Abstract
In a murine model of platelet alloimmunization, we examined the definitive role that mononuclear cells (MC) have in modulating platelet immunity by using platelets from severe combined immunodeficient (SCID) mice. CB.17 (H-2d) SCID or BALB/c (H-2d) mouse platelets were transfused weekly into fully allogeneic CBA (H-2k) mice and antidonor antibodies measured by flow cytometry. MC levels in BALB/c platelets were 1.1 ± 0.6/μL and SCID mouse platelets could be prepared to have significantly lower (<0.05/μL) MC numbers. Transfusions with 108 BALB/c platelets (containing ≈100 MC/transfusion) stimulated IgG antidonor antibodies in 100% of the recipients by the fifth transfusion, whereas 108 SCID mouse platelets (containing ≈5 MC/transfusion) stimulated higher-titered IgG alloantibodies by the second transfusion. When titrations of BALB/c peripheral blood MC were added to the SCID mouse platelets, levels approaching 1 MC/μL reduced SCID platelet immunity to levels similar to BALB/c platelets. Characterization of the alloantibodies showed that the low levels of MC significantly influenced the isotype of the antidonor IgG; the presence of 1 MC/μL was associated with induction of noncomplement fixing IgG1 antidonor antibodies, whereas platelet transfusions, devoid of MC (<0.05/μL), were responsible for complement-fixing IgG2a production. When magnetically sorted defined subpopulations of MC were added to the SCID platelets, major histocompatability complex (MHC) class II positive populations, particularly B cells, were found to be primarily responsible for the reduced SCID mouse platelet immunity. The presence of low numbers of MC within the platelets was also associated with an age-dependent reduction in platelet immunogenicity; this relationship however, was not observed with SCID mouse platelets devoid of MC. The results suggest that a residual number of MHC class II positive B cells within allogeneic platelets are required for maximally reducing alloimmunization.
PRODUCTION OF ANTIDONOR major histocompatibility complex (MHC) class I antibodies is a frequent complication of platelet transfusions. This response can be initiated by direct allorecognition where the T-cell receptors of recipient CD4+ T helper (Th) cells directly recognize intact donor MHC class II molecules on the surface of donor antigen-presenting cells (APC).1-10 Hence, leukoreduction strategies such as leukofiltration have been used to remove the contaminating APC from platelet concentrates.11-21 A number of clinical studies11-21 including the recent large multicenter Trial to Reduce Alloimmunization against Platelets (TRAP),20 have confirmed that leukofiltration of platelets significantly reduces alloimmunization, although a percentage of transfusion recipients still become alloimmunized. In the patients receiving leukofiltered blood products, the mechanism of alloimmunization is unknown, but because the TRAP study showed few failed leukofiltration episodes, it suggests that MHC class I positive, class II negative platelets may, in themselves, stimulate MHC alloimmunization.
It remains unclear what is the actual extent of leukocyte removal required to achieve maximum benefit to the recipient. In this case, benefit can be defined by the clinical outcome of reduced alloimmunization and platelet refractoriness. In an early experimental murine model, Claas et al22 showed that allogeneic platelets could induce IgG alloantibody formation only if at least 103 contaminating leukocytes were present. Based on an approximate murine blood volume of 2 mL, this dosage translated to a human transfusion of 2.5 × 106 leukocytes. Clinical studies subsequently similarly suggested that the minimal threshold of leukocyte contamination in human platelet concentrates to prevent alloimmunization should be less than 1 to 5 × 106leukocytes.21 There is however, little experimental evidence to support whether this level of leukoreduction is optimal for completely preventing alloimmunization or whether lower levels may be required. Several reports have indicated that in healthy mice, rats and humans transfused with leukoreduced allogeneic platelets, leukocyte levels as low as 1/μL can activate recipient T cells23and stimulate IgG antidonor alloantibody responses.24-27Thus, an experimental model in which platelets for transfusion can be consistently prepared with none, or few, leukocytes would be advantageous to understanding the relationship between platelets and leukocytes in modulating recipient immunity.
One potential way to achieve this is to use platelets derived from genetically immunodeficient animals. CB.17 severe combined immunodeficient (SCID) mice were derived from BALB/c mice and have a point mutation on chromosome 16, which inhibits their ability to repair double-stranded DNA breaks.28 Because the proper gene rearrangements for T-cell and B-cell receptors is critically dependent on this DNA repair process, these cells are deleted early in ontogeny. Although myeloid cell numbers are relatively normal, these mice contain no detectible T or B cells in their peripheral blood and can be further depleted of natural killer (NK) cells and some monocytes by in vivo treatment with anti-asialo Gm1 (AsGm 1) antibody. Thus, platelets prepared from the platelet-rich-plasma of antibody-treated SCID mouse blood can be consistently rendered extremely leukoreduced (<0.05 mononuclear cells [MC]/μL). We present evidence that SCID mouse platelets, despite greater MC reduction, are significantly more immunogenic than BALB/c platelets in allogeneic CBA recipients and that levels of approximately 1 MC/μL are optimal for maximal suppression of platelet immunity.
MATERIALS AND METHODS
Animals and cell lines.
CBA (H-2k) female mice, 8 weeks of age were used as the transfusion recipients and female BALB/c (H-2d) and CB.17 (H-2d) SCID mice were used as donors and purchased from commercial breeders. EL-4 (H-2b) C57BL/6 thymoma, P815 (H-2d) DBA mastocytoma, and RT 1.1 (H-2k) CBA lymphoma cell lines were used for serological typing of the recipient sera. All cell lines and cell culture assays were maintained in RPMI-1640 with 5% fetal calf serum (FCS), 100 μg/mL penicillin/streptomycin/fungizone, 100 mmol/L L-glutamine and 5 × 10-5 mol/L 2-mercaptoethanol.
Antibodies.
Antiasialo Gm1 (AsGm 1) antibody was obtained from Waco Laboratories (Waco, TX). Fluorescein isothiocyanate (FITC)-labeled antimurine-CD45, -CD3, -CD4 and -F4/80 antibodies were obtained from Cedarlane Laboratories (Hornsby, Ontario). Phycoerythrin (PE)-labeled antimurine-CD45, -CD8, -B220, -Ly 6G (CD89) and -CD16 antibodies were obtained from PharMingen (Cedarlane Laboratories). Monoclonal FITC- and PE-labeled isotype control reagents were obtained from Cedarlane Laboratories. Biotinylated antimurine-CD3, -B220 and -I-Ad MHC class II antibodies were obtained from PharMingen and biotinylated antimurine-NK (catalog #CL8994B) antibody was obtained from Cedarlane Laboratories.
SCID mouse peripheral blood characterization and treatment.
To ensure complete penetrance of the SCID mutation, peripheral blood was screened for residual B and T cells by flow cytometry. Briefly, mice were bled via a tail-nick procedure into microvettes (Sarstedt, Montreal, Quebec) containing 1.0% (vol/vol) EDTA and 15 U/mL heparin (final concentrations) in saline. Blood counts were performed on a Toa hematology analyzer (Kobe, Japan) calibrated to rodent settings. Red blood cells (RBC) within the whole blood were removed by incubation with RBC lysing solution (Becton Dickinson, Mississauga, Ontario), and the leukcocytes were labeled with the indicated combinations of FITC- and PE-labeled monoclonal antibodies for 45 minutes at room temperature in the dark. The labeled blood was then analyzed by flow cytometry. In indicated experiments, to additionally deplete peripheral blood NK cells and some monocytes, SCID mice were injected intraperitoneally with 100 μg of anti-AsGm1 antibody 48 to 72 hours before bleeding. Table 1 summarizes the peripheral blood leukocyte composition of the murine platelet donors.
Flow Cytometric Analysis of Peripheral Blood and Percolled Leukocyte Subpopulations (Percentage ± SD of Total Leukocytes)
| Cell Population . | Platelet Donors (n = 6) . | Percoll Preparation (n = 6) . | ||
|---|---|---|---|---|
| BALB/c Mice* (%) . | SCID Mice† (%) . | AsGm1-Treated SCID Mice‡ (%) . | BALB/c MC1-153 (%) . | |
| T cells (CD3+) | 40 ± 9 | <0.6 | <0.5 | 64 ± 7 |
| CD4+ T cells | 30 ± 7 | <0.3 | <0.3 | 50 ± 4 |
| CD8+ T cells | 10 ± 2 | <0.2 | <0.2 | 13 ± 1 |
| B cells (B220+) | 15 ± 6 | <0.3 | <0.3 | 18 ± 3 |
| NK cells (CD3−CD16+) | 9 ± 0.5 | 26 ± 7 | 4 ± 2 | 10 ± 3 |
| Monocytes (F4/80+) | 5 ± 0.6 | 10 ± 3 | 5 ± 2 | 6 ± 2 |
| Granulocytes (CD89+) | 28 ± 3 | 58 ± 54 | 77 ± 10 | <0.6 |
| Cell Population . | Platelet Donors (n = 6) . | Percoll Preparation (n = 6) . | ||
|---|---|---|---|---|
| BALB/c Mice* (%) . | SCID Mice† (%) . | AsGm1-Treated SCID Mice‡ (%) . | BALB/c MC1-153 (%) . | |
| T cells (CD3+) | 40 ± 9 | <0.6 | <0.5 | 64 ± 7 |
| CD4+ T cells | 30 ± 7 | <0.3 | <0.3 | 50 ± 4 |
| CD8+ T cells | 10 ± 2 | <0.2 | <0.2 | 13 ± 1 |
| B cells (B220+) | 15 ± 6 | <0.3 | <0.3 | 18 ± 3 |
| NK cells (CD3−CD16+) | 9 ± 0.5 | 26 ± 7 | 4 ± 2 | 10 ± 3 |
| Monocytes (F4/80+) | 5 ± 0.6 | 10 ± 3 | 5 ± 2 | 6 ± 2 |
| Granulocytes (CD89+) | 28 ± 3 | 58 ± 54 | 77 ± 10 | <0.6 |
Total peripheral blood leukocyte counts for BALB/c mice were 8.1 ± 2.2 × 103/μL.
Total peripheral blood leukocyte counts for SCID mice were 4.8 ± 1.2 × 103/μL.
Total peripheral blood leukocyte counts for anti-AsGm1–treated SCID mice were 4.1 ± 2.1 × 103/μL. SCID mice were injected IP with 100 μg antibody 72 hours before analysis.
BALB/c peripheral blood mononuclear cells (PBMC) were separated using 1.077 g/mL Percoll.
Platelet preparation.
Mice were bled by the procedure described above. The whole blood was pooled, centrifuged at 120xg, and platelet rich plasma (PRP) aspirated off; care was taken not to disturb the buffy coat. The platelets were washed once in EDTA/heparin-saline, adjusted to 109 cells/mL (stock solution), and leukocytes were enumerated. The stock platelet solutions were stored for 18 hours at room temperature before transfusion. In some experiments, platelets were transfused fresh; within 4 hours of collection. By flow cytometry, there was <0.01% of RBC and CD89+ granulocytes were not detected in the stock solutions of platelets. Murine RBC were not immunogenic at these levels (JWS, unpublished).
Enumeration of contaminating leukocytes.
Contaminating MC in the platelet concentrates were enumerated by flow cytometry using hypotonic lysis, propidium iodide (PI), and counting beads as an internal standard. Briefly, 100 μL of platelets (109/mL) were incubated with 95 μL of buffer (1 mg/mL sodium citrate, 0.03% (vol/vol) Triton X-100, 50 μg/mL PI, 10 mg/mL RNase), and 5 μL of counting beads (Flow Count Fluorospheres, Coulter Electronics, Hialeah, FL) at 25°C in the dark. Within 30 minutes, the suspension was acquired on a FACSort flow cytometer with an electronic gate set around the counting beads and acquisition was stopped when 5,000 beads were acquired. Standard curves were generated using 10-fold dilutions of BALB/c Percolled MC (5 × 103/μL to 0.005/μL). For analysis, gates were set around the MC nuclei based on forward scatter and FL2 (PI) fluorescence. The number of contaminating MC/μL was determined by the formula:
This method could consistently detect as low as 0.05 MC/μL. The average nucleated MC contamination in the stock solutions of BALB/c platelets (109 platelets/mL) was 1.1 ± 0.6 MC/μL (n = 67), <0.3 MC/μL (n = 20) for CB.17 SCID mice and <0.05 MC/μL (n = 20) in the anti-AsGm1–treated SCID mice.
Transfusion protocol.
In each transfusion protocol, groups of 10 mice were bled 24 hours before the first transfusion and then injected with 100 μL of platelets weekly via the tail vein. Sera were collected at weekly intervals and tested for the presence of antidonor MHC alloantibodies.
MC preparation.
For SCID mouse platelet dosing, MC were prepared from the peripheral blood of BALB/c mice by centrifugation on a 1.077 g/mL Percoll cushion at 2,800g for 30 minutes. The collected MC were washed twice before use. Flow cytometric analysis of the MC are shown in Table 1.
Depletion of selected MC by magnetic activated cell sorter.
To analyze the effect of MC subpopulations on platelet immunity, BALB/c MC were first depleted of the indicated MC populations by a magnetic activated cell sorter (MACS, Miltenyi Biotech, Auburn, CA) using biotinylated antibodies and streptavidin-magnetic beads (Becton-Dickinson) as previously described.29 Briefly, 106 MC were incubated with 5 μg of antibody for 45 minutes at 4°C, washed once, and then incubated with a 10 μL of streptavidin beads for 30 minutes at 4°C. The labeled cells were passed over a cooled MACS column (Miltenyi Biotech), and the unbound cells collected and analyzed by flow cytometry. For all of the depletions, this method removed >90% of the positively selected cells. Depletion with anti-MHC class II also depleted >90% of B220 B cells. The unbound MC cells were washed twice, adjusted to 105/mL, and 10 μL were added to 990 μL of 109 SCID mouse platelets (to make a final concentration of approximately 1 MC/μL).
Flow cytometric analyses.
For detection and characterization of antidonor antibodies, 105 donor MC were incubated with serial dilutions of recipient sera for 45 minutes at 20°C, washed once, and labeled with FITC-conjugated goat antimouse IgG (Fc-specific, Cedarlane Laboratories) for 30 minutes at 20°C in the dark. Cells were analyzed on a FACSort flow cytometer (Becton Dickinson, San Jose, CA) operating with an argon ion laser at 15 mW; 10,000 events were acquired using an electronic cellular (lymphocyte) gate based on forward and side scatter and were analyzed using LYSYS II software (Becton Dickinson). Matched prebleed serum was used as the negative control in all experiments. Antidonor specificity of the antibodies was confirmed by positive reactivity with donor cells, but absence of reactivity with recipient or third party typing cells. Isotype characterization of the antidonor antibodies was performed using FITC-conjugated goat antimouse IgG1, 2a, 2b and 3 antibodies (Cedarlane Laboratories).
To measure MHC class I expression on SCID and BALB/c mouse platelets, flow cytometric analysis was performed using PE-labeled anti-Dd MHC class I antibody (Cedarlane Laboratories). Briefly, 106 platelets were incubated with 5 μL of the antibody for 45 minutes at room temperature and cells were analyzed on markers set with fluorescently-labeled isotype control antibodies as previously described.25
RESULTS
The immunogenicity of BALB/c and SCID mouse platelets.
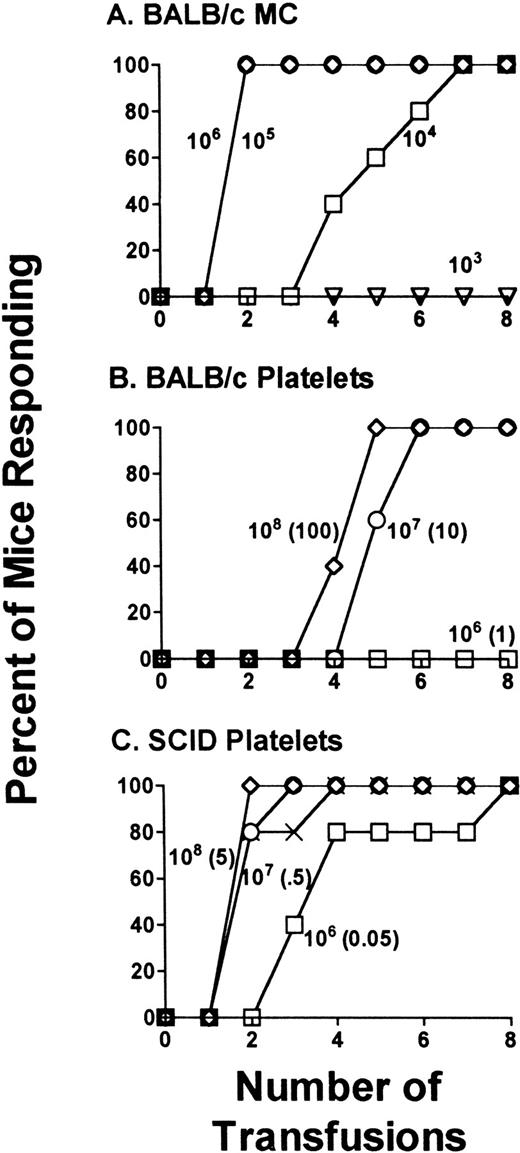
Serial 10-fold dilutions of either BALB/c or SCID mouse platelets were transfused into allogeneic CBA mice and the number of transfusions, which induced antidonor antibodies in 100% of the recipients, was compared. CBA mice did not develop antibodies after transfusions with either syngeneic platelets or syngeneic MC at any time during the 8-week transfusion protocol. In control allogeneic experiments, weekly transfusions of either 106 or 105 BALB/c MC induced high titered IgG antidonor antibodies in all mice by the second transfusion (Fig 1A), whereas a dose of 103 MC/transfusion did not induce antibodies during the protocol (Fig 1A). For platelet transfusions, significant changes in antidonor immunity were observed depending on the platelet donor. When titrations of BALB/c platelets were transfused into CBA mice, 108 platelets/transfusion (containing ≈100 MC/transfusion) induced IgG antidonor antibodies in all mice by the fifth transfusion (Fig 1B). In contrast, 108 platelets (containing approximately 5 MC/transfusion) from SCID mice induced IgG antibodies in all recipient mice by the second transfusion (Fig 1C). The lowest SCID mouse platelet dose capable of inducing IgG antibodies in all recipient mice was eight transfusions of 106platelets (containing <0.05 MC/transfusion, Fig 1C). Anti-AsGm 1 treatment of the SCID mouse donors did not affect their platelet immunity (Fig 1C).
SCID mouse platelets are more immunogenic compared with the platelets of BALB/c mice. CBA recipient mice were transfused weekly with titrations of either (A) control transfusions with BALB/c MC, (B) BALB/c mouse platelets, or (C) platelets from anti-AsGm1–treated SCID mice and their sera tested for antidonor antibodies. The data is expressed as the percentage of mice responding (n = 20) by detection of IgG antidonor antibody during the transfusion protocol. Each line represents a group of mice receiving: (A) (◊) 106, (○) 105, (□) 104, and (▹) 103control MC per transfusion; (B) (◊) 108, (○) 107, and (□) 106 BALB/c platelets per transfusion; (C) (◊) 108, (○) 107 and (□) 106 antibody-treated SCID platelets per transfusion, (X) 108 platelets from antibody nontreated SCID mice. For B and C, the numbers in brackets correspond to the number of MC transfused with the platelets.
SCID mouse platelets are more immunogenic compared with the platelets of BALB/c mice. CBA recipient mice were transfused weekly with titrations of either (A) control transfusions with BALB/c MC, (B) BALB/c mouse platelets, or (C) platelets from anti-AsGm1–treated SCID mice and their sera tested for antidonor antibodies. The data is expressed as the percentage of mice responding (n = 20) by detection of IgG antidonor antibody during the transfusion protocol. Each line represents a group of mice receiving: (A) (◊) 106, (○) 105, (□) 104, and (▹) 103control MC per transfusion; (B) (◊) 108, (○) 107, and (□) 106 BALB/c platelets per transfusion; (C) (◊) 108, (○) 107 and (□) 106 antibody-treated SCID platelets per transfusion, (X) 108 platelets from antibody nontreated SCID mice. For B and C, the numbers in brackets correspond to the number of MC transfused with the platelets.
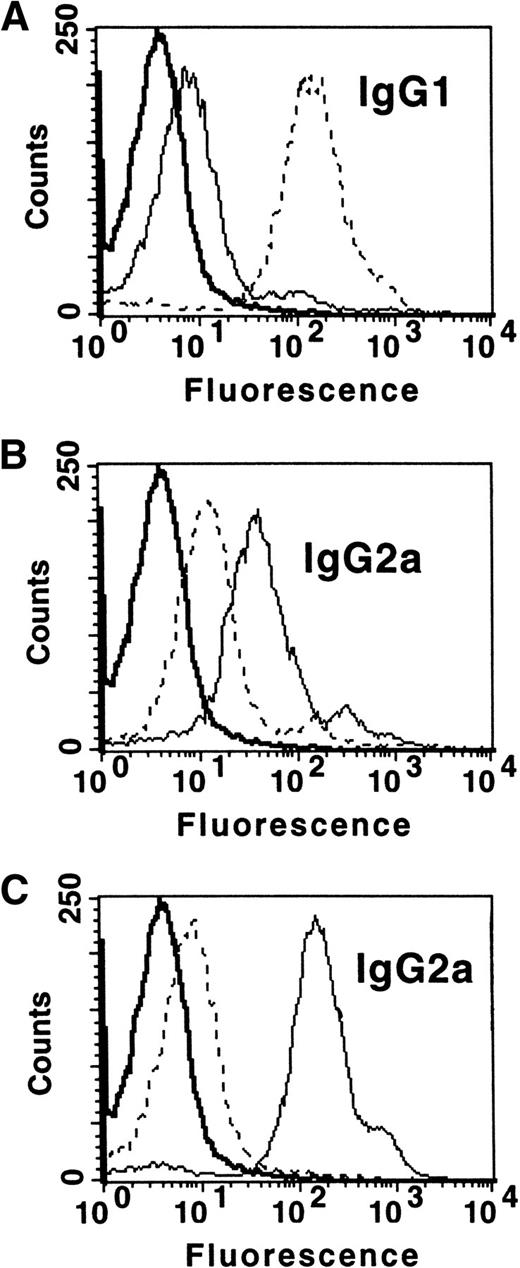
Characterization of the serum IgG antibodies showed that they reacted strongly with donor MHC-matched typing cells, but not with recipient or third party MHC-matched cell lines. Th1 and Th2 associated-isotype analysis of IgG2a and IgG1, respectively, showed that the control MC transfusions (105/transfusion) induced predominantly IgG1 antidonor antibodies with detectible, but lower, levels of IgG2a (Th2>Th1, Fig 2A), whereas BALB/c platelets induced a strong IgG2a response with weaker IgG1 antibodies (Th1, Fig 2B). The platelets from SCID mice, on the other hand, stimulated the production of predominantly IgG2a antibodies with only small amounts of IgG1 detected (Th1>Th2, Fig 2C). IgG2b, but not IgG3 antidonor antibodies, were detected, but at a lower level compared with IgG2a. The increased SCID mouse platelet immunity was not due to increased MHC antigen dosing, as all of the platelet preparations had similar levels of high MHC class I expression. Additionally, none of the platelet preparations had any APC, which could stimulate, at least, proliferation of recipient spleen cells in mixed lymphocyte cultures.
MC affect platelet-induced IgG antidonor isotype production. CBA recipient mice (n = 10) were transfused weekly with either (A) control transfusions with 105 BALB/c MC, (B) 108 BALB/c mouse platelets, or (C) 108platelets from SCID mice and their sera tested for the presence of IgG1 and IgG2a antidonor antibodies. The data are representative flow cytometric histogram analyses of IgG1 and IgG2a antidonor isotypes in a recipient’s serum (1/25) at week 8 transfusion; all mice gave similar results. In all panels, prebleed IgG antidonor (), IgG1 (- - -), and IgG2a () reactivity.
MC affect platelet-induced IgG antidonor isotype production. CBA recipient mice (n = 10) were transfused weekly with either (A) control transfusions with 105 BALB/c MC, (B) 108 BALB/c mouse platelets, or (C) 108platelets from SCID mice and their sera tested for the presence of IgG1 and IgG2a antidonor antibodies. The data are representative flow cytometric histogram analyses of IgG1 and IgG2a antidonor isotypes in a recipient’s serum (1/25) at week 8 transfusion; all mice gave similar results. In all panels, prebleed IgG antidonor (), IgG1 (- - -), and IgG2a () reactivity.
The role of contaminating MC in modulating platelet immunity.
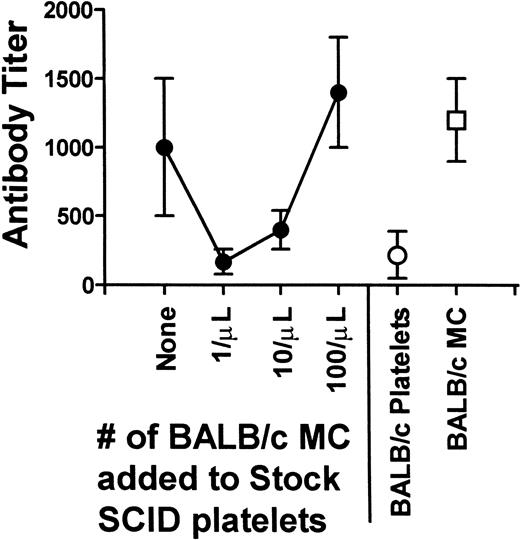
To determine the effect of MC numbers on the enhanced immunogenicity of SCID mouse platelets and the IgG antidonor isotype modulation, titrations of BALB/c MC were added to platelets prepared from anti-AsGm1–treated SCID mice and transfused into CBA recipients. Compared with SCID platelets alone, the addition of MC at 1/μL prolonged the time to formation of antidonor antibodies in all mice to 7 weeks (Fig 3). As the levels of added MC approached 1/μL, platelet-induced antidonor IgG titers were reduced to levels similar to those induced by BALB/c platelets (Fig 4). Additionally, the presence of MC (at 1/μL) was associated with the production of IgG1 antidonor antibodies at levels similar to those in BALB/c platelet recipient mice (not shown). Thus, MC at levels of 1/μL suppressed recipient immunity against allogeneic platelets and was primarily responsible for inducing noncomplement fixing IgG1 antidonor antibodies.
MC can reduce recipient IgG antidonor platelet immunity in CBA mice. The data is presented as the percentage of mice (n = 10), which become IgG antidonor antibody positive during the transfusion protocol. (□) Transfusions of 108 BALB/c mouse platelets; (◊) transfusions with 108 platelets from anti-AsGm1–treated SCID mice; (○) transfusion with 108platelets from anti-AsGm1–treated SCID mice with added BALB/c MC (at 1/μL).
MC can reduce recipient IgG antidonor platelet immunity in CBA mice. The data is presented as the percentage of mice (n = 10), which become IgG antidonor antibody positive during the transfusion protocol. (□) Transfusions of 108 BALB/c mouse platelets; (◊) transfusions with 108 platelets from anti-AsGm1–treated SCID mice; (○) transfusion with 108platelets from anti-AsGm1–treated SCID mice with added BALB/c MC (at 1/μL).
MC at 1/μL maximally inhibit platelet-induced IgG antidonor antibody titers. Titrations of BALB/c MC were added to stock platelets from anti-AsGm1–treated SCID mice and transfused into CBA recipients weekly. Control transfusions with 108 BALB/c platelets or 105 BALB/c MC are shown. The data is expressed as the mean ± standard deviation (SD) of week 8 titers from 10 recipient CBA mice.
MC at 1/μL maximally inhibit platelet-induced IgG antidonor antibody titers. Titrations of BALB/c MC were added to stock platelets from anti-AsGm1–treated SCID mice and transfused into CBA recipients weekly. Control transfusions with 108 BALB/c platelets or 105 BALB/c MC are shown. The data is expressed as the mean ± standard deviation (SD) of week 8 titers from 10 recipient CBA mice.
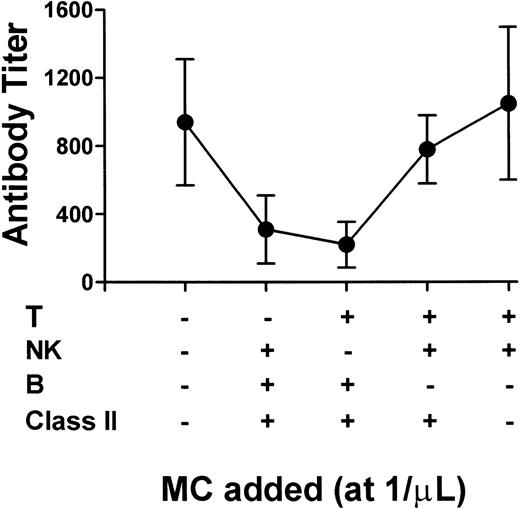
To identify the MC subpopulation(s) responsible for the reduced immunity, BALB/c MC were first depleted of various MC by MACS before being added to the SCID mouse platelets. SCID platelet immunity was reduced only when B220+ B cells (Fig 5, columns 2 and 3,) were present. The presence or absence of CD3+ T cells or asialo Gm 1+ NK cells had no affect on the SCID mouse platelet immunity (Fig 5). Depletion of MHC class II+ MC (which also depleted B cells) also did not affect the SCID platelet immunity (Fig5). Thus, MHC class II positive B cells were primarily responsible for reducing allogeneic platelet immunity.
Residual MHC class II positive B cells are responsible for the reduced platelet immunity. BALB/c MC were depleted (-) of either T cells (second column), NK cells (third column), B cells (fourth column), or MHC class II+ cells (fifth column) by MACS, added to platelets (at 1/μL) from anti-AsGm1–treated SCID mice, and transfused weekly into CBA recipients. The data is expressed as the mean ± SD of the week 8 titers from 10 recipient mice. The first column represents control transfusions with anti-AsGm1–treated SCID mouse platelets alone.
Residual MHC class II positive B cells are responsible for the reduced platelet immunity. BALB/c MC were depleted (-) of either T cells (second column), NK cells (third column), B cells (fourth column), or MHC class II+ cells (fifth column) by MACS, added to platelets (at 1/μL) from anti-AsGm1–treated SCID mice, and transfused weekly into CBA recipients. The data is expressed as the mean ± SD of the week 8 titers from 10 recipient mice. The first column represents control transfusions with anti-AsGm1–treated SCID mouse platelets alone.
To determine if storage of the platelets had an effect on their immunogenicity, they were either transfused fresh (within 4 hours of collection) or after 18 hours of storage. Fresh BALB/c platelets were more immunogenic compared with 18-hour stored BALB/c platelets (Fig 6A). This was not seen when either fresh or stored platelets from SCID mice were transfused (Fig 6B). Thus, the presence of MC at levels of approximately 1/μL is associated with a storage-induced reduction in platelet immunity.
Residual MC are associated with a storage-induced reduction in platelet immunity. A total of 108 platelets from (A) BALB/c mice or (B) anti-AsGm1–treated SCID mice were either transfused fresh (within 4 hours of collection, ○) of after 18 hours of storage (□) into CBA recipients (n = 10). Data is expressed as the percentage of mice which become IgG antidonor antibody positive during the weekly transfusion protocol.
Residual MC are associated with a storage-induced reduction in platelet immunity. A total of 108 platelets from (A) BALB/c mice or (B) anti-AsGm1–treated SCID mice were either transfused fresh (within 4 hours of collection, ○) of after 18 hours of storage (□) into CBA recipients (n = 10). Data is expressed as the percentage of mice which become IgG antidonor antibody positive during the weekly transfusion protocol.
DISCUSSION
Alloimmunization induced by platelet transfusions is defined by the presence of anti-MHC class I antibodies and is thought to be due to the direct recognition of donor MC within the platelet concentrates. Leukoreduction of platelet concentrates has been shown to be effective in reducing the incidence of alloimmunization.11-21Nonetheless, some patients still become alloimmunized, and there is evidence that leukodepletion may be ineffective for reducing alloimmunization in those patients previously sensitized against HLA (eg, due to pregnancy),16 although this is controversial.20 It has been suggested that the minimum immunizing dose of contaminating MC within a 300-mL pooled platelet concentrate is approximately 3 to 17 MC/μL (≈1 to 5 × 106 cells/concentrate) and leukofilters have been designed to remove MC to levels below this concentration. Although there is little experimental data that has confirmed these MC numbers, a previous murine study demonstrated that allogeneic platelets could only induce antidonor alloantibodies if 103 contaminating MC were present.22 Based on comparative blood volumes, 103 MC in mice translates to 2.5 × 106 MC in humans. However, several reports have demonstrated that in healthy recipients, leukoreduced allogeneic platelets stimulate the formation of IgG antidonor alloantibodies23-27 due to an indirect allorecognition mechanism.25 26 The current study was designed to characterize the relationship between allogeneic platelets and contaminating MC in modulating recipient immunity. The results indicate that as MC levels are reduced to levels of 1 MC/μL, the recipient’s immune response against platelets is suppressed, whereas further MC reduction to levels approaching <0.05/μL enhances allogeneic platelet immunity.
When serial 10-fold dilutions of the allogeneic platelets were transfused into recipient mice, SCID mouse platelets were found to be significantly more immunogenic than BALB/c mouse platelets (Fig 1). Removing NK cells from the SCID mouse platelet donors with anti-AsGm 1 antibody did not affect their higher immunity (Fig 1C), suggesting that NK cells, at least, are not responsible. Furthermore, the increased recipient immunity was not due to differences in the amount of MHC class I expression on the murine mouse platelets, but appeared to be due to the absence of MHC class II positive B cells. The recipient immune mechanism(s) responsible for the altered antiplatelet immunity induced by the added MC is unknown, but several possibilities exist. One explanation may be due to the difference in host immunity against a purely MHC class I positive product versus one that contains small numbers of MHC class II positive APC, which can directly interact with recipient CD4+ T helper cells. In support of this, there is accumulating evidence in experimental transplantation, which suggests that the type and quantity of passenger leukocytes in MHC class I positive allografts is important in determining the outcome of the recipient’s antidonor immune response, which can significantly affect the graft’s survival. For example, MHC class I positive liver grafts, which are comparatively rich in leukocytes, are generally considered to be the least immunogenic of organ allografts.30-35 In many species, fully allogeneic liver allografts are permanently accepted without any requirement for recipient immunosuppression,32whereas prior deletion of the passenger leukocytes by irradiation results in enhanced antidonor immunity and rapid graft rejection.33,34 Recently, Steptoe et al35 have demonstrated that dendritic cell numbers were responsible for modulating liver rejection; this suggests that modulating the number of MHC class II APC within allogenic tissue significantly influences the recipient antidonor immune response. Our results with MHC class I positive allogeneic platelets suggests an analogous pattern in that MHC class II positive B cell numbers within the platelets are critical for maximally inhibiting platelet-induced immunity (Figs 4 and5).
Other mechanisms have been postulated to be responsible for the effect of passenger leukocytes on recipient immune responsiveness. These include the long-term engraftment of donor-derived hematopoietic cells (microchimerism) or, alternatively, the transfer of potentially toleragenic costimulatory molecule-deficient APC resulting in operational tolerance via clonal anergy.36 37 Possibly related to operational tolerance is the data in Fig 6. In the platelet preparations which contained MC, fresh (within 4 hours of collection) transfused platelets were significantly more immunogenic than aged platelets (18 hours). This was not seen for the SCID mouse platelets devoid of MC. This suggests that not only are a certain number of MC cells required for modulating platelet immunity but, in addition, storage may enhance their inhibitory effect; it may be that storage induces age-related changes in APC, which causes them to induce a recipient immune tolerant-like state. We are currently studying this possibility.
The data in Fig 2 show that the contaminating MC differentially regulated the recipient IgG isotype response; SCID mouse platelets, devoid of MC, induced high-titered complement-fixing IgG2a antidonor antibodies, whereas the presence of low numbers of MC (1/μL) within the platelets were associated with the production noncomplement-fixing IgG1 antibodies. We have previously shown that these types of platelet-induced IgG2a/IgG1 antidonor isotypes can induce a transient pancytopenia when infused into donor mice indicating their ability to cause thrombocytopenia.38 Furthermore, because murine IgG2a and IgG1 antidonor isotype responses are strongly associated with the presence of Th1 and Th2 cytokines respectively,39 our data also support previous results showing an increase in recipient serum levels of interferon (IFN)-γ associated with leukoreduced platelet transfusions28 and suggest that residual MC populations within platelets may be important in modulating Th1 and Th2 alloimmune responses. More striking, however, was that as the MC levels approached 1/μL, maximal suppression of both IgG1 and IgG2a antidonor immunity occurred. Overall, these results suggest that the platelet:MC ratio transfused may be critical to the quantitative (titer) and qualitative (isotype) outcome of the recipient antidonor immune response. For example, compared with platelets at 20 × 106platelets:1 MC (eg, anti-AsGm1–treated SCID mouse platelets), those at 106 platelets:1MC (eg, BALB/c mouse platelets) are optimal for maximally suppressing platelet immunity. Whether these findings in our murine model can be applied to human transfusions remains unknown, but suggests, at least, that in immunocompetent recipients, excessive leukoreduction may be undesirable.
Although no animal model of transfusion will completely mimic the human situation, these data suggest that in mammalian species with a functioning immune system, allogeneic platelets, independently of leukocytes, are potent immunogens with respect to MHC class I antigens. The data further suggest that residual numbers of MHC class II positive B cells within allogeneic platelets significantly modulate both the quality (isotype) and quantity (titer) of the recipient’s antidonor immunity. These findings may have relevance to the development of technologies capable, for example, of selective leukoreduction, which may have benefit for those recipients who become alloimmunized despite receiving standard leukodepleted products. More importantly, however, we have established a murine model of MC-independent platelet immunity which will allow for the examination of antigen-specific immunotherapies for platelet alloimmunization.
ACKNOWLEDGMENT
We thank Dr Fraser Wright (Connaught Laboratories, Willowdale, Ontario) for his invaluable discussions.
Supported by Grant No. TO. 22929 from the Canadian Red Cross Blood Services.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John W. Semple, PhD, Division of Hematology, St. Michael’s Hospital, 30 Bond St, Toronto, Ontario, Canada, M5B 1W8.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal