that the NO response was transduced by means of PT-inhibitable G-proteins, is similar to the activation of the NADPH oxidase. It has previously been demonstrated that the cytoskeleton and actin levels are important for receptor expression in PMN.35 Depolymerization of the actin cytoskeleton with cytochalasin B enhanced the LTB4-induced NO response in the same manner as for superoxide production (and homotypic aggregation). These results show that the events leading to the activation of the NOS studied here are highly regulated and follow similar principles as aggregation and superoxide forming reactions to LTB4 and fMLP.
To assess a key step of the signal transduction in PMN, we studied the significance of cytosolic Ca2+, [Ca2+]i transients for the leukotriene-induced NO release. Indeed, the leukotriene-induced NO release showed a similar dependence on [Ca2+]i as that for fMLP-induced NADPH oxidase activation.25 Previous studies have found that LTB4 is a substantially more potent activator of [Ca2+]i release in human PMN than LTC4 or LTD4.24 25 That finding is still consistent with our data showing that the NO release activated by cysteinyl leukotrienes has a similar requirement for access to cytosolic Ca2+ as that in response to LTB4. However, the nature of the NOS activated in these cells, constitutive or inducible, is not possible to predict from the present data.
Thus, in the present study, we have demonstrated that LTB4activates human PMN to release NO in a highly regulated receptor-dependent process involving cytosolic Ca2+transients, in apparent analogy with other secretory events induced in PMN by LTB4, such as superoxide anion production.18,25,28 However, the cysteinyl-leukotrienes, LTC4 and LTD4, act in a similar manner as LTB4 and to virtually the same extent to activate NO release from PMN, but do not induce a measurable activation of the NADPH oxidase, as found here and in previous studies by others.25 This is, to the best of our knowledge, the first demonstration of an effect of cysteinyl-leukotrienes for secretory events in PMN. Consequently, the human PMN seems capable to tailor its production of radicals, NO, or O2-, in a stimulus-dependent fashion. The mechanism for this selective response pattern remains to be elucidated. Another question that is raised is the possible biological importance of these findings. Although the amount of NO produced in each granulocyte is very low, the total number of cells and their tendency to migrate towards inflammatory and infectious foci could imply that a NO production is induced in affected tissues and that it is important for the functional responses in human PMN.
ACKNOWLEDGMENT
The authors wish to thank Anette Landström, Annie Brunet, and Hussein Fazipour for skillful technical assistance.
Supported by Grants No. 19X-05991 and 19P-8884 from the Swedish Medical Research Council and the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (grants Acc-SV 11-n° 9511021), the Swedish Heart and Lung Association, the Swedish Association against Rheumatism, and The Funds of the Karolinska Institute, King Gustaf V, Tore Nilson, Börje Dahlin, Inga-Britt, and Arne Lundberg.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Gerd Lärfars MD, Department of Hematology, M54, Huddinge University Hospital, S-141 86 Huddinge, Sweden.


![Fig. 4. Effect of [Ca2+]i on the release of NO from PMN, with Ca2+ present, assessed with the HbO2 method as detailed in Materials and Methods, activated with LTC4, LTD4, and LTB4(all at 100 nmol/L) compared with PMN treated with the Ca2+ chelator BAPTA-AM (5 μmol/L) in nominally Ca2+-free HBSS or with unstimulated PMN in HBSS with Ca2+. * .01 < P < .05; ** P< .01; mean ± SEM; n = 4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1399/5/m_blod40407004x.jpeg?Expires=1767846524&Signature=RtSOf-2-HBlCvYp6Ivbjqaf2HeLUkhMHfRV0Z5vjhLL2v3V4Ve2tPda-ABtVFqMtOdvCTTEsxspN4l8PcgQuLZU4acN-cKoDZNIcEWgshI64u5geVyTHJC8v9qJcZpKraGlUwcr4AeHPrQOWiFwa70cVqu4F1K~INlHy7PeuQ8DZnLGwZKj1dJAD5cO3zy4S3HI1M19kkAvdJa2j4tVEs7Wybjsh67nl6iDTJ6hwnxZ1fmtDnxUpPC-uiaRPwuVXi2cKTW25scy~ZmEkGrRr~c1yeGhqmvVxWKw5XZzbfQsaNqGKhlDC50lfQDyV3LEiViBxRenY4uHrCUB-sqqxKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal