Abstract
The function of the β-globin locus control region (LCR) has been studied both in cell lines and in transgenic mice. We have previously shown that when a 248-kb β-locus YAC was first microinjected into L-cells and then transferred into MEL cells by fusion, the YAC loci of the LxMEL hybrids displayed normal expression and developmental regulation.To test whether direct transfer of a β-globin locus (β-YAC) into MEL cells could be used for studies of the function of the LCR, a 155-kb β-YAC that encompasses the entire β-globin locus was used. This YAC was retrofitted with a PGK-neo selectable marker and with two I-PpoI sites at the vector arm-cloned insert junctions, allowing detection of the intact globin loci on a single I-PpoI fragment by pulsed field gel electrophoresis (PFGE). ThePpo-155 β-YAC was used to directly lipofect MEL 585 cells. In 7 β-YAC MEL clones with at least one intact copy of the YAC, the levels of total human globin mRNA (ie, ɛ + γ + β) per copy of integrated β-YAC varied more than 97-fold between clones. These results indicated that globin gene expression was strongly influenced by the position of integration of the β-YAC into the MEL cell genome and suggested that the LCR cannot function properly when the locus is directly transferred into an erythroid cell environment as naked β-YAC DNA. To test whether passage of the β-YAC through L-cells before transfer into MEL cells was the reason for the previously observed correct developmental regulation of human globin genes in the LxMEL hybrid cells, we transfected the YAC into L-cells by lipofection. Three clones carried the intact 144-kb I-PpoI fragment and transcribed the human globin genes with a fetal-like pattern. Subsequent transfer of the YAC of these L(β-YAC) clones into MEL cells by fusion resulted in LxMEL hybrids that synthesized human globin mRNA. The variation in human β-globin mRNA (ie, ɛ + γ + β) levels between hybrids was 2.5-fold, indicating that globin gene expression was independent of position of integration of the transgene, as expected for normal LCR function. The correct function of the LCR when the YAC is first transferred into the L-cell environment raises the possibility that normal activation of the LCR requires interaction with the transcriptional environment of an uncommitted, nonerythroid cell. We propose that the activation of the LCR may represent a multistep process initiated by the binding of ubiquitous transcription factors early during the differentiation of hematopoietic stem cells and completed with the binding of erythroid type of factors in the committed erythroid progenitors.
THE HUMAN β-GLOBIN locus spans 82 kb on chromosome 11 and contains a powerful regulatory element, the locus control region (LCR), located between 5 to 22 kb upstream of the ε-globin gene and composed of a series of DNaseI hypersensitive sites (5′HS1 to 5).1,2 The importance of this element for globin gene function became apparent from naturally occuring mutations that remove the LCR and result in silencing of all the downstream globin genes and a phenotype of γδβ-thalassemia.3-5The function of the LCR has been best defined through studies in transgenic mice.6,7 When human globin gene constructs lacking the LCR are transferred into the murine genome, the majority of the transgenes fail to express,8-10 indicating that the function of such LCR-less constructs is strongly influenced by the position of integration of the transgene. In contrast, constructs that link the globin genes with the complete LCR or cassetes containing combinations of HS sites or individually HS2, or 3 or 4, are reproducibly expressed in transgenic mice,1,11-13indicating that a major function of the LCR is to shield the globin genes from the effect of position of integration of the transgene. This property of the LCR, the provision of position-independent expression in transgenic mice, has been used in the functional recognition of LCRs in several other loci.14-16 The LCR also acts as a powerful globin gene enhancer and is responsible for the abundant synthesis of globin mRNA in the cells of the erythroid lineage.1 The current model of the function of the LCR implies that the HSs form a structure that loops to interact with the promoters of the downstream globin genes, thus activating globin gene transcription.17 18
Analyses of the structure-function relationships of the LCR are optimally performed in transgenic mice carrying constructs containing the whole human β-globin locus. Mice carrying human β-globin locus yeast artificial chromosomes (β-YACs) have been used for this purpose.19-21 Such transgenic mice show correct developmental regulation of the locus and levels of globin gene expression that are independent of the position of integration of the transgene into the murine genome; these results are characteristic of normal function of the LCR and of the other regulatory elements of the β-locus.1,6,7 To ask whether transfer of β-YACs into MEL cells could be used for the analysis of structure-function relationships of the human β-globin locus, we have previously transferred the β-YAC into L-cells by microinjection and then to MEL cells by LxMEL fusion.22 These L(β-YAC)xMEL hybrids initially expressed ε, γ, and β globin mRNA but subsequently switched to predominantly β-globin expression. When the β-YAC was transferred by L-cell fusion into GM979 cells (an MEL line expressing adult as well as embryonic mouse globins), there was continued γ-globin expression along with β-globin expression, indicating that the genes of the β-YAC respond to the trans-acting factors present in the GM979 line. These results suggested that transfer of β-YACs into MEL cells could be used for the analysis of structure-function relationships of the β-globin locus.
The purpose of this study was to examine whether direct transfer of a β-YAC into MEL cells (instead of the indirect transfer through the L-cells) could be used for the analysis of the function of the LCR and its interactions with the genes of the β-globin locus. If this was the case, YACs transferred into MEL cells could substitute for transgenic mice in structure-function studies of the LCR. Our results indicate that the direct transfer of the β-YAC into MEL cells is characterized by globin gene expression that is strongly influenced by the position of integration of the transgene. However, these position effects were eliminated when the β-YAC was first transferred into L-cells and subsequently into MEL cells by fusion. We explain these findings with the hypothesis that correct function of the LCR may require a stepwise activation that starts in the transcriptional environment of an uncommitted cell and it is completed when the locus finds itself in the transcriptional environment of the cells of the erythroid lineage.
MATERIALS AND METHODS
Cell cultures and transfections.
Semiadherent diploid MEL585 cells (a kind gift from W. Wood, Oxford, UK) were maintained in RPMI 1640 medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; HyClone). One day before transfection, 1 × 106 cells were plated in a 35-mm tissue culture dish and, by the time of lipofection, they reached a confluency of approximately 80%. The adherent LA9 mouse fibroblast line was purchased from ATCC (Rockville, MD) and maintained in Dulbecco’s modified Eagle’s medium (DMEM)-high glucose medium (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% FBS. LA9 cells were seeded at 0.5 × 106 cells/35-mm tissue culture plate the day before transfection. For transfection of the β-YAC, the cells were washed twice with phosphate-buffered saline (PBS) and resupended in 800 μL of Opti-Mem (GIBCO-BRL). Approximately 100 ng of gel-purified YAC-DNA was mixed with 5 μL of lipofectin (GIBCO-BRL) in a total volume of 200 μL in OptiMEM medium. Lipid-DNA complexes were allowed to form for 30 minutes at room temperature. The mixture was then added to the cells and transfection was performed for 6 hours in a tissue culture incubator. At the end, normal medium was added to the cells supplemented with FBS to a final concentration of 20% and the cells were cultured for another 24 hours. The cells were then collected, counted, and plated in 24-well plates at a concentration of 20 × 103 cells/mL/well in medium containing 700 μg/mL of active G418 (GIBCO-BRL) for selection. Calculation of the transfection efficiency was performed by dividing the number of G418-resistant colonies with the number of transfected cells. Because the doubling time for MEL cells is between 16 and 18 hours, the number of transfected cells on transfection day was calculated from the number of cells initially plated multiplied by two. Individual G418-resistant colonies were expanded and induced to terminally differentiate with 3 mmol/L hexamethylene-bis-acetamide (HMBA) and 10 μmol/L hemin. Induction of differentiation was performed with cells plated at 2 × 105 cells/mL in 10-mL cultures. Cells were collected for RNA analysis and for globin staining on the days 3 and 4 of induction, respectively. For lipofection of the LA9 cells with the β-YAC, the same protocol was applied, but the cells were plated for G418 selection in 100-mm TC plates and individual G418-resistant colonies were picked up with cloning rings. The cells were trypsinized in the well and expanded under selection. L(β-YAC)xMEL hybrids were generated as previously described.23 Briefly, 107 MEL 585 cells (suspension phenotype) and 5 × 106 G418-resistant LA-9 cells (adherent phenotype) carrying the β-YAC were fused by polyethyleneglycol-mediated fusion. After fusion and every 2 to 3 days (depending on the cell growth), G418-resistant suspension cells were transferred to a new flask and grown under further selection. Parental MEL cells (G418-sensitive) were killed by G418, whereas parental L-cells (adherent) were removed each time the suspension cells were transferred to a new flask. After 2 weeks of selection, the hybrids were induced to differentiate with HMBA and hemin. Generation of hygromycin-resistant LA9 cells with an SV-Hygro plasmid was also performed by lipofection using the same protocol that was used for the β-YAC but with different amounts of DNA (5 μg) and lipofectin (15 μL). The cells were selected with 1 mg/mL hygromycin (GIBCO-BRL) and a hygromycin-resistant cell pool was used for fusion with the MEL(β-YAC) clones. Fusion between MEL(β-YAC)-G418–resistant clones and LA9-hygromycin–resistant cells was performed as described above and the hybrids were selected by dual resistance to both hygromycin and G418. These are referred to as reverse hybrids.
DNA constructs.
The hygromycin resistance gene was isolated as a SalI-Sma I fragment from the plasmid pCEP4 (Invitrogen, Carlsbad, CA). The pSV-β-Gal plasmid (Promega, Madison, WI) was cut with Sal I and HindIII to remove the β-Gal gene. TheHindIII site was blunted and ligated to the SalI-Sma I fragment from plasmid pCEP4. The resulting construct has the SV40 promoter and enhancer linked to the hygromycin resistance gene.
Production and purification of the 155-kb β-locus YAC.
The 150-kb β-locus YAC (clone A201F4)24 was retrofitted with two I-PpoI sites at the vector-insert junctions using two retrofitting vectors.25 The selectable marker PGKneo that confers resitance to G418 was inserted at the right vector arm. This YAC is 5 kb larger in size from the original clone and will be referred to as Ppo-155 β-YAC. The YAC was purified according to the earlier protocol, with slight modifications.19 21Preparative agarose blocks with high molecular weight DNA from the yeast carrying the 155-kb β-YAC were fractionated by pulsed field gel electrophoresis (PFGE) on a 0.5% agarose MP gel (Boehhringer Mannheim, Indianapolis, IN) in 0.5× TBE under the following conditions: 200 V, 60 seconds switch at 12°C for 18 to 24 hours. The slice that contained the β-YAC DNA was located on the gel by cutting and staining separately the marker lanes at the edge of the gel. The agarose slice was then cut together with a slice containing one of the yeast chromosomes. To concentrate the β-YAC DNA, the agarose slice with the β-YAC and the yeast chromosome slice were rotated vertically relative to their original electrophoresis migration and subjected to electrophoresis in 4% low-melting point agarose (LMPA; NuSieve GTG; FMC, Rockland, ME) in 0.5× TBE at 47 V for 16 hours. The yeast lane was stained to determine the relative migration of the β-YAC DNA into the LMPA gel. The slice with the YAC DNA was equilibrated in 40 to 100× volume of transfection buffer (10 mmol/L Tris, 250 mmol/L EDTA, 100 mmol/L NaCl) for 1 hour. The agarose slice was then melted at 68°C for 10 minutes, transferred in a 42.5°C water bath, and digested with agarase (New England Biolabs, Beverly, MA) overnight with 2 U of the enzyme per 100 mg of melted gel. The DNA concentration of the YAC was estimated by standard agarose electrophoresis with a λHindIII marker and integrity was determined by PFGE. The DNA was diluted to 2 ng/μL in transfection buffer and filtered through a 0.22-mm Acrodisk (Gelman Sciences, Ann Arbor, MI) before transfection.
Structural analysis of the transfected Ppo-155 β-YAC.
Transfected MEL cells were washed twice in ice-cold PBS and resupended at 3 × 107 cells/mL of PBS. An equal volume of 2% low temperature melting agarose (LTMA; Seaplaque GTG agarose; FMC) was added to the cells and plugs were cast. The plugs were incubated in LDS solution (1% lithium dodecyl sulfate, 100 mmol/L EDTA, 10 mmol/L Tris-HCl) at 37°C for 1 hour, followed by a second incubation overnight with fresh solution. After two 30-minute washes in 0.2% NDS (0.2% lauryl sarcosinate, 100 mmol/L EDTA, 2 mmol/L Tris, pH 9.5) and three 30-minute washes in Tris EDTA (TE), pH 8.0, the plugs were stored at 4°C in TE. For structural analysis, portions of the agarose plugs were equilibrated with 1× I-PpoI buffer in a volume of 0.2 mL and digested with 360 U of I-PpoI (Promega) overnight. A total of 12 digested agarose slices were fractionated on an agarose gel by PFGE. The DNA was then transferred by capillary blot onto a nylon membrane (Hybond N+; Amersham, Arlington Heights, IL). Individual strips that represent the lanes of the agarose gel were cut from the blot and hybridized separately to 12 probes that span the entire β-globin locus. The probes used were as follows:BamHI-HindII 5′HS5, Stu I-Sph I 1.1-kb 5′HS4, 0.7-kb Pst I 5′HS3, 1.9-kbHindIII 5′HS2, Dra I-HindIII 5′HS1, 3.7-kb EcoRI ε-globin gene, 2.4-kb EcoRIAγ globin gene, 1.0-kb EcoRV ψβ, 2.1-kbPst I δ-globin gene, 0.9-kb EcoRI-BamHI β-globin gene, 1.4-kb Xba I DF-10 (3′HS1), and a 1.9-kbBgl II HPFH3 (a gift from N. Anagnou, University of Crete, Crete, Greece). All fragments were radiolabeled using a Decaprime II random labeling kit (Ambion, Austin, TX). After hybridization and washing, the blot was reassembled by aligning the individual strips and autoradiographed. The hybridization profile reflects the extent of the locus for each YAC copy.
Southern analysis and copy number determination.
Genomic DNA was isolated by standard procedures26 and digested overnight with EcoRI. Ten micrograms of the digested product was fractionated on a 0.8% agarose gel and transferred by alkaline capillary blot on a nitrocellulose membrane (ZetaProbeGT; Bio-Rad, Hercules, CA). The blot was hybridized to the pPN201 probe27 that consists of the human HS3 core 780-bpPst I fragment linked to a murine 544-bp BamHI Thy1.1 cDNA fragment that served as the endogenous murine two-copy control. To correct for any differences in the specific activity between the two DNA fragments, the plasmid pPN201 was digested with SmaI/Sca I to yield a 2.6-kb HS3 and a 1.7-kb Thy1.1 fragment. Twenty picograms of the plasmid digest was run along with the genomic digests in the same gel. The value of the HS3 to Thy1.1 ratio in the plasmid lanes was used to multiply the Thy1.1 genomic signal; this corrected value, divided by two, was then compared with the HS3 signal. All of the signals were quantitated on a phosphoimager (Molecular Dynamics, Sunnyvale, CA). β-YAC copies were calculated by comparing the human 5′HS3 with the mouse two-copy Thy1.1 gene on a conventional Southern analysis. Fragmented YACs that hybridized to the HS3 probe in the PFGE analysis were subtracted so that only intact copies were accounted in the per copy gene expression.
RNA analysis.
Total cellular RNA was isolated by the method of Chomczynski and Sacchi.28 Human globin mRNA was analyzed by RNase protection assay with probes that gave protected fragments from the second exon of the respective genes (the size of the protected band is in parentheses): pT7β(205), pT7Aγ(170), and pT7ε(188). Mouse RNA was analyzed with a murine pT7α(128) probe from exon I; a T7γ-actin(217) probe (a gift from Dr S. Sakiyama, Chiba Cancer Research Institute, Nitoya, Japan) was used as a gel-loading control. The probes were synthesized with T7 RNA polymerase (Promega). Two micrograms of RNA was hybridized overnight in a 47°C oven with 106 cpm from each radioactive probe; after digestion with a cocktail of RNases (Ambion, Austin, TX), the protected fragments were separated on 6% polyacrylamide-8 mol/L urea gel and autoradiographed. The signals were quantitated by phosphorimage analysis.
RESULTS
Construction of the Ppo-155 β-YAC.
We have previously shown that transfer of a 248-kb β-YAC in transgenic mice is associated with structural rearrangements that can have detrimental effects on the analysis of structure-function relationships of the β-like globin gene expression.29,30Conventional Southern blots for examining the presence of sequences of the β-globin locus are not adequate for determining the structure of the β-YAC, because different parts of the β-globin locus may be contained in different fragments. These fragments will give positive hybridization signals providing false information that the β-globin locus of the YAC is intact. For structural analysis of the 248-kb β-YAC, the structure of a 140-kb Sfi I fragment that contains most of the β-globin locus is analyzed in detail.29 30Using this approach, Sfi I-digested agarose plugs containing murine genomic DNA are subjected to PFGE, the gel is blotted, and each individual lane of the blot is probed for sequences extending from the 5′HS3 to the 3′ of the β-globin locus. Upon reassembly of the blot and autoradiography, the structure of each locus copy can be reconstructed; any deletions or rearrangements of the β-globin locus contained in the Sfi I fragment become readily visible.
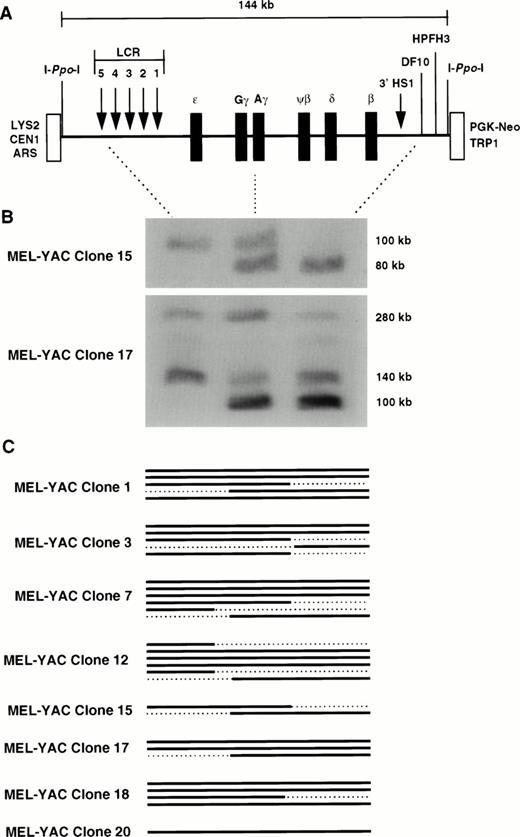
A 150-kb β-YAC was identified and has been used for production of transgenic mice.21 This YAC contains the entire β-globin locus; it has the same 5′ end as the 248-kb β-YAC but a shorter 3′ end that extends 23 kb downstream of 3′HS1 relative to the 109 kb of the 248-kb β-YAC. This 150-kb β-YAC displays correct developmental control of globin gene expression in transgenic mice.30,31 Although per copy expression has been reported to range threefold between transgenic lines, this YAC is considered to display position-independent expression.30 Because of its smaller size, this β-YAC may be less prone to rearrangement32; thus, it may be preferable to the 248-kb β-YAC for gene transfer experiments in cell lines. However, unlike the 248-kb β-YAC, it lacks one of the two Sfi I sites that flank most of the β-globin locus (it lacks the 3′ SfiI site downstream of 3′HS1); therefore, it is not useful for detailed structural analysis. To facilitate its use, two I-PpoI sites were introduced at the junctions of the pYAC4 vector sequence and the globin insert (Fig 1A). Digestion with I-PpoI yields a 144-kb fragment that contains the entire globin insert. A selectable marker (PGK-neo) was concommitantly retrofitted in the right vector arm to allow selection of the transfected cells with G418.
Structural analysis of the Ppo-155 β-YAC tranferred into MEL cells by lipofection. (A) Diagram of thePpo-155 β-YAC. The 144-kb I-Ppo-I fragment used to assess YAC integrity is shown. This YAC contains an intact β-locus with 39 kb of DNA upstream of the 5′HS5 of the LCR and 23 kb of DNA downstream of 3′HS1. The HS sites of the LCR and the 3′HS1 are displayed as arrows. The location of the I-Ppo-I sites are shown as straight lines next to the vector sequences (open boxes). YAC-vector sequences: TRP1 and LYS2, YAC selectable markers for tryptophane and lysine prototrophy, respectively; ARS1, yeast autonomous replicating sequence; CEN1, centromere; PGK-Neo, selectable marker for G418 resistance. (B) Structural analysis of the MEL(Ppo-155 β-YAC) clones 15 and 17; I-Ppo-I digestion, PFGE, and capillary transfer were performed as described in Materials and Methods. The blot was cut into strips and each strip was hybridized with one of the three probes indicated by the dotted lines between the YAC diagram and the autoradiograph (ie, HS5, Aγ-globin, and DF-10). The strips were realigned to reconstruct the original membrane, and a hybridization profile for each I-PpoI fragment was obtained. Fragments with a positive hybridization signal in all three lanes were considered as intact. Notice that clone 15 has two fragmented copies, whereas clone 17 has two intact copies at 140 and 280 kb and a third that extends from Aγ to the 3′ end of the β globin locus. (C) Graphic representation of the β-YACs in the MEL(β-YAC) clones according to their hybridization profile. Solid lines represent intact β globin loci, whereas the dotted lines indicate lack of hybridization and fragmented β-YAC copies.
Structural analysis of the Ppo-155 β-YAC tranferred into MEL cells by lipofection. (A) Diagram of thePpo-155 β-YAC. The 144-kb I-Ppo-I fragment used to assess YAC integrity is shown. This YAC contains an intact β-locus with 39 kb of DNA upstream of the 5′HS5 of the LCR and 23 kb of DNA downstream of 3′HS1. The HS sites of the LCR and the 3′HS1 are displayed as arrows. The location of the I-Ppo-I sites are shown as straight lines next to the vector sequences (open boxes). YAC-vector sequences: TRP1 and LYS2, YAC selectable markers for tryptophane and lysine prototrophy, respectively; ARS1, yeast autonomous replicating sequence; CEN1, centromere; PGK-Neo, selectable marker for G418 resistance. (B) Structural analysis of the MEL(Ppo-155 β-YAC) clones 15 and 17; I-Ppo-I digestion, PFGE, and capillary transfer were performed as described in Materials and Methods. The blot was cut into strips and each strip was hybridized with one of the three probes indicated by the dotted lines between the YAC diagram and the autoradiograph (ie, HS5, Aγ-globin, and DF-10). The strips were realigned to reconstruct the original membrane, and a hybridization profile for each I-PpoI fragment was obtained. Fragments with a positive hybridization signal in all three lanes were considered as intact. Notice that clone 15 has two fragmented copies, whereas clone 17 has two intact copies at 140 and 280 kb and a third that extends from Aγ to the 3′ end of the β globin locus. (C) Graphic representation of the β-YACs in the MEL(β-YAC) clones according to their hybridization profile. Solid lines represent intact β globin loci, whereas the dotted lines indicate lack of hybridization and fragmented β-YAC copies.
Transfer of the Ppo-155 β-YAC in MEL cells.
The Ppo-155 β-YAC was transferred into MEL cells by lipofection. Two variants of the 585 MEL cell line were used, one semiadherent (585A) and one growing in suspension. Cells were plated in culture medium containing 700 μg/mL of G418 and visible colonies were counted between days 10 and 12. The observed distribution of G418-resistant colonies was very close to a Poisson distribution, with an m value of 0.7 (where m is the number G418-resistant cells present in every well). The transfection efficiency for the semiadherent MEL cells was 1:104 cells and 1:2 × 104 for experiments performed with 5 and 10 μg of lipofectin, respectively. Transfection efficiency of MEL cells growing in suspension was 1:7 × 104 cells for either 5 or 10 μg of lipofectin.
Structural analysis of the transfected Ppo-155 β-YACs.
For structural analysis, 3 × 107 cells from 20 randomly selected G418-resistant MEL clones were embedded in agarose and the plugs were digested with I-PpoI and subjected to PFGE as described in Materials and Methods. I-PpoI is a rare cutting enzyme; for example, it cuts only once in S cerevisiae chromosome 12. Therefore, if YAC copies are rearranged upon integration into the mouse genome losing one or both I-PpoI sites, it is highly unlikely that the new I-PpoI fragment will be 144 kb in size. Thus, the presence of the 144-kb fragment indicates that the β-locus is intact. Fragments smaller or larger than 144 kb have deletions of one or both I-PpoI sites and possibly additional variable length deletions of sequences in the β-globin locus. Whether these aberrant PFGE fragments contain all or part of the β-globin locus can be determined with the method of structural analysis that we used.
The initial screen of the 20 clones identified 8 clones with at least one I-PpoI fragment of approximately 140 kb in size that hybridized with the 0.9-kb β-globin gene probe (data not shown). Additional bands were present in 7 of these clones, and a complete structural analysis of all the clones was undertaken to precisely map the integrated β-YAC fragments. The DNA was hybridized either with 12 probes spanning the entire β-globin locus or with 3 probes located at the 5′ end, middle, and 3′ end of the locus (5′HS5,Aγ, and DF-10, respectively; Fig 1A). The latter method has been tested in Ppo-155 β-YAC transgenic mice and has been shown to depict as accurately the extent of the β-globin locus as the complete structural analysis with the 12 probes.30
The hybridization pattern of two MEL-YAC clones (15 and 17) with the 3 probes is shown in the autoradiograph of Fig 1B; the results from the other clones are depicted diagrammaticaly in the same figure. Fragments hybridizing with all three probes were considered to contain an intact β locus and were included when per copy expression of the globin genes was calculated. Clone 15 demonstrates why the analysis with the 3 probes is necessary for delineating the size of the β-YAC fragments; this clone at the initial PFGE screen was considered to contain an intact fragment. However, as shown in Fig 1B, it clearly has two incomplete fragments; one extends from 5′HS5 to Aγ and the other from Aγ to 3′ end of the locus. This was verified by hybridization of a PFGE blot with all 12 probes (data not shown). Clone 17 has an intact fragment of approximately 140 kb and another fragment of about 280 kb that has lost one or both I-PpoI sites but contains an intact β-globin locus.
The remaining clones have at least one intact β-YAC fragment; the structure of the integrated β-globin loci is shown diagrammatically in Fig 1C. Clone 1 has three intact β-globin loci 200, 150, and 90 kb in size; clone 3 has two intact loci at 280 and 140 kb; clone 7 has three intact loci at 280, 140, and 100 kb; clone 12 has three intact loci at 180, 140, and 100 kb; clone 18 has three intact loci at 240, 220, and 200 kb; and clone 20 has one intact locus at 140 kb.
Expression of the globin genes of the Ppo-155 β-YAC is strongly influenced by the position of integration into the MEL cell genome.
The 7 clones that contained intact β-globin loci by structural studies were used for analysis of human β-like and murine α-globin mRNA by RNase protection at several intervals between 20 and 120 days of continuous culture. The results are shown in Fig 2. In Fig 2A, the mRNA is hybridized with a β-globin probe. In Fig 2B, the mRNA is hybridized with the ε- and Aγ globin probes. Two clones synthesized only β-globin mRNA (3 and18) during the 4 months of culture and 2 clones synthesized β and γ mRNA (12 and 20), whereas 1 clone (17) synthesized ε, γ, and β mRNA. Clone 1 failed to express any human mRNA, and clone 7 had minimal γ- and β-globin expression at levels less than 2% of murine α (data not shown).
Expression of the human globin genes in MEL(Ppo-155 β-YAC) clones. Total RNA was isolated from MEL cells at the days shown above the autoradiogram and was subjected to RNase protection assay as described in Materials and Methods. In (A), the RNA was hybridized with the human β-globin probe. In (B), the RNA was hybridized with both γ- and ɛ-globin probes. The location of the protected fragments is shown next to the autoradiogram. Notice the striking variation between human globin mRNA levels between clones.
Expression of the human globin genes in MEL(Ppo-155 β-YAC) clones. Total RNA was isolated from MEL cells at the days shown above the autoradiogram and was subjected to RNase protection assay as described in Materials and Methods. In (A), the RNA was hybridized with the human β-globin probe. In (B), the RNA was hybridized with both γ- and ɛ-globin probes. The location of the protected fragments is shown next to the autoradiogram. Notice the striking variation between human globin mRNA levels between clones.
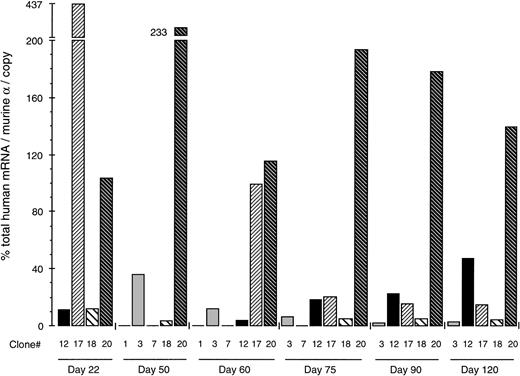
Table 1 shows the levels of total (ie, β + γ + ε) human globin mRNA for each clone as the percentage of mouse-α (corrected for copy number) at different culture days; due to different growth rates among clones, not all of them were available for analysis on the particular days. The results are shown as a diagram in Fig 3. The striking dispersion of the human mRNA values is characteristic. Thus, the mRNA levels of the 4 clones available for analysis on day 22 range from 11.5% to 437% of mouse-α (38-fold variation), on day 60 from 0% to 115%, on day 75 from less than 2% to 193.5% (97-fold), on day 90 from 2% to 178% (89-fold), and on day 120 from 2.5% to 139% (55.6-fold). These results indicated that the expression of the Ppo-155 β-YAC strongly depends on the position of integration of the β-YAC into the MEL cell genome.
Total Human mRNA Levels (ɛ + γ + β) in MEL(β-YAC) Clones
| Clone . | Copies of Intact β-YACs . | Culture Days . | |||||
|---|---|---|---|---|---|---|---|
| 22 . | 50 . | 60 . | 75 . | 90 . | 120 . | ||
| 1 | 3 | — | 0 | 0 | — | — | — |
| 3 | 2 | — | 35.5 | 12 | 6.5 | 2 | 2.5 |
| 7 | 3 | — | <2 | <2 | — | — | — |
| 12 | 3 | 11.5 | — | 4 | 18.5 | 22.5 | 47.5 |
| 17 | 2 | 437 | — | 99.5 | 20 | 15.5 | 15 |
| 18 | 3 | 12 | 3.5 | — | 5 | 5 | 4 |
| 20 | 1 | 103.5 | 233 | 115 | 193.5 | 178 | 139 |
| Clone . | Copies of Intact β-YACs . | Culture Days . | |||||
|---|---|---|---|---|---|---|---|
| 22 . | 50 . | 60 . | 75 . | 90 . | 120 . | ||
| 1 | 3 | — | 0 | 0 | — | — | — |
| 3 | 2 | — | 35.5 | 12 | 6.5 | 2 | 2.5 |
| 7 | 3 | — | <2 | <2 | — | — | — |
| 12 | 3 | 11.5 | — | 4 | 18.5 | 22.5 | 47.5 |
| 17 | 2 | 437 | — | 99.5 | 20 | 15.5 | 15 |
| 18 | 3 | 12 | 3.5 | — | 5 | 5 | 4 |
| 20 | 1 | 103.5 | 233 | 115 | 193.5 | 178 | 139 |
Values are expressed as the percentage of murine α mRNA, corrected for the number of copies of the β-YAC integrated into each MEL cell line.
Abbreviation: —, no data.
Position-dependent expression of human globin genes after direct transfer of the YAC into MEL cells. Total human globin mRNA levels (ie, ɛ + γ + β) of the MEL (β-YAC) clones are expressed as the percentage of the endogenous mouse -gene corrected for copy number. Each column represents results obtained by a single clone identified with the number below the column. Clone 1 had 0% and clone 7 less than 2% of mouse mRNA and are shown as flat lines. Results from 6 culture days are shown. Notice the striking variation in human globin gene expression between clones. Such findings indicate that the expression of the globin genes is strongly influenced by the position of integration of the β-YAC into the mouse genome.
Position-dependent expression of human globin genes after direct transfer of the YAC into MEL cells. Total human globin mRNA levels (ie, ɛ + γ + β) of the MEL (β-YAC) clones are expressed as the percentage of the endogenous mouse -gene corrected for copy number. Each column represents results obtained by a single clone identified with the number below the column. Clone 1 had 0% and clone 7 less than 2% of mouse mRNA and are shown as flat lines. Results from 6 culture days are shown. Notice the striking variation in human globin gene expression between clones. Such findings indicate that the expression of the globin genes is strongly influenced by the position of integration of the β-YAC into the mouse genome.
Developmental expression of the human globin genes in the MEL(Ppo-155 β-YAC) cells is random.
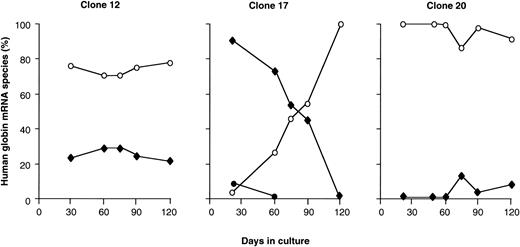
As mentioned earlier, clones 3 and 18 synthesize only β-globin mRNA, whereas clones 12, 17, and 20 synthesize more than one globin mRNA. The changes in human globin gene expression during the 120 days in culture for the latter 3 clones are shown in Fig 4. Clone 20 expressed only β-globin initially, but after 60 days in culture, small levels of γ-globin mRNA appeared. In clone 12, similar levels of γ- and β-globin mRNA were present from 30 to 120 days in culture so that the γ/γ + β mRNA ratio remained constant. Initially, in clone 17, there was predominantly γ- and some ε-globin mRNA; as the culture time advanced, ε and γ mRNA decreased, and by day 60 the expression pattern had switched to β-globin only. Thus, several patterns of globin gene expression were observed: an adult pattern from the onset of observation (clones 3 and 18), an initial β and later partial γ activation pattern (clone 20), a nonswitching pattern of globin gene expression (clone 12), and a pattern typical of the γ to β switch (clone 17). These results suggest that the globin genes of the β-YAC are activated randomly when the β-YAC is directly transferred into the MEL cells.
Developmental regulation of the globin genes in the MEL(β-YAC) clones. Human ɛ-, γ-, and β-globin gene expression levels are plotted as a percentage of total human mRNA output from the β-locus. Results are shown from three MEL(β-YAC) clones that express more than one mRNA species. (○) β-Globin mRNA; (⧫) γ-globin mRNA; (•) ɛ-globin mRNA. Notice the highly inconsistent pattern. In clone 12 there is no change in γ or β gene expression. Clone 17 shows a typical ɛ → γ → β switch. Clone 20 displays a delayed γ gene expression.
Developmental regulation of the globin genes in the MEL(β-YAC) clones. Human ɛ-, γ-, and β-globin gene expression levels are plotted as a percentage of total human mRNA output from the β-locus. Results are shown from three MEL(β-YAC) clones that express more than one mRNA species. (○) β-Globin mRNA; (⧫) γ-globin mRNA; (•) ɛ-globin mRNA. Notice the highly inconsistent pattern. In clone 12 there is no change in γ or β gene expression. Clone 17 shows a typical ɛ → γ → β switch. Clone 20 displays a delayed γ gene expression.
Transfer of the Ppo-155 β-YAC in L-cells.
The results obtained in the MEL-YAC cells were surprising, because the 155-kb β-YAC contains an intact LCR. Several previous studies in transgenic mice have clearly shown that an intact LCR protects the globin gene from effects of position of integration.1,12,33Therefore, the manifestation of strong position effects in the presence of an intact LCR was unexpected. Also, previous studies in which a 248-kb β-YAC was transfected into L-cells and subsequently into MEL cells by LxMEL cell fusion have shown that the globin genes of the β-YAC were correctly developmentally regulated.22 There are two explanations for the difference between the results of the previous and the present study: (1) the Ppo-155 β-YAC may be missing sequences that are present in the 248-kb β-YAC and are essential for protecting the β-globin locus from position effects; or (2) the transfer of the YAC into L-cells first may have affected the locus in a manner that allowed normal function of the LCR when the 248-kb β-YAC was subsequently transferred in the MEL cells by fusion.
To test the latter hypothesis, the Ppo-155 β-YAC was transfected into L-cells by lipofection. After 12 days in culture, 14 G418-resistant clones were randomly picked and expanded under further selection. The clones were screened for the presence of β-YAC DNA by conventional Southern analysis. Three clones were identified (LY2, 5, and 7) that carried the correct size fragments from the β-locus (data not shown). The structural integrity of the transfected β-YACs was analyzed by I-PpoI digestion and PFGE; all 3 clones carried at least one intact copy of the YAC (data not shown).
Fetal/embryonic pattern of globin gene expression of thePpo-155 β-YAC transferred into L-cells.
Previous studies have shown that human globin gene transcripts are present in L-cells transfected with the 248-kb β-YAC. The pattern of β-YAC gene expression was predominantly fetal-embryonic, with about 80% γ-globin mRNA, 15% ε, and 5% β.22 There was no transcription of the endogenous murine globin genes.
In the present study, the 14 L(β-YAC) clones were screened for globin gene expression by RNase protection. Globin mRNA was present only in the 3 clones that had an I-Ppo-I fragment containing the entire β-globin locus (LY2, 5, and 7). Globin gene expression patterns varied between clones. Clone 2 synthesized exclusively γ mRNA; in clone 5, 85% of the transcripts were γ and 15% ε, and clone 7 had 72% γ, 23% β, and 5% ε mRNA. These results provide further evidence that the genes of the β-YAC are activated when transferred into the nonerythroid environment of the L-cells. In addition, the fetal pattern of expression of the Ppo-155 β-YAC in this study is similar to the expression pattern of the 248-kb β-YAC.22
Position-independent human globin expression in L(β-YAC)x MEL cell hybrids.
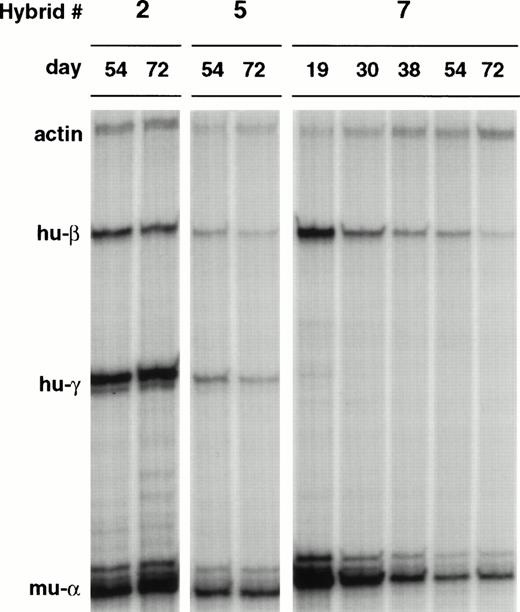
The three L-cell clones containing structurally normal β-YACs were fused with MEL-585, and L(β-YAC)xMEL cell hybrids were obtained by selecting cells growing in suspension and in the presence of G418. Parental cells were eliminated because of their G418 sensitivity (MEL cells) or adherent growth (L-cells). Figure 5 shows the results of RNase protection after 54 and 72 days in culture. Notice that in hybrids 2 and 5, both γ and β globin genes are transcribed at similar levels so that the γ/γ + β ratios are 0.68 and 0.67, respectively. Hybrid 7 had only β-globin mRNA on days 54 and 72. This was a robustly growing hybrid and cells for RNA studies were available from day 19 postfusion; at this time-point, a small amount of γ mRNA was present (γ/γ + β ratio of 3.7%), raising the possibility that this hybrid underwent a rapid γ to β switch.
Human globin gene expression of β-YACs of LxMEL hybrids. The β-YACs were first transferred into L-cells by lipofection and subsequently into MEL cells by cell fusion. Globin mRNA expression was analyzed in L(Ppo-155 β-YAC)xMEL hybrids at various time points after hybrid formation. Hybrid 7 shows extinction of expression of the human and murine globin genes. The visual differences in expression levels in the autoradiogram were diminished when the human mRNA levels were corrected for the copy number of the integrated β-YACs and the level of expression of the murine globin gene. Quantitative data are shown in Fig 6.
Human globin gene expression of β-YACs of LxMEL hybrids. The β-YACs were first transferred into L-cells by lipofection and subsequently into MEL cells by cell fusion. Globin mRNA expression was analyzed in L(Ppo-155 β-YAC)xMEL hybrids at various time points after hybrid formation. Hybrid 7 shows extinction of expression of the human and murine globin genes. The visual differences in expression levels in the autoradiogram were diminished when the human mRNA levels were corrected for the copy number of the integrated β-YACs and the level of expression of the murine globin gene. Quantitative data are shown in Fig 6.
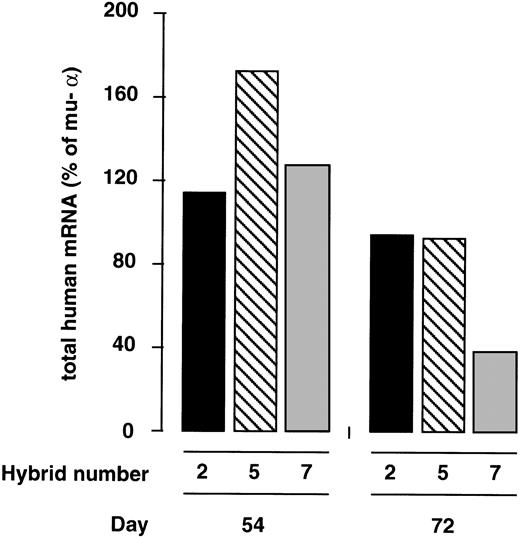
The total human globin mRNA (ie, ε + γ + β) synthesized at culture days 54 and 72 is expressed in Fig6 as the percentage of murine α-globin mRNA. Notice that at day 54 postfusion, the levels of expression per copy of the YAC in the three hybrids are very close to the levels of expression of the endogenous α gene. Human mRNA per copy ranged from 112.3% to 170.4% of murine α; ie, there was only a 1.5-fold variation in expression between the three hybrids. By day 72, the levels of human mRNA decreased and ranged from 37.6% to 92.5% of murine α, ie, they displayed a 2.5-fold variation. Thus, in sharp contrast to the position-dependent expression pattern obtained after the direct transfer of the Ppo-155 β-YAC into MEL cells, the genes of the chromosomally transferred YAC were transcribed in a position-independent manner after transfer into MEL cells.
Position-independent expression of globin genes of the YAC in L(β-YAC)xMEL hybrids. Total human mRNA levels of L(Ppo-155 β-YAC)xMEL hybrids shown in Fig 5 were corrected for the number of copies of the integrated human globin genes present in the hybrids and expressed as the percentage of one copy of the murine- gene. Results from culture days 54 and 72 postfusion are shown. Notice the small degree of variation in human globin expression between the three hybrids. Globin mRNA levels varied 1.5-fold on day 54 and 2.5-fold on day 72.
Position-independent expression of globin genes of the YAC in L(β-YAC)xMEL hybrids. Total human mRNA levels of L(Ppo-155 β-YAC)xMEL hybrids shown in Fig 5 were corrected for the number of copies of the integrated human globin genes present in the hybrids and expressed as the percentage of one copy of the murine- gene. Results from culture days 54 and 72 postfusion are shown. Notice the small degree of variation in human globin expression between the three hybrids. Globin mRNA levels varied 1.5-fold on day 54 and 2.5-fold on day 72.
Activation of the silent globin genes of a MEL(β-YAC) clone after fusion with L-cells.
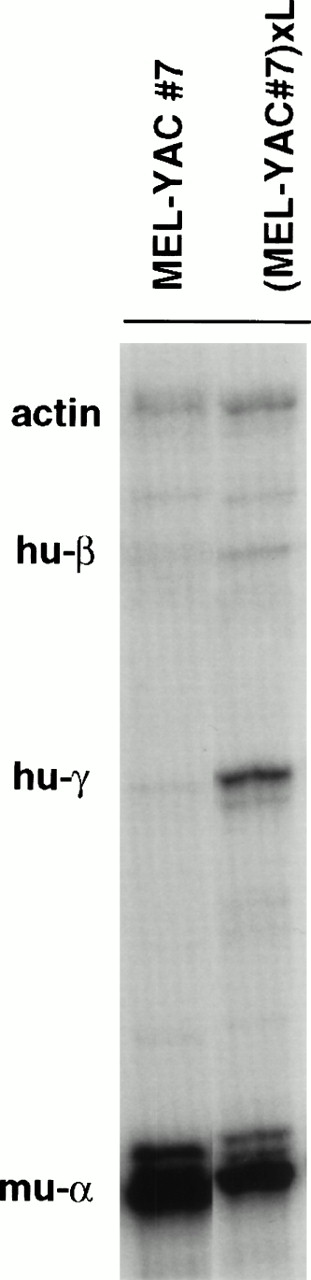
Our results so far have shown that passage of the β-YAC through the L-cells resulted in position-independent expression in the LxMEL hybrids. This was interpreted as evidence of the ability of the L-cells to modify the incoming naked DNA in a way that resulted in proper function of the LCR when the locus was chromosomally transferred into MEL cells. We then asked whether L-cells could also influence the expression of a β-YAC that was first transferred into MEL cells; to this effect, hygromycin-resistant L-cells were generated and fused with three MEL(β-YAC) clones. Hybrids were selected on the basis of dual resistance to G418 and hygromycin. We analyzed hybrid pools that maintained their ability for erythroid differentiation as evidenced by exposure to HMBA. One hybrid, formed from the nearly silent MEL(β-YAC) clone 7, fulfilled the above-noted criteria and was further analyzed. The expression levels for the β and γ globin genes increased 2.7- and 34-fold, respectively, in the hybrid cells (Fig 7). The level of total human mRNA output from the locus increased 13-fold (from 1.6% to 21% of murine α), indicating that a silenced β-YAC can be reactivated upon transfer into L-cells.
Activation of human globin gene expression of a silent MEL(β-YAC) clone after transfer into L-cells. The nearly silent MEL(β-YAC) clone 7 (see Fig 3) was fused with HygroR L-cells. A hybrid cell population was selected on the basis of its dual resistance to G418 (from the MEL βYAC cells) and hygromycin (from the L-cells). This hybrid retained the potential for globin gene expression upon induction of erythroid differentiation with hemin-HMBA. Total human globin mRNA expression of the parental MEL clone at the time of fusion was 1.6% of murine-. Notice that fusion with L-cells resulted in MEL(β-YAC)xL cell hybrids in which globin gene expression was activated with a fetal-like pattern. Total mRNA output from the locus was increased to 21% of the murine gene. RNase protection was performed on day 30 postfusion.
Activation of human globin gene expression of a silent MEL(β-YAC) clone after transfer into L-cells. The nearly silent MEL(β-YAC) clone 7 (see Fig 3) was fused with HygroR L-cells. A hybrid cell population was selected on the basis of its dual resistance to G418 (from the MEL βYAC cells) and hygromycin (from the L-cells). This hybrid retained the potential for globin gene expression upon induction of erythroid differentiation with hemin-HMBA. Total human globin mRNA expression of the parental MEL clone at the time of fusion was 1.6% of murine-. Notice that fusion with L-cells resulted in MEL(β-YAC)xL cell hybrids in which globin gene expression was activated with a fetal-like pattern. Total mRNA output from the locus was increased to 21% of the murine gene. RNase protection was performed on day 30 postfusion.
DISCUSSION
In this study, we have analyzed the expression pattern of a 155-kb human β-globin locus YAC in MEL cells and in L(β-YAC)xMEL hybrids. Our results show that the direct transfer of the β-YAC in MEL cells was associated with wide variation in expression between clones, indicating that the expression of the globin genes of the YAC was strongly influenced by the position of integration of the YAC into the mouse genome. When we introduced the 155-kb β-YAC first into L-cells and subsequently produced L(β-YAC)xMEL hybrids, the expression of globin genes of the β-YAC was independent of the position of integration into the murine genome. These results provide insights on the mechanisms whereby the LCR is activated during the course of hematopoietic cell differentiation.
The expression pattern of the 155-kb β-YAC that we used has been analyzed in transgenic mice.30,31 In this system, the level of expression of the globin genes of the YAC was similar to that of the endogenous murine globin genes, and there was only a 2.2-fold variation of β-globin mRNA levels between lines of transgenic mice.30 Small variation in human globin mRNA levels between transgenic lines indicates that globin gene expression is independent of position of integration, a finding that is charecteristic of normal function of the LCR. The apparent contrast between our findings in MEL cells and those in transgenic mice carrying the Ppo-155 β-YAC indicates that the direct transfer of the YAC into MEL cells disrupts the function of the LCR, resulting in loss of position-independent expression of the globin genes. The fact that position-independent expression of globin genes was restored when the β-YAC was first introduced into L-cells and then passed into MEL cells suggests that a common mechanism underlies the normal function of the LCR in the L(β-YAC)xMEL hybrids and in the transgenic mice. In both experimental settings, the β-YAC DNA is exposed to the environment of an uncommitted nonerythroid cell line (L-cell) or to that of a pluripotent stem cell before it is transferred into differentiated erythroid cells (like the MEL or the in vivo erythroblasts). It is possible that this interaction of the LCR with nonerythroid transcription factors before commitment to the erythroid lineage has occured is a crucial requirement for the subsequent orderly activation of the LCR when erythroid differentiation takes place.
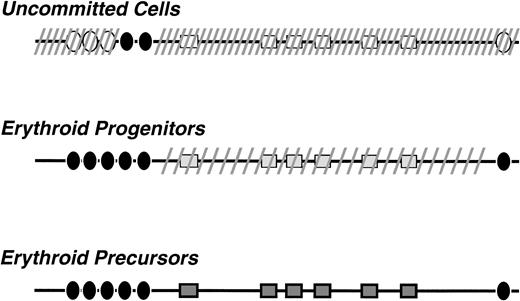
The possibilty that the LCR is active in uncommitted cells of the hematopoietic lineage was first proposed by Jimenez et al34 to interpret the finding of DNaseI sensitivity studies in a line considered to have pluripotent stem cell characteristics. In vivo evidence was provided by Papayannopoulou et al35 with studies of transgenic mice carrying a μLCR-β promoter-lacZ recombinant; these studies showed that the LCR is active in multipotent progenitors (as evidenced by LacZ expression in these cells), but it is silenced after commitment of these cells to the myeloid lineage.35 These results and the findings of the present study provide the basis for the model for activation of the LCR that is depicted in Fig 8.
Model for β-LCR activation during hematopoietic cell differentiation. It is proposed that, in the uncommitted cells, the locus is in a closed chromatin configuration, shown in the diagram by the dashed lines. However, constitutive transcription factors are bound to LCR sequences and certain DNaseI hypersensitive sites (shown as • in the diagram) are formed. In erythroid progenitors all the DNaseI HS sites are formed and the LCR holocomplex is generated. The locus attains an intermediate stage of DNaseI sensitivity (shown by the sparse dashed lines). The locus becomes fully active when, in the erythroblasts, the transcriptional complexes interact with the globin gene promoters and the promoter-specific DNaseI hypersensitive sites are formed.
Model for β-LCR activation during hematopoietic cell differentiation. It is proposed that, in the uncommitted cells, the locus is in a closed chromatin configuration, shown in the diagram by the dashed lines. However, constitutive transcription factors are bound to LCR sequences and certain DNaseI hypersensitive sites (shown as • in the diagram) are formed. In erythroid progenitors all the DNaseI HS sites are formed and the LCR holocomplex is generated. The locus attains an intermediate stage of DNaseI sensitivity (shown by the sparse dashed lines). The locus becomes fully active when, in the erythroblasts, the transcriptional complexes interact with the globin gene promoters and the promoter-specific DNaseI hypersensitive sites are formed.
According to this model, in uncommitted progenitor cells, certain sequences of the LCR are already occupied by transcription factors and certain DNaseI hypersensitive sites (HSs) are formed. The enhancer activity of the so-formed HSs is sufficient to enhance transcription from the downstream promoters, as our findings from the L-cells and those from the LCR-β-promoter LacZ mice35 indicate. Upon erythroid differentiation, erythroid lineage-specific transcription factors interact with the LCR and an erythroid lineage-specific LCR holocomplex is formed. This complex has a configuration that allows an optimal interaction with the downstream globin gene promoters. The formation of this normal LCR holocomplex requires the stepwise interaction with the LCR sequences, first of factors present in uncommitted cells and subsequently of factors present in the cells committed to the erythroid lineage. When this hierarchical interaction of factors with the LCR does not take place, as is the case of direct transfer into MEL cells, there is a random binding of factors with LCR sequences, resulting in dysfunctional LCR complexes.
Supported by National Institutes of Health Grants No. HL53750, DK30852, and DK 45365; an International Fogarty Fellowship Award to G.V.; and a Cooley’s Anemia Foundation Fellowship Award to E.S.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to George Stamatoyannopoulos, MD, Dr Sci, Medical Genetics, Box 357720, University of Washington, Seattle, WA 98195; e-mail: gstam@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal