Abstract
Chemokines play an important role in attracting granulocytes into sites of inflammation. Two chemokine subfamilies differ in their biologic activity for different granulocyte subsets. Whereas CXC chemokines such as interleukin-8 (IL-8) activate predominantly neutrophils, CC chemokines such as RANTES and eotaxin activate predominantly eosinophils. However, controversial results have been published in the past regarding the biologic role of IL-8 in eosinophil activation, particularly in allergic diseases. In this study, we investigated the functional evidence and expression of both IL-8 receptors, CXCR1 and CXCR2, on highly purified human eosinophils. In the first set of experiments, a chemotaxis assay was performed showing that IL-8 did not induce chemotaxis of eosinophils. In addition, and in contrast to neutrophils and lymphocytes, IL-8 did not induce a rapid and transient release of cytosolic free Ca2+([Ca2+]i) in eosinophils, even after preincubation with TH1- and TH2-like cytokines. To investigate whether neutrophil contamination might be responsible for the reported IL-8 effects on eosinophils, neutrophils were added to highly purified eosinophils from the same donor in different concentrations. Interestingly, as little as 5% of neutrophil contamination was sufficient to induce an increase of [Ca2+]iafter stimulation with IL-8. Flow cytometry experiments with monoclonal antibodies against both IL-8 receptors demonstrated no expression of CXCR1 and CXCR2 on eosinophils before or after cytokine activation. Reverse transcriptase-polymerase chain reaction experiments showed that eosinophils, in contrast to neutrophils and lymphocytes, did not express mRNA for CXCR1 and CXCR2. In summary, this study clearly demonstrates that CXCR1 and CXCR2 are not expressed on human eosinophils, even after priming with different bioactive cytokines. Because the CXC chemokine IL-8 did not induce in vitro effects on human eosinophils, IL-8 may also not contribute in vivo to the influx of eosinophil granulocytes into sites of allergic inflammation. Our results suggest that CC chemokines such as eotaxin, eotaxin-2, and MCP-4 are predominant for the activation of eosinophils.
CHEMOKINES PLAY an important role in attracting granulocytes into sites of inflammation. Up to now, four different subfamilies of chemokines have been identified according to highly conserved cysteine motifs in their aminoterminal domain.1,2 The two major subfamilies of chemokines, CXC and CC chemokines, differ in their biologic activity to stimulate different kinds of effector cells. Whereas CXC chemokines such as interleukin-8 (IL-8), NAP-2, GRO-α, -β, -γ, and ENA-78 activate predominantly neutrophils,3 CC chemokines such as RANTES, MCP-4, and eotaxin activate eosinophils, basophils, and, as described recently, T-lymphocyte subsets.4-6 However, controversial results have been published in the past regarding the biologic importance of IL-8 in eosinophil activation, particularly in allergic diseases such as bronchial asthma and atopic dermatitis but also in hypereosinophilia, eg, in Hodgkin’s disease.
Competition-binding studies showed that human neutrophil granulocytes bear two classes of IL-8 receptors, CXCR1 (IL-8RA)7 and CXCR2 (IL-8RB).8,9 Both receptors are binding IL-8 with high affinity in contrast to the other CXC chemokines, NAP-2 and GRO-α, which bind with high affinity only to CXCR2.10Thus, CXCR1 is referred to as an IL-8–specific receptor, whereas CXCR2 is regarded as a promiscuous receptor responding to various CXC chemokines. Jones et al11 demonstrated that CXCR1 and CXCR2 are functionally different due to aminoacid sequence differences clustered at the NH2- and COOH-terminal domains. Transient changes of cytosolic free Ca2+ and the release of granular enzymes were mediated by both receptor types, whereas the production of reactive oxygen species via the NADPH oxydase depended exclusively on stimulation through CXCR1.11 Wuyts et al12characterized granulocyte chemotactic protein 2 (GCP-2) as another CXC chemokine signaling through CXCR1 and CXCR2 and being nearly as effective as IL-8. As opposed to human neutrophils, they found no evidence for any activity on human eosinophils.12
Donnelly et al13 reported that, in patients with an early stage of adult respiratory distress syndrome, serum concentrations of IL-8 could be detected in picomolar ranges and, therefore, initiate the migration of granulocytes towards the inflamed area. In the sputum of patients with chronic inflammatory airways disease, typically associated with serum and tissue eosinophilia, concentrations of IL-8 were reported to range from 1 to 9 nmol/L. But these studies did not verify a direct effect of IL-8 on human eosinophils. The importance of the CXC chemokine IL-8 for eosinophil activation and the expression of CXCR1 and CXCR2 on human eosinophils are, therefore, still a matter of debate.
After priming with the eosinophil-specific cytokine IL-5, Schweizer et al14 reported that stimulation of human eosinophils with IL-8 did induce chemotaxis and actin polymerization as related events. Their cell preparations consisted of up to 95% eosinophils.14 In another study, IL-8 was found to be a chemoattractant for eosinophils purified from patients with blood eosinophilia. It was proposed that this might be due to in vivo priming mechanisms.15 These data are in contrast to previous reports in which the effect of IL-8 on human eosinophils was found to be negligible.16 17
Eosinophils are known to produce and secrete IL-8,18-21which can be stimulated by TH2 cell-derived cytokines.22Therefore, it may be assumed that the enhanced production of bioactive IL-8 after priming the cells with cytokines results in a downregulation of CXCR1 and CXCR2 on eosinophils.23 Schnyder-Candrian et al24 found that interferon γ (INFγ) inhibits the production of IL-8 and ENA-78 in human monocytes.
In this study, we investigated the functional evidence and expression of both IL-8 receptor types, CXCR1 and CXCR2, on human eosinophils from healthy nonatopic volunteers. In addition, purified eosinophils were primed with TH1 and TH2 cell-derived cytokines. To investigate whether neutrophil contamination might be responsible for the reported IL-8 in vitro effects on eosinophils, neutrophils were added to highly purified human eosinophils from the same donor in various concentrations and functional assays were performed. Therefore, this study helps to understand the effects of IL-8 on human eosinophils and may help to get insight into the physiologic role of this chemokine during the inflammatory process.
MATERIALS AND METHODS
Isolation of human eosinophils.
Human granulocytes were isolated from heparin-anticoagulated venous blood from normal nonatopic healthy European donors without signs of bacterial or viral infections. All donors were nonsmokers and did not take any medicine. The isolation was performed using Ficoll (Pharmacia, Uppsala, Sweden) density gradient centrifugation as described previously.25 For further purification, granulocytes were resuspended in HEPES-buffered Hanks’ Balanced Salt Solution (HBSS; GIBCO, Grand Island, NY), pH 7.4, containing 1 mg/mL bovine serum albumin (BSA; HBSS + BSA). Eosinophils were purified by negative selection with anti-CD16 antibody (clone 3G8; Immunotech, Hamburg, Germany) coated Dynabeads M-450 (Dynal, Hamburg, Germany), as described previously.25 The resulting eosinophil purity was ≥99.5% as determined by flow cytometrical analysis (FACScan; Becton Dickinson, Heidelberg, Germany) using phycoerythrin-conjugated anti-CD16 antibody (clone 3G8; Immunotech).
Priming of eosinophils with different cytokines.
For some experiments, highly purified human eosinophils were incubated for 24 or 36 hours at 37°C with 50 ng/mL IL-4, 50 ng/mL IL-5, 30 ng/mL tumor necrosis factor α (TNFα), 100 ng/mL INFγ, 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), or medium alone; washed; and resuspended in assay buffer before the assessment of their functional response to IL-8. Viability after incubation was determined by Kimura staining and always ≥85%.
Monoclonal antibodies (MoAbs).
The human anti-CXCR1 MoAb (MoAb SE2) and human anti-CXCR2 MoAb (MoAb HC2) were used as described previously.26 The human IgG1 and IgG1-fluorescein isothiocyanate (FITC)-conjugated isotype control; human anti-CD3, anti-CD4, anti-CD8, and anti-CD19 MoAbs; and also the human IgG2b and IgG2b-FITC-conjugated isotype control were obtained from Sigma Chemicals (Deisenhofen, Germany). The humanized anti-CD52 MoAb (Campath-1H monomer) was a kind gift from Wellcome Foundation (London, UK).27
Immunofluorescence of granulocytes and eosinophils.
Immunofluorescence of granulocytes was performed with standard techniques. In brief, granulocytes and eosinophils were adjusted to a density of 1 × 107 cells/mL. Aliquots (20 μL) containing 2 × 105 cells were incubated at 4°C for 30 minutes with the indicated antibody. Thereafter, cells were washed twice with cold phosphate-buffered saline. For indirect immunofluorescence, cells were stained in a second step with an FITC-conjugated mouse antihuman antibody (Immunotech) and subsequently washed twice. In some experiments, double staining with FITC-conjugated antibodies and anti-CD16 phycoerythrin (PE)-conjugated antibody or anti-CD3, CD4, CD8, and CD19 PE-conjugated antibodies were performed. Thereafter, cells were analyzed by flow cytometry (FACScan). The sample was excited at 488 nm and emission was measured at 530 nm (FITC-labeled antibodies, Fluorescence 1, green fluorescence) and at 585 nm (anti-CD16 PE, Fluorescence 2, red fluorescence).
Eosinophils were preincubated for 24 and 36 hours in the presence of 100 ng/mL INFγ, 50 ng/mL IL-4, 50 ng/mL IL-5, 30 ng/mL TNFα, and 100 ng/mL GM-CSF (Genzyme, Rüsselshiver, Germany) and RPMI medium, respectively. Thereafter, cells were washed and stained by anti-CXCR1 MoAb, CXCR2 MoAb, or isotype control.
CXCR1 and CXCR2 mRNA expression.
Total RNA was isolated from eosinophils, lymphocytes, and neutrophils using TRIzol (GIBCO) according to the manufacturer’s instructions based on the guanidine isothiocyanate method. First-strand cDNA synthesis was performed in a 20 μL reaction mixture containing 5 μL RNA, 1 mmol/L dNTP, 1.6 μg Oligo-p(dT)15 primer, 50 U RNase inhibitor, 20 U avian myeloblastosis virus (AMV) reverse transcriptase (Boehringer Mannheim, Mannheim, Germany), incubated at 25°C for 10 minutes, and then incubated at 42°C for 1 hour. The AMV reverse transcriptase was denatured by 99°C for 5 minutes and then placed on ice. Primers for the amplification of CXCR1 (sense, 5′-CGACTGTGGGCGGATTCTTG-3′; and antisense, 5′-AGACCGATACCATGTGCTCT-3′), CXCR2 (sense, 5′-ACGCATGTTGCTGTCTCTGG-3′; and antisense, 5′-TGTTGGTCCTAGGGCGTAG-3′), CD16 (sense, 5′-GGCCTCGAGCTACTTCATTG-3′; and antisense, 5′-GGAGCCGCTATCTTTGAGTG-3′), and CD52 (sense, 5′-GCCACGAAGATCCTACCAAA-3′; and antisense, 5′-GCTTGGCCCCTACATCATTA-3′) were designed according to the published sequences (accession numbers are as follows: CXCR1, L19591; CXCR2, M73969; CD16, M24854; CD52, A23013). Reverse transcriptase reaction mixture was used in the polymerase chain reaction (PCR) in a 50 μL final volume, 0.2 mmol/L of each dNTP, 0.4 μmol/L of each primer (CXCR1, CXCR2, CD16, or CD52), and 1.3 U Taq DNA polymerase (Boehringer Mannheim). The mixture was incubated in a thermocycler using the following temperature profile: initial denaturation step at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72° C for 1 minute. The final extension step was at 72°C for 5 minutes. PCR samples were run on a 1.8% agarose gel stained with 0.2 μg/mL ethidium bromide, and the PCR products were visualized with UV light and photographed.
Chemotaxis assay.
In analogy to the previously described modified Boyden chamber technique,16 28-30 the chemotaxis of human eosinophils, neutrophils, and lymphocytes was determined by filling the lower chamber with the stimuli and the upper chamber with the cells. A polycarbonate membrane of 3 μm pore size was used for eosinophils and neutrophils. For lymphocytes, the polycarbonate membrane had a pore size of 1 μm. Human eosinophil, neutrophil, or lymphocyte suspensions of 100 μL at a concentration of 5 × 105/mL cells were placed in the upper part of each chamber and migration was allowed to proceed for 1 hour in a humidified atmosphere at 37°C. The lower part of the Boyden chambers contained the migrated cells that were subsequently lysed by adding 0.1% Triton X-100. Usingp-nitrophenyl β-D-glucuronide (Sigma Chemicals) as a substrate, the β-glucuronidase activity in the lysates was measured photometrically. Fluorescence readings were taken on a Titertek Twinreader Plus (EFLAB, Finland) with 405 nm emission wave length. For calculation of the number of migrated cells based on the β-glucuronidase activity determined in the lower part of the Boyden chamber, values were calculated by a computer-assisted technique from a standard curve using known numbers of unchallenged eosinophils. The assays were performed in duplicates or triplicates. The results were expressed as a ratio between the number of migration cells in the sample versus the control medium, which reflects spontaneous migration. This ratio is referred to as chemotactic index (CI).
Measurement of [Ca2+]i in spectrofluorometry.
For the measurement of the cytosolic free Ca2+concentration ([Ca2+]i) of human eosinophils, neutrophils, and lymphocytes, the fluorescence Ca2+indicator Fura-2 (Molecular Probes, Eugene, OR) was used at a concentration of 2 μmol/L. Fluorescence was detected in an Aminco Bowman Series 2 spectrofluorometer (SLM-Aminco, Urbana, IL), as described previously.31,32 In brief, after addition of each stimulus and subsequent measurement, maximal and minimal fluorescence intensities were calibrated by the addition of 0.2% Triton X-100 leading to 100% Fura-2 saturation followed by a subsequent quenching of the fluorescence with 2 mmol/L EGTA. Fura-2 fluorescence changes were continuously monitored at a dual excitation spectra at λ1 = 340 nm and λ2 = 380 nm; the emission wavelength was fixed at 510 nm.32 Absolute [Ca2+]i was calculated automatically by AB 2 series 2 software (SLM-Aminco) according to the equation described by Grynkiewicz et al.33
Statistical analysis.
Unless otherwise stated, the data in the text and figures are expressed as the mean ± SEM analysis of variance (ANOVA). Newman-Keuls tests were used for comparing experimental groups to control values.P values less than .05 were accepted as significant. When the global test of differences was significant at the 5% level, pairwise tests of differences between groups were applied (Student’st-test for paired data using 5% significance level, closed test procedure).
RESULTS
IL-8 does not induce chemotaxis of human eosinophils, whereas human neutrophils and lymphocytes are stimulated.
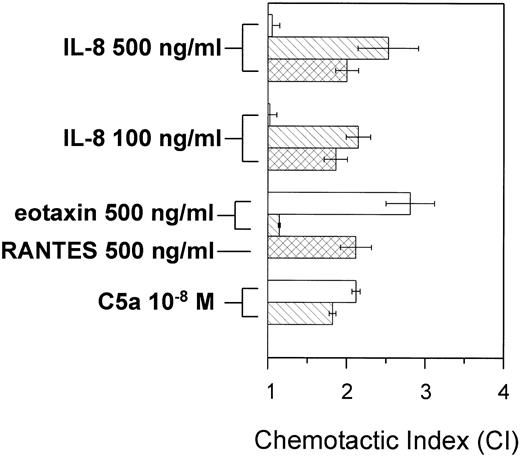
To investigate whether IL-8 stimulates highly purified human eosinophils, the modified Boyden chamber technique was performed to detect the chemotactic activity of IL-8 in comparison to the potent eosinophil activator eotaxin and C5a. As seen in Fig 1, IL-8 did not induce a significant response of eosinophils at concentrations known to be potent for lymphocytes and neutrophils. Also, higher or lower concentrations of IL-8 did not induce a chemotactic response in eosinophils. In contrast to eosinophils, IL-8 induced significantly chemotaxis of human neutrophils and lymphocytes (Fig 1). The CC chemokines eotaxin, RANTES, and C5a were used as positive controls. They induced chemotaxis in the different cell types in expected patterns. Therefore, the CXC-chemokine IL-8 is not a chemotactic stimulus for human eosinophils.
IL-8 induces chemotaxis of human neutrophils and lymphocytes but does not stimulate eosinophils. The chemotactic activity was measured using the modified Boyden chamber technique and expressed as the CI, defined as the ratio of the number of migrating cells in presence of stimulus versus migrating cells in the presence of medium. Cells were incubated with the indicated stimuli. The results are presented as the mean ± SEM of five different experiments. (□) Eosinophils; () neutrophils; (▩) lymphocytes.
IL-8 induces chemotaxis of human neutrophils and lymphocytes but does not stimulate eosinophils. The chemotactic activity was measured using the modified Boyden chamber technique and expressed as the CI, defined as the ratio of the number of migrating cells in presence of stimulus versus migrating cells in the presence of medium. Cells were incubated with the indicated stimuli. The results are presented as the mean ± SEM of five different experiments. (□) Eosinophils; () neutrophils; (▩) lymphocytes.
Effect of IL-8 on [Ca2+]i in human eosinophils.
To further evaluate whether IL-8 induces eosinophil activation, changes in [Ca2+]i were investigated using the fluorescence Ca2+ indicator Fura-2. Stimulation of highly purified human eosinophils with IL-8 did not induce an increase in [Ca2+]i (Fig 2). In addition, preincubation of highly purified eosinophils with 50 ng/mL IL-4, 50 ng/mL IL-5, 30 ng/mL TNFα, 100 ng/mL GM-CSF, or 100 ng/mL INFγ for 24 or 36 hours, respectively, and subsequent stimulation with IL-8 with various concentrations also did not induce [Ca2+]i transients in eosinophils (data not shown). In contrast to eosinophils, human neutrophils and lymphocytes showed rapid and transient changes of [Ca2+]iafter stimulation with IL-8 (Fig 2).
IL-8 induces [Ca2+]itransients in human neutrophils and lymphocytes but not in eosinophils. Spectrofluorometric measurements of [Ca2+]iin Fura-2–loaded purified human eosinophils, neutrophils, and lymphocytes were performed. Cells were stimulated at the indicated time points; C5a or RANTES was used as a positive control. One representative experiment of six performed is shown.
IL-8 induces [Ca2+]itransients in human neutrophils and lymphocytes but not in eosinophils. Spectrofluorometric measurements of [Ca2+]iin Fura-2–loaded purified human eosinophils, neutrophils, and lymphocytes were performed. Cells were stimulated at the indicated time points; C5a or RANTES was used as a positive control. One representative experiment of six performed is shown.
The purity of eosinophil preparations is important for receptor studies.
To rule out the influence of neutrophils contaminating the preparation of eosinophil granulocytes on measurements of [Ca2+]i, further experiments with Fura-2–loaded cells were performed. Different amounts of human neutrophils were added to a granulocyte suspension of highly purified human eosinophils from the same donor and subsequently stimulated with IL-8 and eotaxin. Changes in [Ca2+]i were detected in cell suspensions containing 100% highly purified human eosinophils down to 0%, containing only purified neutrophils. As seen in Fig 3A, a detectable but low neutrophil contamination of 5% in the granulocyte suspension resulted in [Ca2+]i transients. Therefore, 50,000 contaminating neutrophils do induce detectable differences in [Ca2+]i. In contrast to IL-8, eotaxin was highly effective to induce [Ca2+]i transients in this granulocyte suspension (Fig 3A). Increasing numbers of contaminating neutrophils, up to 100% in the granulocyte suspension, resulted in higher increase of [Ca2+]i in response to IL-8 but not to eotaxin (Fig 3A and B).
(A and B) Contaminating neutrophils within preparations of human eosinophils are leading to [Ca2+]itransients. Spectrofluorometric measurements of [Ca2+]i in Fura-2–loaded unprimed human eosinophils between 100% and 0% purity were performed. All contaminating cells were neutrophils as detected by flow cytometry. (A) Eosinophils contaminated with different percentages of neutrophils were stimulated with 100 ng/mL IL-8 and subsequently received 500 ng/mL eotaxin as a positive control. One representative experiment of five performed is shown. (B) Statistical analysis of [Ca2+]i in human eosinophils contaminated with different amounts of purified human neutrophils (0% up to 100%). Cells were stimulated with 100 ng/mL IL-8 or 100 ng/mL eotaxin as a positive control. The results are presented as the mean ± SEM of five experiments.
(A and B) Contaminating neutrophils within preparations of human eosinophils are leading to [Ca2+]itransients. Spectrofluorometric measurements of [Ca2+]i in Fura-2–loaded unprimed human eosinophils between 100% and 0% purity were performed. All contaminating cells were neutrophils as detected by flow cytometry. (A) Eosinophils contaminated with different percentages of neutrophils were stimulated with 100 ng/mL IL-8 and subsequently received 500 ng/mL eotaxin as a positive control. One representative experiment of five performed is shown. (B) Statistical analysis of [Ca2+]i in human eosinophils contaminated with different amounts of purified human neutrophils (0% up to 100%). Cells were stimulated with 100 ng/mL IL-8 or 100 ng/mL eotaxin as a positive control. The results are presented as the mean ± SEM of five experiments.
CXCR1 and CXCR2 are not expressed on the surface of human eosinophils.
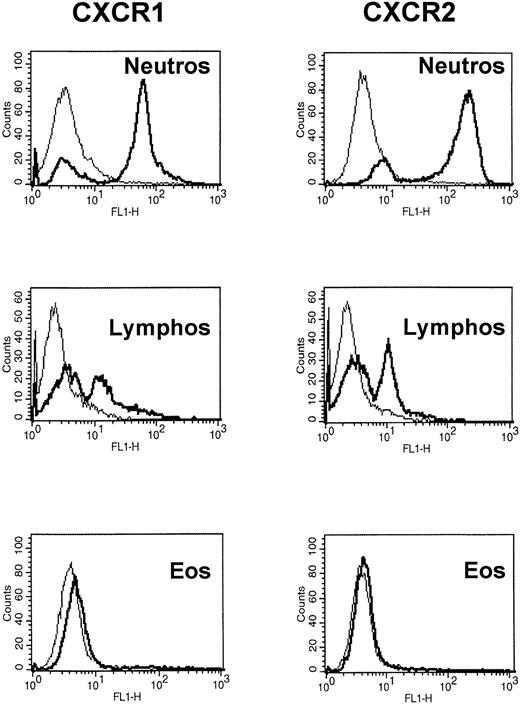
In the next set of experiments, the expression of both IL-8 receptor types, CXCR1 and CXCR2, on human eosinophils was investigated by flow cytometry. Highly purified human neutrophils were used as a positive control and stained with anti-CXCR1 MoAb (SE 2) and anti-CXCR2 MoAb (HC 2) in different concentrations (0.5 to 50 μg/mL). Histogram analysis showed binding of both MoAbs to human neutrophils. Maximal binding of anti-CXCR1/CXCR2 MoAbs was reached at 20 μg/mL (Fig 4) and showed that CXCR1 and CXCR2 are expressed on human neutrophils. The second proportion of cells appearing negative for CXCR1 and CXCR2 were human eosinophils, as detected by staining with anti-CD52 MoAb. In addition, highly purified human lymphocytes could also be stained with CXCR1/CXCR2 MoAbs indicating the expression of both IL-8 receptor types (Fig 4). Two cell populations are seen with a proportion of lymphocyte subsets negative for CXCR1 and CXCR2. Double-color flow cytometric analysis showed that CXCR1 and CXCR2 are expressed on CD8+ T cells, but not on CD19+ B lymphocytes and CD4+ T cells (data not shown). These data are in accordance with previous findings.34-37 In contrast to human neutrophils and lymphocytes, highly purified CD16− selected human eosinophils did not express CXCR1 and CXCR2 (Fig 4). Furthermore, priming of human eosinophils for 24 or 36 hours, respectively, with 50 ng/mL IL-4, 50 ng/mL IL-5, 30 ng/mL TNFα, 100 ng/mL GM-CSF, or 100 ng/mL INFγ, respectively, did not induce the expression of CXCR1 or CXCR2 on the surface of eosinophils (Table1).
CXCR1 and CXCR2 are expressed on human neutrophils and lymphocytes but not on human eosinophils. Flow cytometric analysis of human neutrophils, lymphocytes, and eosinophils. Cells were double-stained with the anti-CXCR1 or anti-CXCR2 MoAbs, respectively, and anti-CD3, CD4, CD8, and CD19 MoAbs for lymphocyte subsets; anti-CD16 MoAb for neutrophils; or anti-CD52 MoAb for eosinophils, respectively. One representative experiment of eight performed is shown.
CXCR1 and CXCR2 are expressed on human neutrophils and lymphocytes but not on human eosinophils. Flow cytometric analysis of human neutrophils, lymphocytes, and eosinophils. Cells were double-stained with the anti-CXCR1 or anti-CXCR2 MoAbs, respectively, and anti-CD3, CD4, CD8, and CD19 MoAbs for lymphocyte subsets; anti-CD16 MoAb for neutrophils; or anti-CD52 MoAb for eosinophils, respectively. One representative experiment of eight performed is shown.
Expression of CXCR1 and CXCR2 on Human Neutrophils, Lymphocyte Subsets, and Eosinophils From Healthy Nonatopic Donors Stimulated by the Indicated Cytokines
| . | CXCR1 . | CXCR2 . |
|---|---|---|
| Eosinophils | ||
| Medium | 5.3 ± 0.3 | 4.8 ± 0.3 |
| 50 ng/mL IL-4 | 6.1 ± 0.4 | 5.6 ± 0.6 |
| 50 ng/mL IL-5 | 4.9 ± 0.4 | 4.4 ± 0.3 |
| 100 ng/mL TNFα | 5.5 ± 0.8 | 4.9 ± 0.8 |
| 30 ng/mL INFγ | 5.4 ± 0.6 | 5.0 ± 1.3 |
| 100 ng/mL GM-CSF | 4.7 ± 0.3 | 4.5 ± 0.3 |
| Isotype IgG2b | 4.9 ± 1.0 | 0 |
| Isotype IgG1 | 0 | 4.7 ± 0.3 |
| Neutrophils | ||
| Medium | 66.8 ± 10.6 | 73.7 ± 18.4 |
| Isotype IgG2b | 5.2 ± 1.2 | 0 |
| Isotype IgG1 | 0 | 4.6 ± 0.9 |
| Lymphocytes | ||
| Medium | 54.2 ± 10.4 | 41.6 ± 19.2 |
| Isotype IgG2b | 3.7 ± 0.6 | 0 |
| Isotype IgG1 | 0 | 4.6 ± 0.7 |
| . | CXCR1 . | CXCR2 . |
|---|---|---|
| Eosinophils | ||
| Medium | 5.3 ± 0.3 | 4.8 ± 0.3 |
| 50 ng/mL IL-4 | 6.1 ± 0.4 | 5.6 ± 0.6 |
| 50 ng/mL IL-5 | 4.9 ± 0.4 | 4.4 ± 0.3 |
| 100 ng/mL TNFα | 5.5 ± 0.8 | 4.9 ± 0.8 |
| 30 ng/mL INFγ | 5.4 ± 0.6 | 5.0 ± 1.3 |
| 100 ng/mL GM-CSF | 4.7 ± 0.3 | 4.5 ± 0.3 |
| Isotype IgG2b | 4.9 ± 1.0 | 0 |
| Isotype IgG1 | 0 | 4.7 ± 0.3 |
| Neutrophils | ||
| Medium | 66.8 ± 10.6 | 73.7 ± 18.4 |
| Isotype IgG2b | 5.2 ± 1.2 | 0 |
| Isotype IgG1 | 0 | 4.6 ± 0.9 |
| Lymphocytes | ||
| Medium | 54.2 ± 10.4 | 41.6 ± 19.2 |
| Isotype IgG2b | 3.7 ± 0.6 | 0 |
| Isotype IgG1 | 0 | 4.6 ± 0.7 |
Results are expressed as the mean channel fluorescence (MCF) and represent data from eight experiments. In the histogram for neutrophils and lymphocyte subsets, MCF was calculated on the second peak. Isotype controls are shown: IgG2b for anti-CXCR1 MoAb and IgG1 for anti-CXCR2 MoAb.
CXCR1 and CXCR2 mRNA are not expressed in human eosinophils.
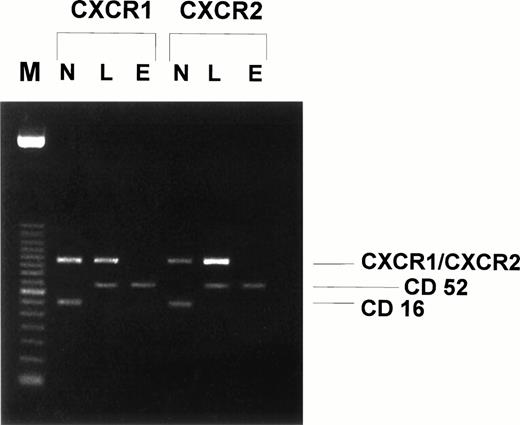
To further confirm the binding results of anti–IL-8 receptor MoAbs, reverse transcriptase-PCR (RT-PCR) was performed to investigate whether purified human eosinophils express mRNA specific for the IL-8 receptors CXCR1 and CXCR2. Again, human neutrophils and lymphocytes were used as positive controls. As seen in Fig 5, no CXCR1 or CXCR2 mRNA could be detected in highly purified human eosinophils. In addition, preincubation of human eosinophils for 24 or 36 hours with 100 ng/mL INFγ, 50 ng/mL IL-4, 50 ng/mL IL-5, 30 ng/mL TNFα, or 100ng/mL GM-CSF, respectively, did not induce the expression of CXCR1/CXCR2 mRNA (data not shown). Therefore, mRNA specific for CXCR1 or CXCR2 is not expressed by human eosinophils. As expected and in contrast to eosinophils, highly purified human neutrophils and lymphocytes did express constitutively CXCR1 and CXCR2 mRNA, as indicated by specific RT-PCR products with an expected size of 507 bp for CXCR1 and 520 bp for CXCR2, respectively (Fig 5). To demonstrate that all mRNA preparations were highly purified and not contaminated with other cell types, RT-PCR was performed simultaneously using primers for CD16 (neutrophils) and CD52 (eosinophils and lymphocytes) (Fig 5).
CXCR1 and CXCR2 mRNA is expressed in human neutrophils and lymphocytes but not in human eosinophils. Human neutrophil, lymphocyte, and eosinophil mRNA was isolated and first-strand cDNA synthesis was performed. PCR was performed with primer pairs specific for CXCR1 (expected size, 507 bp), CXCR2 (expected size, 520 bp), CD52 (expected size, 385 bp), and CD16 (expected size, 297 bp). The amplicons were separated by electrophoresis in 1.8% agarose gel and stained by ethidium bromide. Lane M, 100-bp DNA size marker; lane N, human neutrophil mRNA; lane L, human lymphocyte mRNA; lane E, human eosinophil mRNA. One representative experiment of four performed is shown.
CXCR1 and CXCR2 mRNA is expressed in human neutrophils and lymphocytes but not in human eosinophils. Human neutrophil, lymphocyte, and eosinophil mRNA was isolated and first-strand cDNA synthesis was performed. PCR was performed with primer pairs specific for CXCR1 (expected size, 507 bp), CXCR2 (expected size, 520 bp), CD52 (expected size, 385 bp), and CD16 (expected size, 297 bp). The amplicons were separated by electrophoresis in 1.8% agarose gel and stained by ethidium bromide. Lane M, 100-bp DNA size marker; lane N, human neutrophil mRNA; lane L, human lymphocyte mRNA; lane E, human eosinophil mRNA. One representative experiment of four performed is shown.
DISCUSSION
The CXC chemokine IL-8 has been shown to play a central role in several chronic inflammatory diseases, such as allergic bronchial asthma,38 rheumatoid arthritis,39,40 and psoriasis.41 The recruitment and activation of human neutrophil granulocytes is especially important for the inflammatory response in these disorders. It could be demonstrated that IL-8 acts via specific G-protein–coupled receptors, named CXCR1 and CXCR2, which are expressed on the surface of human neutrophils, lymphocyte subsets, and keratinocytes. In addition, IL-8 has been found in the BAL of patients with allergic bronchial asthma and it has, therefore, been speculated that IL-8 may also be responsible for the attraction of eosinophils that are the predominant cells in BAL of asthmatic patients. To clarify the controversial data concerning the effects of IL-8 on human eosinophils, we investigated the in vitro effect of IL-8 and the expression of both IL-8 receptors on human eosinophils in comparison to neutrophils and lymphocytes.
In the first set of experiments, the functional role of IL-8 on human eosinophils was investigated. We found that IL-8 did not stimulate chemotaxis and [Ca2+]i transients of highly purified eosinophil granulocytes from healthy nonatopic donors. These data indicate that IL-8 does not play a role in the recruitment of eosinophils to the site of inflammation and are contrasting previous results that IL-8 may be a potent chemotaxin for human eosinophils. Erger and Casale42 have demonstrated that IL-8 is a potent mediator of eosinophil chemotaxis through umbilical vein endothelial cell and human pulmonary type II-like epithelial cell monolayers cultured on polycarbonate filters. However, their eosinophil preparations had only an average purity of 78%, with a maximal chemotactic response of 25% of the granulocyte suspension through naked filters. In addition, Sehmi et al15 reported that eosinophils exhibit chemotaxis towards IL-8. Again, the investigators in that study used cell preparations consisting up to only 80% ± 4% and 92% ± 2% eosinophils from normal donors and subjects with hypereosinophilia, respectively. Further studies describing the response of human eosinophils toward IL-8 also used eosinophil preparations with only up to 95% purity.14 43-45
Therefore, it may be speculated that the in vitro effects in these studies could be due to contaminating human neutrophils. To address this issue, neutrophils were added to various preparations of highly enriched human eosinophils from the same donor. We found that as little as 5% neutrophil contamination was enough to transient changes in [Ca2+]i. Thus, these data indicate that the previously published effects of IL-8 on eosinophil chemotaxis may be due to a contamination with neutrophils. They further point out that highly purified eosinophils are essential for studying the in vitro effects of cytokines and receptor expression in these cells.
To investigate whether in vivo priming mechanisms of human eosinophils may be responsible for the expression of CXCR1 and CXCR2, highly purified human eosinophils were preincubated with different cytokines known to alter eosinophil activation: IL-4 and IL-5 were used as TH2 cell-derived cytokines well-known as specific activators of eosinophil granulocytes46,47 and capable of stimulating eosinophils to synthesize and release cytokines and express cytokine receptors on the cell surface. Previously, Sehmi et al15 have reported that eosinophils exhibit functional responses toward IL-8 in contrast to cells from normal healthy donors due to in vivo priming mechanisms and identified IL-5 as a cytokine enhancing the chemotactic response to IL-8. In another study, IL-5–primed eosinophils showed a chemotactic response to IL-8.14 However, our data showed that neither IL-4, TNFα, INFγ, and IL-5 nor GM-CSF was able to prime eosinophils to induce [Ca2+]i transients in response to IL-8.
In the last set of experiments, the expression of IL-8 receptors on the surface of human eosinophils was investigated. Flow cytometric measurements showed that eosinophils did not express CXCR1 or CXCR2 on the cell surface. Moreover, priming of eosinophils with cytokines such as IL-4, IL-5, TNFα, IFNγ, and GM-CSF had no effect on the expression of IL-8 receptors. Furthermore, RT-PCR analysis did not show any mRNA transcripts specific for CXCR1 or CXCR2 in human eosinophils, whereas in human neutrophils and lymphocytes, mRNA specific for both IL-8 receptor types was detected.
In summary, this study demonstrates that CXCR1 and CXCR2 are not expressed on human eosinophils even after priming with different bioactive cytokines. Although eosinophils produce IL-8 itself, the CXC chemokine IL-8 seems to have no direct effect on eosinophils and there is no evidence for an autocrine stimulation of eosinophils in the state of hypereosinophilia. Because the CXC chemokine IL-8 did not induce in vitro effects on human eosinophils, IL-8 may also not contribute in vivo to the influx of eosinophils into sites of allergic inflammation. Our results suggest that CC chemokines such as eotaxin, eotaxin-2, and MCP-4 as well as their receptor CCR3 are predominant for the activation of eosinophils.
Supported by a grant from the Deutsche Forschungsgemeinschaft (EL 160/3-2).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jörn Elsner, MD, Department of Dermatology and Allergology, Hannover Medical University, Ricklinger Str. 5, D-30449 Hannover, Germany; e-mail: jelsner@csi.com.

![Fig. 2. IL-8 induces [Ca2+]itransients in human neutrophils and lymphocytes but not in eosinophils. Spectrofluorometric measurements of [Ca2+]iin Fura-2–loaded purified human eosinophils, neutrophils, and lymphocytes were performed. Cells were stimulated at the indicated time points; C5a or RANTES was used as a positive control. One representative experiment of six performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.694/4/m_blod40231002x.jpeg?Expires=1769193500&Signature=nrCKn3KOLptfqSaVpRTG2WxNkV4aQhCUwLbAsSfnW6ZK48CQj3x5JNOk1X-dhI6HfZoaU5t6jqzW7~8cEgtLuBn2yYU1B~tqXBgdcQ1mdkN1h5XNfhXLBV0rXE4sGH19AKGTiFM4on8PpXlaV30asF9QfP~83-Y0twycOCDKhSRbTL-L5OuKrtXmUVtSsRzNkQvh6rb-m42p6EeiigyA8HytJM-jB2M8j2Km1I82vrGtf-VNGYnI5Pw39kmc0wEZ-isuSeUNifVsMPXXNxQvJ7aq-TCCg4BXk3-RKOENZCkxlhbEpkzoAUihcLd7vCWS-H9RiV1iPDRhYHO3CAQOKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. (A and B) Contaminating neutrophils within preparations of human eosinophils are leading to [Ca2+]itransients. Spectrofluorometric measurements of [Ca2+]i in Fura-2–loaded unprimed human eosinophils between 100% and 0% purity were performed. All contaminating cells were neutrophils as detected by flow cytometry. (A) Eosinophils contaminated with different percentages of neutrophils were stimulated with 100 ng/mL IL-8 and subsequently received 500 ng/mL eotaxin as a positive control. One representative experiment of five performed is shown. (B) Statistical analysis of [Ca2+]i in human eosinophils contaminated with different amounts of purified human neutrophils (0% up to 100%). Cells were stimulated with 100 ng/mL IL-8 or 100 ng/mL eotaxin as a positive control. The results are presented as the mean ± SEM of five experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.694/4/m_blod40231003ax.jpeg?Expires=1769193500&Signature=fG1Dy-SzkKarD8WMpt~FwTVRDE8--cg8bUII-t8dguZL15dJxsJSL77wflHiZydzL-urTCiq6gqqXbWHoXQWtXt3ScHlHSMs7-vgz7eD023u5MM4Zq3VuF6hwMX~H75jiPevc76YvDK3-5ZsZntmat~ENT7R3z7HDQc3awZT6e-CaIPw5jsDC0qrxXwWLr8YXl46gNsjWHXz1BZDdFdPk9lEUVdmWA1zbtDqXmzNdvk-xqZvCTNKlaSSJYKexeTnADxvmh0iOKITH67bBIPo~FSiW1xwR4NGrtq5~W0ZJFnCJLRMPNkUqEdpD3w8TKn3fRFcOq1KlY-kVwWfeKLw4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. (A and B) Contaminating neutrophils within preparations of human eosinophils are leading to [Ca2+]itransients. Spectrofluorometric measurements of [Ca2+]i in Fura-2–loaded unprimed human eosinophils between 100% and 0% purity were performed. All contaminating cells were neutrophils as detected by flow cytometry. (A) Eosinophils contaminated with different percentages of neutrophils were stimulated with 100 ng/mL IL-8 and subsequently received 500 ng/mL eotaxin as a positive control. One representative experiment of five performed is shown. (B) Statistical analysis of [Ca2+]i in human eosinophils contaminated with different amounts of purified human neutrophils (0% up to 100%). Cells were stimulated with 100 ng/mL IL-8 or 100 ng/mL eotaxin as a positive control. The results are presented as the mean ± SEM of five experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.694/4/m_blod40231003bx.jpeg?Expires=1769193500&Signature=rmuQ1DUXIfWGBqSe3vhNv6m0EB8uN2nVXkMrDnvtD6VyOl3VhGGYd5t9bOk90d~-5mGF6RsZwQuwLWHpRy32bfl1DPmFPVRdY54ChJ5d2TRJ34YR6o3H4WNou8nKxZ9aF2Cx5v87mhRf51WyEKs8k6EU7sDDEAELrRYBxwwYvkOBW6siHH5L3~6~9gn6GHWn4X~fNKGWuNjQb5bcxDMOn4qeqY51MDElte3tt8lJC~0tZ8HJhITwA1hXodQRbe1UAd43OyP~pGvtxT6sLRNuGpypKnVXD4duSfwSelGprGO1ejfZOrVKNECFvrXraIpqn01Jxt7gNWBu2~poZG2heQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal